Pharmacological Optimization of PSMA-Based Radioligand Therapy
Abstract
1. Introduction
2. Methods
3. Pharmacokinetics of PSMA-Based Radioligand Therapy
3.1. Distribution
3.2. Elimination (Metabolism and Excretion)
3.3. Variability in Pharmacokinetics
3.4. Optimizing Pharmacokinetic Properties of the Radioligand
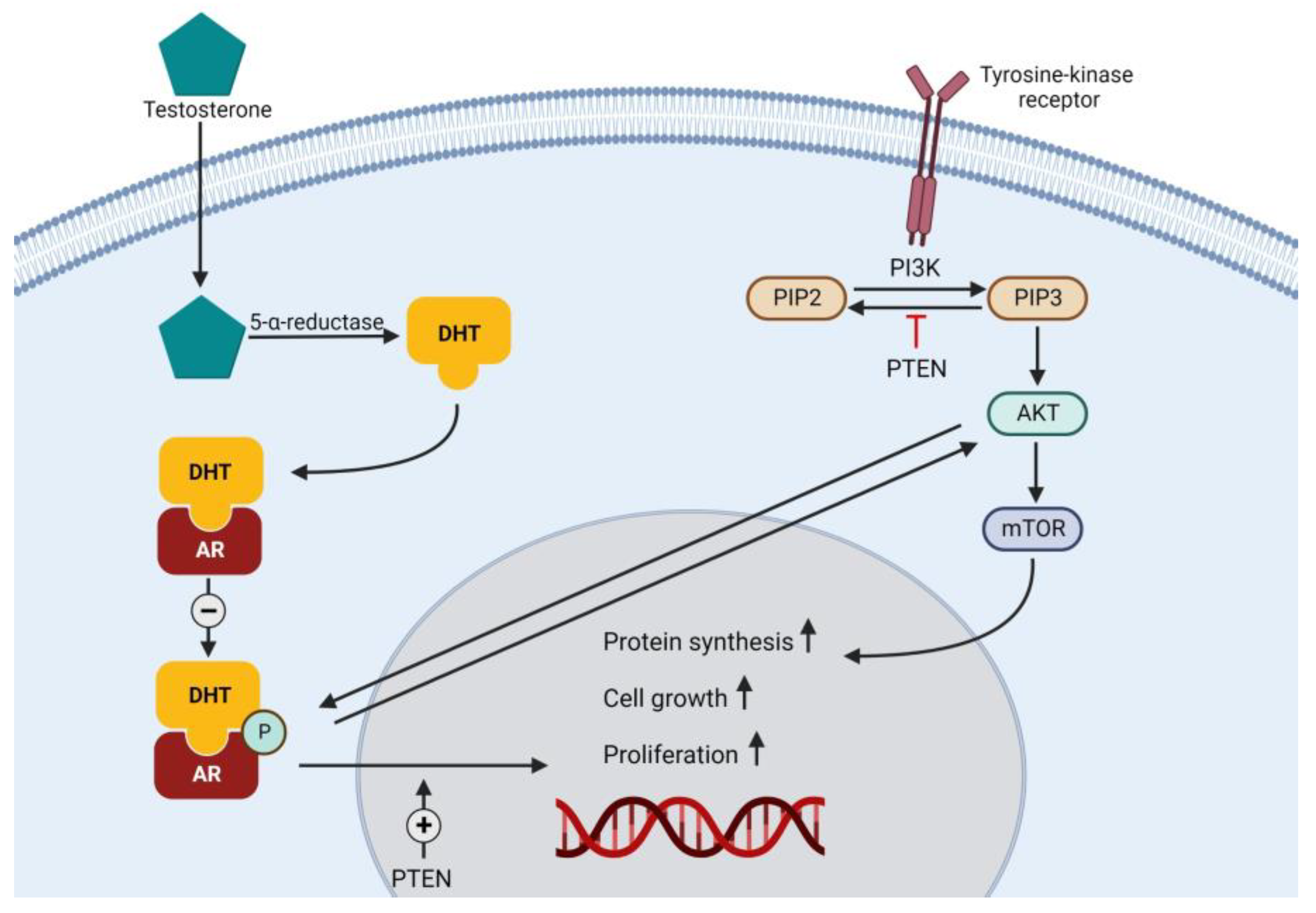
4. Pharmacodynamics of PSMA-Based Radioligand Therapy
4.1. PSMA Receptor as a Target
4.2. Predictors of Response
4.3. Synergistic Effects
4.3.1. Synergy by Upregulation of PSMA
4.3.2. Synergy with Concomitant Agents
4.4. Dosing of PSMA-Based Radioligands
4.4.1. Dose–Response of 177Lu-PSMA Radioligands
4.4.2. Interval of 177Lu-PSMA Radioligands
4.5. Adverse Events
Management of Adverse Events
5. Optimization Strategy and Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Search | Query | Results |
|---|---|---|
| #3 | #1 AND #2 | 1111 |
| #2 | “Randomized Controlled Trial” [Publication Type] OR “Controlled Clinical Trial” [Publication Type] OR “drug therapy” [Subheading] OR “random*” [tiab] OR “crossover*” [tiab] OR “cross over*” [tiab] OR “placebo*” [tiab] OR (“doubl*” [tiab] AND “blind*” [tiab]) OR (“singl*” [tiab] AND “blind*” [tiab]) OR “trial*” [tiab] | 4,277,412 |
| #1 | “prostate specific membrane antigen*” [tiab] OR “PSMA” [tiab] | 5324 |
| Search | Query | Results |
|---|---|---|
| #4 | #3 NOT (‘conference abstract’/it OR ‘conference paper’/it OR ‘conference review’/it OR ‘erratum’/it OR ‘note’/it) | 482 |
| #3 | #1 AND #2 | 1266 |
| #2 | ‘crossover procedure’/exp OR ‘double blind procedure’/exp OR ‘randomized controlled trial’/exp OR ‘single blind procedure’/exp OR (‘random*’ OR ‘crossover*’ OR (‘cross’ NEXT/1 ‘over*’) OR ‘placebo*’ OR (‘doubl*’ AND ‘blind*’) OR (‘singl*’ AND ‘blind*’) OR ‘trial’):ti,ab,kw | 2,585,339 |
| #1 | (‘prostate specific membrane antigen*’ OR ‘PSMA’):ti,ab,kw | 9779 |
| Search | Query | Results |
|---|---|---|
| #3 | #1 AND #2 | 1075 |
| #2 | TS = (“Randomized Controlled Trial” OR “Controlled Clinical Trial” OR “drug therapy” OR “random*” OR “crossover*” OR “cross over*” OR “placebo*” OR (“double” AND “blind”) OR (“singl*” AND “blind*”) OR “trial*”) | 3,357,790 |
| #1 | TS = (“prostate specific membrane antigen*” OR “PSMA”) | 8416 |
| Search | Query | Results |
|---|---|---|
| #3 | #1 AND #2 | 324 |
| #2 | (“Randomized NEXT Controlled NEXT Trial” OR “Controlled NEXT Clinical NEXT Trial” OR “drug NEXT therapy” OR “random*” OR “crossover*” OR “cross NEXT over*” OR “placebo*” OR (“double” AND “blind”) OR (“singl*” AND “blind*”) OR “trial*”):ti,ab,kw | 1,171,083 |
| #1 | (“prostate NEXT specific NEXT membrane NEXT antigen*” OR “PSMA”):ti,ab,kw | 398 |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. FDA Approves Pluvicto for Metastatic Castration-Resistant Prostate Cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pluvicto-metastatic-castration-resistant-prostate-cancer (accessed on 26 October 2022).
- Diao, W.; Cai, H.; Chen, L.; Jin, X.; Liao, X.; Jia, Z. Recent Advances in Prostate-Specific Membrane Antigen-Based Radiopharmaceuticals. Curr. Top. Med. Chem. 2019, 19, 33–56. [Google Scholar] [CrossRef]
- O’Keefe, D.S.; Bacich, D.J.; Huang, S.S.; Heston, W.D.W. A Perspective on the Evolving Story of PSMA Biology, PSMA-Based Imaging, and Endoradiotherapeutic Strategies. J. Nucl. Med. 2018, 59, 1007–1013. [Google Scholar] [CrossRef]
- Sweat, S.D.; Pacelli, A.; Murphy, G.P.; Bostwick, D.G. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology 1998, 52, 637–640. [Google Scholar] [CrossRef]
- Haffner, M.C.; Kronberger, I.E.; Ross, J.S.; Sheehan, C.E.; Zitt, M.; Mühlmann, G.; Öfner, D.; Zelger, B.; Ensinger, C.; Yang, X.J.; et al. Prostate-specific membrane antigen expression in the neovasculature of gastric and colorectal cancers. Hum. Pathol. 2009, 40, 1754–1761. [Google Scholar] [CrossRef]
- Ren, H.; Zhang, H.; Wang, X.; Liu, J.; Yuan, Z.; Hao, J. Prostate-specific membrane antigen as a marker of pancreatic cancer cells. Med. Oncol. 2014, 31, 857. [Google Scholar] [CrossRef]
- Morgantetti, G.; Ng, K.L.; Samaratunga, H.; Rhee, H.; Gobe, G.C.; Wood, S.T. Prostate specific membrane antigen (PSMA) expression in vena cava tumour thrombi of clear cell renal cell carcinoma suggests a role for PSMA-driven tumour neoangiogenesis. Transl. Androl. Urol. 2019, 8, S147–S155. [Google Scholar] [CrossRef]
- Stopa, B.M.; Crowley, J.; Juhász, C.; Rogers, C.M.; Witcher, M.R.; Kiser, J.W. Prostate-Specific Membrane Antigen as Target for Neuroimaging of Central Nervous System Tumors. Mol. Imaging 2022, 2022, 5358545. [Google Scholar] [CrossRef]
- Sartor, O.; De Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Giraudet, A. Radionuclide therapy targeting PSMA for the treatment of metastatic prostate cancer: Current point of view and ways of improvement. Med. Nucl. 2019, 43, 275–279. [Google Scholar] [CrossRef]
- Weineisen, M.; Schottelius, M.; Simecek, J.; Baum, R.P.; Yildiz, A.; Beykan, S.; Kulkarni, H.R.; Lassmann, M.; Klette, I.; Eiber, M.; et al. 68Ga- and 177Lu-Labeled PSMA I&T: Optimization of a PSMA-Targeted Theranostic Concept and First Proof-of-Concept Human Studies. J. Nucl. Med. 2015, 56, 1169–1176. [Google Scholar] [CrossRef]
- Barber, T.W.; Singh, A.; Kulkarni, H.R.; Niepsch, K.; Billah, B.; Baum, R.P. Clinical Outcomes of 177Lu-PSMA Radioligand Therapy in Earlier and Later Phases of Metastatic Castration-Resistant Prostate Cancer Grouped by Previous Taxane Chemotherapy. J. Nucl. Med. 2019, 60, 955–962. [Google Scholar] [CrossRef]
- Lütje, S.; Heskamp, S.; Cornelissen, A.S.; Poeppel, T.D.; van den Broek, S.A.; Rosenbaum-Krumme, S.; Bockisch, A.; Gotthardt, M.; Rijpkema, M.; Boerman, O.C. PSMA ligands for radionuclide imaging and therapy of prostate cancer: Clinical status. Theranostics 2015, 5, 1388–1401. [Google Scholar] [CrossRef]
- Virgolini, I.; Decristoforo, C.; Haug, A.; Fanti, S.; Uprimny, C. Current status of theranostics in prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 471–495. [Google Scholar] [CrossRef]
- Hair, K.; Bahor, Z.; Macleod, M.; Liao, J.; Sena, E.S. The automated systematic search Deduplicator (ASySD): A rapid, open-source, interoperable tool to remove duplicate citations in biomedical systematic reviews. Biorxiv 2021, in press. [Google Scholar] [CrossRef]
- Umbricht, C.A.; Benešová, M.; Schibli, R.; Müller, C. Preclinical Development of Novel PSMA-Targeting Radioligands: Modulation of Albumin-Binding Properties to Improve Prostate Cancer Therapy. Mol. Pharm. 2018, 15, 2297–2306. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Stefanova, M.; Benešová, M.; Bronzel, M.; Afshar-Oromieh, A.; Mier, W.; Eder, M.; Kopka, K.; Haberkorn, U. PSMA-Targeted Radionuclide Therapy of Metastatic Castration-Resistant Prostate Cancer with 177Lu-Labeled PSMA-617. J. Nucl. Med. 2016, 57, 1170–1176. [Google Scholar] [CrossRef]
- Kratochwil, C.; Afshar-Oromieh, A.; Kopka, K.; Haberkorn, U.; Giesel, F.L. Current Status of Prostate-Specific Membrane Antigen Targeting in Nuclear Medicine: Clinical Translation of Chelator Containing Prostate-Specific Membrane Antigen Ligands Into Diagnostics and Therapy for Prostate Cancer. Semin. Nucl. Med. 2016, 46, 405–418. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. PSMA-targeting alpha-Radiation therapy with 225Actinium-PSMA-617: Dosimetry, toxicity and duration of tumor-control. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, S163. [Google Scholar]
- Paganelli, G.; Sarnelli, A.; Severi, S.; Sansovini, M.; Belli, M.L.; Monti, M.; Foca, F.; Celli, M.; Nicolini, S.; Tardelli, E.; et al. Dosimetry and safety of 177Lu PSMA-617 along with polyglutamate parotid gland protector: Preliminary results in metastatic castration-resistant prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 3008–3017. [Google Scholar] [CrossRef]
- Yadav, M.; Ballal, S.; Tripathi, M.; Damle, N.; Sahoo, R.; Roesch, F.; Bal, C. Post-therapeutic dosimetry of 177Lu-DKFZ-PSMA-617 in treatment of patients with castration resistant prostate cancer. J. Nucl. Med. 2016, 57 (Suppl. S2), 1017. [Google Scholar] [CrossRef]
- Baum, R.P.; Kulkarni, H.R.; Schuchardt, C.; Singh, A.; Wirtz, M.; Wiessalla, S.; Schottelius, M.; Mueller, D.; Klette, I.; Wester, H.-J. 177Lu-Labeled Prostate-Specific Membrane Antigen Radioligand Therapy of Metastatic Castration-Resistant Prostate Cancer: Safety and Efficacy. J. Nucl. Med. 2016, 57, 1006–1013. [Google Scholar] [CrossRef]
- Privé, B.M.; Peters, S.M.; Muselaers, C.H.; van Oort, I.M.; Janssen, M.J.; Sedelaar, J.M.; Konijnenberg, M.W.; Zámecnik, P.; Uijen, M.J.; Schilham, M.G.; et al. Lutetium-177-PSMA-617 in Low-Volume Hormone-Sensitive Metastatic Prostate Cancer: A Prospective Pilot Study. Clin. Cancer Res. 2021, 27, 3595–3601. [Google Scholar] [CrossRef]
- Information NCfB. PubChem Compound Summary for CID 122706785, PSMA-617 Lu-177. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/PSMA-617-Lu-177 (accessed on 23 August 2022).
- Chatalic, K.L.; Heskamp, S.; Konijnenberg, M.; Molkenboer-Kuenen, J.D.; Franssen, G.M.; Groningen, M.C.C.-V.; Schottelius, M.; Wester, H.-J.; van Weerden, W.M.; Boerman, O.C.; et al. Towards Personalized Treatment of Prostate Cancer: PSMA I&T, a Promising Prostate-Specific Membrane Antigen-Targeted Theranostic Agent. Theranostics 2016, 6, 849–861. [Google Scholar] [CrossRef]
- Benešová, M.; Schäfer, M.; Bauder-Wüst, U.; Afshar-Oromieh, A.; Kratochwil, C.; Mier, W.; Haberkorn, U.; Kopka, K.; Eder, M. Preclinical Evaluation of a Tailor-Made DOTA-Conjugated PSMA Inhibitor with Optimized Linker Moiety for Imaging and Endoradiotherapy of Prostate Cancer. J. Nucl. Med. 2015, 56, 914–920. [Google Scholar] [CrossRef]
- Kurth, J.; Krause, B.J.; Schwarzenböck, S.M.; Stegger, L.; Schäfers, M.; Rahbar, K. External radiation exposure, excretion, and effective half-life in 177Lu-PSMA-targeted therapies. EJNMMI Res. 2018, 8, 32. [Google Scholar] [CrossRef]
- Kratochwil, C.; Fendler, W.P.; Eiber, M.; Baum, R.; Bozkurt, M.F.; Czernin, J.; Bolton, R.C.D.; Ezziddin, S.; Forrer, F.; Hicks, R.J.; et al. EANM procedure guidelines for radionuclide therapy with 177Lu-labelled PSMA-ligands (177Lu-PSMA-RLT). Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2536–2544. [Google Scholar] [CrossRef]
- Kratochwil, C.; Haberkorn, U.; Giesel, F.L. Radionuclide Therapy of Metastatic Prostate Cancer. Semin. Nucl. Med. 2019, 49, 313–325. [Google Scholar] [CrossRef]
- Kulkarni, H.R.; Singh, A.; Schuchardt, C.; Niepsch, K.; Sayeg, M.; Leshch, Y.; Wester, H.-J.; Baum, R.P. PSMA-Based Radioligand Therapy for Metastatic Castration-Resistant Prostate Cancer: The Bad Berka Experience Since 2013. J. Nucl. Med. 2016, 57 (Suppl. S3), 97S–104S. [Google Scholar] [CrossRef]
- Hohberg, M.; Eschner, W.; Schmidt, M.; Dietlein, M.; Kobe, C.; Fischer, T.; Drzezga, A.; Wild, M. Lacrimal Glands May Represent Organs at Risk for Radionuclide Therapy of Prostate Cancer with [177Lu]DKFZ-PSMA-617. Mol. Imaging Biol. 2016, 18, 437–445. [Google Scholar] [CrossRef]
- Yadav, M.P.; Ballal, S.; Tripathi, M.; Damle, N.A.; Sahoo, R.K.; Seth, A.; Bal, C. Post-therapeutic dosimetry of 177Lu-DKFZ-PSMA-617 in the treatment of patients with metastatic castration-resistant prostate cancer. Nucl. Med. Commun. 2017, 38, 91–98. [Google Scholar] [CrossRef]
- Belli, M.L.; Sarnelli, A.; Mezzenga, E.; Cesarini, F.; Caroli, P.; Di Iorio, V.; Strigari, L.; Cremonesi, M.; Romeo, A.; Nicolini, S.; et al. Targeted Alpha Therapy in mCRPC (Metastatic Castration-Resistant Prostate Cancer) Patients: Predictive Dosimetry and Toxicity Modeling of 225Ac-PSMA (Prostate-Specific Membrane Antigen). Front. Oncol. 2020, 10, 531660. [Google Scholar] [CrossRef]
- Hardiansyah, D.; Kletting, P.; Begum, N.J.; Beer, A.; Pawiro, S.; Glatting, G. Global Sensitivity Analysis of a Physiologically-Based Pharmacokinetic Model used for Treatment Planning in Lu-177-labelled PSMA Therapy. J. Nucl. Med. 2020, 61 (Suppl. S1), 1405. [Google Scholar]
- Hardiansyah, D.; Maass, C.; Attarwala, A.A.; Müller, B.; Kletting, P.; Mottaghy, F.M.; Glatting, G. The role of patient-based treatment planning in peptide receptor radionuclide therapy. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 871–880. [Google Scholar] [CrossRef]
- Begum, N.J.; Thieme, A.; Eberhardt, N.; Tauber, R.; D’Alessandria, C.; Beer, A.J.; Glatting, G.; Eiber, M.; Kletting, P. The Effect of Total Tumor Volume on the Biologically Effective Dose to Tumor and Kidneys for 177Lu-Labeled PSMA Peptides. J. Nucl. Med. 2018, 59, 929–933. [Google Scholar] [CrossRef]
- Begum, N.J.; Glatting, G.; Wester, H.-J.; Eiber, M.; Beer, A.J.; Kletting, P. The effect of ligand amount, affinity and internalization on PSMA-targeted imaging and therapy: A simulation study using a PBPK model. Sci. Rep. 2019, 9, 20041. [Google Scholar] [CrossRef]
- Kletting, P.; Thieme, A.; Eberhardt, N.; Rinscheid, A.; D’Alessandria, C.; Allmann, J.; Wester, H.-J.; Tauber, R.; Beer, A.J.; Glatting, G.; et al. Modeling and Predicting Tumor Response in Radioligand Therapy. J. Nucl. Med. 2019, 60, 65–70. [Google Scholar] [CrossRef]
- Benešová, M.; Bauder-Wüst, U.; Schäfer, M.; Klika, K.D.; Mier, W.; Haberkorn, U.; Kopka, K.; Eder, M. Linker Modification Strategies to Control the Prostate-Specific Membrane Antigen (PSMA)-Targeting and Pharmacokinetic Properties of DOTA-Conjugated PSMA Inhibitors. J. Med. Chem. 2016, 59, 1761–1775. [Google Scholar] [CrossRef]
- Benešová, M.; Umbricht, C.A.; Schibli, R.; Müller, C. Albumin-Binding PSMA Ligands: Optimization of the Tissue Distribution Profile. Mol. Pharm. 2018, 15, 934–946. [Google Scholar] [CrossRef]
- Choy, C.; Ling, X.; Geruntho, J.; Beyers, S.; Latoche, J.; Langton-Webster, B.; Anderson, C.; Berkman, C. Improving the in vitro and in vivo performance of a 177Lu-labeled phosphoramidate-based PSMA inhibitor with an albumin-binding motif. In Abstracts of Papers of the American Chemical Society; American Chemical Society: New York, NY, USA, 2017; Volume 253. [Google Scholar]
- Kopka, K.; Benešová, M.; Bařinka, C.; Haberkorn, U.; Babich, J. Glu-Ureido–Based Inhibitors of Prostate-Specific Membrane Antigen: Lessons Learned During the Development of a Novel Class of Low-Molecular-Weight Theranostic Radiotracers. J. Nucl. Med. 2017, 58 (Suppl. S2), 17S–26S. [Google Scholar] [CrossRef]
- Ling, X.; Choy, C.; Geruntho, J.; Beyer, S.; Latoche, J.; Langton-Webster, B.; Anderson, C.; Berkman, C. Comparison of traditional and albumin-binding Lu-177-labeled phosphoramidate-based PSMA inhibitors for targeted radionuclide therapy of prostate cancer. J. Nucl. Med. 2017, 58 (Suppl. S1), 317. [Google Scholar]
- Wang, Z.; Tian, R.; Niu, G.; Ma, Y.; Lang, L.; Szajek, L.P.; Kiesewetter, D.O.; Jacobson, O.; Chen, X. Single Low-Dose Injection of Evans Blue Modified PSMA-617 Radioligand Therapy Eliminates Prostate-Specific Membrane Antigen Positive Tumors. Bioconj. Chem. 2018, 29, 3213–3221. [Google Scholar] [CrossRef] [PubMed]
- Zang, J.; Fan, X.; Wang, H.; Liu, Q.; Wang, J.; Li, H.; Li, F.; Jacobson, O.; Niu, G.; Zhu, Z.; et al. First-in-human study of 177Lu-EB-PSMA-617 in patients with metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Zang, J.; Liu, Q.; Sui, H.; Wang, R.; Jacobson, O.; Fan, X.; Zhu, Z.; Chen, X. 177Lu-EB-PSMA Radioligand Therapy with Escalating Doses in Patients with Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2020, 61, 1772–1778. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Latoche, J.D.; Choy, C.J.; Kurland, B.F.; Laymon, C.M.; Wu, Y.; Salamacha, N.; Shen, D.; Geruntho, J.J.; Rigatti, L.H.; et al. Preclinical Dosimetry, Imaging, and Targeted Radionuclide Therapy Studies of Lu-177-Labeled Albumin-Binding, PSMA-Targeted CTT1403. Mol. Imaging Biol. 2020, 22, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Choy, C.J.; Ling, X.; Geruntho, J.J.; Beyer, S.K.; Latoche, J.D.; Langton-Webster, B.; Anderson, C.J.; Berkman, C.E. 177Lu-Labeled Phosphoramidate-Based PSMA Inhibitors: The Effect of an Albumin Binder on Biodistribution and Therapeutic Efficacy in Prostate Tumor-Bearing Mice. Theranostics 2017, 7, 1928–1939. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, S.; Riondato, M.; Uccelli, L.; Giovacchini, G.; Giovannini, E.; Duce, V.; Ciarmiello, A. Toward the Discovery and Development of PSMA Targeted Inhibitors for Nuclear Medicine Applications. Curr. Radiopharm. 2020, 13, 63–79. [Google Scholar] [CrossRef]
- Pu, F.; Salarian, M.; Xue, S.; Qiao, J.; Feng, J.; Tan, S.; Patel, A.; Li, X.; Mamouni, K.; Hekmatyar, K.; et al. Prostate-specific membrane antigen targeted protein contrast agents for molecular imaging of prostate cancer by MRI. Nanoscale 2016, 8, 12668–12682. [Google Scholar] [CrossRef]
- Goodman, O.B., Jr.; Barwe, S.P.; Ritter, B.; McPherson, P.S.; Vasko, A.J.; Keen, J.H.; Nanus, D.M.; Bander, N.H.; Rajasekaran, A.K. Interaction of prostate specific membrane antigen with clathrin and the adaptor protein complex-2. Int. J. Oncol. 2007, 31, 1199–1203. [Google Scholar]
- Tönnesmann, R.; Meyer, P.T.; Eder, M.; Baranski, A.-C. [177Lu]Lu-PSMA-617 Salivary Gland Uptake Characterized by Quantitative In Vitro Autoradiography. Pharmaceuticals 2019, 12, 18. [Google Scholar] [CrossRef]
- Kuban, D.A.; El-Mahdi, A.M.; Schellhammer, P.F. PSA for Outcome Prediction and Posttreatment Evaluation Following Radiation for Prostate Cancer: Do We Know How to Use It? Semin. Radiat. Oncol. 1998, 8, 72–80. [Google Scholar] [CrossRef]
- Roviello, G. Role of PSA response as a marker for efficacy of patients with castration-resistant prostate cancer treated with novel hormonal therapies. Int. J. Biol. Mark. 2018, 33, 132–133. [Google Scholar] [CrossRef] [PubMed]
- Asif, S.; Teply, B.A. Biomarkers for Treatment Response in Advanced Prostate Cancer. Cancers 2021, 13, 5723. [Google Scholar] [CrossRef] [PubMed]
- Emmett, L.; Crumbaker, M.; Ho, B.; Willowson, K.; Eu, P.; Ratnayake, L.; Epstein, R.; Blanksby, A.; Horvath, L.; Guminski, A.; et al. Results of a Prospective Phase 2 Pilot Trial of 177Lu–PSMA-617 Therapy for Metastatic Castration-Resistant Prostate Cancer Including Imaging Predictors of Treatment Response and Patterns of Progression. Clin. Genitourin. Cancer 2019, 17, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sawyers, C.L.; Scher, H.I. Targeting the androgen receptor pathway in prostate cancer. Curr. Opin. Pharmacol. 2008, 8, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Rosar, F.; Dewes, S.; Ries, M.; Schaefer, A.; Khreish, F.; Maus, S.; Bohnenberger, H.; Linxweiler, J.; Bartholomä, M.; Ohlmann, C.; et al. New insights in the paradigm of upregulation of tumoral PSMA expression by androgen receptor blockade: Enzalutamide induces PSMA upregulation in castration-resistant prostate cancer even in patients having previously progressed on enzalutamide. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Manafi-Farid, R.; Harsini, S.; Saidi, B.; Ahmadzadehfar, H.; Herrmann, K.; Briganti, A.; Walz, J.; Beheshti, M. Factors predicting biochemical response and survival benefits following radioligand therapy with [177Lu]Lu-PSMA in metastatic castrate-resistant prostate cancer: A review. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4028–4041. [Google Scholar] [CrossRef] [PubMed]
- Violet, J.; Jackson, P.; Ferdinandus, J.; Sandhu, S.; Akhurst, T.; Iravani, A.; Kong, G.; Kumar, A.R.; Thang, S.P.; Eu, P.; et al. Dosimetry of 177Lu-PSMA-617 in Metastatic Castration-Resistant Prostate Cancer: Correlations Between Pretherapeutic Imaging and Whole-Body Tumor Dosimetry with Treatment Outcomes. J. Nucl. Med. 2019, 60, 517–523. [Google Scholar] [CrossRef]
- Meller, B.; Bremmer, F.; Sahlmann, C.O.; Hijazi, S.; Bouter, C.; Trojan, L.; Meller, J.; Thelen, P. Alterations in androgen deprivation enhanced prostate-specific membrane antigen (PSMA) expression in prostate cancer cells as a target for diagnostics and therapy. EJNMMI Res. 2015, 5, 66. [Google Scholar] [CrossRef]
- Murga, J.D.; Moorji, S.M.; Han, A.Q.; Magargal, W.W.; DiPippo, V.A.; Olson, W.C. Synergistic co-targeting of prostate-specific membrane antigen and androgen receptor in prostate cancer. Prostate 2015, 75, 242–254. [Google Scholar] [CrossRef]
- Kranzbühler, B.; Salemi, S.; Umbricht, C.A.; Müller, C.; Burger, I.A.; Sulser, T.; Eberli, D. Pharmacological upregulation of prostate-specific membrane antigen (PSMA) expression in prostate cancer cells. Prostate 2018, 78, 758–765. [Google Scholar] [CrossRef]
- Kranzbühler, B.; Salemi, S.; Umbricht, C.A.; Deberle, L.M.; Müller, C.; Burger, I.A.; Hermanns, T.; Sulser, T.; Eberli, D. Concentration-dependent effects of dutasteride on prostate-specific membrane antigen (PSMA) expression and uptake of 177Lu-PSMA-617 in LNCaP cells. Prostate 2019, 79, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Mathy, C.S.; Mayr, T.; Kürpig, S.; Meisenheimer, M.; Dolscheid-Pommerich, R.C.; Stoffel-Wagner, B.; Kristiansen, G.; Essler, M.; Muders, M.H.; Bundschuh, R.A. Antihormone treatment differentially regulates PSA secretion, PSMA expression and 68Ga–PSMA uptake in LNCaP cells. J. Cancer Res. Clin. Oncol. 2021, 147, 1733–1743. [Google Scholar] [CrossRef] [PubMed]
- Staniszewska, M.; Costa, P.F.; Eiber, M.; Klose, J.; Wosniack, J.; Reis, H.; Szarvas, T.; Hadaschik, B.; Lückerath, K.; Herrmann, K.; et al. Enzalutamide Enhances PSMA Expression of PSMA-Low Prostate Cancer. Int. J. Mol. Sci. 2021, 22, 7431. [Google Scholar] [CrossRef] [PubMed]
- Hope, T.A.; Truillet, C.C.; Ehman, E.C.; Afshar-Oromieh, A.; Aggarwal, R.; Ryan, C.J.; Carroll, P.R.; Small, E.J.; Evans, M.J. 68Ga-PSMA-11 PET Imaging of Response to Androgen Receptor Inhibition: First Human Experience. J. Nucl. Med. 2017, 58, 81–84. [Google Scholar] [CrossRef]
- Lückerath, K.; Wei, L.; Fendler, W.P.; Evans-Axelsson, S.; Stuparu, A.D.; Slavik, R.; Mona, C.E.; Calais, J.; Rettig, M.; Reiter, R.E.; et al. Preclinical evaluation of PSMA expression in response to androgen receptor blockade for theranostics in prostate cancer. EJNMMI Res. 2018, 8, 96. [Google Scholar] [CrossRef]
- Emmett, L.M.; Yin, C.; Crumbaker, M.; Hruby, G.; Kneebone, A.; Epstein, R.; Nguyen, Q.; Hickey, A.; Ihsheish, N.; O’Neill, G.; et al. Rapid Modulation of PSMA Expression by Androgen Deprivation: Serial 68Ga-PSMA-11 PET in Men with Hormone-Sensitive and Castrate-Resistant Prostate Cancer Commencing Androgen Blockade. J. Nucl. Med. 2019, 60, 950–954. [Google Scholar] [CrossRef]
- Rosar, F.; Neher, R.; Burgard, C.; Linxweiler, J.; Schreckenberger, M.; Hoffmann, M.A.; Bartholomä, M.; Khreish, F.; Ezziddin, S. Upregulation of PSMA Expression by Enzalutamide in Patients with Advanced mCRPC. Cancers 2022, 14, 1696. [Google Scholar] [CrossRef]
- Emmett, L.; Subramaniam, S.; Joshua, A.M.; Crumbaker, M.; Martin, A.; Zhang, A.Y.; Rana, N.; Langford, A.; Mitchell, J.; Yip, S.; et al. ENZA-p trial protocol: A randomized phase II trial using prostate-specific membrane antigen as a therapeutic target and prognostic indicator in men with metastatic castration-resistant prostate cancer treated with enzalutamide (ANZUP 1901). Br. J. Urol. 2021, 128, 642–651. [Google Scholar] [CrossRef]
- Serda, R.E.; Bisoffi, M.; Thompson, T.A.; Ji, M.; Omdahl, J.L.; Sillerud, L.O. 1α,25-Dihydroxyvitamin D3 down-regulates expression of prostate specific membrane antigen in prostate cancer cells. Prostate 2008, 68, 773–783. [Google Scholar] [CrossRef]
- Ruigrok, E.A.; van Weerden, W.M.; Nonnekens, J.; de Jong, M. The Future of PSMA-Targeted Radionuclide Therapy: An Overview of Recent Preclinical Research. Pharmaceutics 2019, 11, 560. [Google Scholar] [CrossRef]
- Hong, S.-W.; Lee, S.-H.; Moon, J.-H.; Hwang, J.J.; E Kim, D.; Ko, E.; Kim, H.-S.; Cho, I.J.; Kang, J.S.; Kim, J.-E.; et al. SVCT-2 in breast cancer acts as an indicator for L-ascorbate treatment. Oncogene 2013, 32, 1508–1517. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Espey, M.G.; Krishna, M.C.; Mitchell, J.B.; Corpe, C.P.; Buettner, G.R.; Shacter, E.; Levine, M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues. Proc. Natl. Acad. Sci. USA 2005, 102, 13604–13609. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Cieslak, J.A., 3rd; Welsh, J.L.; Sibenaller, Z.A.; Allen, B.G.; Wagner, B.A.; Kalen, A.L.; Doskey, C.M.; Strother, R.K.; Button, A.M.; et al. Pharmacological ascorbate radiosensitizes pancreatic cancer. Cancer Res. 2015, 75, 3314–3326. [Google Scholar] [CrossRef]
- Alexander, M.S.; Wilkes, J.G.; Schroeder, S.R.; Buettner, G.R.; Wagner, B.A.; Du, J.; Gibson-Corley, K.; O’Leary, B.R.; Spitz, D.R.; Buatti, J.M.; et al. Pharmacologic Ascorbate Reduces Radiation-Induced Normal Tissue Toxicity and Enhances Tumor Radiosensitization in Pancreatic Cancer. Cancer Res. 2018, 78, 6838–6851. [Google Scholar] [CrossRef] [PubMed]
- Jafari, E.; Ahmadzadehfar, H.; Bagheri, D.; Amini, A.; Assadi, M. Assessment of early oxidative stress following the use of radiotheranostics agents 177Lu-PSMA for prostate cancer and 177Lu-DOTATATE for neuroendocrine tumors; radioprotective effect of vitamin C. Nucl. Med. Commun. 2021, 42, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Lamoureux, F.; Crafter, C.; Davies, B.R.; Beraldi, E.; Fazli, L.; Kim, S.; Thaper, D.; Gleave, M.E.; Zoubeidi, A. Synergistic Targeting of PI3K/AKT Pathway and Androgen Receptor Axis Significantly Delays Castration-Resistant Prostate Cancer Progression In Vivo. Mol. Cancer Ther. 2013, 12, 2342–2355. [Google Scholar] [CrossRef]
- Carver, B.S.; Chapinski, C.; Wongvipat, J.; Hieronymus, H.; Chen, Y.; Chandarlapaty, S.; Arora, V.K.; Le, C.; Koutcher, J.; Scher, H.; et al. Reciprocal Feedback Regulation of PI3K and Androgen Receptor Signaling in PTEN-Deficient Prostate Cancer. Cancer Cell 2011, 19, 575–586. [Google Scholar] [CrossRef]
- Lee, S.H.; Johnson, D.; Luong, R.; Sun, Z. Crosstalking between Androgen and PI3K/AKT Signaling Pathways in Prostate Cancer Cells. J. Biol. Chem. 2015, 290, 2759–2768. [Google Scholar] [CrossRef]
- Yao, E.; Zhou, W.; Lee-Hoeflich, S.T.; Truong, T.; Haverty, P.M.; Eastham-Anderson, J.; Lewin-Koh, N.; Gunter, B.; Belvin, M.; Murray, L.J.; et al. Suppression of HER2/HER3-mediated growth of breast cancer cells with combinations of GDC-0941 PI3K inhibitor, trastuzumab, and pertuzumab. Clin. Cancer Res. 2009, 15, 4147–4156. [Google Scholar] [CrossRef]
- Pezaro, C. PARP inhibitor combinations in prostate cancer. Ther. Adv. Med. Oncol. 2020, 12, 1758835919897537. [Google Scholar] [CrossRef]
- Ruigrok, E.A.M.; Verkaik, N.S.; de Blois, E.; de Ridder, C.; Stuurman, D.; Roobol, S.J.; Van Gent, D.C.; de Jong, M.; Van Weerden, W.M.; Nonnekens, J. Preclinical Assessment of the Combination of PSMA-Targeting Radionuclide Therapy with PARP Inhibitors for Prostate Cancer Treatment. Int. J. Mol. Sci. 2022, 23, 8037. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V.; Zengerling, F.; Steinacker, J.P.; Bolenz, C.; Beer, M.; Wiegel, T.; Eiber, M.; Fleshner, N.; Beer, A.J. First experiences with Lu-177 PSMA therapy in combination with Pembrolizumab or after pretreatment with Olaparib in single patients. J. Nucl. Med. 2020, 62, 975–978. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, S.K.; Joshua, A.M.; Emmett, L.; Spain, L.; Horvath, L.G.; Crumbaker, M.; Anton, A.; Wallace, R.; Pasam, A.; Bressel, M.; et al. PRINCE: Interim Analysis of the Phase Ib Study of 177Lu-PSMA-617 in Combination with Pembrolizumab for Metastatic Castration Resistant Prostate Cancer. Ann. Oncol. 2021, 32, S626–S627. [Google Scholar] [CrossRef]
- Sandhu, S.; Joshua, A.M.; Emmett, L.; Spain, L.A.; Horvath, L.; Crumbaker, M.; Anton, A.; Wallace, R.; Pasam, A.; Bressel, M.; et al. PRINCE: Phase I Trial of 177Lu-PSMA-617 in Combination with Pembrolizumab in Patients with mCRPC. J. Clin. Oncol. 2022, 40, 5017. [Google Scholar] [CrossRef]
- Sweeney, C.; Percent, I.J.; Babu, S.; Cultrera, J.; Mehlhaff, B.A.; Goodman, O.B., Jr.; Morris, D.; Schnadig, I.D.; Albany, C.; Shore, N.D.; et al. Phase 1b/2 study of enzalutamide (ENZ) with LY3023414 (LY) or placebo (PL) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) after progression on abiraterone. J. Clin. Oncol. 2019, 37, 5009. [Google Scholar] [CrossRef]
- Zhang, H.; Abou, D.; Lu, P.; Hasson, A.M.; Villmer, A.; Benabdallah, N.; Jiang, W.; Ulmert, D.; Carlin, S.; Rogers, B.E.; et al. [18F]-Labeled PARP-1 PET imaging of PSMA targeted alpha particle radiotherapy response. Sci. Rep. 2022, 12, 13034. [Google Scholar] [CrossRef]
- Derlin, T.; Sommerlath Sohns, J.M.; Schmuck, S.; Henkenberens, C.; von Klot, C.A.J.; Ross, T.L.; Bengel, F.M. Influence of short-term dexamethasone on the efficacy of 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer. Prostate 2020, 80, 619–631. [Google Scholar] [CrossRef]
- Aghdam, R.A.; Amoui, M.; Ghodsirad, M.; Khoshbakht, S.; Mofid, B.; Kaghazchi, F.; Tavakoli, M.; Pirayesh, E.; Ahmadzadehfar, H. Efficacy and safety of 177Lutetium-prostate-specific membrane antigen therapy in metastatic castration-resistant prostate cancer patients: First experience in West Asia—A prospective study. World J. Nucl. Med. 2019, 18, 258–265. [Google Scholar] [CrossRef]
- Ahmadzadehfar, H.; Rahbar, K.; Kürpig, S.; Bögemann, M.; Claesener, M.; Eppard, E.; Gärtner, F.; Rogenhofer, S.; Schäfers, M.; Essler, M. Early side effects and first results of radioligand therapy with 177Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: A two-centre study. EJNMMI Res. 2015, 5, 36. [Google Scholar] [CrossRef]
- Ahmadzadehfar, H.; Eppard, E.; Kürpig, S.; Fimmers, R.; Yordanova, A.; Schlenkhoff, C.; Rogenhofer, S.; Essler, M. Therapeutic response and side effects of repeated radioligand therapy with 177Lu-PSMA-DKFZ-617 of castrate-resistant metastatic prostate cancer. Oncotarget 2016, 7, 12477. [Google Scholar] [CrossRef]
- Ahmadzadehfar, H.; Wegen, S.; Yordanova, A.; Fimmers, R.; Kürpig, S.; Eppard, E.; Wei, X.; Schlenkhoff, C.; Hauser, S.; Essler, M. Overall survival and response pattern of castration-resistant metastatic prostate cancer to multiple cycles of radioligand therapy using [177Lu]Lu-PSMA-617. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzadehfar, H.; Zimbelmann, S.; Yordanova, A.; Fimmers, R.; Kürpig, S.; Eppard, E.; Gaertner, F.C.; Wei, X.; Hauser, S.; Essler, M. Radioligand therapy of metastatic prostate cancer using 177Lu-PSMA-617 after radiation exposure to 223Ra-dichloride. Oncotarget 2017, 8, 55567–55574. [Google Scholar] [CrossRef] [PubMed]
- Assadi, M.; Rezaei, S.; Jafari, E.; Rekabpour, S.J.; Ravanbod, M.R.; Zohrabi, F.; Amini, A.; Keshmiri, S.; Dadgar, H.; Ahmadzadehfar, H. Potential application of lutetium-177-labeled prostate-specific membrane antigen-617 radioligand therapy for metastatic castration-resistant prostate cancer in a limited resource environment: Initial clinical experience after 2 years. World J. Nucl. Med. 2020, 19, 15–20. [Google Scholar] [CrossRef]
- Bräuer, A.; Grubert, L.S.; Roll, W.; Schrader, A.J.; Schäfers, M.; Bögemann, M.; Rahbar, K. 177Lu-PSMA-617 radioligand therapy and outcome in patients with metastasized castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Crumbaker, M.; Pathmanandavel, S.; Yam, A.O.; Nguyen, A.; Ho, B.; Chan, L.; Ende, J.A.; Rofe, C.; Kongrak, K.; Kwan, E.M.; et al. Phase I/II Trial of the Combination of 177Lutetium Prostate specific Membrane Antigen 617 and Idronoxil (NOX66) in Men with End-stage Metastatic Castration-resistant Prostate Cancer (LuPIN). Eur. Urol. Oncol. 2021, 4, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Delker, A.; Fendler, W.P.; Kratochwil, C.; Brunegraf, A.; Gosewisch, A.; Gildehaus, F.J.; Tritschler, S.; Stief, C.G.; Kopka, K.; Haberkorn, U.; et al. Dosimetry for 177Lu-DKFZ-PSMA-617: A new radiopharmaceutical for the treatment of metastatic prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 42–51. [Google Scholar] [CrossRef]
- Von Eyben, F.E.; Singh, A.; Zhang, J.; Nipsch, K.; Meyrick, D.; Lenzo, N.; Kairemo, K.; Joensuu, T.; Virgolini, I.; Soydal, C.; et al. 177Lu-PSMA radioligand therapy of predominant lymph node metastatic prostate cancer. Oncotarget 2019, 10, 2451–2461. [Google Scholar] [CrossRef][Green Version]
- Fendler, W.P.; Reinhardt, S.; Ilhan, H.; Delker, A.; Böning, G.; Gildehaus, F.J.; Stief, C.; Bartenstein, P.; Gratzke, C.; Lehner, S.; et al. Preliminary experience with dosimetry, response and patient reported outcome after 177Lu-PSMA-617 therapy for metastatic castration-resistant prostate cancer. Oncotarget 2019, 8, 3581–3590. [Google Scholar] [CrossRef]
- Ferdinandus, J.; Eppard, E.; Gaertner, F.C.; Kürpig, S.; Fimmers, R.; Yordanova, A.; Hauser, S.; Feldmann, G.; Essler, M.; Ahmadzadehfar, H. Predictors of Response to Radioligand Therapy of Metastatic Castrate-Resistant Prostate Cancer with 177Lu-PSMA-617. J. Nucl. Med. 2017, 58, 312–319. [Google Scholar] [CrossRef]
- Gupta, M.; Choudhury, P.; Karthikeyan, G.; Talwar, V.; Rawal, S. Clinical outcome of radioligand therapy with Lu-177 PSMA in metastatic prostate cancer: An initial experience from a tertiary care cancer hospital. Eur. J. Nucl. Med. Mol. Imaging 2020, 47 (Suppl. S1), S424–S425. [Google Scholar]
- Gupta, M.; Karthikeyan, G.; Choudhury, P.S.; Sharma, A.; Singh, A.; Rawal, S. Is 177Lu-PSMA an effective treatment modality for mCRPC patients with bone and visceral metastasis? Hell J. Nucl. Med. 2020, 23, 312–320. [Google Scholar] [PubMed]
- Heck, M.M.; Retz, M.; D’Alessandria, C.; Rauscher, I.; Scheidhauer, K.; Maurer, T.; Storz, E.; Janssen, F.; Schottelius, M.; Wester, H.-J.; et al. Systemic Radioligand Therapy with 177Lu Labeled Prostate Specific Membrane Antigen Ligand for Imaging and Therapy in Patients with Metastatic Castration Resistant Prostate Cancer. J. Urol. 2016, 196, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Heck, M.M.; Tauber, R.; Schwaiger, S.; Retz, M.; D’Alessandria, C.; Maurer, T.; Gafita, A.; Wester, H.J.; Gschwend, J.E.; Weber, W.A.; et al. Treatment Outcome, Toxicity, and Predictive Factors for Radioligand Therapy with 177Lu-PSMA-I&T in Metastatic Castration-resistant Prostate Cancer. Eur. Urol. 2019, 75, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhurst, T.; Iravani, A.; Kong, G.; Kumar, A.R.; Murphy, D.G.; et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; Ng, S.; et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomised, open-label, phase 2 trial. Lancet 2021, 397, 797–804. [Google Scholar] [CrossRef] [PubMed]
- van Kalmthout, L.; Braat, A.; Lam, M.; van Leeuwaarde, R.; Krijger, G.; Ververs, T.; Mehra, N.; Bins, A.; Hunting, J.; de Keizer, B. First Experience With 177Lu-PSMA-617 Therapy for Advanced Prostate Cancer in the Netherlands. Clin. Nucl. Med. 2019, 44, 446–451. [Google Scholar] [CrossRef]
- Kesavan, M.; Turner, J.H.; Meyrick, D.; Yeo, S.; Cardaci, G.; Lenzo, N.P. Salvage Radiopeptide Therapy of Advanced Castrate-Resistant Prostate Cancer with Lutetium-177-Labeled Prostate-Specific Membrane Antigen: Efficacy and Safety in Routine Practice. Cancer Biother. Radiopharm. 2018, 33, 274–281. [Google Scholar] [CrossRef]
- Kessel, K.; Seifert, R.; Schäfers, M.; Weckesser, M.; Schlack, K.; Boegemann, M.; Rahbar, K. Second line chemotherapy and visceral metastases are associated with poor survival in patients with mCRPC receiving 177Lu-PSMA-617. Theranostics 2019, 9, 4841–4848. [Google Scholar] [CrossRef]
- Khreish, F.; Ebert, N.; Ries, M.; Maus, S.; Rosar, F.; Bohnenberger, H.; Stemler, T.; Saar, M.; Bartholomä, M.; Ezziddin, S. 225Ac-PSMA-617/177Lu-PSMA-617 tandem therapy of metastatic castration-resistant prostate cancer: Pilot experience. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 721–728. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Eder, M.; Afshar-Oromieh, A.; Benešová, M.; Mier, W.; Kopka, K.; Haberkorn, U. [177Lu]Lutetium-labelled PSMA ligand-induced remission in a patient with metastatic prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 987–988. [Google Scholar] [CrossRef]
- Maffey-Steffan, J.; Scarpa, L.; Svirydenka, A.; Nilica, B.; Mair, C.; Buxbaum, S.; Bektic, J.; von Guggenberg, E.; Uprimny, C.; Horninger, W.; et al. The 68Ga/177Lu-theragnostic concept in PSMA-targeting of metastatic castration–resistant prostate cancer: Impact of post-therapeutic whole-body scintigraphy in the follow-up. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 695–712. [Google Scholar] [CrossRef] [PubMed]
- Meyrick, D.; Gallyamov, M.; Sabarimurugan, S.; Falzone, N.; Lenzo, N. Real-World Data Analysis of Efficacy and Survival After Lutetium-177 Labelled PSMA Ligand Therapy in Metastatic Castration-Resistant Prostate Cancer. Target. Oncol. 2021, 16, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, S.; Thieme, A.; Allmann, J.; D’Alessandria, C.; Maurer, T.; Retz, M.; Tauber, R.; Heck, M.M.; Wester, H.-J.; Tamaki, N.; et al. Radiation Dosimetry for 177Lu-PSMA I&T in Metastatic Castration-Resistant Prostate Cancer: Absorbed Dose in Normal Organs and Tumor Lesions. J. Nucl. Med. 2017, 58, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, K.; Bode, A.; Weckesser, M.; Avramovic, N.; Claesener, M.; Stegger, L.; Bögemann, M. Radioligand Therapy With 177Lu-PSMA-617 as A Novel Therapeutic Option in Patients with Metastatic Castration Resistant Prostate Cancer. Clin. Nucl. Med. 2016, 41, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, K.; Schmidt, M.; Heinzel, A.; Eppard, E.; Bode, A.; Yordanova, A.; Claesener, M.; Ahmadzadehfar, H. Response and Tolerability of a Single Dose of 177Lu-PSMA-617 in Patients with Metastatic Castration-Resistant Prostate Cancer: A Multicenter Retrospective Analysis. J. Nucl. Med. 2016, 57, 1334–1338. [Google Scholar] [CrossRef]
- Rahbar, K.; Ahmadzadehfar, H.; Kratochwil, C.; Haberkorn, U.; Schäfers, M.; Essler, M.; Baum, R.P.; Kulkarni, H.R.; Schmidt, M.; Drzezga, A.; et al. German Multicenter Study Investigating 177Lu-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. J. Nucl. Med. 2017, 58, 85–90. [Google Scholar] [CrossRef]
- Rahbar, K.; Boegemann, M.; Yordanova, A.; Eveslage, M.; Schäfers, M.; Essler, M.; Ahmadzadehfar, H. PSMA targeted radioligandtherapy in metastatic castration resistant prostate cancer after chemotherapy, abiraterone and/or enzalutamide. A retrospective analysis of overall survival. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 12–19. [Google Scholar] [CrossRef]
- Rasul, S.; Hacker, M.; Kretschmer-Chott, E.; Leisser, A.; Grubmüller, B.; Kramer, G.; Shariat, S.; Wadsak, W.; Mitterhauser, M.; Hartenbach, M.; et al. Clinical outcome of standardized 177Lu-PSMA-617 therapy in metastatic prostate cancer patients receiving 7400 MBq every 4 weeks. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 713–720. [Google Scholar] [CrossRef]
- Rathke, H.; Giesel, F.L.; Flechsig, P.; Kopka, K.; Mier, W.; Hohenfellner, M.; Haberkorn, U.; Kratochwil, C. Repeated 177Lu-Labeled PSMA-617 Radioligand Therapy Using Treatment Activities of Up to 9.3 GBq. J. Nucl. Med. 2018, 59, 459–465. [Google Scholar] [CrossRef]
- Sarnelli, A.; Belli, M.L.; Di Iorio, V.; Mezzenga, E.; Celli, M.; Severi, S.; Tardelli, E.; Nicolini, S.; Oboldi, D.; Uccelli, L.; et al. Dosimetry of 177Lu-PSMA-617 after Mannitol Infusion and Glutamate Tablet Administration: Preliminary Results of EUDRACT/RSO 2016-002732-32 IRST Protocol. Molecules 2019, 24, 621. [Google Scholar] [CrossRef]
- Scarpa, L.; Buxbaum, S.; Kendler, D.; Fink, K.; Bektic, J.; Gruber, L.; Decristoforo, C.; Uprimny, C.; Lukas, P.; Horninger, W.; et al. The 68Ga/177Lu theragnostic concept in PSMA targeting of castration-resistant prostate cancer: Correlation of SUV(max) values and absorbed dose estimates. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Seifert, R.; Kessel, K.; Schlack, K.; Weckesser, M.; Bögemann, M.; Rahbar, K. Radioligand therapy using [177Lu]Lu-PSMA-617 in mCRPC: A pre-VISION single-center analysis. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2106–2112. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, S.T.; Osborne, J.R.; Hackett, A.; Niaz, M.J.; Cooley, V.; Christos, P.; Vlachostergios, P.J.; Thomas, C.; Gracey, L.; Beltran, H.; et al. Preliminary results of a phase I/II dose-escalation study of fractionated dose 177Lu-PSMA-617 for progressive metastatic castration resistant prostate cancer (mCRPC). Ann. Oncol. 2019, 30, 329–330. [Google Scholar] [CrossRef]
- Yadav, M.P.; Ballal, S.; Bal, C.; Sahoo, R.K.; Damle, N.A.; Tripathi, M.; Seth, A. Efficacy and Safety of 177Lu-PSMA-617 Radioligand Therapy in Metastatic Castration-Resistant Prostate Cancer Patients. Clin. Nucl. Med. 2020, 45, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Yordanova, A.; Becker, A.; Eppard, E.; Kürpig, S.; Fisang, C.; Feldmann, G.; Essler, M.; Ahmadzadehfar, H. The impact of repeated cycles of radioligand therapy using [177Lu]Lu-PSMA-617 on renal function in patients with hormone refractory metastatic prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1473–1479. [Google Scholar] [CrossRef]
- Violet, J.; Sandhu, S.; Iravani, A.; Ferdinandus, J.; Thang, S.-P.; Kong, G.; Kumar, A.R.; Akhurst, T.; Pattison, D.; Beaulieu, A.; et al. Long-Term Follow-up and Outcomes of Retreatment in an Expanded 50-Patient Single-Center Phase II Prospective Trial of 177Lu-PSMA-617 Theranostics in Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2020, 61, 857–865. [Google Scholar] [CrossRef]
- Moghadam, S.Z.; Askari, E.; Divband, G.; Shakeri, S.; Aryana, K. Efficacy, safety and prognostic factors affecting overall survival among metastatic prostate cancer patients undergoing treatment with 177Lu-PSMA-617: A single center study. Rev. Esp. Med. Nucl. Imagen Mol. 2022, 41, 239–246. [Google Scholar] [CrossRef]
- Schneider, C.A.; Täger, P.; Hammes, J.; Fischer, T.; Drzezga, A.; Pfister, D.; Heidenreich, A.; Schmidt, M. Treatment outcome and identification of factors influencing overall survival after Lu-177-PSMA-617 radioligand therapy in metastatic prostate cancer. Nuklearmedizin-NuclearMedicine 2022, 61, 25–32. [Google Scholar] [CrossRef]
- Rathke, H.; Giesel, F.; Haberkorn, U.; Kratochwil, C. Dose escalation of Lu-177-PSMA-617 from 4 to 9.3 GBq per cycle in patients with mCRPC. J. Nucl. Med. 2017, 58, 313. [Google Scholar]
- Barnum, K.J.; O’Connell, M.J. Cell Cycle Regulation by Checkpoints. Methods Mol. Biol. 2014, 1170, 29–40. [Google Scholar]
- Foray, N.; Charvet, A.-M.; Duchemin, D.; Favaudon, V.; Lavalette, D. The repair rate of radiation-induced DNA damage: A stochastic interpretation based on the Gamma function. J. Theor. Biol. 2005, 236, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Z.; Selby, C.P.; Sancar, A. Long-term, genome-wide kinetic analysis of the effect of the circadian clock and transcription on the repair of cisplatin-DNA adducts in the mouse liver. J. Biol. Chem. 2019, 294, 11960–11968. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Oromieh, A.; Haberkorn, U.; Zechmann, C.; Armor, T.; Mier, W.; Spohn, F.; Debus, N.; Holland-Letz, T.; Babich, J.; Kratochwil, C. Repeated PSMA-targeting radioligand therapy of metastatic prostate cancer with 131I-MIP-1095. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Gafita, A.; Fendler, W.P.; Hui, W.; Sandhu, S.; Weber, M.; Esfandiari, R.; Calais, J.; Rauscher, I.; Rathke, H.; Tauber, R.; et al. Efficacy and Safety of 177Lu-labeled Prostate-specific Membrane Antigen Radionuclide Treatment in Patients with Diffuse Bone Marrow Involvement: A Multicenter Retrospective Study. Eur. Urol. 2020, 78, 148–154. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Rathke, H.; Bronzel, M.; Apostolidis, C.; Weichert, W.; Haberkorn, U.; Giesel, F.L.; Morgenstern, A. Targeted α-Therapy of Metastatic Castration-Resistant Prostate Cancer with 225Ac-PSMA-617: Dosimetry Estimate and Empiric Dose Finding. J. Nucl. Med. 2017, 58, 1624–1631. [Google Scholar] [CrossRef]
- Leibowitz, R.; Davidson, T.; Gadot, M.; Aharon, M.; Malki, A.; Levartovsky, M.; Oedegaard, C.; Saad, A.; Sandler, I.; Ben-Haim, S.; et al. A Retrospective Analysis of the Safety and Activity of Lutetium-177-Prostate-Specific Membrane Antigen Radionuclide Treatment in Older Patients with Metastatic Castration-Resistant Prostate Cancer. Oncologist 2020, 25, 787–792. [Google Scholar] [CrossRef]
- Sathekge, M.; Bruchertseifer, F.; Knoesen, O.; Reyneke, F.; Lawal, I.; Lengana, T.; Davis, C.; Mahapane, J.; Corbett, C.; Vorster, M.; et al. 225Ac-PSMA-617 in chemotherapy-naive patients with advanced prostate cancer: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 129–138. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; Babich, J.W.; Kratochwil, C.; Giesel, F.L.; Eisenhut, M.; Kopka, K.; Haberkorn, U. The Rise of PSMA Ligands for Diagnosis and Therapy of Prostate Cancer. J. Nucl. Med. 2016, 57 (Suppl. S3), 79S–89S. [Google Scholar] [CrossRef]
- Haberkorn, U.; Eder, M.; Kopka, K.; Babich, J.W.; Eisenhut, M. New Strategies in Prostate Cancer: Prostate-Specific Membrane Antigen (PSMA) Ligands for Diagnosis and Therapy. Clin. Cancer Res. 2016, 22, 9–15. [Google Scholar] [CrossRef]
- Kratochwil, C.; Haberkorn, U.; Giesel, F.L. 225Ac-PSMA-617 for Therapy of Prostate Cancer. Semin. Nucl. Med. 2020, 50, 133–140. [Google Scholar] [CrossRef]
- Iravani, A.; Violet, J.; Azad, A.; Hofman, M.S. Lutetium-177 prostate-specific membrane antigen (PSMA) theranostics: Practical nuances and intricacies. Prostate Cancer Prostatic Dis. 2020, 23, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Rosar, F.; Kochems, N.; Bartholomä, M.; Maus, S.; Stemler, T.; Linxweiler, J.; Khreish, F.; Ezziddin, S. Renal Safety of [177Lu]Lu-PSMA-617 Radioligand Therapy in Patients with Compromised Baseline Kidney Function. Cancers 2021, 13, 3095. [Google Scholar] [CrossRef] [PubMed]
- Baum, R.P.; Langbein, T.; Singh, A.; Shahinfar, M.; Schuchardt, C.; Volk, G.F.; Kulkarni, H. Injection of Botulinum Toxin for Preventing Salivary Gland Toxicity after PSMA Radioligand Therapy: An Empirical Proof of a Promising Concept. Nucl. Med. Mol. Imaging 2018, 52, 80–81. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, E.; Lau, J.; Kuo, H.-T.; Zhang, Z.; Merkens, H.; Hundal-Jabal, N.; Colpo, N.; Lin, K.-S.; Bénard, F. Monosodium Glutamate Reduces 68Ga-PSMA-11 Uptake in Salivary Glands and Kidneys in a Preclinical Prostate Cancer Model. J. Nucl. Med. 2018, 59, 1865–1868. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Giesel, F.L.; Leotta, K.; Eder, M.; Hoppe-Tich, T.; Youssoufian, H.; Kopka, K.; Babich, J.W.; Haberkorn, U. PMPA for Nephroprotection in PSMA-Targeted Radionuclide Therapy of Prostate Cancer. J. Nucl. Med. 2015, 56, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Harsini, S.; Saprunoff, H.; Alden, T.M.; Mohammadi, B.; Wilson, D.; Bénard, F. The effects of monosodium glutamate on PSMA radiotracer uptake in men with recurrent prostate cancer: A prospective, randomized, double-blind, placebo-controlled intraindividual imaging study. J. Nucl. Med. 2021, 62, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Sarnelli, A.; Belli, M.L.; Di Iorio, V.; Mezzenga, E.; Celli, M.; Severi, S.; Tardelli, E.; Nicolini, S.; Oboldi, D.; Uccelli, L.; et al. Dosimetry of 177Lu-PSMA 617 after Mannitol infusion and Glutamate candies administration: A strategy to reduce adsorbed dose to kidneys and parotid glands. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, S52–S53. [Google Scholar]
- Yilmaz, B.; Nisli, S.; Ergul, N.; Gursu, R.U.; Acikgoz, O.; Çermik, T.F. Effect of External Cooling on 177Lu-PSMA Uptake by the Parotid Glands. J. Nucl. Med. 2019, 60, 1388–1393. [Google Scholar] [CrossRef]
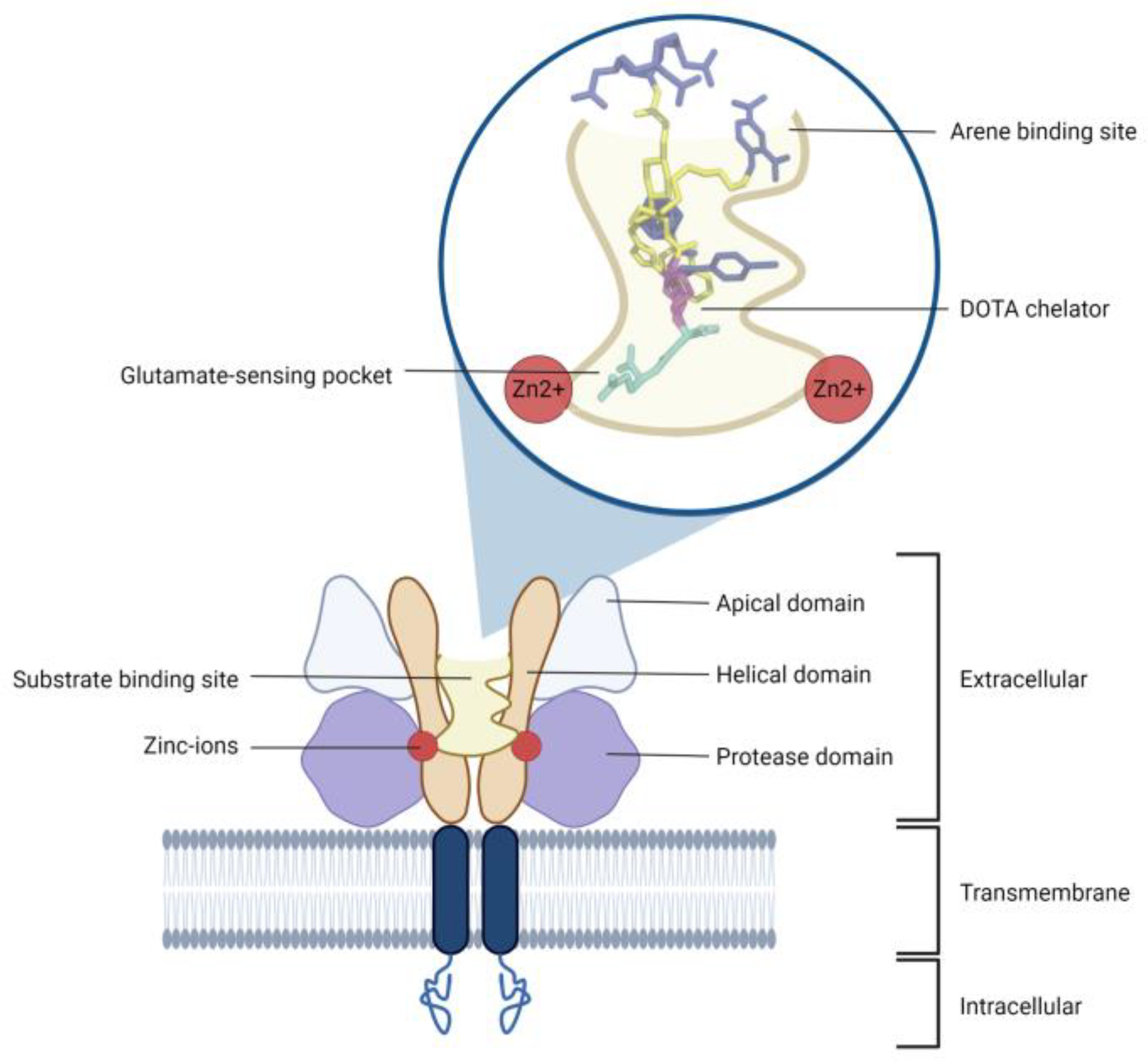

| Study Design | Therapy | Patients | PSA Response | Survival | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of Study | Follow-Up Period (After Last Cycle) | Agent | Treatment Dose (GBq) | No. of Cycles | Interval (Weeks) | Population | Prior Therapy | No. of Patients | Mean Age | Any Decrease | Decrease ≥50% | Median PFS (Weeks) | Median OS (Weeks) | Ref |
| P, SS | 8 weeks | Lu-PSMA-617 | 4.4–6.6 | 1–6 | mCRPC | AA, CTx | 14 | 70.57 | 78.6% | 45.4% | - | - | [92] | |
| P, MS | 8 weeks | Lu-PSMA-617 | 5.6 | 1 | n/a | mCRPC | AA, CTx | 10 | 73.5 | 70% | 50% | - | - | [93] |
| R, SS | 8 weeks | Lu-PSMA-617 | 6 | 2 | 12 | mCRPC | AA, CTx | 24 | 75.2 | 79.1% | 41.6% | - | - | [94] |
| P, SS | 8 weeks | Lu-PSMA-617 | 6.0 | 1–6 | 8 | CRPC | AA, CTx, 223Ra | 52 | 70.9 | 80.8% | 44.2% | - | 60 (CI 44.2–75.8) | [95] |
| R, SS, C | Med. 3.4 months | Lu-PSMA-617 | 6 | 1–3 | 8 | mCRPC | AA, CTx | 49 | 71.25 | 67.3% | 53.1% | - | - | [96] |
| P, SS | 9 months (range 1–25) | Lu-PSMA-617 | 3.7–7.4 | 1–4 | 8 | mCRPC | AA, CTx | 21 | 70.3 | 76% | 62% | - | 62.7 (CI 42.1–83.3) | [97] |
| R, SS, C | Med. 10.3 months | Lu-PSMA-I&T + 617 | 3.6–8.6 | 1–10 | ≥6 | mCRPC | AA, CTx, 223Ra | 83 1 | 69.3 | 40% | - | 26.1 (CI 13.9–38.3) | 46.5 (CI 34.3–58.7) | [13] |
| R, SS, C | Med. 10.3 months | Lu-PSMA-I&T + 617 | 3.6–8.6 | 1–10 | ≥6 | mCRPC | AA, CTx, 223Ra | 84 1 | 70.8 | 57% | - | 38.3 (CI 30.9–46.1) | 117.8 (CI 80.0–155.7) | [13] |
| P, SS | 15 months (range 6–28) | Lu-PSMA-I&T | 5.76 | 1–5 | Personalized | mCRPC | AA, CTx, 223Ra | 56 | 72 | 80.4% | 58.9% | 59.6 | NR | [23] |
| R, SS | 24 weeks (range 15–36) | Lu-PSMA-617 | 6.1 | 1–7 | 8 | mCRPC | AA, CTx, 223Ra | 59 | 72 | 91% | 53% | 18.0 (CI 13.6–22.4) | 32.0 (CI 21.1–42.9) | [98] |
| P, SS, C, phase I/II | Med. 16.3 months | Lu-PSMA-617 | 7.5 | 1–6 | 6 | mCRPC | AA, CTx | 32 | 69 | 91% | 62.5% | 26.5 (CI 12.2–40.0) | 74.4 (CI 28.3–117.8) | [99] |
| R, SS | 4 weeks | Lu-PSMA-617 | 3.6 | 2 | 10 | mCRPC | AA, CTx | 5 | 68 | - | - | - | - | [100] |
| R, MS, C | 26 months (range 18–38) | Lu-PSMA-I&T + 617 | >14.8, cum | mCRPC | AA, CTx | 45 | 70 | 92% | 80% | 69.6 | NR | [101] | ||
|
P, SS, phase II | - | Lu-PSMA-617 | 6–8 | 1–4 | 6 | mCRPC | AA, CTx | 14 | 69.5 | 71% | 36% | - | 50 ± 33 | [57] |
| R, SS | 8–10 weeks | Lu-PSMA-617 | 6.0 | 2 | 8–10 | mCRPC | AA, CTx, 223Ra | 10 | 73 | 85% | 60% | - | - | [102] |
| R, SS | 8 weeks | Lu-PSMA-617 | 4.1–7.1 | 1 | n/a | CRPC | AA, CTx, 223Ra | 40 | 71.4 | 67.5% | 32.5% | - | - | [103] |
| P, SS | 8–10 weeks | Lu-PSMA-617 | 7.4 | 1–4 | mCRPC | AA, CTx | 25 | 69 | 84% | - | 24 (CI 9–52) | - | [104] | |
| R, SS | 8–10 weeks | Lu-PSMA-617 | 3.7–7.4 | 1 | n/a | mCRPC | AA, CTx | 10 | 67.1 | 70% | - | 24.0 (CI 8.0–36.0) | - | [105] |
| R, SS | 8 weeks | Lu-PSMA-I&T | 7.4 | 1–4 | 8 | mCRPC | AA, CTx, 223Ra | 22 | 71 | 81.8% | 27.3% | - | - | [106] |
| R, SS | 9.5 months (range 7.0–16.3) | Lu-PSMA-I&T | 7.4 | 1–6 | 6–8 | mCRPC | AA, CTx, 223Ra | 100 | 72 | - | 38% | 17.8 (CI 10.4–24.8) | 56.1 (CI 43.0–69.1) | [107] |
|
P, SS, phase II | 25.0 months (range 12.7–25.2) | Lu-PSMA-617 | 4–8 | 1–4 | 6 | mCRPC | AA, CTx | 30 | 71 | 97% | 57% | 33.0 (CI 27.4–39.1) | 58.7 (CI 45.2–98.7) | [108] |
|
P, MS, C, phase II | 6 months | Lu-PSMA-617 | 6.0–8.5 | 1–6 | 6 | mCRPC | AA | 200 | 71.6 | 89.8% | 66% | 22.2 (CI 14.8–24.8) | NR | [109] |
| R, SS | 7 days | Lu-PSMA-617 | 5.52 | 1 | n/a | mCRPC | AA, CTx | 9 | 69.0 | - | - | - | - | [32] |
| R, SS | 48 h | Lu-PSMA-617 | 7.2 | 1–4 | mCRPC | AA, CTx | 20 | 65 | - | - | - | - | [79] | |
| R, SS | 13.7 months (range 9.8–32.3) | Lu-PSMA-617 | 6 | 1–6 | 6 | mCRPC | AA, CTx, 223Ra | 30 | 70 | - | 57% | - | 49.1 (CI 6.1–140.4) | [110] |
| R, SS | 17 months | Lu-PSMA-I&T | 5.5 | 1–4 | 8 | mCRPC | AA, CTx | 20 | 71 | 40% | - | - | - | [111] |
| R, SS | - | Lu-PSMA-617 | 6.2 | 1–9 | 6–8 | mCRPC | AA, CTx, 223Ra | 109 | 72 | 70% | 32% | - | 43.0 (CI 31.3–54.4) | [112] |
| R, SS | 22 weeks (range 14–63) | Lu-PSMA-617 | 6.9 | 1 | n/a | mCRPC | AA, CTx, 223Ra | 20 | 72 | 90% | 65% | 19 (CI 12–26) | 49 (CI 4–92) | [113] |
| R, SS | - | Lu-PSMA-617 | 7.4, cum | 2 | 12 | mCRPC | AA, CTx | 1 | - | 100% | 100% | - | - | [114] |
| R, SS | 24 weeks | Lu-PSMA-617 | 4–6 | 3 | 8 | CRPC | AA, CTx, 223Ra | 30 | 71.9 | 70% | 43.3% | - | - | [18] |
| R, SS | Med. 19.0 months | Lu-PSMA-I&T | 6.0 | 1–7 | mCRPC | AA, CTx, 223Ra | 119 | 71 | 76.3% | 57.5% | 46.5 | NR | [31] | |
| P, SS | Until death | Lu-PSMA-617 | 6 | 1–6 | 6–10 | mCRPC | AA, CTx, 223Ra | 32 | 71.4 | 71.9% | 37.5% | 30.4 | 52.2 | [115] |
| R, SS | 7 months (range 3–14) | Lu-PSMA-I&T + 617 | 6.1 | 1–5 | mCRPC | AA, CTx | 191 | 70 | 75% | 56% | 17.4 (CI 13.0–34.8) | 52.2 (CI 21.7–78.3) | [116] | |
| R, SS | - | Lu-PSMA-I&T | 7.4 | 1–4 | mCRPC | AA, CTx | 18 | 68 | - | - | - | - | [117] | |
|
P, SS, phase II | - | Lu-PSMA-617 | 3.7–5.5 | 1–2 | 8–12 | mCRPC | AA, CTx | 43 | 73 | - | 30.8% | - | - | [21] |
| P, SS | 10.6 months (range 8.3–21) | Lu-PSMA-617 | 3–6 | 2 | 8 | mHSPC | local | 10 | 67.2 | 100% | 50% | - | - | [24] |
| R, SS | 8 weeks | Lu-PSMA-617 | 5.9 | 8 | mCRPC | AA, CTx | 28 | 73.4 | 59% | 32% | - | 29.4 | [118] | |
| R, MS | 8 weeks | Lu-PSMA-617 | 5.9 | 1 | n/a | mCRPC | AA, CTx, 223Ra | 82 | 73 | 64% | 31% | - | - | [119] |
| R, MS | 16 weeks (range 2–30) | Lu-PSMA-617 | 2–8 | 1–4 | 8–12 | mCRPC | AA, CTx, 223Ra | 145 | 73 | 60% | 45% | - | - | [120] |
| R, MS | Until death | Lu-PSMA-617 | 6.1 | 1–8 | 8 | CRPC | AA, CTx, 223Ra | 104 | 70 | 66% | 56% | - | 56 (CI 50.5–61.5) | [121] |
| R, SS | 24 months (range 6–40) | Lu-PSMA-617 | 7.3 | 3 | 4 | mCRPC | AA, CTx, 223Ra | 54 | 71.6 | 79% | 58% | - | 2 | [122] |
| R, SS | 8 weeks | Lu-PSMA-617 | 4.0 | 1–3 | 8 | mCRPC | AA, CTx, 223Ra | 10 | 75.5 | 90%1 | 40% 1 | - | - | [123] |
| R, SS | 8 weeks | Lu-PSMA-617 | 6.0 | 1–3 | 8 | mCRPC | AA, CTx, 223Ra | 10 | 70.5 | 70% 1 | 30% 1 | - | - | [123] |
| R, SS | 8 weeks | Lu-PSMA-617 | 7.4 | 1–3 | 8 | mCRPC | AA, CTx | 10 | 70.5 | 70% 1 | 50% 1 | - | - | [123] |
| R, SS | 8 weeks | Lu-PSMA-617 | 9.3 | 1–3 | 8 | mCRPC | AA, CTx, 223Ra | 10 | 73.5 | 80% 1 | 30% 1 | - | - | [123] |
|
P, SS, phase II | - | Lu-PSMA-617 | 3.7–5.5 | 1–4 | 8–12 | mCRPC | AA, CTx | 9 | 68.3 | - | - | - | - | [124] |
|
P, MS, phase III | Med. 20.9 months | Lu-PSMA-617 | 7.4 | 1–6 | 6 | mCRPC | AA, CTx, 223Ra | 831 | 71 | 71.5% | 46.0% | 37.8 | 66.5 | [10] |
| P, SS | Until death | Lu-PSMA-617 | 6.1 | 1–3 | 6–10 | mCRPC | AA, CTx, 223Ra | 10 | 68 | 60% | 20% | - | - | [125] |
| R, SS, C | Until death | Lu-PSMA-617 | 6.1 | 1–4 | 6–8 | mCRPC | AA, CTx | 41 | 72.7 | 40.2% 1 | 35.1% 1 | 53.5 1 (CI 44.3–62.6) | 55.2 1 (CI 44.3–66.1) | [126] |
| R, SS, C | Until death | Lu-PSMA-617 | 7.4 | 1–4 | 6–8 | mCRPC | AA, CTx | 37 | 68.7 | 57.8% 1 | 53.7% 1 | 41.3 1 (CI 32.2–50.4) | 49.1 1 (CI 33.5–65.2) | [126] |
|
P, SS, phase I | Ongoing | Lu-PSMA-617 | 7.4–22.2, fr | 1 | n/a | mCRPC | AA, CTx, 223Ra | 44 | 69 | - | 41% | - | 69.6 (CI 47.8–NR) | [127] |
| P, SS | 11 months | Lu-PSMA-617 | 1.11–5.55 | 1 | n/a | mCRPC | AA, CTx | 26 | 66.3 | 40.4% | 10.6% | - | - | [33] |
| P, SS | 2–3 months | Lu-PSMA-617 | 3.7–8 | 1–7 | 8–12 | mCRPC | AA, CTx | 90 | 66.5 | 62.2% | 32.2% | 47.8 (CI 39.1–56.5) | 60.9 (CI 56.5–69.6) | [128] |
| R, SS | At least 2 months | Lu-PSMA-617 | 4.0–7.1 | 1–6 | 8 | mCRPC | AA, CTx, 223Ra | 55 | 72 | - | - | - | - | [129] |
| R, SS | - | Lu-PSMA-617 | 5.7–8.7 | 1–4 | 6 | mCRPC | AA, CTx | 30 | 70.5 | - | - | - | - | [61] |
|
P, SS, phase II | 31.4 months (range 25.1–36.3) | Lu-PSMA-617 | 7.5 | 1–4 | 6 | mCRPC | AA, CTx | 50 | 71 | - | 64% | 30.0 (CI 26.1–37.8) | 57.8 (CI 45.7–81.3) | [130] |
| Adverse Event | Grade I/II | Grade III/IV |
|---|---|---|
| Xerostomia | 6–88.2% | 1.2% 1 |
| Hematological toxicity | ||
| Thrombocytopenia | 4.8–20.6% | 0.9–27% |
| Leukopenia | 6.9–38.2% | 0.9–13% |
| Anemia | 14–98% | 1–22% |
| Fatigue | 5.9–70% | 3–5.9% |
| Nausea | 12.5–71% | 1.3% |
| Nephrotoxicity | 19.5–24% | 2–5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Gaag, S.; Bartelink, I.H.; Vis, A.N.; Burchell, G.L.; Oprea-Lager, D.E.; Hendrikse, H. Pharmacological Optimization of PSMA-Based Radioligand Therapy. Biomedicines 2022, 10, 3020. https://doi.org/10.3390/biomedicines10123020
van der Gaag S, Bartelink IH, Vis AN, Burchell GL, Oprea-Lager DE, Hendrikse H. Pharmacological Optimization of PSMA-Based Radioligand Therapy. Biomedicines. 2022; 10(12):3020. https://doi.org/10.3390/biomedicines10123020
Chicago/Turabian Stylevan der Gaag, Suzanne, Imke H. Bartelink, André N. Vis, George L. Burchell, Daniela E. Oprea-Lager, and Harry Hendrikse. 2022. "Pharmacological Optimization of PSMA-Based Radioligand Therapy" Biomedicines 10, no. 12: 3020. https://doi.org/10.3390/biomedicines10123020
APA Stylevan der Gaag, S., Bartelink, I. H., Vis, A. N., Burchell, G. L., Oprea-Lager, D. E., & Hendrikse, H. (2022). Pharmacological Optimization of PSMA-Based Radioligand Therapy. Biomedicines, 10(12), 3020. https://doi.org/10.3390/biomedicines10123020






