Serotonin Receptor Gene Polymorphisms Are Associated with Cerebrospinal Fluid, Genetic, and Neuropsychological Biomarkers of Alzheimer’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Neuropsychological Testing
2.3. Analysis of CSF Biomarkers
2.4. Determination of Polymorphisms
2.5. Statistical Analysis
3. Results
3.1. Polymorphisms in 5HT Receptor Genes and CSF Biomarkers
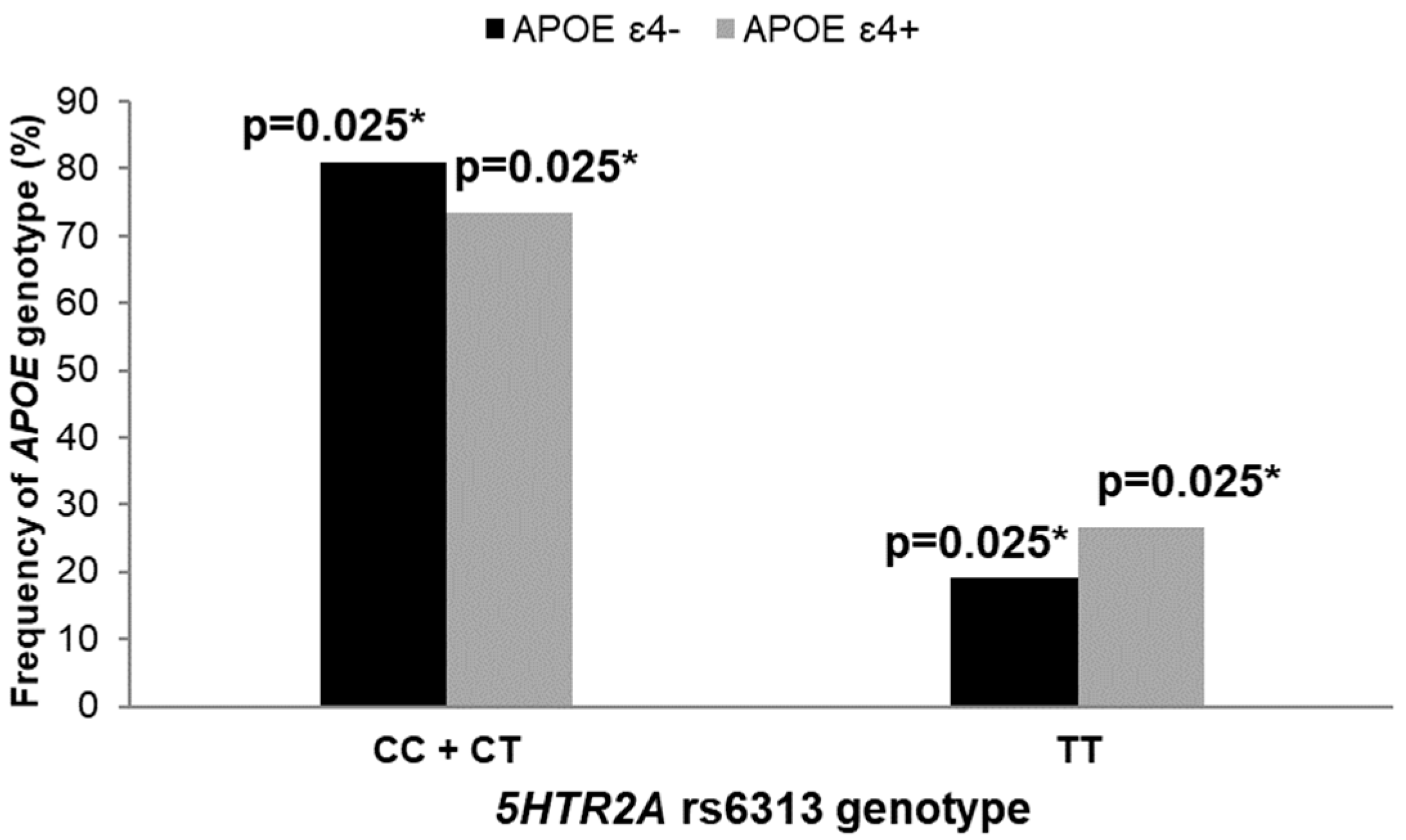
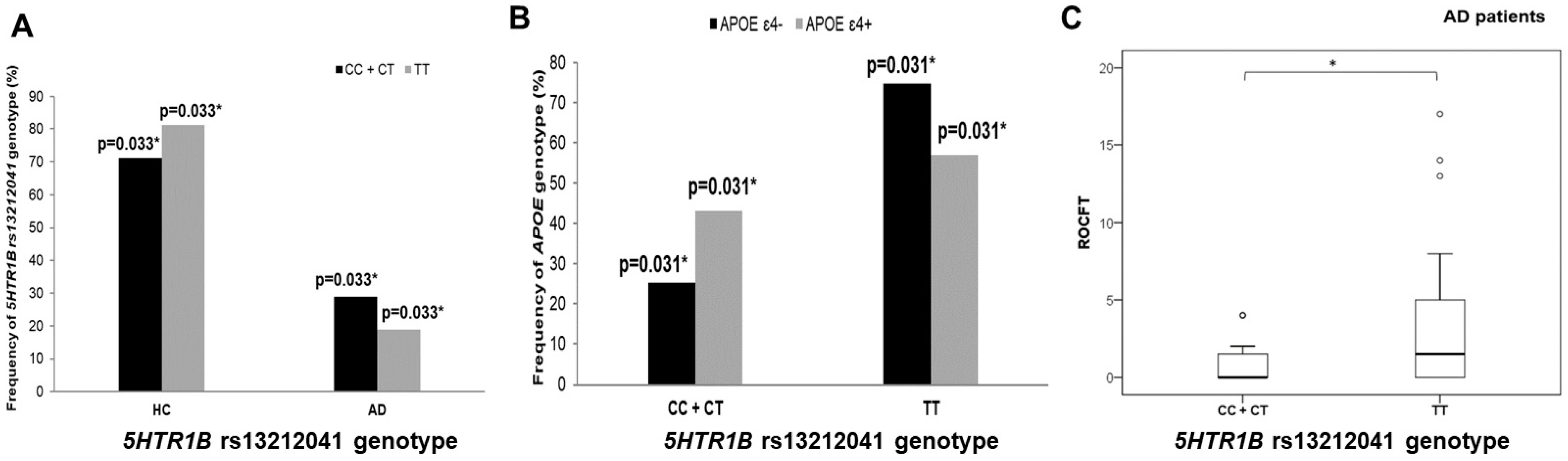
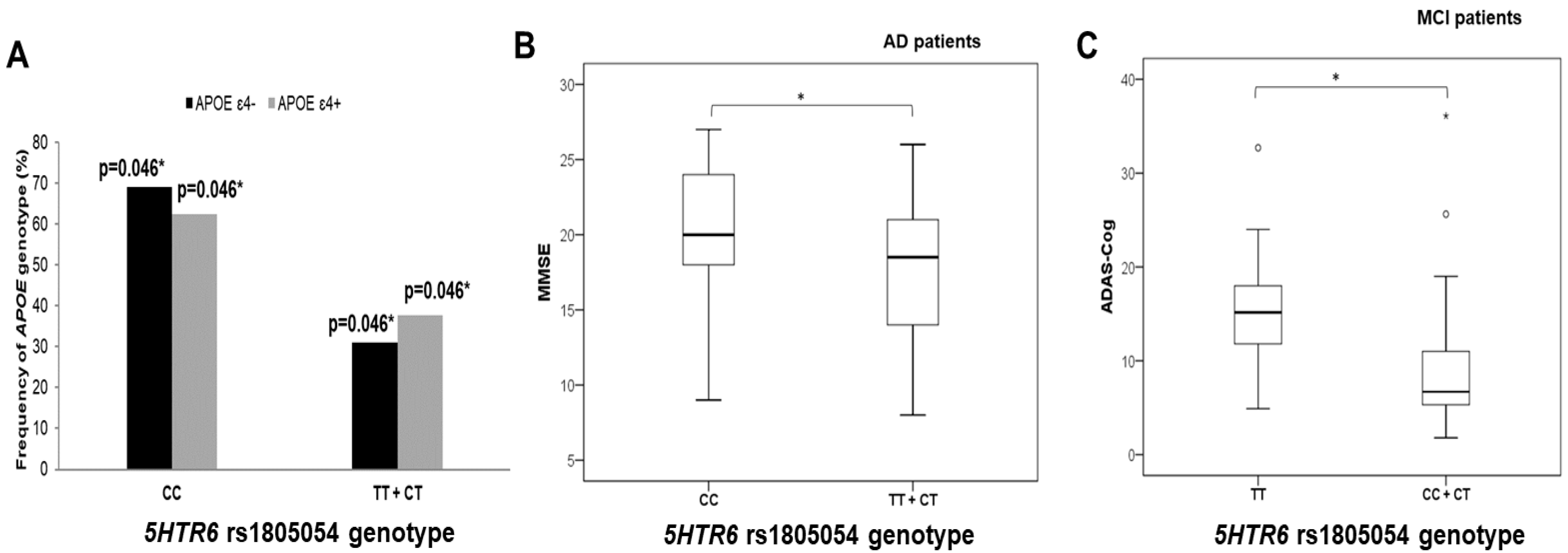
3.2. Polymorphisms in 5HT Receptor Genes, APOE Genotype, and AD Diagnosis
3.3. Polymorphisms in 5HT Receptors, Genes, and Neuropsychological Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Šimić, G.; Stanić, G.; Mladinov, M.; Jovanov-Milošević, N.; Kostović, I.; Hof, P. Does Alzheimer’s disease begin in the brainstem? Annotation. Neuropathol. Appl. Neurobiol. 2009, 35, 532–554. [Google Scholar] [CrossRef] [PubMed]
- Trillo, L.; Das, D.; Hsieh, W.; Medina, B.; Moghadam, S.; Lin, B.; Dang, V.; Sanchez, M.M.; De Miguel, Z.; Ashford, J.W.; et al. Ascending monoaminergic systems alterations in Alzheimer’s disease. Translating basic science into clinical care. Neurosci. Biobehav. Rev. 2013, 37, 1363–1379. [Google Scholar] [CrossRef] [PubMed]
- Babić Leko, M.; Hof, P.R.; Šimić, G. Alterations and interactions of subcortical modulatory systems in Alzheimer’s disease. Prog. Brain Res. 2021, 261, 379–421. [Google Scholar] [CrossRef] [PubMed]
- Šimić, G.; Babić Leko, M.; Wray, S.; Harrington, C.R.; Delalle, I.; Jovanov-Milošević, N.; Bažadona, D.; Buée, L.; de Silva, R.; Di Giovanni, G.; et al. Monoaminergic neuropathology in Alzheimer’s disease. Prog. Neurobiol. 2017, 151, 101–138. [Google Scholar] [CrossRef]
- Takahashi, H.; Nakashima, S.; Ohama, E.; Takeda, S.; Ikuta, F. Distribution of serotonin-containing cell bodies in the brainstem of the human fetus determined with immunohistochemistry using antiserotonin serum. Brain Dev. 1986, 8, 355–365. [Google Scholar] [CrossRef]
- Halliday, G.M.; Törk, I. Serotonin-like immunoreactive cells and fibres in the rat ventromedial mesencephalic tegmentum. Brain Res. Bull. 1989, 22, 725–735. [Google Scholar] [CrossRef]
- Baker, K.; Halliday, G.; Törk, I. Cytoarchitecture of the human dorsal raphe nucleus. J. Comp. Neurol. 1990, 301, 147–161. [Google Scholar] [CrossRef]
- Nieuwenhuys, R.; Voogd, J.; van Huijzen, C. The Human Central Nervous System, 4th ed.; Springer: New York, NY, USA, 2008. [Google Scholar]
- Seyedabadi, M.; Fakhfouri, G.; Ramezani, V.; Mehr, S.E.; Rahimian, R. The role of serotonin in memory: Interactions with neurotransmitters and downstream signaling. Exp. Brain Res. 2014, 232, 723–738. [Google Scholar] [CrossRef]
- Darmon, M.; Al Awabdh, S.; Emerit, M.-B.; Masson, J. Insights into serotonin receptor trafficking: Cell membrane targeting and internalization. Prog. Mol. Biol. Transl. Sci. 2015, 132, 97–126. [Google Scholar] [CrossRef]
- Curcio, C.A.; Kemper, T. Nucleus raphe dorsalis in dementia of the Alzheimer type: Neurofibrillary changes and neuronal packing density. J. Neuropathol. Exp. Neurol. 1984, 43, 359–368. [Google Scholar] [CrossRef]
- Halliday, G.M.; McCann, H.L.; Pamphlett, R.; Brooks, W.S.; Creasey, H.; McCusker, E.; Cotton, R.G.; Broe, G.A.; Harper, C.G. Brain stem serotonin-synthesizing neurons in Alzheimer’s disease: A clinicopathological correlation. Acta Neuropathol. 1992, 84, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.P.L.-H.; Eastwood, S.L.; Hope, T.; McDonald, B.; Francis, P.T.; Esiri, M.M. Immunocytochemical study of the dorsal and median raphe nuclei in patients with Alzheimer’s disease prospectively assessed for behavioural changes. Neuropathol. Appl. Neurobiol. 2000, 26, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Nazarali, A.J.; Reynolds, G.P. Monoamine neurotransmitters and their metabolites in brain regions in Alzheimer’s disease: A postmortem study. Cell. Mol. Neurobiol. 1992, 12, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alloza, M.; Gil-Bea, F.J.; Diez-Ariza, M.; Chen, C.P.L.-H.; Francis, P.T.; Lasheras, B.; Ramirez, M.J. Cholinergic–serotonergic imbalance contributes to cognitive and behavioral symptoms in Alzheimer’s disease. Neuropsychologia 2005, 43, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Grinberg, L.T.; Rüb, U.; Ferretti, R.E.L.; Nitrini, R.; Farfel, J.M.; Polichiso, L.; Gierga, K.; Jacob-Filho, W.; Heinsen, H. The dorsal raphe nucleus shows phospho-tau neurofibrillary changes before the transentorhinal region in Alzheimer’s disease. A precocious onset? Neuropathol. Appl. Neurobiol. 2009, 35, 406–416. [Google Scholar] [CrossRef]
- Garcia-Alloza, M.; Hirst, W.D.; Chen, C.P.L.-H.; Lasheras, B.; Francis, P.T.; Ramírez, M.J. Differential involvement of 5-HT1B/1D and 5-HT6 receptors in cognitive and non-cognitive symptoms in Alzheimer’s disease. Neuropsychopharmacology 2004, 29, 410–416. [Google Scholar] [CrossRef]
- Truchot, L.; Costes, N.; Zimmer, L.; Laurent, B.; Le Bars, D.; Thomas-Antérion, C.; Mercier, B.; Hermier, M.; Vighetto, A.; Krolak-Salmon, P. A distinct [18F]MPPF PET profile in amnestic mild cognitive impairment compared to mild Alzheimer’s disease. Neuroimage 2008, 40, 1251–1256. [Google Scholar] [CrossRef]
- Marner, L.; Frokjaer, V.G.; Kalbitzer, J.; Lehel, S.; Madsen, K.; Baaré, W.F.C.; Knudsen, G.M.; Hasselbalch, S.G. Loss of serotonin 2A receptors exceeds loss of serotonergic projections in early Alzheimer’s disease: A combined [11C]DASB and [18F]altanserin-PET study. Neurobiol. Aging 2012, 33, 479–487. [Google Scholar] [CrossRef]
- Holmes, C.; Arranz, M.; Powell, J.; Collier, D.; Lovestone, S. 5-HT2A and 5-HT2C receptor polymorphisms and psychopathology in late onset Alzheimer’s disease. Hum. Mol. Genet. 1998, 7, 1507–1509. [Google Scholar] [CrossRef]
- Holmes, C.; Arranz, M.; Collier, D.; Powell, J.; Lovestone, S. Depression in Alzheimer’s disease: The effect of serotonin receptor gene variation. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2003, 119B, 40–43. [Google Scholar] [CrossRef]
- Pritchard, A.L.; Harris, J.; Pritchard, C.W.; Coates, J.; Haque, S.; Holder, R.; Bentham, P.; Lendon, C.L. Role of 5HT 2A and 5HT 2C polymorphisms in behavioural and psychological symptoms of Alzheimer’s disease. Neurobiol. Aging 2008, 29, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Assal, F.; Alarcón, M.; Solomon, E.C.; Masterman, D.; Geschwind, D.H.; Cummings, J.L. Association of the serotonin transporter and receptor gene polymorphisms in neuropsychiatric symptoms in Alzheimer’s disease. Arch. Neurol. 2004, 61, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- Lam, L.C.W.; Tang, N.L.S.; Ma, S.L.; Zhang, W.; Chiu, H.F.K. 5-HT2A T102C receptor polymorphism and neuropsychiatric symptoms in Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2004, 19, 523–526. [Google Scholar] [CrossRef]
- Angelucci, F.; Bernardini, S.; Gravina, P.; Bellincampi, L.; Trequattrini, A.; Di Iulio, F.; Vanni, D.; Federici, G.; Caltagirone, C.; Bossù, P.; et al. Delusion symptoms and response to antipsychotic treatment are associated with the 5-HT2A receptor polymorphism (102T/C) in Alzheimer’s disease: A 3-year follow-up longitudinal study. J. Alzheimers. Dis. 2009, 17, 203–211. [Google Scholar] [CrossRef]
- Tang, L.; Wang, Y.; Chen, Y.; Chen, L.; Zheng, S.; Bao, M.; Xiang, J.; Luo, H.; Li, J.; Li, Y. The association between 5HT2A T102C and behavioral and psychological symptoms of dementia in Alzheimer’s disease: A meta-analysis. Biomed Res. Int. 2017, 2017, 5320135. [Google Scholar] [CrossRef] [PubMed]
- Babić Leko, M.; Willumsen, N.; Nikolac Perković, M.; Klepac, N.; Borovečki, F.; Hof, P.R.; Sonicki, Z.; Pivac, N.; de Silva, R.; Šimić, G. Association of MAPT haplotype-tagging polymorphisms with cerebrospinal fluid biomarkers of Alzheimer’s disease: A preliminary study in a Croatian cohort. Brain Behav. 2018, 8, e01128. [Google Scholar] [CrossRef] [PubMed]
- Boban, M.; Malojčić, B.; Mimica, N.; Vuković, S.; Zrilić, I.; Hof, P.R.; Šimić, G. The reliability and validity of the Mini-Mental State Examination in the elderly Croatian population. Dement. Geriatr. Cogn. Disord. 2012, 33, 385–392. [Google Scholar] [CrossRef]
- Grimmer, T.; Riemenschneider, M.; Förstl, H.; Henriksen, G.; Klunk, W.E.; Mathis, C.A.; Shiga, T.; Wester, H.-J.; Kurz, A.; Drzezga, A. Beta amyloid in Alzheimer’s disease: Increased deposition in brain is reflected in reduced concentration in cerebrospinal fluid. Biol. Psychiatry 2009, 65, 927–934. [Google Scholar] [CrossRef]
- Bürger, K.; Ewers, M.; Pirttila, T.; Zinkowski, R.; Alafuzoff, I.; Teipel, S.J.; DeBernardis, J.; Kerkman, D.; McCulloch, C.; Soininen, H.; et al. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer’s disease. Brain 2006, 129, 3035–3041. [Google Scholar] [CrossRef]
- Babić Leko, M.; Borovečki, F.; Dejanović, N.; Hof, P.R.; Šimić, G. Predictive value of cerebrospinal fluid visinin-like protein-1 levels for Alzheimer’s disease early detection and differential diagnosis in patients with mild cognitive impairment. J. Alzheimers Dis. 2016, 50, 765–778. [Google Scholar] [CrossRef]
- Babić Leko, M.; Krbot Skorić, M.; Klepac, N.; Borovečki, F.; Langer Horvat, L.; Vogrinc, Ž.; Sonicki, Z.; Hof, P.R.; Šimić, G. Event-related potentials improve the efficiency of cerebrospinal fluid biomarkers for differential diagnosis of Alzheimer’s disease. Curr. Alzheimer Res. 2018, 15, 1244–1260. [Google Scholar] [CrossRef] [PubMed]
- Rudan, I.; Marušić, A.; Janković, S.; Rotim, K.; Boban, M.; Lauc, G.; Grković, I.; Dogaš, Z.; Zemunik, T.; Vatavuk, Z.; et al. “10 001 Dalmatians:” Croatia launches its national biobank. Croat. Med. J. 2009, 50, 4–6. [Google Scholar] [CrossRef] [PubMed]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Smith, G.E.; Waring, S.C.; Ivnik, R.J.; Tangalos, E.G.; Kokmen, E. Mild cognitive impairment: Clinical characterization and outcome. Arch. Neurol. 1999, 56, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Miller, S.A.; Dykes, D.D.; Polesky, H.F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988, 16, 1215. [Google Scholar] [CrossRef]
- Borroni, B.; Costanzi, C.; Padovani, A. Genetic susceptibility to behavioral and psychological symptoms in Alzheimer’s disease. Curr. Alzheimer Res. 2010, 7, 158–164. [Google Scholar] [CrossRef]
- Martorana, A.; Di Lorenzo, F.; Esposito, Z.; Lo Giudice, T.; Bernardi, G.; Caltagirone, C.; Koch, G. Dopamine D2-agonist Rotigotine effects on cortical excitability and central cholinergic transmission in Alzheimer’s disease patients. Neuropharmacology 2013, 64, 108–113. [Google Scholar] [CrossRef]
- Stefani, A.; Olivola, E.; Liguori, C.; Hainsworth, A.H.; Saviozzi, V.; Angileri, G.; D’Angelo, V.; Galati, S.; Pierantozzi, M. Catecholamine-based treatment in AD patients: Expectations and delusions. Front. Aging Neurosci. 2015, 7, 67. [Google Scholar] [CrossRef]
- Kepe, V.; Barrio, J.R.; Huang, S.-C.; Ercoli, L.; Siddarth, P.; Shoghi-Jadid, K.; Cole, G.M.; Satyamurthy, N.; Cummings, J.L.; Small, G.W.; et al. Serotonin 1A receptors in the living brain of Alzheimer’s disease patients. Proc. Natl. Acad. Sci. USA 2006, 103, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.K.P.; Tsang, S.W.Y.; Francis, P.T.; Esiri, M.M.; Keene, J.; Hope, T.; Chen, C.P.L.H. Reduced serotonin 5-HT1A receptor binding in the temporal cortex correlates with aggressive behavior in Alzheimer’s disease. Brain Res. 2003, 974, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Hu, Y. Activation of 5-HT4 receptors inhibits secretion of β-amyloid peptides and increases neuronal survival. Exp. Neurol. 2007, 203, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.R.; Wallace, C.E.; Tripoli, D.L.; Sheline, Y.I.; Cirrito, J.R. Redundant Gs-coupled serotonin receptors regulate amyloid-β metabolism in vivo. Mol. Neurodegener. 2016, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Baranger, K.; Giannoni, P.; Girard, S.D.; Girot, S.; Gaven, F.; Stephan, D.; Migliorati, M.; Khrestchatisky, M.; Bockaert, J.; Marchetti-Gauthier, E.; et al. Chronic treatments with a 5-HT 4 receptor agonist decrease amyloid pathology in the entorhinal cortex and learning and memory deficits in the 5xFAD mouse model of Alzheimer’s disease. Neuropharmacology 2017, 126, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Tesseur, I.; Pimenova, A.A.; Lo, A.C.; Ciesielska, M.; Lichtenthaler, S.F.; De Maeyer, J.H.; Schuurkes, J.A.J.; D’Hooge, R.; De Strooper, B. Chronic 5-HT4 receptor activation decreases Aβ production and deposition in hAPP/PS1 mice. Neurobiol. Aging 2013, 34, 1779–1789. [Google Scholar] [CrossRef]
- Christensen, D.Z.; Kraus, S.L.; Flohr, A.; Cotel, M.-C.; Wirths, O.; Bayer, T.A. Transient intraneuronal Aβ rather than extracellular plaque pathology correlates with neuron loss in the frontal cortex of APP/PS1KI mice. Acta Neuropathol. 2008, 116, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Holm, P.; Ettrup, A.; Klein, A.B.; Santini, M.A.; El-Sayed, M.; Elvang, A.B.; Stensbøl, T.B.; Mikkelsen, J.D.; Knudsen, G.M.; Aznar, S. Plaque deposition dependent decrease in 5-HT2A serotonin receptor in AβPPswe/PS1dE9 amyloid overexpressing mice. J. Alzheimers Dis. 2010, 20, 1201–1213. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Wang, D.-H.; Watterson, S.; McClean, P.L.; Behera, C.K.; Sharp, T.; Wong-Lin, K. Opportunities for multiscale computational modelling of serotonergic drug effects in Alzheimer’s disease. Neuropharmacology 2020, 174, 108118. [Google Scholar] [CrossRef]
- Polter, A.M.; Li, X. Glycogen synthase kinase-3 is an intermediate modulator of serotonin neurotransmission. Front. Mol. Neurosci. 2011, 4, 31. [Google Scholar] [CrossRef]
- Shinohara, M.; Shinohara, M.; Zhao, J.; Fu, Y.; Liu, C.C.; Kanekiyo, T.; Bu, G. 5-HT3 antagonist ondansetron increases apoE secretion by modulating the LXR-ABCA1 pathway. Int. J. Mol. Sci. 2019, 20, 1488. [Google Scholar] [CrossRef] [PubMed]
- Chhibber, A.; Zhao, L. ERβ and ApoE isoforms interact to regulate BDNF–5-HT2A signaling and synaptic function in the female brain. Alzheimers. Res. Ther. 2017, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- Bundo, M.; Iwamoto, K.; Yamada, K.; Yoshikawa, T.; Kato, T. Mutation screening and assessment of the effect of genetic variations on expression and RNA editing of serotonin receptor 2C in the human brain. Psychiatry Clin. Neurosci. 2010, 64, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Lonsdale, J.; Thomas, J.; Salvatore, M.; Phillips, R.; Lo, E.; Shad, S.; Hasz, R.; Walters, G.; Garcia, F.; Young, N.; et al. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 2013, 45, 580–585. [Google Scholar] [CrossRef]
- Buckland, P.R.; Hoogendoorn, B.; Guy, C.A.; Smith, S.K.; Coleman, S.L.; O’Donovan, M.C. Low gene expression conferred by association of an allele of the 5-HT2C receptor gene with antipsychotic-induced weight gain. Am. J. Psychiatry 2005, 162, 613–615. [Google Scholar] [CrossRef]
- Nitsch, R.M.; Deng, M.; Growdon, J.H.; Wurtman, R.J. Serotonin 5-HT2a and 5-HT2c receptors stimulate amyloid precursor protein ectodomain secretion. J. Biol. Chem. 1996, 271, 4188–4194. [Google Scholar] [CrossRef]
- Arjona, A.A.; Pooler, A.M.; Lee, R.K.; Wurtman, R.J. Effect of a 5-HT2C serotonin agonist, dexnorfenfluramine, on amyloid precursor protein metabolism in guinea pigs. Brain Res. 2002, 951, 135–140. [Google Scholar] [CrossRef]
- Orlacchio, A.; Kawarai, T.; Paciotti, E.; Stefani, A.; Orlacchio, A.; Sorbi, S.; St George-Hyslop, P.; Bernardi, G. Association study of the 5-hydroxytryptamine6 receptor gene in Alzheimer’s disease. Neurosci. Lett. 2002, 325, 13–16. [Google Scholar] [CrossRef]
- Tsai, S.; Liu, H.; Liu, T.; Wang, Y.; Hong, C. Association analysis of the 5-HT6 receptor polymorphism C267T in Alzheimer’s disease. Neurosci. Lett. 1999, 276, 138–139. [Google Scholar] [CrossRef]
- Kan, R.; Wang, B.; Zhang, C.; Yang, Z.; Ji, S.; Lu, Z.; Zheng, C.; Jin, F.; Wang, L. Association of the HTR6 polymorphism C267T with late-onset Alzheimer’s disease in Chinese. Neurosci. Lett. 2004, 372, 27–29. [Google Scholar] [CrossRef]
- Thome, J.; Retz, W.; Baader, M.; Pesold, B.; Hu, M.; Cowen, M.; Durany, N.; Adler, G.; Henn, F.; Rösler, M. Association analysis of HTR6 and HTR2A polymorphisms in sporadic Alzheimer’s disease. J. Neural Transm. 2001, 108, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Alvarez, M.; Galdos, L.; Fernández-Martínez, M.; Gómez-Busto, F.; García-Centeno, V.; Arias-Arias, C.; Sánchez-Salazar, C.; Rodríguez-Martínez, A.B.; Zarranz, J.J.; de Pancorbo, M.M. 5-Hydroxytryptamine 6 receptor (5-HT6) receptor and apolipoprotein E (ApoE) polymorphisms in patients with Alzheimer’s disease in the Basque Country. Neurosci. Lett. 2003, 339, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Khoury, R.; Grysman, N.; Gold, J.; Patel, K.; Grossberg, G.T. The role of 5 HT6-receptor antagonists in Alzheimer’s disease: An update. Expert Opin. Investig. Drugs 2018, 27, 523–533. [Google Scholar] [CrossRef] [PubMed]
- de Jong, I.E.M.; Mørk, A. Antagonism of the 5-HT6 receptor—Preclinical rationale for the treatment of Alzheimer’s disease. Neuropharmacology 2017, 125, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.P.; Covault, J.; Conner, T.S.; Tennen, H.; Kranzler, H.R.; Furneaux, H.M. A common polymorphism in serotonin receptor 1B mRNA moderates regulation by miR-96 and associates with aggressive human behaviors. Mol. Psychiatry 2009, 14, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Bortolato, M.; Pivac, N.; Mück Šeler, D.; Nikolac Perković, M.; Pessia, M.; Di Giovanni, G. The role of the serotonergic system at the interface of aggression and suicide. Neuroscience 2013, 236, 160–185. [Google Scholar] [CrossRef]
- Li, L.; Yang, Y.; Zhang, Q.; Wang, J.; Jiang, J. Use of deep-learning genomics to discriminate healthy individuals from those with Alzheimer’s disease or mild cognitive impairment. Behav. Neurol. 2021, 2021, 3359103. [Google Scholar] [CrossRef]
- Micheli, D.; Bonvicini, C.; Rocchi, A.; Ceravolo, R.; Mancuso, M.; Tognoni, G.; Gennarelli, M.; Siciliano, G.; Murri, L. No evidence for allelic association of serotonin 2A receptor and transporter gene polymorphisms with depression in Alzheimer disease. J. Alzheimers. Dis. 2006, 10, 371–378. [Google Scholar] [CrossRef]
- Fehér, Á.; Juhász, A.; László, A.; Pákáski, M.; Kálmán, J.; Janka, Z. Serotonin transporter and serotonin receptor 2A gene polymorphisms in Alzheimer’s disease. Neurosci. Lett. 2013, 534, 233–236. [Google Scholar] [CrossRef]
- Craig, D.; Donnelly, C.; Hart, D.; Carson, R.; Passmore, P. Analysis of the 5HT-2A T102C receptor polymorphism and psychotic symptoms in Alzheimer’s disease. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2007, 144B, 126–128. [Google Scholar] [CrossRef]
- Wilkosz, P.A.; Kodavali, C.; Weamer, E.A.; Miyahara, S.; Lopez, O.L.; Nimgaonkar, V.L.; DeKosky, S.T.; Sweet, R.A. Prediction of psychosis onset in Alzheimer disease: The role of depression symptom severity and the HTR2A T102C polymorphism. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2007, 144B, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Damoiseaux, J.S.; Seeley, W.W.; Zhou, J.; Shirer, W.R.; Coppola, G.; Karydas, A.; Rosen, H.J.; Miller, B.L.; Kramer, J.H.; Greicius, M.D. Gender modulates the APOE ε4 eEffect in healthy older adults: Convergent evidence from functional brain connectivity and spinal fluid tau levels. J. Neurosci. 2012, 32, 8254–8262. [Google Scholar] [CrossRef] [PubMed]
- Perry, L.A.M.; Goldstein-Piekarski, A.N.; Williams, L.M. Sex differences modulating serotonergic polymorphisms implicated in the mechanistic pathways of risk for depression and related disorders: A mini-review: Sex Modulation of Genes in Depression. J. Neurosci. Res. 2017, 95, 737–762. [Google Scholar] [CrossRef] [PubMed]
- Payami, H.; Zareparsi, S.; Montee, K.R.; Sexton, G.J.; Kaye, J.A.; Bird, T.D.; Yu, C.E.; Wijsman, E.M.; Heston, L.L.; Litt, M.; et al. Gender difference in apolipoprotein E-associated risk for familial Alzheimer disease: A possible clue to the higher incidence of Alzheimer disease in women. Am. J. Hum. Genet. 1996, 58, 803–811. [Google Scholar] [PubMed]
- Mortensen, E.L.; Høgh, P. A gender difference in the association between APOE genotype and age-related cognitive decline. Neurology 2001, 57, 89–95. [Google Scholar] [CrossRef]
- Sampedro, F.; Vilaplana, E.; de Leon, M.J.; Alcolea, D.; Pegueroles, J.; Montal, V.; Carmona-Iragui, M.; Sala, I.; Sánchez-Saudinos, M.B.; Antón-Aguirre, S.; et al. APOE-by-sex interactions on brain structure and metabolism in healthy elderly controls. Oncotarget 2015, 6, 26663–26674. [Google Scholar] [CrossRef]
- Cacciottolo, M.; Christensen, A.; Moser, A.; Liu, J.; Pike, C.J.; Smith, C.; LaDu, M.J.; Sullivan, P.M.; Morgan, T.E.; Dolzhenko, E.; et al. The APOE4 allele shows opposite sex bias in microbleeds and Alzheimer’s disease of humans and mice. Neurobiol. Aging 2016, 37, 47–57. [Google Scholar] [CrossRef]
- Molina-Guzman, G.; González-Castro, T.B.; Hernández Díaz, Y.; Tovilla-Zárate, C.A.; Juárez-Rojop, I.E.; Guzmán-Priego, C.G.; Genis, A.; Pool García, S.; López-Narvaez, M.L.; Rodriguez-Perez, J.M. Gender differences in the association between HTR2C gene variants and suicidal behavior in a Mexican population: A case & ndash; control study. Neuropsychiatr. Dis. Treat. 2017, 13, 559–566. [Google Scholar] [CrossRef][Green Version]
- Xia, X.; Ding, M.; Xuan, J.F.; Xing, J.X.; Pang, H.; Wang, B.J.; Yao, J. Polymorphisms in the human serotonin receptor 1B (HTR1B) gene are associated with schizophrenia: A case control study. BMC Psychiatry 2018, 18, 303. [Google Scholar] [CrossRef]

| AD | MCI | HC | ||
|---|---|---|---|---|
| Measured biomarkers | CSF | + | + | - |
| Genetic | + | + | + | |
| Neuropsychological | + | + | − | |
| n | 115 | 53 | 2701 | |
| Age | Median | 73 | 70 | 55 |
| (25–75th percentile) | (67–77) | (60–75) | (43–66) | |
| Sex | F/M | 62/53 | 27/26 | 1714/987 |
| MMSE | Mean ± SD | 19.6 ± 5.2 | 25.1 ± 3 | − |
| Aβ1–42 (pg/mL) | Mean ± SD | 536.9 ± 296.9 | 723.4 ± 371.9 | − |
| T-tau (pg/mL) | 520.0 ± 394.4 | 246.4 ± 158.0 | − | |
| p-tau181 (pg/mL) | 80.0 ± 47.8 | 57.6 ± 30.9 | − | |
| p-tau199 (pg/mL) | 4.4 ± 3.5 | 3.4 ± 2.4 | − | |
| p-tau231 (U/mL) | 3.9 ± 5.5 | 1.8 ± 3.2 | − | |
| VILIP-1 (pg/mL) | 138.3 ± 88.5 | 94.9 ± 78.1 | − | |
| AD | MCI | HC | ||
|---|---|---|---|---|
| APOE | ε2ε2 | 10 | ||
| ε3ε2 | 9 | 1 | 252 | |
| ε3ε3 | 58 | 36 | 1966 | |
| ε4ε3 | 36 | 14 | 421 | |
| ε4ε4 | 7 | 2 | 28 | |
| ε4ε2 | 5 | 24 | ||
| 5HTR2C rs3813929 (−759C/T) | CC | 79 | 37 | |
| CT | 24 | 12 | − | |
| TT | 12 | 4 | ||
| 5HTR2A rs6313 | CC | 40 | 18 | 911 |
| CT | 56 | 27 | 1267 | |
| TT | 19 | 8 | 523 | |
| 5HTR1B rs13212041 | CC | 6 | 1 | 87 |
| CT | 38 | 16 | 648 | |
| TT | 71 | 36 | 1966 | |
| 5HTR6 rs1805054 (C267T) | CC | 59 | 28 | 1834 |
| CT | 33 | 18 | 768 | |
| TT | 2 | 1 | 99 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babić Leko, M.; Nikolac Perković, M.; Španić, E.; Švob Štrac, D.; Pleić, N.; Vogrinc, Ž.; Gunjača, I.; Bežovan, D.; Nedić Erjavec, G.; Klepac, N.; et al. Serotonin Receptor Gene Polymorphisms Are Associated with Cerebrospinal Fluid, Genetic, and Neuropsychological Biomarkers of Alzheimer’s Disease. Biomedicines 2022, 10, 3118. https://doi.org/10.3390/biomedicines10123118
Babić Leko M, Nikolac Perković M, Španić E, Švob Štrac D, Pleić N, Vogrinc Ž, Gunjača I, Bežovan D, Nedić Erjavec G, Klepac N, et al. Serotonin Receptor Gene Polymorphisms Are Associated with Cerebrospinal Fluid, Genetic, and Neuropsychological Biomarkers of Alzheimer’s Disease. Biomedicines. 2022; 10(12):3118. https://doi.org/10.3390/biomedicines10123118
Chicago/Turabian StyleBabić Leko, Mirjana, Matea Nikolac Perković, Ena Španić, Dubravka Švob Štrac, Nikolina Pleić, Željka Vogrinc, Ivana Gunjača, Dora Bežovan, Gordana Nedić Erjavec, Nataša Klepac, and et al. 2022. "Serotonin Receptor Gene Polymorphisms Are Associated with Cerebrospinal Fluid, Genetic, and Neuropsychological Biomarkers of Alzheimer’s Disease" Biomedicines 10, no. 12: 3118. https://doi.org/10.3390/biomedicines10123118
APA StyleBabić Leko, M., Nikolac Perković, M., Španić, E., Švob Štrac, D., Pleić, N., Vogrinc, Ž., Gunjača, I., Bežovan, D., Nedić Erjavec, G., Klepac, N., Borovečki, F., Zemunik, T., Pivac, N., Hof, P. R., & Šimić, G. (2022). Serotonin Receptor Gene Polymorphisms Are Associated with Cerebrospinal Fluid, Genetic, and Neuropsychological Biomarkers of Alzheimer’s Disease. Biomedicines, 10(12), 3118. https://doi.org/10.3390/biomedicines10123118








