Anti-Inflammatory Activity of Olive Oil Polyphenols—The Role of Oleacein and Its Metabolites
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
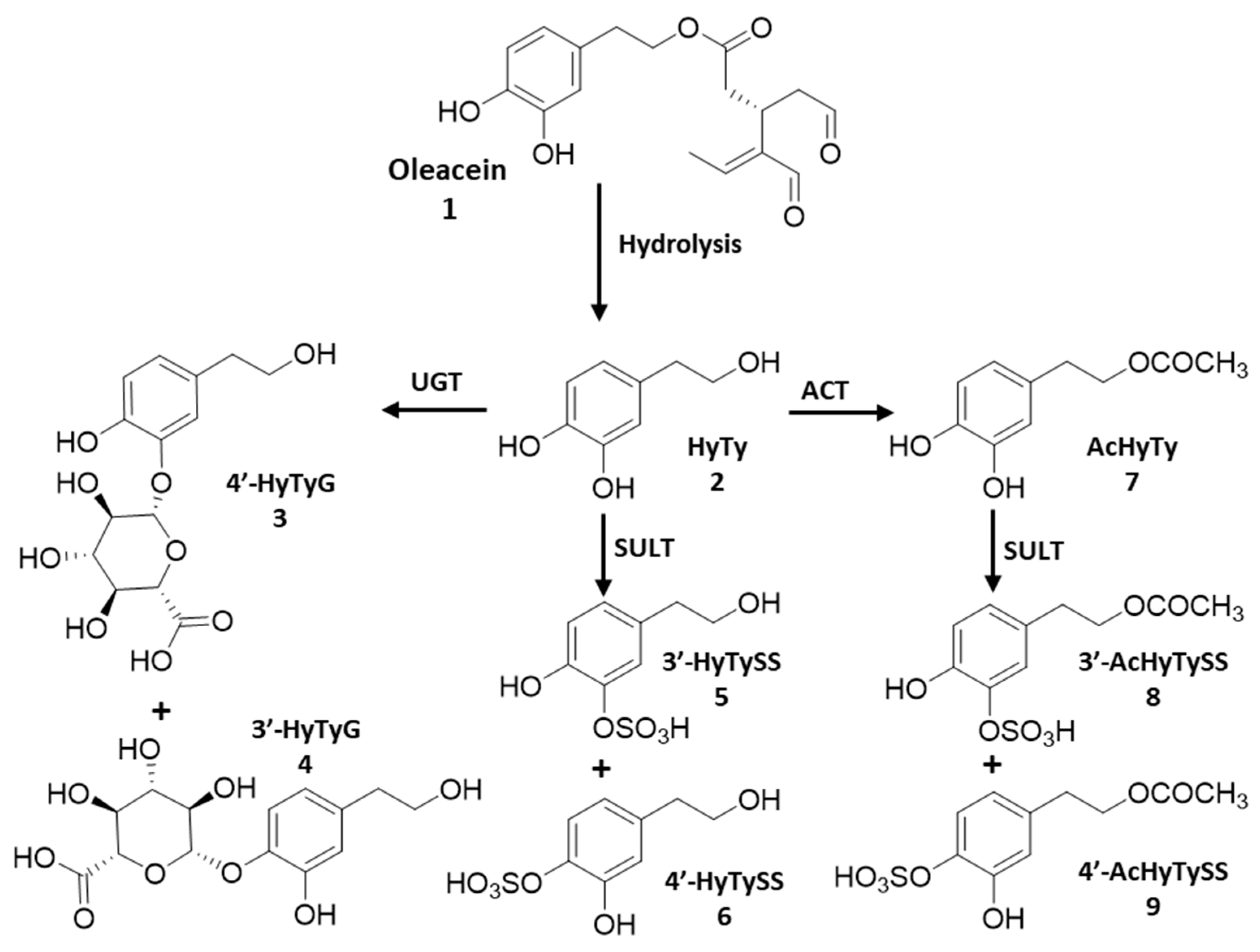
2.2. Phenolic Compounds and Metabolites
2.3. RAW 264.7 Macrophages
2.3.1. Cell Culture
2.3.2. MTT Cell Viability Assay
2.3.3. Determination of ●NO Levels in Cell Culture Medium
2.3.4. Determination of L-Citrulline and ●NO Levels in Extracellular Medium
2.4. Arachidonic Acid Cascade Enzymes
2.4.1. PLA2 Inhibition Assay
2.4.2. 5-LOX Inhibition Assay
2.4.3. COX-1 and COX-2 Inhibition Assay
2.5. Statistical Analysis
3. Results
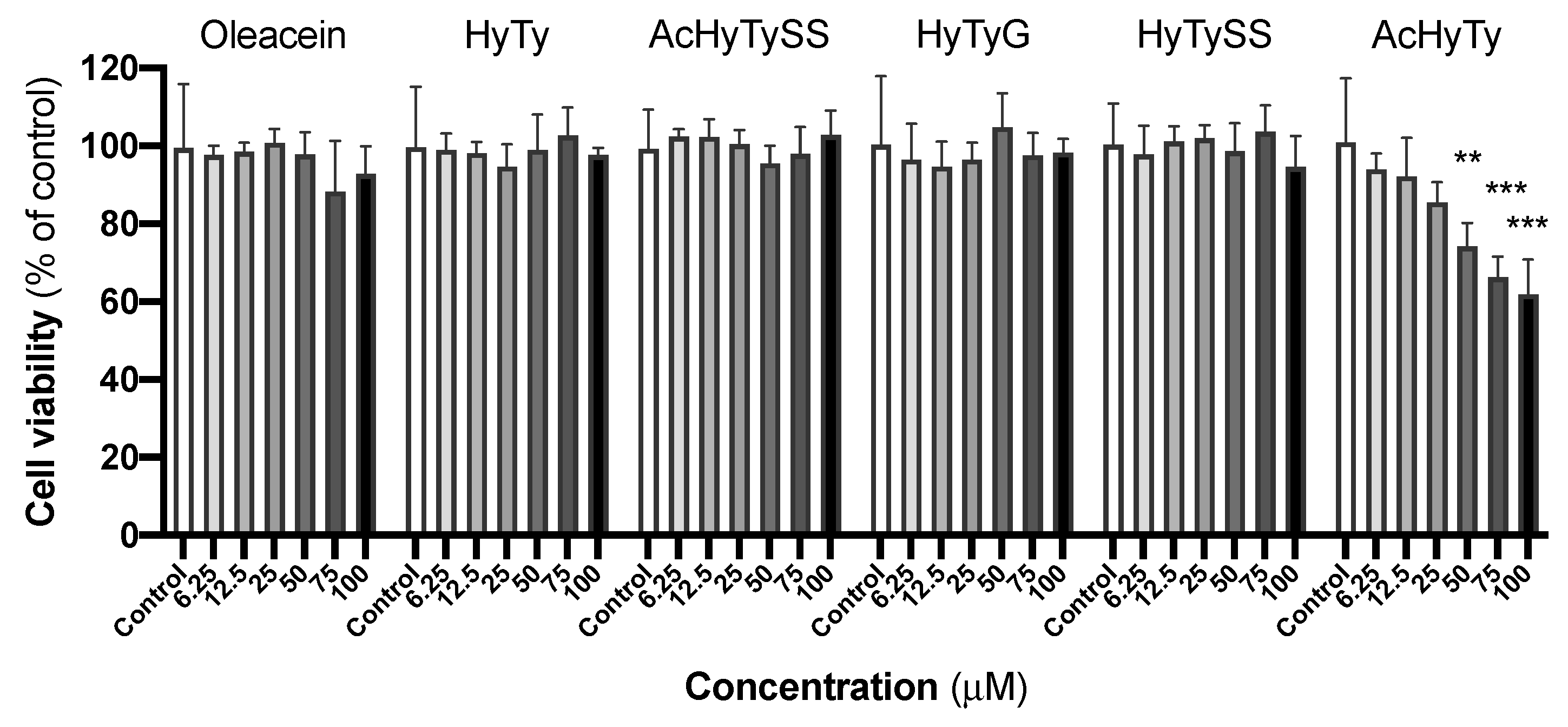
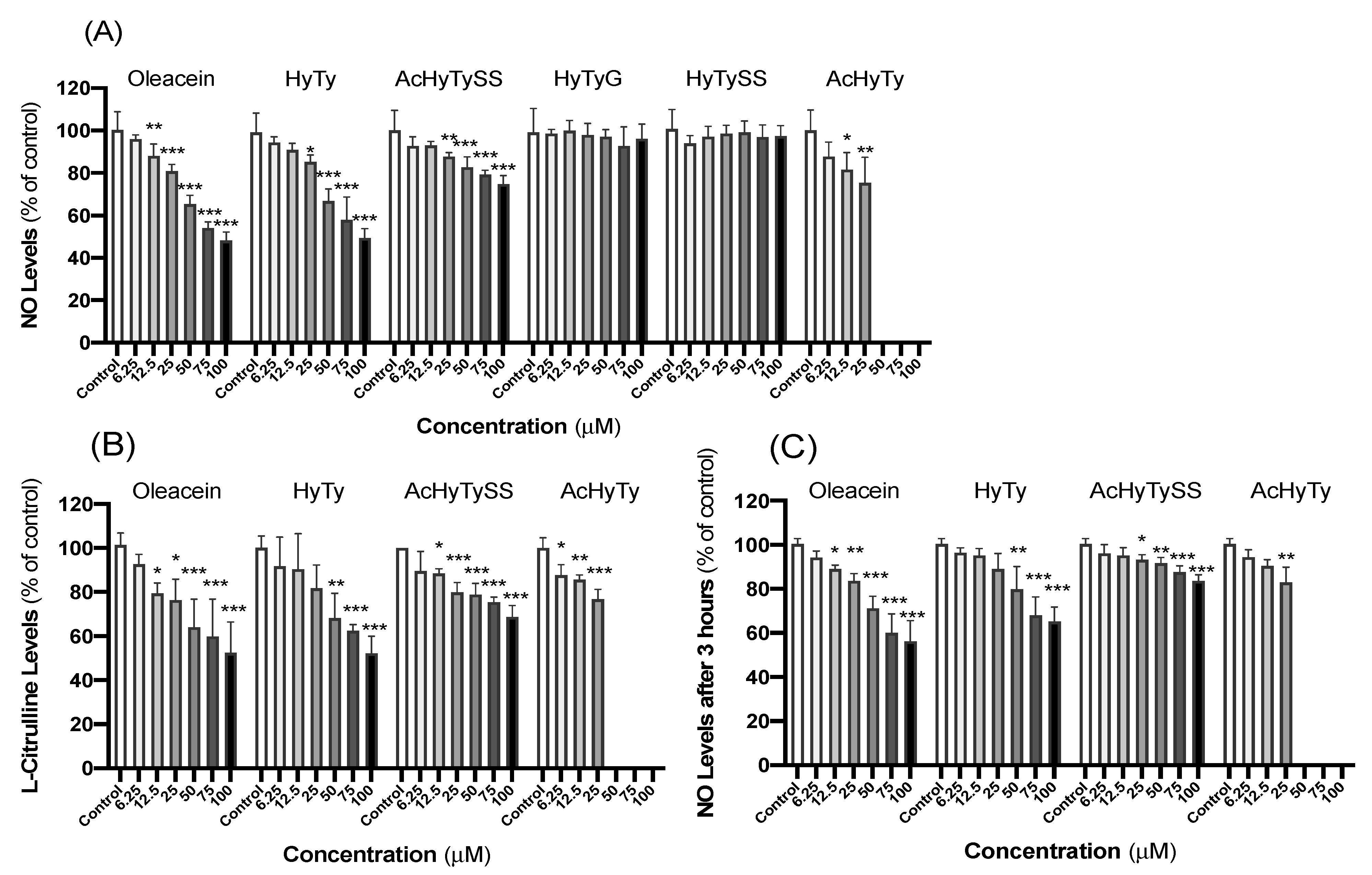
3.1. Effects on RAW 264.7 Macrophages
3.2. Effects on Arachidonic Acid Cascade
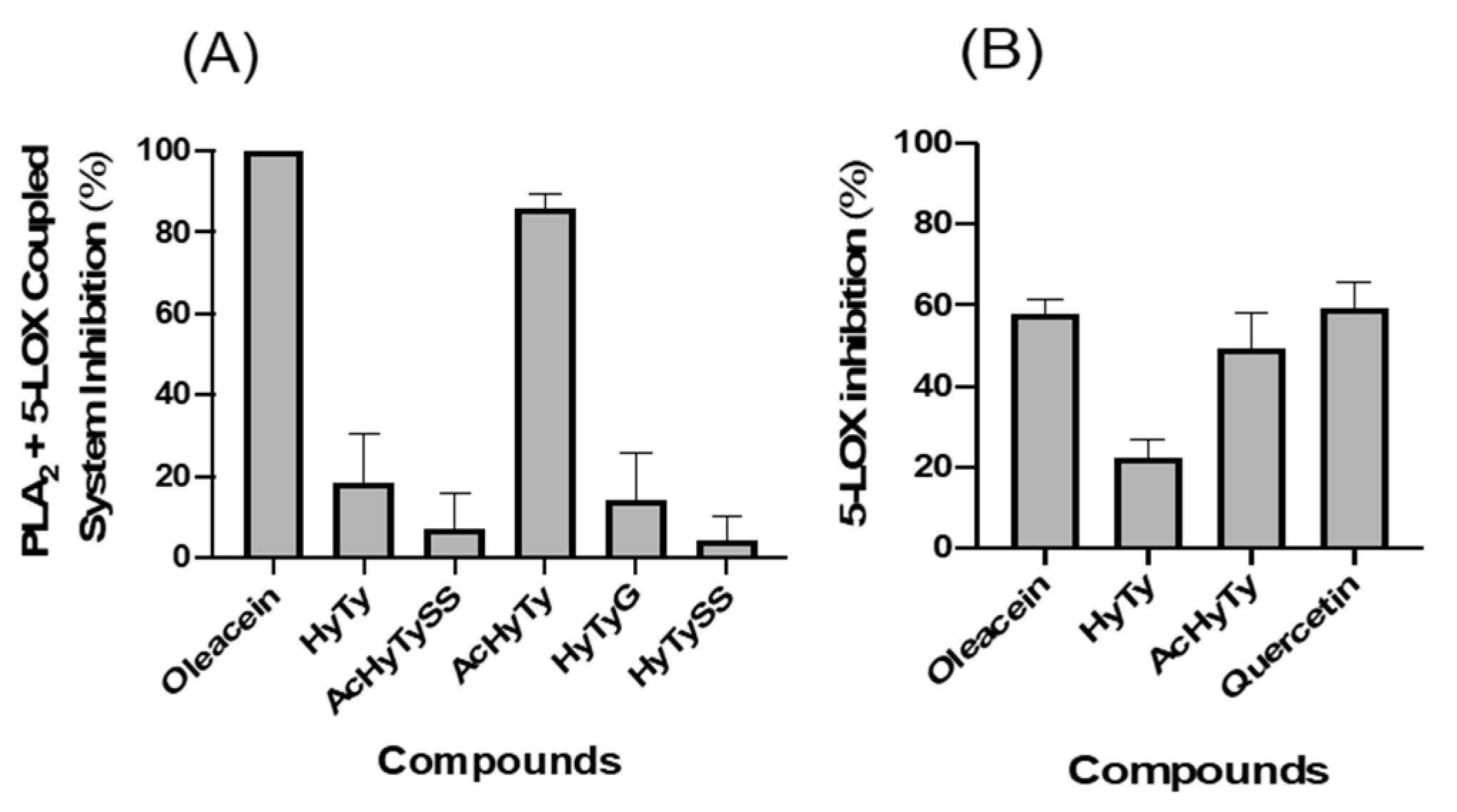
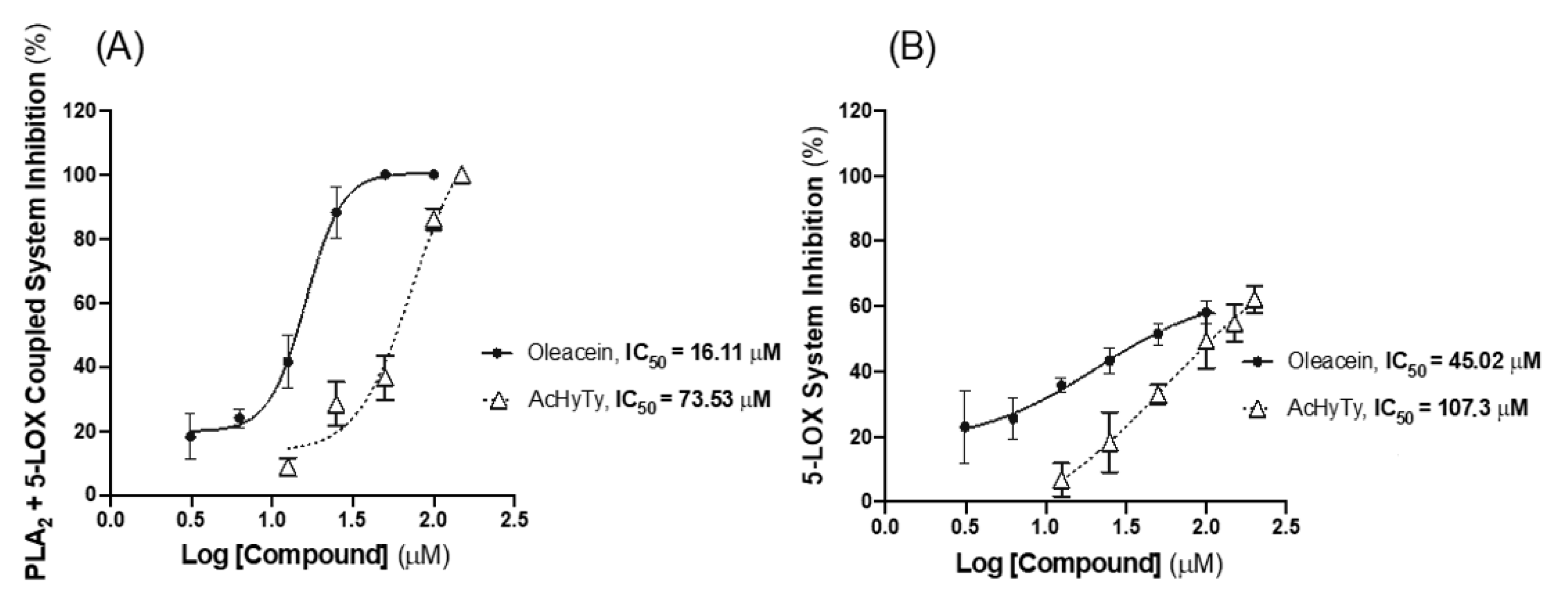
3.2.1. Effects on PLA2 + 5-LOX Coupled System and on 5-LOX Pure Enzyme
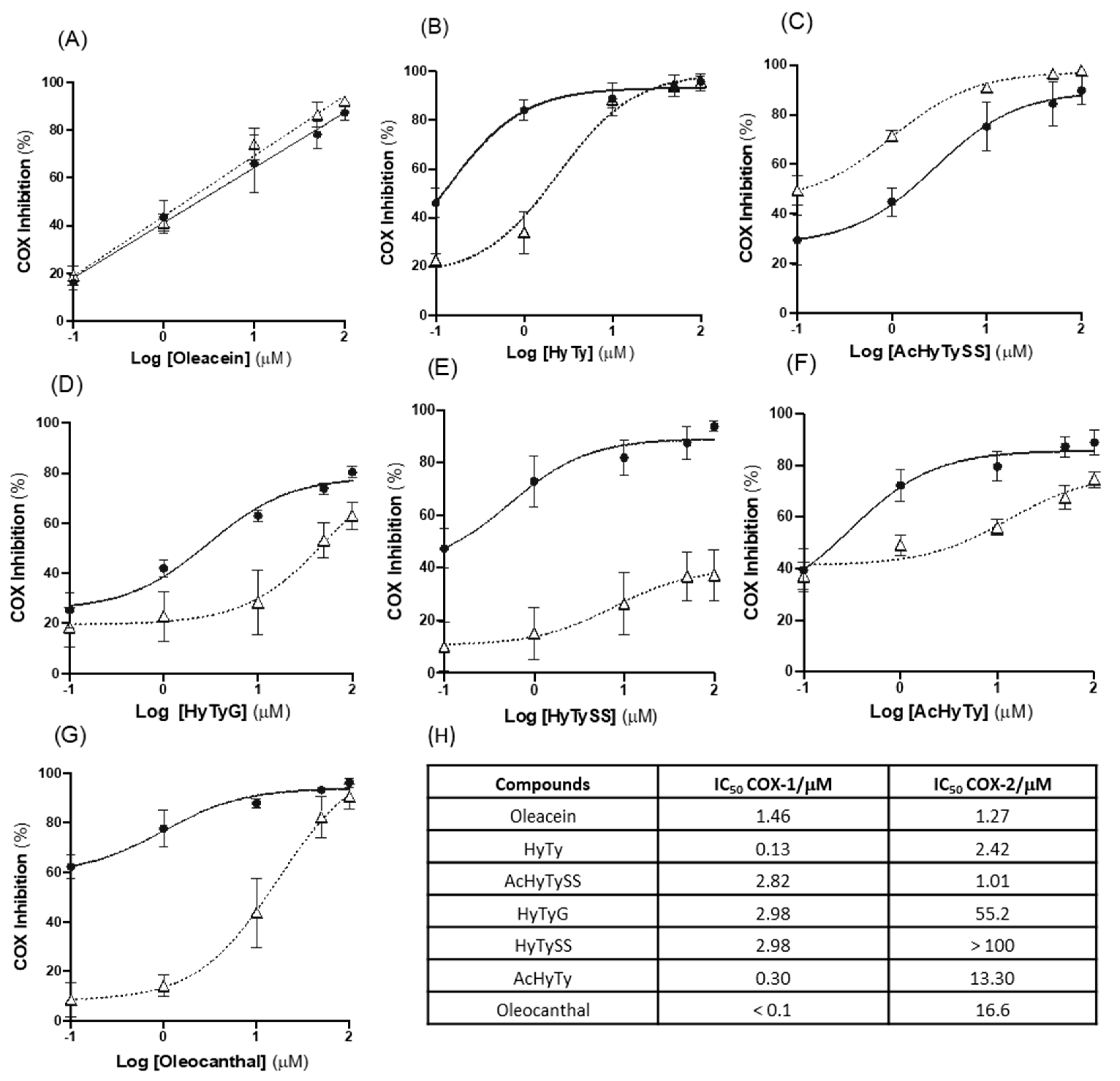
3.2.2. Effects on COX-1 and COX-2 Inhibition
4. Discussion
4.1. Effects on RAW 264.7 Macrophages
4.2. Effects on Arachidonic Acid Cascade
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carluccio, M.A.; Calabriso, N.; Scoditti, E.; Massaro, M.; De Caterina, R. Chapter 27—Mediterranean Diet Polyphenols. In The Mediterranean Diet; Preedy, V.R., Watson, R.R., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 291–300. [Google Scholar] [CrossRef]
- Boskou, D. 1—Olive Fruit, Table Olives, and Olive Oil Bioactive Constituents. In Olive and Olive Oil Bioactive Constituents; Boskou, D., Ed.; Academic Press: San Diego, CA, USA; AOCS Press: Champaign, IL, USA, 2015; pp. 1–30. [Google Scholar] [CrossRef]
- Visioli, F.; Galli, C. Olive Oil Phenols and Their Potential Effects on Human Health. J. Agric. Food Chem. 1998, 46, 4292–4296. [Google Scholar] [CrossRef]
- Ghanbari, R.; Anwar, F.; Alkharfy, K.M.; Gilani, A.H.; Saari, N. Valuable nutrients and functional bioactives in different parts of olive (Olea europaea L.)—A review. Int. J. Mol. Sci. 2012, 13, 3291–3340. [Google Scholar] [CrossRef] [PubMed]
- Paiva-Martins, F.; Kiritsakis, A. Olive fruit and olive oil composition and their functional compounds. In Olives and Olive Oil as Functional Foods: Bioactivity, Chemistry and Processing; Kiritsakis, A., Shahidi, F., Eds.; John Wiley & Sons: New York, NY, USA, 2017; pp. 81–115. [Google Scholar] [CrossRef]
- Boskou, D.; Blekas, G.; Tsimidou, M. 4—Olive Oil Composition. In Olive Oil, 2nd ed.; Boskou, D., Ed.; AOCS Press: Champaign, IL, USA, 2006; pp. 41–72. [Google Scholar] [CrossRef]
- Kiritsakis, K.; Gerasopoulos, D. Production of Phenol-Enriched Olive Oil. In Olives and Olive Oil as Functional Foods: Bioactivity, Chemistry and Processing; Kiritsakis, A., Shahidi, F., Eds.; John Wiley & Sons: New York, NY, USA, 2017; pp. 401–415. [Google Scholar] [CrossRef]
- Paiva-Martins, F.; Gordon, M.H. Isolation and characterization of the antioxidant component 3,4-dihydroxyphenylethyl 4-formyl-3-formylmethyl-4-hexenoate from olive (Olea europaea) leaves. J. Agric. Food Chem. 2001, 49, 4214–4219. [Google Scholar] [CrossRef] [PubMed]
- Jaismy Jacob, P.; Manju, S.L.; Ethiraj, K.R.; Elias, G. Safer anti-inflammatory therapy through dual COX-2/5-LOX inhibitors: A structure-based approach. Eur. J. Pharm. Sci. 2018, 121, 356–381. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Vu, T.-Y.; Chandi, V.; Polimati, H.; Tatipamula, V.B. Dual COX and 5-LOX inhibition by clerodane diterpenes from seeds of Polyalthia longifolia (Sonn.) Thwaites. Sci. Rep. 2020, 10, 15965. [Google Scholar] [CrossRef]
- Vissers, M.N.; Zock, P.L.; Katan, M.B. Bioavailability and antioxidant effects of olive oil phenols in humans: A review. Eur. J. Clin. Nutr. 2004, 58, 955–965. [Google Scholar] [CrossRef]
- Corona, G.; Tzounis, X.; Assunta DessÌ, M.; Deiana, M.; Debnam, E.S.; Visioli, F.; Spencer, J.P.E. The fate of olive oil polyphenols in the gastrointestinal tract: Implications of gastric and colonic microflora-dependent biotransformation. Free Radic. Res. 2006, 40, 647–658. [Google Scholar] [CrossRef]
- Covas, M.-I.; Fitó, M.; Khymenets, O.; de la Torre, R. Chapter 73—The Bioavailability of Olive Oil Phenolic Compounds. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Academic Press: San Diego, CA, USA, 2010; pp. 699–703. [Google Scholar] [CrossRef]
- Pinto, J.; Paiva-Martins, F.; Corona, G.; Debnam, E.S.; Jose Oruna-Concha, M.; Vauzour, D.; Gordon, M.H.; Spencer, J.P.E. Absorption and metabolism of olive oil secoiridoids in the small intestine. Br. J. Nutr. 2011, 105, 1607–1618. [Google Scholar] [CrossRef]
- Gomes, V.P.M.; Torres, C.; Rodríguez-Borges, J.E.; Paiva-Martins, F. A Convenient Synthesis of Hydroxytyrosol Monosulfate Metabolites. J. Agric. Food Chem. 2015, 63, 9565–9571. [Google Scholar] [CrossRef]
- Paiva-Martins, F.; Silva, A.; Almeida, V.; Carvalheira, M.; Serra, C.; Rodrígues-Borges, J.E.; Fernandes, J.; Belo, L.; Santos-Silva, A. Protective Activity of Hydroxytyrosol Metabolites on Erythrocyte Oxidative-Induced Hemolysis. J. Agric. Food Chem. 2013, 61, 6636–6642. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Pereira, R.B.; Pereira, D.M.; Jiménez, C.; Rodríguez, J.; Nieto, R.M.; Videira, R.A.; Silva, O.; Andrade, P.B.; Valentão, P. Anti-Inflammatory Effects of 5α,8α-Epidioxycholest-6-en-3β-ol, a Steroidal Endoperoxide Isolated from Aplysia depilans, Based on Bioguided Fractionation and NMR Analysis. Mar. Drugs 2019, 17, 330. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, G.K.; Keast, R.S.; Morel, D.; Lin, J.; Pika, J.; Han, Q.; Lee, C.H.; Smith, A.B.; Breslin, P.A. Phytochemistry: Ibuprofen-like activity in extra-virgin olive oil. Nature 2005, 437, 45–46. [Google Scholar] [CrossRef]
- Veloso, C.; Videira, R.A.; Andrade, P.B.; Cardoso, C.; Vitorino, C. Topical fixed-dose combinations: Current in vitro methodologies for pre-clinical development. Int. J. Pharm. 2022, 617, 121621. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Kobayashi, K. Macrophages in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef]
- Andrade, C.; Ferreres, F.; Gomes, N.G.M.; Duangsrisai, S.; Srisombat, N.; Vajrodaya, S.; Pereira, D.M.; Gil-Izquierdo, A.; Andrade, P.B.; Valentão, P. Phenolic Profiling and Biological Potential of Ficus curtipes Corner Leaves and Stem Bark: 5-Lipoxygenase Inhibition and Interference with NO Levels in LPS-Stimulated RAW 264.7 Macrophages. Biomolecules 2019, 9, 400. [Google Scholar] [CrossRef] [PubMed]
- Bigagli, E.; Cinci, L.; Paccosi, S.; Parenti, A.; D’Ambrosio, M.; Luceri, C. Nutritionally relevant concentrations of resveratrol and hydroxytyrosol mitigate oxidative burst of human granulocytes and monocytes and the production of pro-inflammatory mediators in LPS-stimulated RAW 264.7 macrophages. Int. Immunopharmacol. 2017, 43, 147–155. [Google Scholar] [CrossRef]
- Costa, M.; Sezgin-Bayindir, Z.; Losada-Barreiro, S.; Paiva-Martins, F.; Saso, L.; Bravo-Díaz, C. Polyphenols as Antioxidants for Extending Food Shelf-Life and in the Prevention of Health Diseases: Encapsulation and Interfacial Phenomena. Biomedicines 2021, 9, 1909. [Google Scholar] [CrossRef]
- Menendez, J.A.; Joven, J.; Aragonès, G.; Barrajón-Catalán, E.; Beltrán-Debón, R.; Borrás-Linares, I.; Camps, J.; Corominas-Faja, B.; Cufí, S.; Fernández-Arroyo, S.; et al. Xenohormetic and anti-aging activity of secoiridoid polyphenols present in extra virgin olive oil: A new family of gerosuppressant agents. Cell Cycle 2013, 12, 555–578. [Google Scholar] [CrossRef]
- Parkinson, L.; Cicerale, S. The Health Benefiting Mechanisms of Virgin Olive Oil Phenolic Compounds. Molecules 2016, 21, 1734. [Google Scholar] [CrossRef]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, K.W.; Lee, H.J. Chapter 29—Polyphenols Suppress and Modulate Inflammation: Possible Roles in Health and Disease. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 393–408. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; González-Sarrías, A.; Laparra-Llopis, J.M.; Schneider, C.; Espín, J.C. Targeting Mammalian 5-Lipoxygenase by Dietary Phenolics as an Anti-Inflammatory Mechanism: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 7937. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Gálvez, M.; González-Sarrías, A.; Martínez-Díaz, F.; Abellán, B.; Martínez-Torrano, A.J.; Fernández-López, A.J.; Giménez-Bastida, J.A.; Espín, J.C. Disposition of Dietary Polyphenols in Breast Cancer Patients’ Tumors, and Their Associated Anticancer Activity: The Particular Case of Curcumin. Mol. Nutr. Food Res. 2021, 65, e2100163. [Google Scholar] [CrossRef]
- Shimoi, K.; Saka, N.; Nozawa, R.T.; Satô, M.; Amano, I.; Nakayama, T.; Kinae, N. Deglucuronidation of a flavonoid, luteolin monoglucuronide, during inflammation. Drug Metab. Dispos. Biol. Fate Chem. 2001, 29, 1521–1524. [Google Scholar] [PubMed]
- Galindo, P.; Rodriguez-Gómez, I.; González-Manzano, S.; Dueñas, M.; Jiménez, R.; Menéndez, C.; Vargas, F.; Tamargo, J.; Santos-Buelga, C.; Pérez-Vizcaíno, F.; et al. Glucuronidated quercetin lowers blood pressure in spontaneously hypertensive rats via deconjugation. PLoS ONE 2012, 7, e32673. [Google Scholar] [CrossRef] [PubMed]
- Ishisaka, A.; Kawabata, K.; Miki, S.; Shiba, Y.; Minekawa, S.; Nishikawa, T.; Mukai, R.; Terao, J.; Kawai, Y. Mitochondrial dysfunction leads to deconjugation of quercetin glucuronides in inflammatory macrophages. PLoS ONE 2013, 8, e80843. [Google Scholar] [CrossRef]
- Kawai, Y.; Nishikawa, T.; Shiba, Y.; Saito, S.; Murota, K.; Shibata, N.; Kobayashi, M.; Kanayama, M.; Uchida, K.; Terao, J. Macrophage as a target of quercetin glucuronides in human atherosclerotic arteries: Implication in the anti-atherosclerotic mechanism of dietary flavonoids. J. Biol. Chem. 2008, 283, 9424–9434. [Google Scholar] [CrossRef]
- Menendez, C.; Dueñas, M.; Galindo, P.; González-Manzano, S.; Jimenez, R.; Moreno, L.; Zarzuelo, M.J.; Rodríguez-Gómez, I.; Duarte, J.; Santos-Buelga, C.; et al. Vascular deconjugation of quercetin glucuronide: The flavonoid paradox revealed? Mol. Nutr. Food Res. 2011, 55, 1780–1790. [Google Scholar] [CrossRef]
- Ávila-Gálvez, M.A.; Giménez-Bastida, J.A.; González-Sarrías, A.; Espín, J.C. Tissue deconjugation of urolithin A glucuronide to free urolithin A in systemic inflammation. Food Funct. 2019, 10, 3135–3141. [Google Scholar] [CrossRef]
- López-Yerena, A.; Pérez, M.; Vallverdú-Queralt, A.; Miliarakis, E.; Lamuela-Raventós, R.M.; Escribano-Ferrer, E. Oleacein Intestinal Permeation and Metabolism in Rats Using an In Situ Perfusion Technique. Pharmaceutics 2021, 13, 719. [Google Scholar] [CrossRef]
- Zidar, N.; Odar, K.; Glavac, D.; Jerse, M.; Zupanc, T.; Stajer, D. Cyclooxygenase in normal human tissues—Is COX-1 really a constitutive isoform, and COX-2 an inducible isoform? J. Cell. Mol. Med. 2009, 13, 3753–3763. [Google Scholar] [CrossRef] [PubMed]
- Arora, M.; Choudhary, S.; Singh, P.K.; Sapra, B.; Silakari, O. Structural investigation on the selective COX-2 inhibitors mediated cardiotoxicity: A review. Life Sci. 2020, 251, 117631. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Mincione, E.; Barontini, M.; Crisante, F. Convenient synthesis of hydroxytyrosol and its lipophilic derivatives from tyrosol or homovanillyl alcohol. J. Agric. Food Chem. 2008, 56, 8897–8904. [Google Scholar] [CrossRef]
- Macedo, T.; Ferreres, F.; Pereira, D.M.; Oliveira, A.P.; Gomes, N.G.M.; Gil-Izquierdo, Á.; Valentão, P.; Araújo, L.; Andrade, P.B. Cassia sieberiana DC. leaves modulate LPS-induced inflammatory response in THP-1 cells and inhibit eicosanoid-metabolizing enzymes. J. Ethnopharmacol. 2021, 269, 113746. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, V.; Costa, M.; Videira, R.A.; Andrade, P.B.; Paiva-Martins, F. Anti-Inflammatory Activity of Olive Oil Polyphenols—The Role of Oleacein and Its Metabolites. Biomedicines 2022, 10, 2990. https://doi.org/10.3390/biomedicines10112990
Costa V, Costa M, Videira RA, Andrade PB, Paiva-Martins F. Anti-Inflammatory Activity of Olive Oil Polyphenols—The Role of Oleacein and Its Metabolites. Biomedicines. 2022; 10(11):2990. https://doi.org/10.3390/biomedicines10112990
Chicago/Turabian StyleCosta, Vânia, Marlene Costa, Romeu António Videira, Paula Branquinho Andrade, and Fátima Paiva-Martins. 2022. "Anti-Inflammatory Activity of Olive Oil Polyphenols—The Role of Oleacein and Its Metabolites" Biomedicines 10, no. 11: 2990. https://doi.org/10.3390/biomedicines10112990
APA StyleCosta, V., Costa, M., Videira, R. A., Andrade, P. B., & Paiva-Martins, F. (2022). Anti-Inflammatory Activity of Olive Oil Polyphenols—The Role of Oleacein and Its Metabolites. Biomedicines, 10(11), 2990. https://doi.org/10.3390/biomedicines10112990




