Neopterin Levels in Periodontitis and after Nonsurgical Periodontal Therapy: Evaluation of Gingival Crevicular Fluid, Oral Fluid, Serum and Urinary Samples—A Case-Control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Dental Examination
2.3. Sample Collection, Preparation, and Analysis
2.4. Statistical Analysis
3. Results
3.1. Description of Study and Control Group
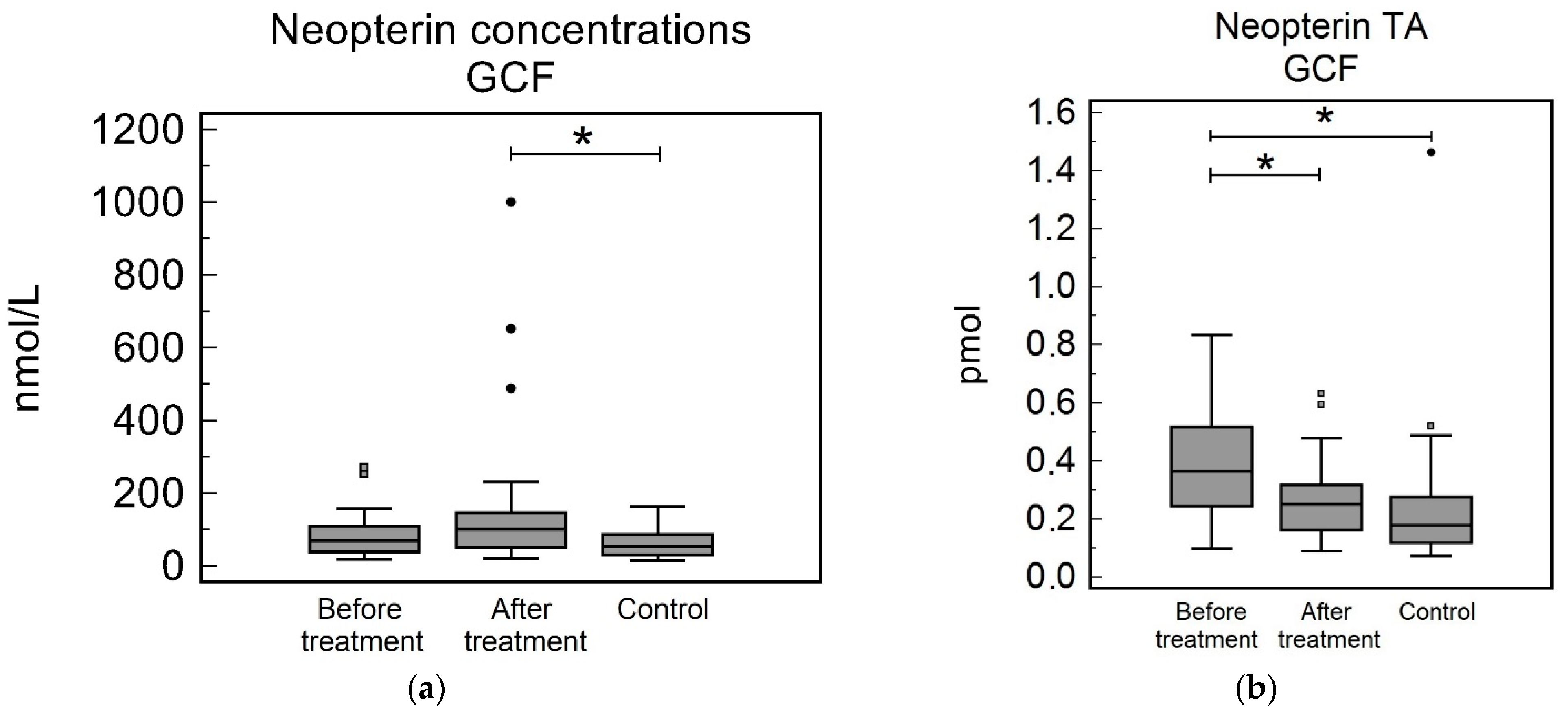
3.2. Gingival Crevicular Fluid
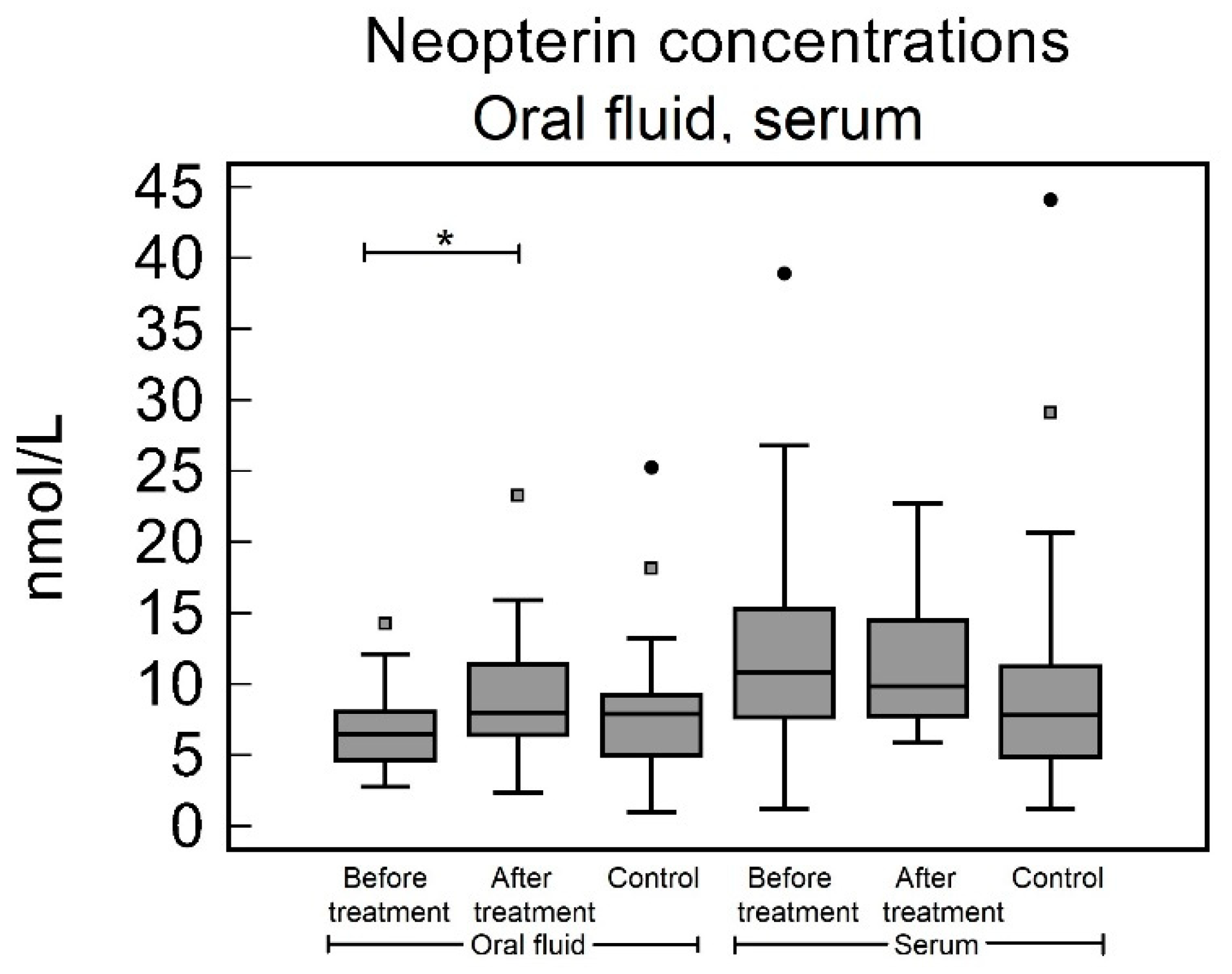
3.3. Oral Fluid
3.4. Serum
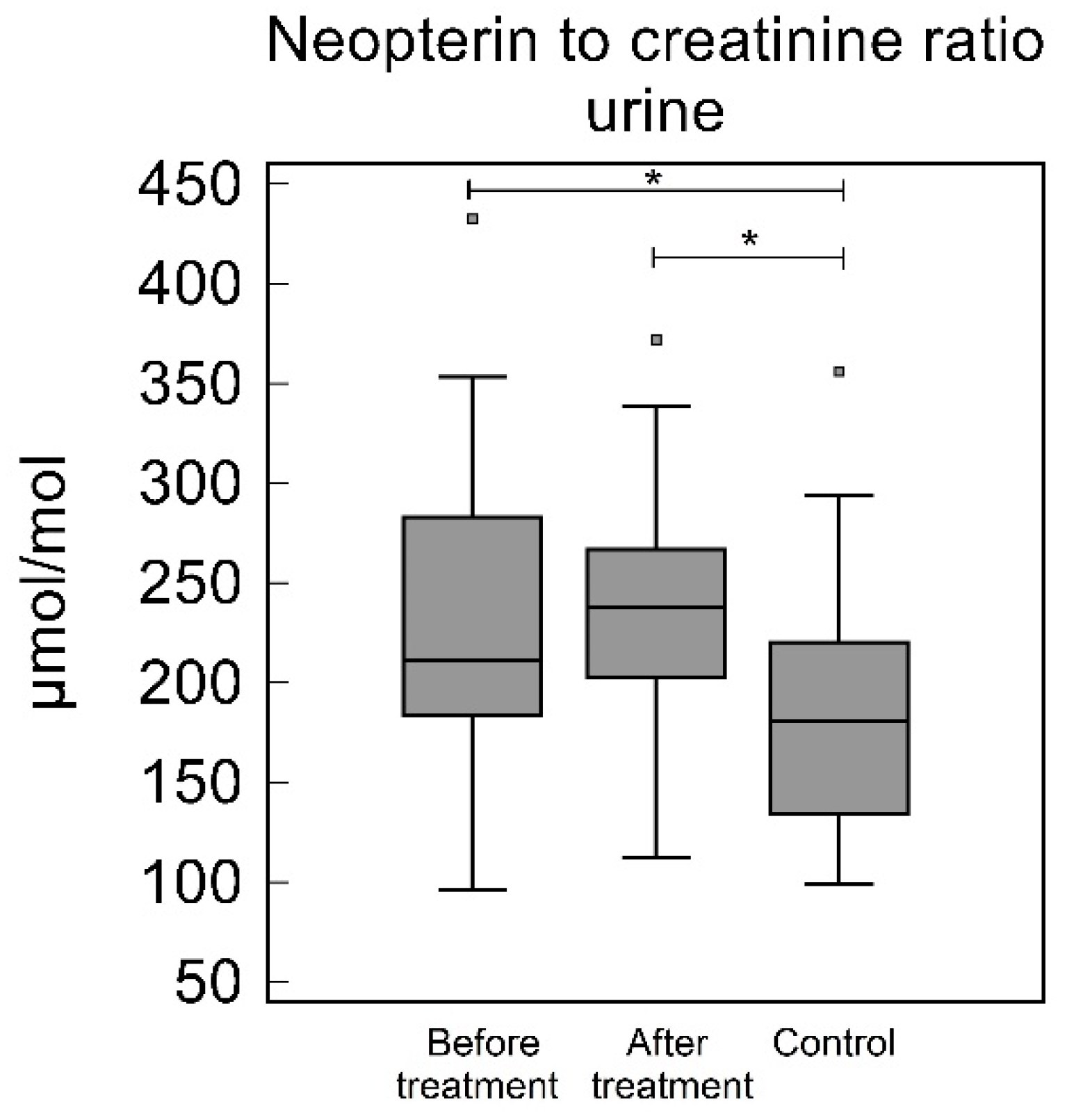
3.5. Urine
3.6. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus Report of Workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions: Classification and Case Definitions for Periodontitis. J. Clin. Periodontol. 2018, 45, S162–S170. [Google Scholar] [CrossRef] [PubMed]
- Meyle, J.; Chapple, I. Molecular Aspects of the Pathogenesis of Periodontitis. Periodontology 2000 2015, 69, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Grossi, S.G.; Zambon, J.J.; Ho, A.W.; Koch, G.; Dunford, R.G.; Machtei, E.E.; Norderyd, O.M.; Genco, R.J. Assessment of Risk for Periodontal Disease. I. Risk Indicators for Attachment Loss. J. Periodontol. 1994, 65, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Bártová, J.; Krátká-Opatrná, Z.; Procházková, J.; Krejsa, O.; Dušková, J.; Mrklas, L.; Tlaskalová, H.; Cukrowská, B. Th1 and Th2 Cytokine Profile in Patients with Early Onset Periodontitis and Their Healthy Siblings. Mediat. Inflamm. 2000, 9, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Sommer, M.E.L.; Dalia, R.A.; Nogueira, A.V.B.; Cirelli, J.A.; Vinolo, M.A.R.; Fachi, J.L.; Oliveira, C.A.; Andrade, T.A.M.; Mendonça, F.A.S.; Santamaria, M.; et al. Immune Response Mediated by Th1/IL-17/Caspase-9 Promotes Evolution of Periodontal Disease. Arch. Oral Biol. 2019, 97, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Ruiz, J.S.; Guerrero-Velázquez, C.; Martínez-Esquivias, F.; Martínez-Pérez, L.A.; Guzmán-Flores, J.M. Innate and Adaptive Immunity of Periodontal Disease. From Etiology to Alveolar Bone Loss. Oral Dis. 2022, 28, 1441–1447. [Google Scholar] [CrossRef]
- Zhou, L.; Bi, C.; Gao, L.; An, Y.; Chen, F.; Chen, F. Macrophage Polarization in Human Gingival Tissue in Response to Periodontal Disease. Oral Dis. 2019, 25, 265–273. [Google Scholar] [CrossRef]
- Ozmeric, N.; Baydar, T.; Bodur, A.; Engin, A.B.; Uraz, A.; Eren, K.; Sahin, G. Level of Neopterin, a Marker of Immune Cell Activation in Gingival Crevicular Fluid, Saliva, and Urine in Patients with Aggressive Periodontitis. J. Periodontol. 2002, 73, 720–725. [Google Scholar] [CrossRef]
- Pradeep, A.R.; Kumar, M.S.; Ramachandraprasad, M.V.; Shikha, C. Gingival Crevicular Fluid Levels of Neopterin in Healthy Subjects and in Patients with Different Periodontal Diseases. J. Periodontol. 2007, 78, 1962–1967. [Google Scholar] [CrossRef]
- Plata-Nazar, K.; Jankowska, A. Clinical Usefulness of Determining the Concentration of Neopterin. Pteridines 2011, 22, 77–89. [Google Scholar] [CrossRef]
- Turgut Cankaya, Z.; Bodur, A.; Tacoy, G.; Erguder, I.; Aktuna, D.; Cengel, A. The Effect of Periodontal Therapy on Neopterin and Vascular Cell Adhesion Molecule-1 Levels in Chronic Periodontitis Patients with and without Acute Myocardial Infarction: A Case-Control Study. J. Appl. Oral Sci. Rev. FOB 2018, 26, e20170199. [Google Scholar] [CrossRef] [PubMed]
- Arjunkumar, R.; Sudhakar, U.; Jayakumar, P.; Arunachalam, L.; Suresh, S.; Virupapuram, P. Comparative Analysis of Gingival Crevicular Fluid Neopterin Levels in Health and Periodontal Disease: A Biochemical Study. Indian J. Dent. Res. Off. Publ. Indian Soc. Dent. Res. 2013, 24, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Bodur, A.; Baydar, T.; Ozmeric, N.; Engin, A.; Uraz, A.; Eren, K.; Sahin, G. Neopterin Profile to Evaluate the Effectiveness of Treatment in Aggressive Periodontitis. Pteridines 2003, 14, 77–81. [Google Scholar] [CrossRef]
- Prasanna, J.S.; Sumadhura, C.; Karunakar, P.; Rohini, N. Comparative Evaluation of Salivary Neopterin Levels and Its Effects to Periodontal Therapy in Pre- and Post-Menopausal Women. J. Menopausal Med. 2017, 23, 32–41. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mahendra, L.; Mahendra, J.; Borra, S.K.; Nagarajan, A. Estimation of Salivary Neopterin in Chronic Periodontitis. Indian J. Dent. Res. Off. Publ. Indian Soc. Dent. Res. 2014, 25, 794–796. [Google Scholar] [CrossRef] [PubMed]
- Vrecko, K.; Staedtler, P.; Mischak, I.; Maresch, L.; Reibnegger, G. Periodontitis and Concentrations of the Cellular Immune Activation Marker Neopterin in Saliva and Urine. Clin. Chim. Acta Int. J. Clin. Chem. 1997, 268, 31–40. [Google Scholar] [CrossRef]
- Prasanna, J.S.; Sumadhura, C.; Karunakar, P.; Rekharani, K.; Himabindu, G.; Manasa, A. Correlative Analysis of Plasma and Urine Neopterin Levels in the Pre- and Post-Menopausal Women with Periodontitis, Following Nonsurgical Periodontal Therapy. J. Indian Soc. Periodontol. 2017, 21, 276–284. [Google Scholar] [CrossRef]
- Ren, J.; Chen, Y.-B.; Zhang, Y.-Y.; Zhou, Q.-B.; Chen, S.; Yang, J.-Y.; Tao, J. Decreased Circulating Neopterin Is Associated with Increased Arterial Elasticity: A Beneficial Role of Periodontal Treatment. Aust. Dent. J. 2016, 61, 76–83. [Google Scholar] [CrossRef][Green Version]
- Löe, H.; Silness, J. Periodontal Disease in Pregnancy I. Prevalence and Severity. Acta Odontol. Scand. 1963, 21, 533–551. [Google Scholar] [CrossRef]
- Silness, J.; Löe, H. Periodontal Disease in Pregnancy II. Correlation Between Oral Hygiene and Periodontal Condition. Acta Odontol. Scand. 1964, 22, 121–135. [Google Scholar] [CrossRef]
- Schmidt, J.; Guder, U.; Kreuz, M.; Löffler, M.; Kiess, W.; Hirsch, C.; Ziebolz, D.; Haak, R. AMMP-8 in Correlation to Caries and Periodontal Condition in Adolescents—Results of the Epidemiologic LIFE Child Study. Clin. Oral Investig. 2018, 22, 449–460. [Google Scholar] [CrossRef]
- Robinson, P.G. Dental Epidemiology. In International Encyclopedia of Public Health; Elsevier: Amsterdam, The Netherlands, 2017; pp. 258–263. ISBN 978-0-12-803708-9. [Google Scholar]
- Wassall, R.R.; Preshaw, P.M. Clinical and Technical Considerations in the Analysis of Gingival Crevicular Fluid. Periodontology 2000 2016, 70, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.G.; Takei, H.; Klokkevold, P.R.; Carranza, F.A. Carranza’s Clinical Periodontology, 12th ed.; Newman, M.G., Takei, H.H., Klokkevold, P.R., Carranza, F.A., Eds.; Elsevier Saunders: St. Louis, MO, USA, 2015; ISBN 978-0-323-22799-5. [Google Scholar]
- Vernerová, A.; Krčmová, L.K.; Heneberk, O.; Radochová, V.; Švec, F. Liquid Chromatography Method with Tandem Mass Spectrometry and Fluorescence Detection for Determination of Inflammatory Biomarkers in Gingival Crevicular Fluid as a Tool for Diagnosis of Periodontal Disease. J. Pharm. Biomed. Anal. 2022, 212, 114644. [Google Scholar] [CrossRef] [PubMed]
- Vernerová, A.; Krčmová, L.K.; Heneberk, O.; Radochová, V.; Strouhal, O.; Kašparovský, A.; Melichar, B.; Švec, F. Chromatographic Method for the Determination of Inflammatory Biomarkers and Uric Acid in Human Saliva. Talanta 2021, 233, 122598. [Google Scholar] [CrossRef] [PubMed]
- Krcmova, L.; Solichova, D.; Melichar, B.; Kasparova, M.; Plisek, J.; Sobotka, L.; Solich, P. Determination of Neopterin, Kynurenine, Tryptophan and Creatinine in Human Serum by High Throuput HPLC. Talanta 2011, 85, 1466–1471. [Google Scholar] [CrossRef] [PubMed]
- Zezulová, M.; Bartoušková, M.; Hlídková, E.; Adam, T.; Krčmová, L.K.; Červinková, B.; Solichová, D.; Zlevorová, M.; Cwiertka, K.; Friedecký, D.; et al. Citrulline as a Biomarker of Gastrointestinal Toxicity in Patients with Rectal Carcinoma Treated with Chemoradiation. Clin. Chem. Lab. Med. CCLM 2016, 54, 305–314. [Google Scholar] [CrossRef]
- Barros, S.P.; Williams, R.; Offenbacher, S.; Morelli, T. Gingival Crevicular Fluid as a Source of Biomarkers for Periodontitis. Periodontology 2000 2016, 70, 53–64. [Google Scholar] [CrossRef]
- Fenol, A.; Swetha, V.R.; Krishnan, S.; Perayil, J.; Vyloppillil, R.; Bhaskar, A.; Shereef, M.; Balakrishnan, B.; Puzhankara, L. Correlation of Salivary Neopterin and Plasma Fibrinogen Levels in Patients with Chronic Periodontitis and/or Type 2 Diabetes Mellitus. Pteridines 2017, 28, 177–183. [Google Scholar] [CrossRef]
- Huang, X.; Yu, T.; Ma, C.; Wang, Y.; Xie, B.; Xuan, D.; Zhang, J. Macrophages Play a Key Role in the Obesity-Induced Periodontal Innate Immune Dysfunction via Nucleotide-Binding Oligomerization Domain-Like Receptor Protein 3 Pathway. J. Periodontol. 2016, 87, 1195–1205. [Google Scholar] [CrossRef]
- Italiani, P.; Boraschi, D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front. Immunol. 2014, 5, 514. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, Y.; Duan, D.; Wang, P.; Xin, Y.; Bai, L.; Liu, Y.; Xu, Y. Enhanced Activity of Macrophage M1/M2 Phenotypes in Periodontitis. Arch. Oral Biol. 2018, 96, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Arango Duque, G.; Descoteaux, A. Macrophage Cytokines: Involvement in Immunity and Infectious Diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the Full Spectrum of Macrophage Activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Gieseg, S.P.; Baxter-Parker, G.; Lindsay, A. Neopterin, Inflammation, and Oxidative Stress: What Could We Be Missing? Antioxidants 2018, 7, 80. [Google Scholar] [CrossRef] [PubMed]
- Murr, C.; Widner, B.; Wirleitner, B.; Fuchs, D. Neopterin as a Marker for Immune System Activation. Curr. Drug Metab. 2002, 3, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Duggan, S.; Rait, C.; Gebicki, J.M.; Gieseg, S.P. Inhibition of Protein Oxidation by the Macrophage-Synthesised Antioxidant 7,8-Dihydroneopterin. Redox Rep. 2001, 6, 188–190. [Google Scholar] [CrossRef]
- Shchepetkina, A.A.; Hock, B.D.; Miller, A.; Kennedy, M.A.; Gieseg, S.P. Effect of 7,8-Dihydroneopterin Mediated CD36 down Regulation and Oxidant Scavenging on Oxidised Low-Density Lipoprotein Induced Cell Death in Human Macrophages. Int. J. Biochem. Cell Biol. 2017, 87, 27–33. [Google Scholar] [CrossRef]
- Griffiths, G.S. Formation, Collection and Significance of Gingival Crevice Fluid: Formation, Collection and Significance of Gingival Crevice Fluid. Periodontology 2000 2003, 31, 32–42. [Google Scholar] [CrossRef]
- Bibi, T.; Khurshid, Z.; Rehman, A.; Imran, E.; Srivastava, K.C.; Shrivastava, D. Gingival Crevicular Fluid (GCF): A Diagnostic Tool for the Detection of Periodontal Health and Diseases. Molecules 2021, 26, 1208. [Google Scholar] [CrossRef]
- Nair, V.; Grover, V.; Arora, S.; Das, G.; Ahmad, I.; Ohri, A.; Sainudeen, S.; Saluja, P.; Saha, A. Comparative Evaluation of Gingival Crevicular Fluid Interleukin-17, 18 and 21 in Different Stages of Periodontal Health and Disease. Medicina 2022, 58, 1042. [Google Scholar] [CrossRef]
- Nijakowski, K.; Ortarzewska, M.; Morawska, A.; Brożek, A.; Nowicki, M.; Formanowicz, D.; Surdacka, A. Bruxism Influence on Volume and Interleukin-1β Concentration of Gingival Crevicular Fluid: A Preliminary Study. Appl. Sci. 2022, 12, 2089. [Google Scholar] [CrossRef]
- Go, H.; Park, T.; Shin, A.; Jung, Y.; Amano, A.; Song, K.; Choi, Y. Validity of a Combination of Periodontal Pathogens and Salivary Biomarkers as Predictors of Periodontitis. J. Periodontal Res. 2022, 57, 1083–1092. [Google Scholar] [CrossRef] [PubMed]

| Study Group before Treatment Median (IQR) | Study Group after Treatment Median (IQR) | Control Group Median (IQR) | Before vs. Control p-Value | Before vs. after p-Value | After vs. Control p-Value | |
|---|---|---|---|---|---|---|
| Age | 50 (47–54) | 50 (47–54) | 46 (44–54) | 0.263 | ||
| Full mouth evaluation | ||||||
| PPD | 3.91 (3.55–4.93) | 1.70 (1.30–1.94) | 1.17 (1.125–1.23) | <0.001 | <0.001 | <0.001 |
| GR | 0.17 (0–0.37 | 0.213 (0.045–1.937) | 0.036 (0.0062–0.77) | 0.053 | 0.015 | <0.001 |
| CAL | 4.35 (3.90–5.01) | 1.86 (1.56–2.51) | 1.20 (0–0.083) | <0.001 | <0.001 | <0.001 |
| PLI | 2.39 (1.02–2.95) | 0.095 (0.052–0.134) | 0.018 (0–0.083) | <0.001 | 0.006 | <0.001 |
| GI | 2.60 (1.82–2.95) | 0.082 (0.048–0.153) | 0.090 (0.060–0.12) | <0.001 | <0.001 | 1.00 |
| BOP % | 100 (95.65–100) | 0.00 (0.00–0.15) | 0.00 (0.00–0.00) | <0.001 | <0.001 | 0.23 |
| Sampled teeth evaluation | ||||||
| PPD | 5.67 (5–6.5) | 2.33 (1.62–2.71) | 1 (1–1.67) | <0.001 | <0.001 | <0.001 |
| GR | 0.17 (0–0.37) | 0.33 (0.00–0.92) | 0.00 (0.00–0.00) | 0.004 | <0.001 | 0.001 |
| CAL | 6 (5.24–7) | 2.67 (1.63–3.21) | 1.17 (1–2.5) | <0.001 | <0.001 | <0.001 |
| PLI | 2.5 (1.33–3) | 0.17 (0.00–0.17) | 0.00 (0.00–0.00) | <0.001 | <0.001 | 0.001 |
| GI | 3 (2.17–3) | 0.083 (0.00–0.167) | 0.00 (0.00–0.33) | <0.001 | <0.001 | 0.035 |
| BOP % | 1 (0.83–1) | 0.00 (0.00–0.00) | 0.00 (0.000.00) | <0.001 | <0.001 | >0.999 |
| DMF index * | 15 (12–19) | 15 (12–20) | 13 (8–16) | 0.17 | 0.65 | 0.13 |
| Study Group before Treatment | Study Group after Treatment | Control Group | Before vs. Control | Before vs. After | After vs. Control | |
|---|---|---|---|---|---|---|
| Sample | Median (IQR) | Median (IQR) | Median (IQR) | p-Value | p-Value | p-Value |
| GCF Conc. [nmol/L] | 69.87 (39.84–108.37) | 101.03 (50.84–146.58) | 54.36 (31.02–84.34) | 0.322 | 0.31 | 0.038 |
| GCF TA [pmol] | 0.36 (0.24–0.51) | 0.25 (0.16–0.32) | 0.18 (0.12–0.27) | 0.001 | 0.024 | 0.11 |
| Oral fluid [nmol/L] | 6.48 (4.69–8.05) | 8.03 (6.43–11.39) | 7.89 (5.00–9.20) | 0.27 | 0.020 | 0.50 |
| Serum [nmol/L] | 10.83 (7.67–15.34) | 9.86 (7.69–14.17) | 7.83 (4.89–11.27) | 0.23 | 0.72 | 0.14 |
| Urine NC [µmol/mol] | 210.96 (183.60–282.91) | 237.87 (202.46–266.80) | 180.59 (133.97–220.22) | 0.020 | 0.36 | 0.0013 |
| GCF vol. [µL] | 5.41 (3.44–8.42) | 3.53 (1.85–4.75) | 3.86 (2.37–5.65) | 0.095 | 0.0062 | 0.23 |
| p-Value | ρ | |
|---|---|---|
| GCF concentration. vs. oral fluid | 0.35 | 0.11 |
| GCF concentration. vs. serum | 0.51 | 0.078 |
| GCF concentration. vs. urinary NC | 0.11 | 0.19 |
| GCF total amount vs. oral fluid | 0.18 | 0.16 |
| GCF total amount vs. serum | 0.83 | 0.025 |
| GCF total amount vs. urinary NC | 0.13 | 0.157 |
| Serum vs. oral fluid | 0.001 | 0.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heneberk, O.; Vernerova, A.; Kujovska Krcmova, L.; Wurfelova, E.; Radochova, V. Neopterin Levels in Periodontitis and after Nonsurgical Periodontal Therapy: Evaluation of Gingival Crevicular Fluid, Oral Fluid, Serum and Urinary Samples—A Case-Control Study. Biomedicines 2022, 10, 3200. https://doi.org/10.3390/biomedicines10123200
Heneberk O, Vernerova A, Kujovska Krcmova L, Wurfelova E, Radochova V. Neopterin Levels in Periodontitis and after Nonsurgical Periodontal Therapy: Evaluation of Gingival Crevicular Fluid, Oral Fluid, Serum and Urinary Samples—A Case-Control Study. Biomedicines. 2022; 10(12):3200. https://doi.org/10.3390/biomedicines10123200
Chicago/Turabian StyleHeneberk, Ondrej, Andrea Vernerova, Lenka Kujovska Krcmova, Eliska Wurfelova, and Vladimira Radochova. 2022. "Neopterin Levels in Periodontitis and after Nonsurgical Periodontal Therapy: Evaluation of Gingival Crevicular Fluid, Oral Fluid, Serum and Urinary Samples—A Case-Control Study" Biomedicines 10, no. 12: 3200. https://doi.org/10.3390/biomedicines10123200
APA StyleHeneberk, O., Vernerova, A., Kujovska Krcmova, L., Wurfelova, E., & Radochova, V. (2022). Neopterin Levels in Periodontitis and after Nonsurgical Periodontal Therapy: Evaluation of Gingival Crevicular Fluid, Oral Fluid, Serum and Urinary Samples—A Case-Control Study. Biomedicines, 10(12), 3200. https://doi.org/10.3390/biomedicines10123200






