Milmed Yeast Alters the LPS-Induced M1 Microglia Cells to Form M2 Anti-Inflammatory Phenotype
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strains and Growth
2.2. Cell Culture and Treatment
2.3. Cell Viability Assays
2.3.1. Trypan Blue Exclusion Assay
2.3.2. Immunofluorescence Microscopy
2.4. Real-Time Quantitative PRC Analysis
2.5. Statistical Analysis
3. Results
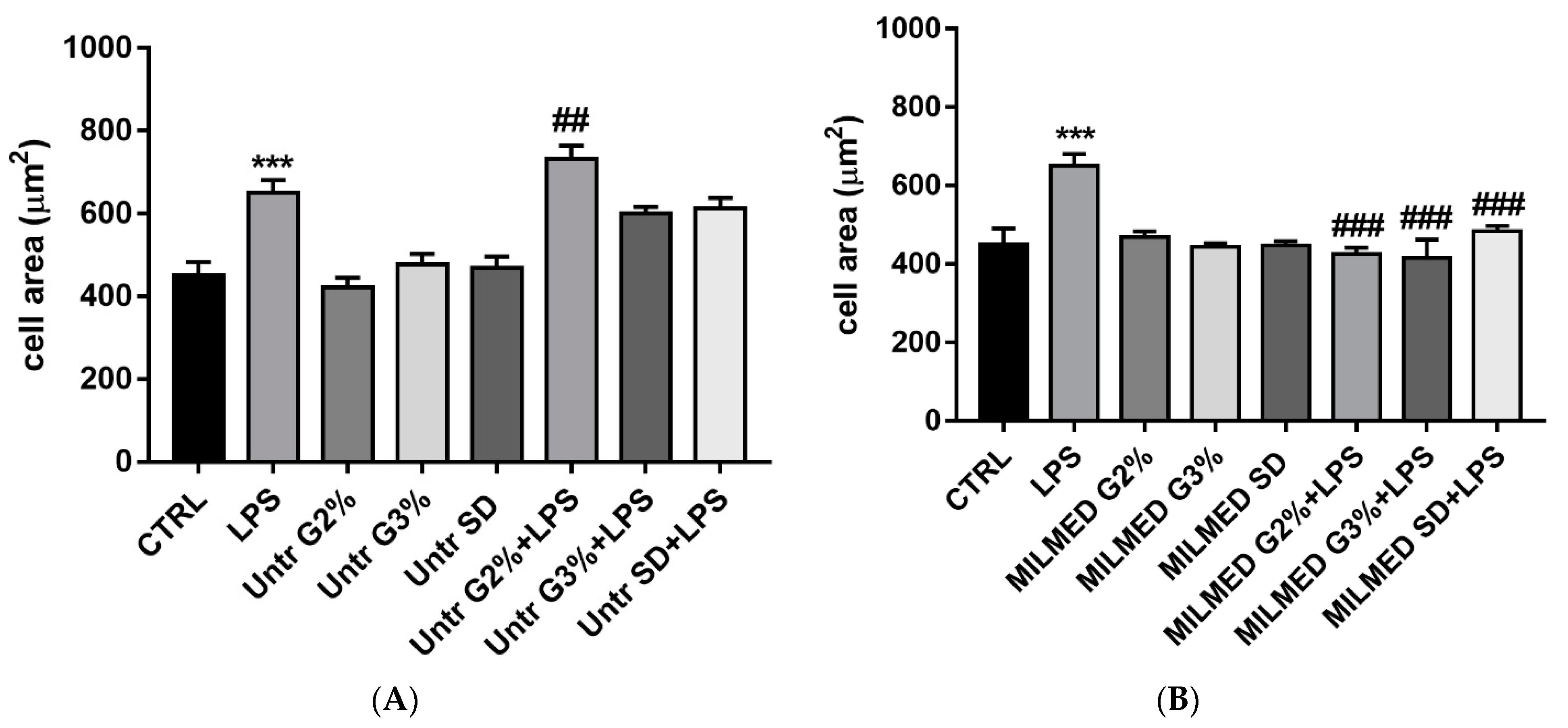
3.1. BV-2 Cellular Areas
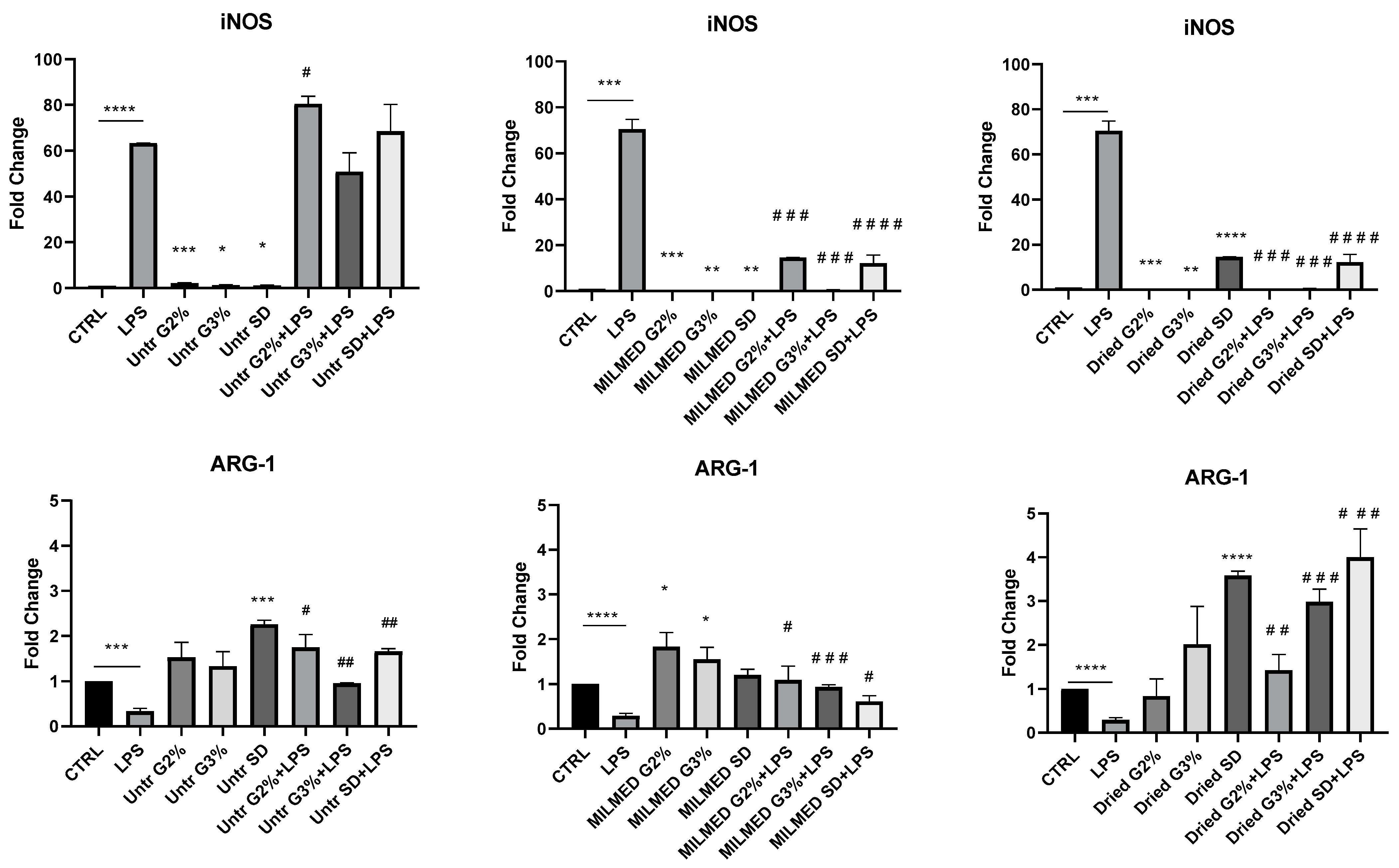
3.2. iNOS and Arg-1 Expression
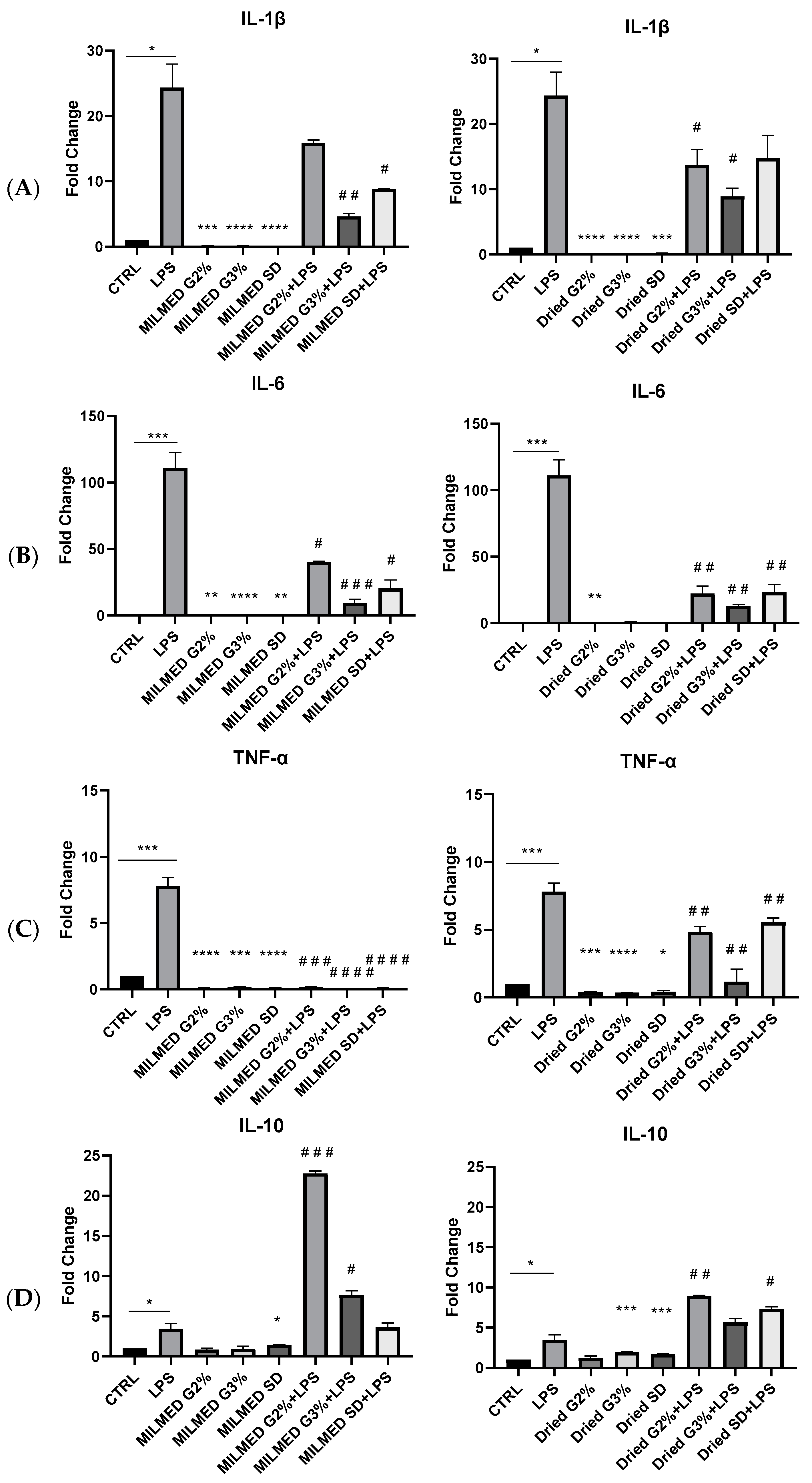
3.3. Cytokines Expression
4. Discussion
5. Conclusions
Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- El Dib, R.; Periyasamy, A.G.; de Barros, J.L.; França, C.G.; Senefonte, F.L.; Vesentini, G.; Alves, M.G.O.; Rodrigues, J.V.D.S.; Gomaa, H.; Júnior, J.R.G.; et al. Probiotics for the Treatment of Depression and Anxiety: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. Nutr. ESPEN 2021, 45, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Maggi, E.; Armeli, F.; Mengoni, B.; Leo, M.; Filetici, P.; Mancini, P.; Lenz, T.; Businaro, R.; Archer, T. Milmed Saccharomyces cerevisiae Activity on Central Nervous System Cells. J. Toxicol. Pharmacol. 2022, 5, 26. [Google Scholar]
- Shabbir, U.; Arshad, M.S.; Sameen, A.; Oh, D.-H. Crosstalk between Gut and Brain in Alzheimer’s Disease: The Role of Gut Microbiota Modulation Strategies. Nutrients 2021, 13, 690. [Google Scholar] [CrossRef] [PubMed]
- Eltokhi, A.; Sommer, I.E. A Reciprocal Link Between Gut Microbiota, Inflammation and Depression: A Place for Probiotics? Front. Neurosci. 2022, 16, 852506. [Google Scholar] [CrossRef]
- Grieco, M.; De Caris, M.G.; Maggi, E.; Armeli, F.; Coccurello, R.; Bisogno, T.; D’Erme, M.; Maccarrone, M.; Mancini, P.; Businaro, R. Fatty Acid Amide Hydrolase (FAAH) Inhibition Modulates Amyloid-Beta-Induced Microglia Polarization. Int. J. Mol. Sci. 2021, 22, 7711. [Google Scholar] [CrossRef]
- Neta, F.I.; de Souza, F.E.S.; Batista, A.L.; Pinheiro, F.I.; Cobucci, R.N.; Guzen, F.P. Effects of Supplementation with Probiotics in Experimental Models OfAlzheimer’s Disease: A Systematic Review of Animal Experiments. Curr. Alzheimer Res. 2022, 19, 188–201. [Google Scholar] [CrossRef]
- Li, K.; Ly, K.; Mehta, S.; Braithwaite, A. Importance of crosstalk between the microbiota and the neuroimmune system for tissue homeostasis. Clin. Transl. Immunol. 2022, 11, e1394. [Google Scholar] [CrossRef]
- Varela-Trinidad, G.U.; Domínguez-Díaz, C.; Solórzano-Castanedo, K.; Íñiguez-Gutiérrez, L.; Hernández-Flores, T.D.J.; Fafutis-Morris, M. Probiotics: Protecting Our Health from the Gut. Microorganisms 2022, 10, 1428. [Google Scholar] [CrossRef]
- Caputi, V.; Giron, M.C. Microbiome-Gut-Brain Axis and Toll-Like Receptors in Parkinson’s Disease. Int. J. Mol. Sci. 2018, 19, 1689. [Google Scholar] [CrossRef]
- Zhuang, Z.-Q.; Shen, L.-L.; Li, W.-W.; Fu, X.; Zeng, F.; Gui, L.; Lü, Y.; Cai, M.; Zhu, C.; Tan, Y.-L.; et al. Gut Microbiota Is Altered in Patients with Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 63, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut Microbiota in Human Metabolic Health and Disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Piekut, T.; Hurła, M.; Banaszek, N.; Szejn, P.; Dorszewska, J.; Kozubski, W.; Prendecki, M. Infectious Agents and Alzheimer’s Disease. J. Integr. Neurosci. 2022, 21, 073. [Google Scholar] [CrossRef] [PubMed]
- Webberley, T.S.; Masetti, G.; Bevan, R.J.; Kerry-Smith, J.; Jack, A.A.; Michael, D.R.; Thomas, S.; Glymenaki, M.; Li, J.; McDonald, J.A.K.; et al. The Impact of Probiotic Supplementation on Cognitive, Pathological and Metabolic Markers in a Transgenic Mouse Model of Alzheimer’s Disease. Front. Neurosci. 2022, 16, 843105. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.A.; McCormick, T.S.; Retuerto, M.; Bebek, G.; Cousineau, S.; Hartman, L.; Barth, C.; Schrom, K. Evaluation of Microbiome Alterations Following Consumption of BIOHM, a Novel Probiotic. Curr. Issues Mol. Biol. 2021, 43, 2135–2146. [Google Scholar] [CrossRef] [PubMed]
- Birmann, P.T.; Casaril, A.M.; Pesarico, A.P.; Caballero, P.S.; Smaniotto, T.Â.; Rodrigues, R.R.; Moreira, Â.N.; Conceição, F.R.; Sousa, F.S.S.; Collares, T.; et al. Komagataella Pastoris KM71H Modulates Neuroimmune and Oxidative Stress Parameters in Animal Models of Depression: A Proposal for a New Probiotic with Antidepressant-like Effect. Pharmacol. Res. 2021, 171, 105740. [Google Scholar] [CrossRef]
- Durmaz, S.; Kurtoğlu, T.; Barbarus, E.; Çetin, N.K.; Yılmaz, M.; Rahman, F.; Abacıgil, F. Probiotic Saccharomyces boulardii Alleviates Lung Injury by Reduction of Oxidative Stress and Cytokine Response Induced by Supraceliac Aortic Ischemia-Reperfusion Injury in Rats. Braz. J. Cardiovasc. Surg. 2020, 36, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Roy Sarkar, S.; Mitra Mazumder, P.; Chatterjee, K.; Sarkar, A.; Adhikary, M.; Mukhopadhyay, K.; Banerjee, S. Saccharomyces Boulardii Ameliorates Gut Dysbiosis Associated Cognitive Decline. Physiol. Behav. 2021, 236, 113411. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, B.; Alimadadi, N.; Attaran, B.; Nasr, S. Yeasts from Iranian Traditional Milk Kefir Samples: Isolation, Molecular Identification and Their Potential Probiotic Properties. Lett. Appl. Microbiol. 2022, 75, 1264–1274. [Google Scholar] [CrossRef]
- Ye, T.; Yuan, S.; Kong, Y.; Yang, H.; Wei, H.; Zhang, Y.; Jin, H.; Yu, Q.; Liu, J.; Chen, S.; et al. Effect of Probiotic Fungi against Cognitive Impairment in Mice via Regulation of the Fungal Microbiota–Gut–Brain Axis. J. Agric. Food Chem. 2022, 70, 9026–9038. [Google Scholar] [CrossRef]
- Archer, T. Milmed Treatment Alleviates Symptoms of Allergy and Improves General Health. J. Immunol. Allergy 2021, 1, 1–11. [Google Scholar] [CrossRef]
- Prokhorov, A.M.; Dianov, E.M. In Memory of Mikhail B. Golan. Tech. Phys. 2001, 46, 1068. [Google Scholar] [CrossRef]
- Betskii, O.V.; Devyatkov, N.D.; Kislov, V.V. Low Intensity Millimeter Waves in Medicine and Biology. Crit. Rev. Biomed. Eng. 2000, 28, 247–268. [Google Scholar] [CrossRef] [PubMed]
- Devyatkov, N.; Golant, M.; Betsky, O. Brief Information for Physicians about the Physical Characteristics of the Processes Occurring in the Body under MM-Wave Therapy Performed by Installations “Jav-1”, and the Associated Effects on the Body of Electromagnetic Millimeter Waves; Radio i Svyaz: Moscow, Russia, 1991. [Google Scholar]
- Devyatkov, N.D.; Gelvich, É.A.; Davydova, I.B.; Kirillov, V.V.; Kolmakov, D.N.; Mazokhin, V.N.; Sinyagovskiĭ, V.I.; Chilikin, P.I. Microwave and radio-frequency apparatus and methods for use in oncology. Sov. Phys. Uspekhi 1981, 24, 432. [Google Scholar] [CrossRef]
- Golant, M.B.; Mudrik, D.G.; Kruglyakova, O.P.; Izvol’skaya, V.E. Effect of EHF-Radiation Polarization on Yeast Cells. Radiophys. Quantum Electron. 1994, 37, 82–84. [Google Scholar] [CrossRef]
- Golant, M.B. Physical Laws of Medicine and Their Use in the Realization of Interaction of Living Organisms with EHF Radiation. Radiophys. Quantum Electron. 1994, 37, 45–47. [Google Scholar] [CrossRef]
- Ragimov, C.R.; Ter-Asaturov, G.P.; Golant, M.B.; Rogov, K.A.; Balakireva, L.Z. Stimulation of Reparative Osteogenesis by Millimeter Band Electromagnetic Radiation in Experimental Traumatic Defects of the Mandible. Bull. Exp. Biol. Med. 1991, 111, 562–565. [Google Scholar] [CrossRef]
- Archer, T.; Fredriksson, A. The Yeast Product Milmed Enhances the Effect of Physical Exercise on Motor Performance and Dopamine Neurochemistry Recovery in MPTP-Lesioned Mice. Neurotox. Res. 2013, 24, 393–406. [Google Scholar] [CrossRef]
- Orihuela, R.; McPherson, C.A.; Harry, G.J. Microglial M1/M2 Polarization and Metabolic States: Microglia Bioenergetics with Acute Polarization. Br. J. Pharmacol. 2016, 173, 649–665. [Google Scholar] [CrossRef]
- Chidambaram, S.B.; Essa, M.M.; Rathipriya, A.G.; Bishir, M.; Ray, B.; Mahalakshmi, A.M.; Tousif, A.H.; Sakharkar, M.K.; Kashyap, R.S.; Friedland, R.P.; et al. Gut Dysbiosis, Defective Autophagy and Altered Immune Responses in Neurodegenerative Diseases: Tales of a Vicious Cycle. Pharmacol. Ther. 2022, 231, 107988. [Google Scholar] [CrossRef]
- Schroeder, B.O.; Bäckhed, F. Signals from the Gut Microbiota to Distant Organs in Physiology and Disease. Nat. Med. 2016, 22, 1079–1089. [Google Scholar] [CrossRef]
- Goyal, D.; Ali, S.A.; Singh, R.K. Emerging Role of Gut Microbiota in Modulation of Neuroinflammation and Neurodegeneration with Emphasis on Alzheimer’s Disease. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 106, 110112. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, K.; Mulak, A. Brain-Gut-Microbiota Axis in Alzheimer’s Disease. J. Neurogastroenterol. Motil. 2019, 25, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 Microglia: The Good, the Bad, and the Inflamed. J. Neuroinflamm. 2014, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef]
- Dubbelaar, M.L.; Kracht, L.; Eggen, B.J.L.; Boddeke, E.W.G.M. The Kaleidoscope of Microglial Phenotypes. Front. Immunol. 2018, 9, 1753. [Google Scholar] [CrossRef]
- Jurga, A.M.; Paleczna, M.; Kuter, K.Z. Overview of General and Discriminating Markers of Differential Microglia Phenotypes. Front. Cell. Neurosci. 2020, 14, 198. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in Neurodegenerative Disorders: The Roles of Microglia and Astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Palepu, M.S.K.; Dandekar, M.P. Remodeling of Microbiota Gut-Brain Axis Using Psychobiotics in Depression. Eur. J. Pharmacol. 2022, 931, 175171. [Google Scholar] [CrossRef]
- Rahman, Z.; Dandekar, M.P. Crosstalk between Gut Microbiome and Immunology in the Management of Ischemic Brain Injury. J. Neuroimmunol. 2021, 353, 577498. [Google Scholar] [CrossRef]
- Chen, Z.; Maqbool, J.; Sajid, F.; Hussain, G.; Sun, T. Human Gut Microbiota and Its Association with Pathogenesis and Treatments of Neurodegenerative Diseases. Microb. Pathog. 2021, 150, 104675. [Google Scholar] [CrossRef]
- Westfall, S.; Pasinetti, G.M. The Gut Microbiota Links Dietary Polyphenols With Management of Psychiatric Mood Disorders. Front. Neurosci. 2019, 13, 1196. [Google Scholar] [CrossRef] [PubMed]
- Rahimian, R.; Belliveau, C.; Chen, R.; Mechawar, N. Microglial Inflammatory-Metabolic Pathways and Their Potential Therapeutic Implication in Major Depressive Disorder. Front. Psychiatry 2022, 13, 871997. [Google Scholar] [CrossRef] [PubMed]
- Princiotta Cariddi, L.; Mauri, M.; Cosentino, M.; Versino, M.; Marino, F. Alzheimer’s Disease: From Immune Homeostasis to Neuroinflammatory Condition. Int. J. Mol. Sci. 2022, 23, 13008. [Google Scholar] [CrossRef]
- Choi, I.; Heaton, G.R.; Lee, Y.-K.; Yue, Z. Regulation of α-Synuclein Homeostasis and Inflammasome Activation by Microglial Autophagy. Sci. Adv. 2022, 8, eabn1298. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Zhenxin, Y.; Chen, S.; Tan, Z.; Zong, Z.; Zhang, H.; Xiong, X. The Innate and Adaptive Immune Cells in Alzheimer’s and Parkinson’s Diseases. Oxidative Med. Cell. Longev. 2022, 2022, 1315248. [Google Scholar] [CrossRef] [PubMed]
- Araújo, B.; Caridade-Silva, R.; Soares-Guedes, C.; Martins-Macedo, J.; Gomes, E.D.; Monteiro, S.; Teixeira, F.G. Neuroinflammation and Parkinson’s Disease—From Neurodegeneration to Therapeutic Opportunities. Cells 2022, 11, 2908. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Dhapola, R.; Sarma, P.; Medhi, B.; Reddy, D.H. Neuroinflammation in Alzheimer’s Disease: Current Progress in Molecular Signaling and Therapeutics. Inflammation 2022, 1–17. [Google Scholar] [CrossRef]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W.J. Psychobiotics and the Manipulation of Bacteria–Gut–Brain Signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef]
- Blander, J.M.; Longman, R.S.; Iliev, I.D.; Sonnenberg, G.F.; Artis, D. Regulation of Inflammation by Microbiota Interactions with the Host. Nat. Immunol. 2017, 18, 851–860. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, F.; Xing, Z.; Chen, J.; Peng, C.; Li, D. Beneficial Effects of Natural Flavonoids on Neuroinflammation. Front. Immunol. 2022, 13, 1006434. [Google Scholar] [CrossRef]
- Marć, M.A.; Jastrząb, R.; Mytych, J. Does the Gut Microbial Metabolome Really Matter? The Connection between GUT Metabolome and Neurological Disorders. Nutrients 2022, 14, 3967. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. Administration of Bifidobacterium Breve Improves the Brain Function of Aβ1-42-Treated Mice via the Modulation of the Gut Microbiome. Nutrients 2021, 13, 1602. [Google Scholar] [CrossRef] [PubMed]
- Akbari, E.; Asemi, Z.; Daneshvar Kakhaki, R.; Bahmani, F.; Kouchaki, E.; Tamtaji, O.R.; Hamidi, G.A.; Salami, M. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer’s disease: A randomized, double-blind and controlled trial. Front. Aging Neurosci. 2016, 8, 256. [Google Scholar] [CrossRef] [PubMed]
- Gur, T.L.; Bailey, M.T. Effects of Stress on Commensal Microbes and Immune System Activity. In Microbial Endocrinology: Interkingdom Signaling in Infectious Disease and Health; Lyte, M., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2016; Volume 874, pp. 289–300. ISBN 978-3-319-20214-3. [Google Scholar]
- Archer, T.; Garcia, D.; Fredriksson, A. Restoration of MPTP-Induced Deficits by Exercise and Milmed® Co-Treatment. PeerJ 2014, 2, e531. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Xie, Q.; Xu, H.; Zhang, T.; Li, X.; Tian, Y.; Lan, H.; Kong, L.; Zhang, Z. Yeast Microcapsule Mediated Natural Products Delivery for Treating Ulcerative Colitis through Anti-Inflammatory and Regulation of Macrophage Polarization. ACS Appl. Mater. Interfaces 2022, 14, 31085–31098. [Google Scholar] [CrossRef]
- Taylor, P.R.; Tsoni, S.V.; Willment, J.A.; Dennehy, K.M.; Rosas, M.; Findon, H.; Haynes, K.; Steele, C.; Botto, M.; Gordon, S.; et al. Dectin-1 Is Required for β-Glucan Recognition and Control of Fungal Infection. Nat. Immunol. 2007, 8, 31–38. [Google Scholar] [CrossRef]
- Ranuh, R.; Athiyyah, A.F.; Darma, A.; Risky, V.P.; Riawan, W.; Surono, I.S.; Sudarmo, S.M. Effect of the Probiotic Lactobacillus Plantarum IS-10506 on BDNF and 5HT Stimulation: Role of Intestinal Microbiota on the Gut-Brain Axis. Iran. J. Microbiol. 2019, 11, 145–150. [Google Scholar] [CrossRef]
- Businaro, R.; Vauzour, D.; Sarris, J.; Münch, G.; Gyengesi, E.; Brogelli, L.; Zuzarte, P. Therapeutic Opportunities for Food Supplements in Neurodegenerative Disease and Depression. Front. Nutr. 2021, 8, 669846. [Google Scholar] [CrossRef]
- De Caris, M.G.; Grieco, M.; Maggi, E.; Francioso, A.; Armeli, F.; Mosca, L.; Pinto, A.; D’Erme, M.; Mancini, P.; Businaro, R. Blueberry Counteracts BV-2 Microglia Morphological and Functional Switch after LPS Challenge. Nutrients 2020, 12, 1830. [Google Scholar] [CrossRef]
- Angeloni, C.; Businaro, R.; Vauzour, D. The Role of Diet in Preventing and Reducing Cognitive Decline. Curr. Opin. Psychiatry 2020, 33, 432–438. [Google Scholar] [CrossRef]
- Businaro, R.; Corsi, M.; Asprino, R.; Di Lorenzo, C.; Laskin, D.; Corbo, R.M.; Ricci, S.; Pinto, A. Modulation of Inflammation as a Way of Delaying Alzheimer’s Disease Progression: The Diet’s Role. Curr. Alzheimer Res. 2018, 15, 363–380. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, H.; Wang, L.; Tao, Y.; Du, G.; Guan, W.; Liu, J.; Brennan, C.; Ho, C.-T.; Li, S. Effects of Selected Resveratrol Analogues on Activation and Polarization of Lipopolysaccharide-Stimulated BV-2 Microglial Cells. J. Agric. Food Chem. 2020, 68, 3750–3757. [Google Scholar] [CrossRef] [PubMed]
- Buttari, B.; Profumo, E.; Segoni, L.; D’Arcangelo, D.; Rossi, S.; Facchiano, F.; Saso, L.; Businaro, R.; Iuliano, L.; Riganò, R. Resveratrol Counteracts Inflammation in Human M1 and M2 Macrophages upon Challenge with 7-Oxo-Cholesterol: Potential Therapeutic Implications in Atherosclerosis. Oxidative Med. Cell. Longev. 2014, 2014, 257543. [Google Scholar] [CrossRef]
- Román, G.C.; Jackson, R.E.; Gadhia, R.; Román, A.N.; Reis, J. Mediterranean Diet: The Role of Long-Chain ω-3 Fatty Acids in Fish; Polyphenols in Fruits, Vegetables, Cereals, Coffee, Tea, Cacao and Wine; Probiotics and Vitamins in Prevention of Stroke, Age-Related Cognitive Decline, and Alzheimer Disease. Rev. Neurol. 2019, 175, 724–741. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.; Bonucci, A.; Maggi, E.; Corsi, M.; Businaro, R. Anti-Oxidant and Anti-Inflammatory Activity of Ketogenic Diet: New Perspectives for Neuroprotection in Alzheimer’s Disease. Antioxidants 2018, 7, 63. [Google Scholar] [CrossRef]
- Versele, R.; Corsi, M.; Fuso, A.; Sevin, E.; Businaro, R.; Gosselet, F.; Fenart, L.; Candela, P. Ketone Bodies Promote Amyloid-Β1–40 Clearance in a Human in Vitro Blood–Brain Barrier Model. Int. J. Mol. Sci. 2020, 21, 934. [Google Scholar] [CrossRef]
- Lazo-Vélez, M.A.; Serna-Saldívar, S.O.; Rosales-Medina, M.F.; Tinoco-Alvear, M.; Briones-García, M. Application of Saccharomyces cerevisiae var. boulardii in Food Processing: A Review. J. Appl. Microbiol. 2018, 125, 943–951. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, E. The Probiotic Effects of the Saccharomyces cerevisiae 28-7 Strain Isolated from Nuruk in a DSS-Induced Colitis Mouse Model. J. Microbiol. Biotechnol. 2022, 32, 877–884. [Google Scholar] [CrossRef]
- Pothoulakis, C. Review Article: Anti-Inflammatory Mechanisms of Action of Saccharomyces boulardii. Aliment. Pharmacol. Ther. 2009, 30, 826–833. [Google Scholar] [CrossRef]
- Wang, C.; Li, W.; Wang, H.; Ma, Y.; Zhao, X.; Zhang, X.; Yang, H.; Qian, J.; Li, J. Saccharomyces boulardii Alleviates Ulcerative Colitis Carcinogenesis in Mice by Reducing TNF-α and IL-6 Levels and Functions and by Rebalancing Intestinal Microbiota. BMC Microbiol. 2019, 19, 246. [Google Scholar] [CrossRef]
- Archer, T. Anti-Inflammatory Action of the Treated-Yeast, Milmed, Under IBS-IBD Conditions. J. Immunol. Allergy 2022, 3. [Google Scholar] [CrossRef] [PubMed]

| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Accession Numbers |
| mIL-1β | GAAATGCCACCTTTTGACAGTG | TGGATGCTCTCATCAGGACAG | NM_008361.4 |
| mTNF-α | CTGAACTTCGGGGTGATCGG | GGCTTGTCACTCGAATTTTGAGA | BC137720.1 |
| mIL-10 | GCCCTTTGCTATGGTGTCCTTTC | TCCCTGGTTTCTCTTCCCAAGAC | NM_010548.2 |
| mARG1 | ATGTGCCCTCTGTCTTTTAGGG | GGTCTCTCACGTCATACTCTGT | NM_007482.3 |
| miNOS | GGCAGCCTGTGAGACCTTTG | GCATTGGAAGTGAAGCGTTTC | AF427516.1 |
| mIL-6 | CGGAGAGGAGACTTCACAGAGGA | TTTCCACGATTTCCCAGAGAACA | NM_001314054.1 |
| mACT-β | GGCTGTATTCCCCTCCATCG | CCAGTTGGTAACAATGCCATGT | NM_007393.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armeli, F.; Mengoni, B.; Maggi, E.; Mazzoni, C.; Preziosi, A.; Mancini, P.; Businaro, R.; Lenz, T.; Archer, T. Milmed Yeast Alters the LPS-Induced M1 Microglia Cells to Form M2 Anti-Inflammatory Phenotype. Biomedicines 2022, 10, 3116. https://doi.org/10.3390/biomedicines10123116
Armeli F, Mengoni B, Maggi E, Mazzoni C, Preziosi A, Mancini P, Businaro R, Lenz T, Archer T. Milmed Yeast Alters the LPS-Induced M1 Microglia Cells to Form M2 Anti-Inflammatory Phenotype. Biomedicines. 2022; 10(12):3116. https://doi.org/10.3390/biomedicines10123116
Chicago/Turabian StyleArmeli, Federica, Beatrice Mengoni, Elisa Maggi, Cristina Mazzoni, Adele Preziosi, Patrizia Mancini, Rita Businaro, Thomas Lenz, and Trevor Archer. 2022. "Milmed Yeast Alters the LPS-Induced M1 Microglia Cells to Form M2 Anti-Inflammatory Phenotype" Biomedicines 10, no. 12: 3116. https://doi.org/10.3390/biomedicines10123116
APA StyleArmeli, F., Mengoni, B., Maggi, E., Mazzoni, C., Preziosi, A., Mancini, P., Businaro, R., Lenz, T., & Archer, T. (2022). Milmed Yeast Alters the LPS-Induced M1 Microglia Cells to Form M2 Anti-Inflammatory Phenotype. Biomedicines, 10(12), 3116. https://doi.org/10.3390/biomedicines10123116









