Tricetin Reduces Inflammation and Acinar Cell Injury in Cerulein-Induced Acute Pancreatitis: The Role of Oxidative Stress-Induced DNA Damage Signaling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Culture of Pancreatic Acinar Cells
2.2. Cell Treatments
2.3. Radical Scavenging Assay
2.4. Cell Viability (Calcein-AM Assay)
2.5. Lactate Dehydrogenase (LDH) Assay
2.6. Propidium Iodide (PI) Uptake
2.7. Caspase Activity Assay
2.8. RNA Extraction, Reverse Transcription, and qPCR Analysis
2.9. NFκB Assay
2.10. PARP Inhibition Assay
2.11. Immunofluorescent (IF) Staining of Acinar Cells
2.12. Western Blot Analysis
2.13. Acute Pancreatitis Model
2.14. Serum α-Amylase and Lipase Determination
2.15. Myeloperoxidase Assay
2.16. Histology
2.17. Immunofluorescent (IF) Detection of PAR Polymers in Tissue Sections
2.18. Statistical Analysis
3. Results
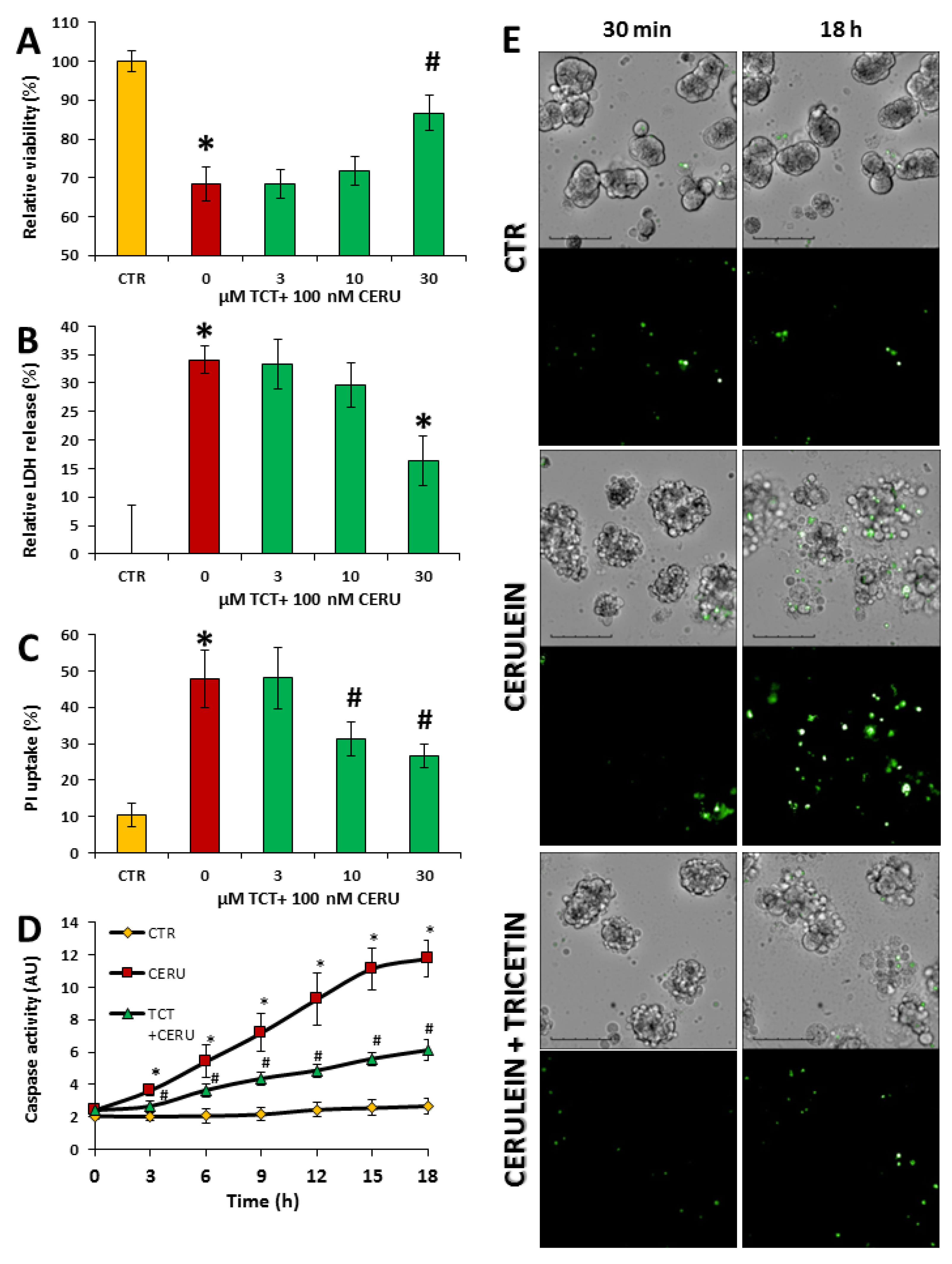
3.1. Tricetin Is Nontoxic to Acinar Cells
3.2. Tricetin Protects Isolated Acinar Cells from Cerulein-Induced but Not from Hydrogen Peroxide-Induced Cell Death
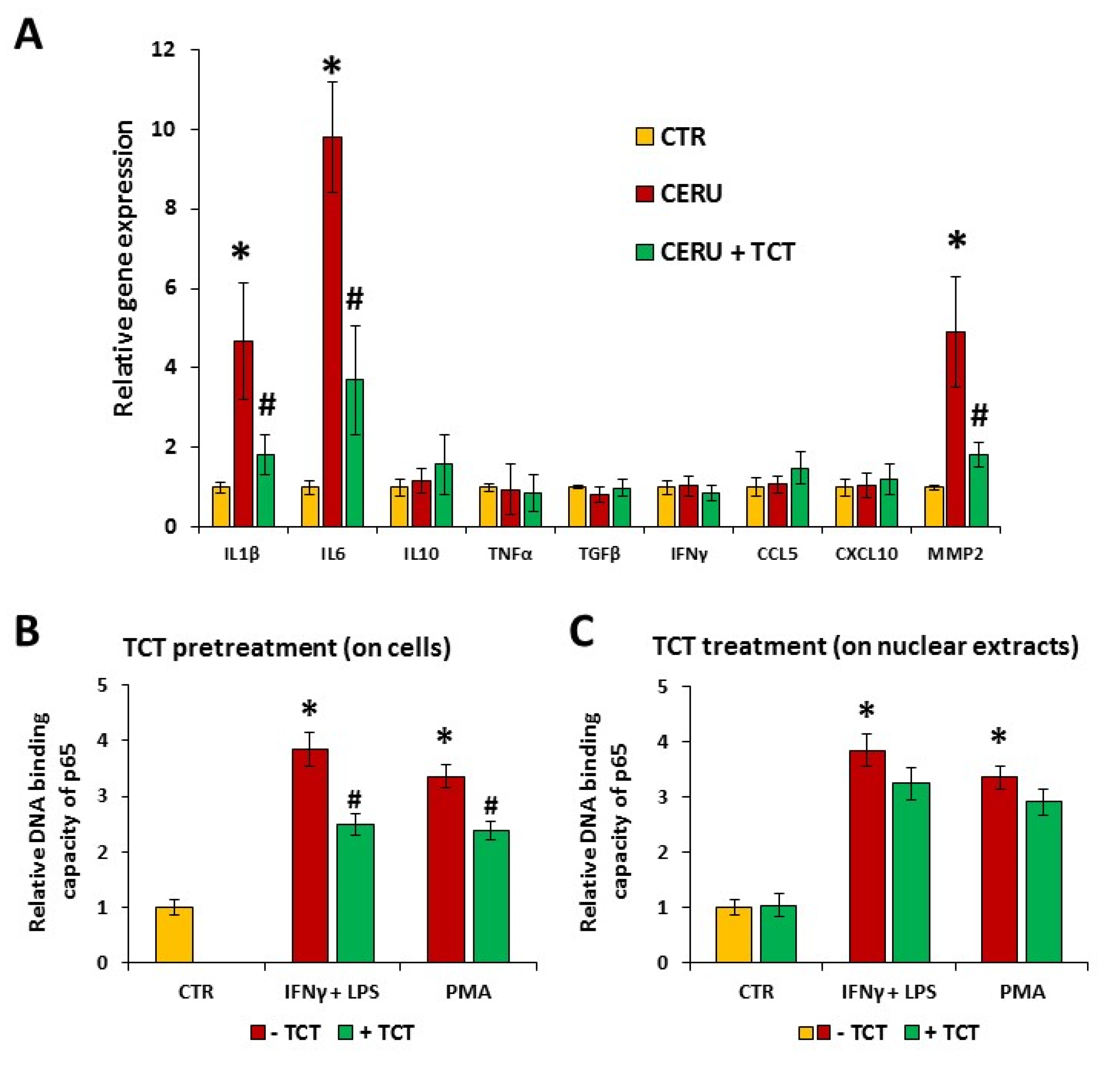
3.3. Cerulein-Induced Expression of Interleukin-1β (IL-1β), Interleukin-6 (IL6), and Matrix Metalloproteinase-2 (MMP2) Was Attenuated by Tricetin in Primary Pancreatic Acinar Cells
3.4. Tricetin Inhibits NFκB Signaling
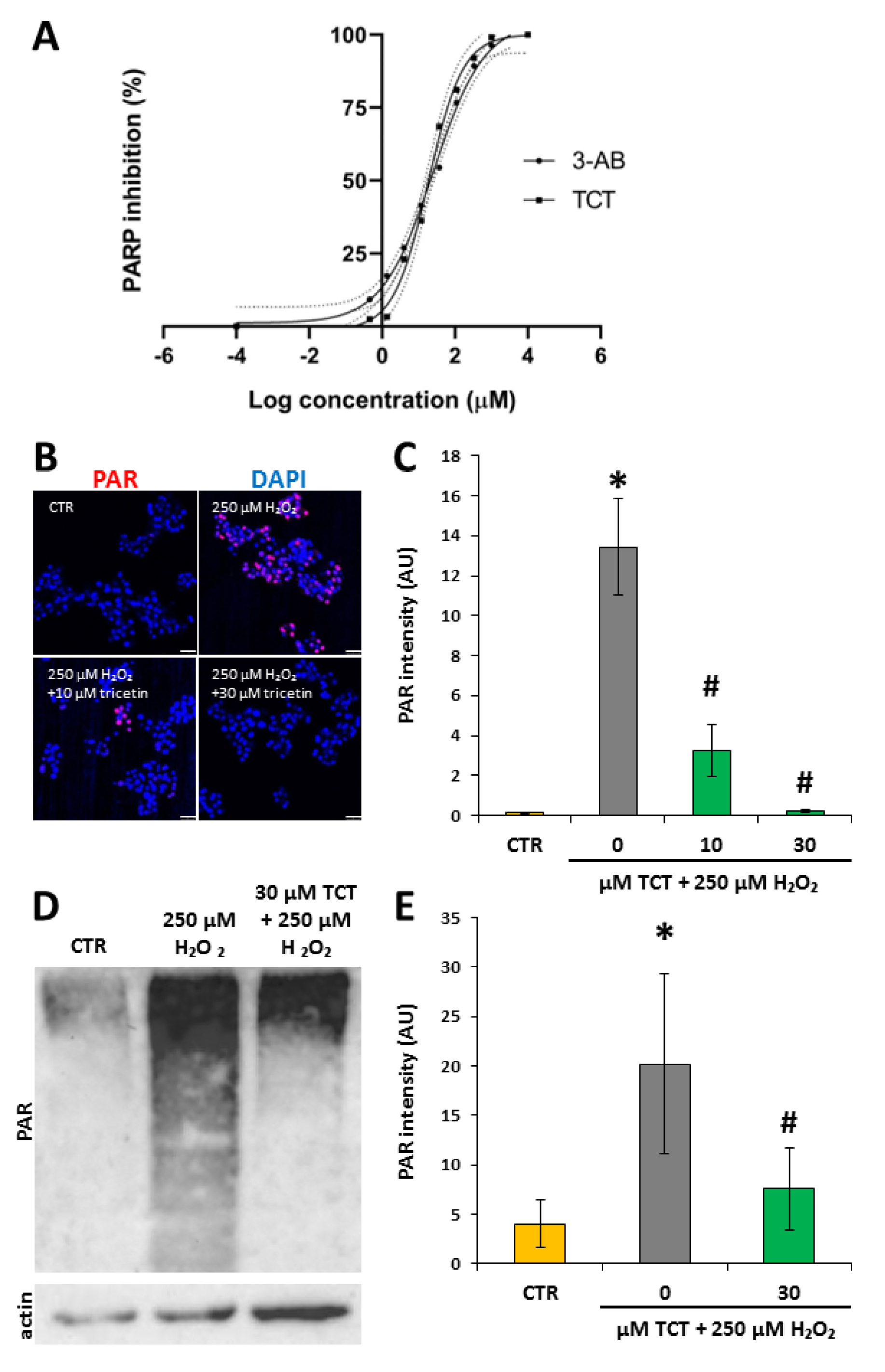
3.5. Tricetin Inhibits PARP1 and Suppresses H2O2-Induced Poly(ADP-ribosyl)ation (PARylation) in Isolated Acinar Cells
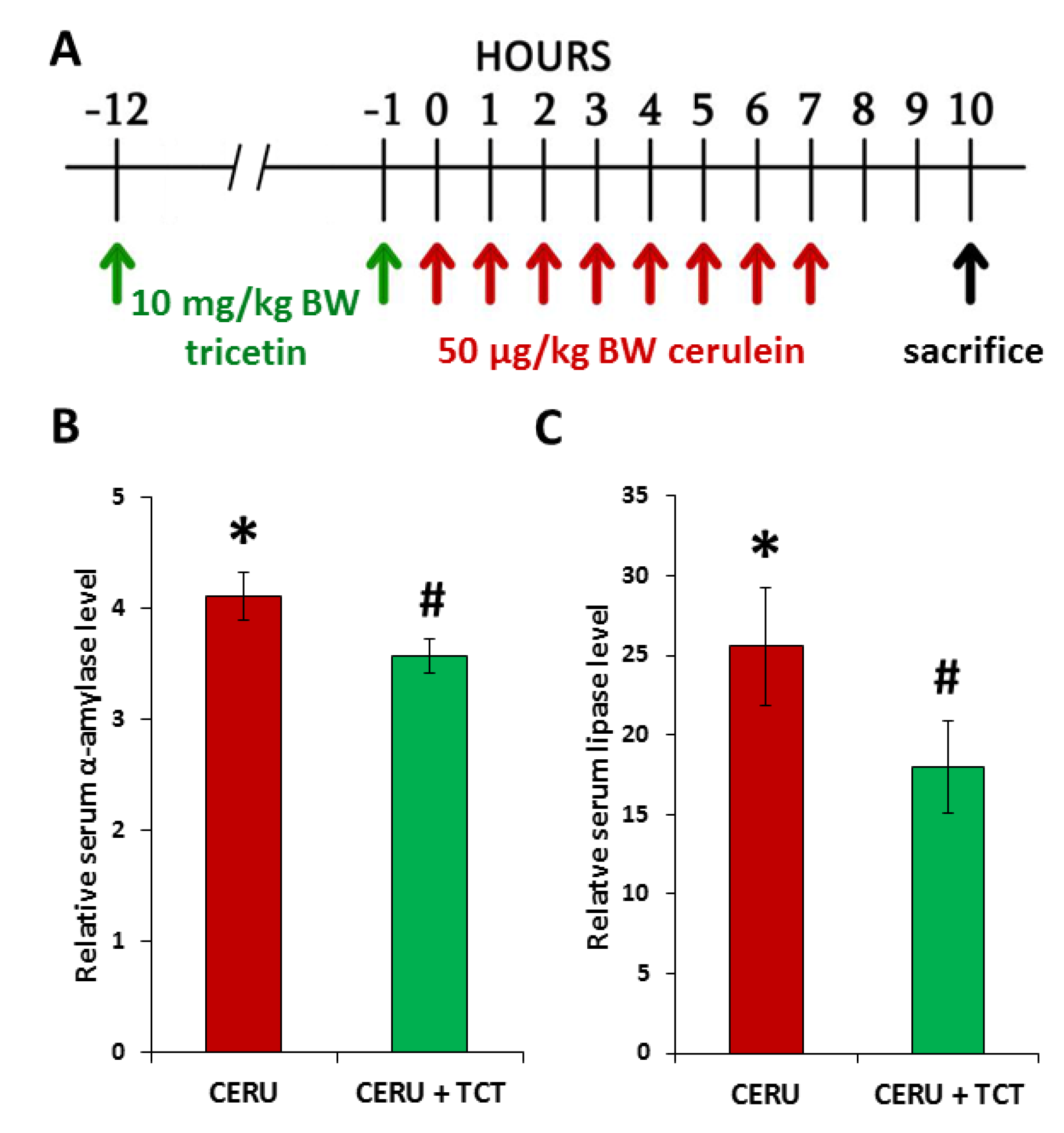
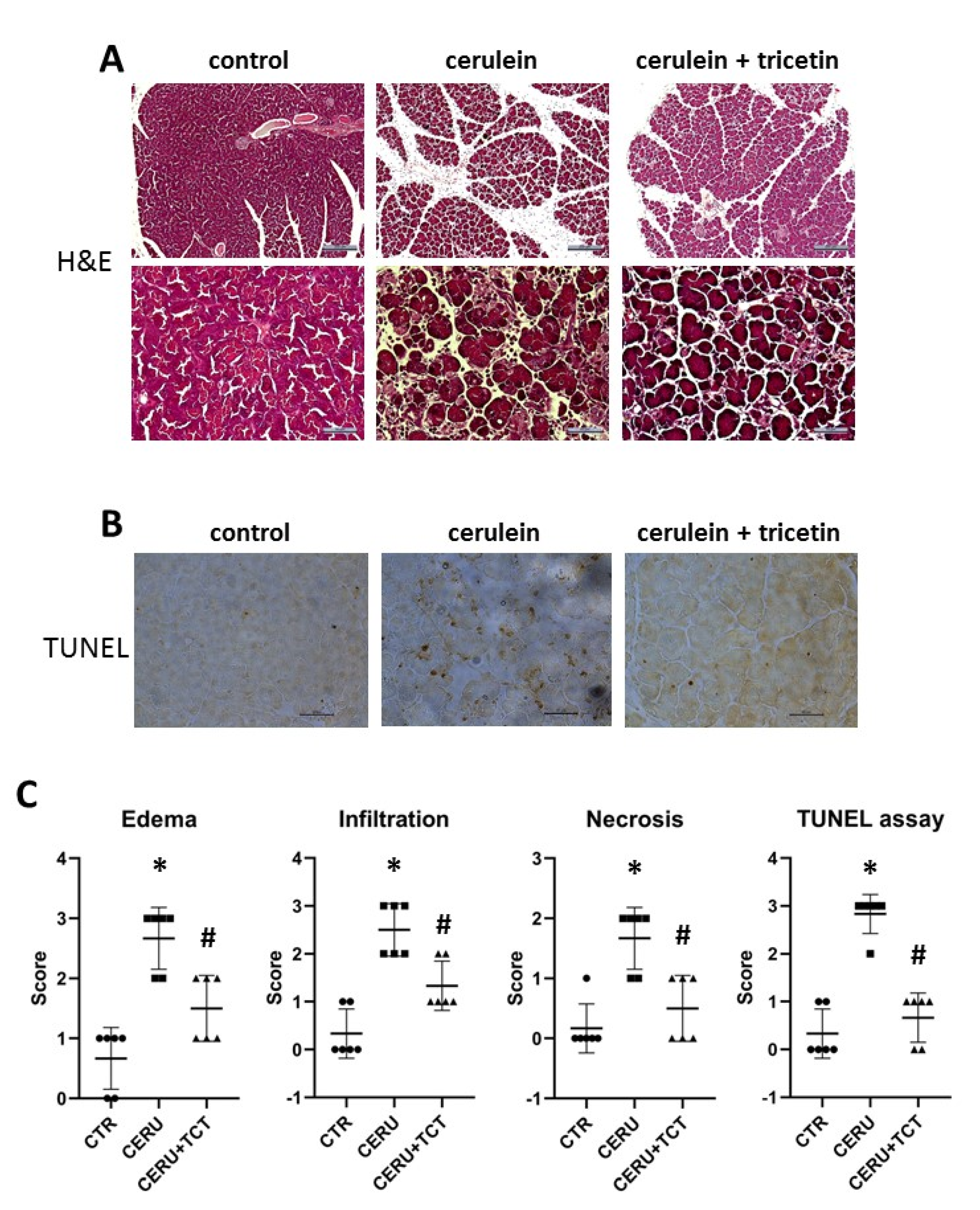
3.6. Tricetin Pretreatment Protects Mice from Cerulein-Induced Acute Pancreatitis
3.7. Tricetin Suppresses Granulocyte Infiltration, Production of Inflammatory Mediators, and Synthesis of Poly(ADP-ribose) in Acute Pancreatitis
3.8. Posttreatment with Tricetin Protects Mice from Cerulein-Induced Acute Pancreatitis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Czako, L.; Hegyi, P.; Rakonczay, Z., Jr.; Wittmann, T.; Otsuki, M. Interactions between the endocrine and exocrine pancreas and their clinical relevance. Pancreatology 2009, 9, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Hegyi, P.; Petersen, O.H. The exocrine pancreas: The acinar-ductal tango in physiology and pathophysiology. Rev. Physiol. Biochem. Pharmacol. 2013, 165, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Hegyi, P.; Maleth, J.; Venglovecz, V.; Rakonczay, Z., Jr. Pancreatic ductal bicarbonate secretion: Challenge of the acinar Acid load. Front. Physiol. 2011, 2, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andralojc, K.M.; Mercalli, A.; Nowak, K.W.; Albarello, L.; Calcagno, R.; Luzi, L.; Bonifacio, E.; Doglioni, C.; Piemonti, L. Ghrelin-producing epsilon cells in the developing and adult human pancreas. Diabetologia 2009, 52, 486–493. [Google Scholar] [CrossRef] [Green Version]
- Lutz, T.A. Creating the amylin story. Appetite 2022, 172, 105965. [Google Scholar] [CrossRef] [PubMed]
- Parniczky, A.; Kui, B.; Szentesi, A.; Balazs, A.; Szucs, A.; Mosztbacher, D.; Czimmer, J.; Sarlos, P.; Bajor, J.; Godi, S.; et al. Prospective, Multicentre, Nationwide Clinical Data from 600 Cases of Acute Pancreatitis. PLoS ONE 2016, 11, e0165309. [Google Scholar] [CrossRef] [Green Version]
- Meczker, A.; Hanak, L.; Parniczky, A.; Szentesi, A.; Eross, B.; Hegyi, P.; Hungarian Pancreatic Study Group. Analysis of 1060 Cases of Drug-Induced Acute Pancreatitis. Gastroenterology 2020, 159, 1958–1961.e8. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Shimosegawa, T.; Masamune, A.; Hirota, M.; Kikuta, K.; Kihara, Y.; Kuriyama, S.; Tsuji, I.; Satoh, A.; Hamada, S.; et al. Nationwide epidemiological survey of acute pancreatitis in Japan. Pancreas 2011, 40, 503–507. [Google Scholar] [CrossRef]
- Mosztbacher, D.; Hanak, L.; Farkas, N.; Szentesi, A.; Miko, A.; Bajor, J.; Sarlos, P.; Czimmer, J.; Vincze, A.; Hegyi, P.J.; et al. Hypertriglyceridemia-induced acute pancreatitis: A prospective, multicenter, international cohort analysis of 716 acute pancreatitis cases. Pancreatology 2020, 20, 608–616. [Google Scholar] [CrossRef]
- Demcsak, A.; Soos, A.; Kincses, L.; Capunge, I.; Minkov, G.; Kovacheva-Slavova, M.; Nakov, R.; Wu, D.; Huang, W.; Xia, Q.; et al. Acid suppression therapy, gastrointestinal bleeding and infection in acute pancreatitis—An international cohort study. Pancreatology 2020, 20, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Parniczky, A.; Lantos, T.; Toth, E.M.; Szakacs, Z.; Godi, S.; Hagendorn, R.; Illes, D.; Koncz, B.; Marta, K.; Miko, A.; et al. Antibiotic therapy in acute pancreatitis: From global overuse to evidence based recommendations. Pancreatology 2019, 19, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Hritz, I.; Czako, L.; Dubravcsik, Z.; Farkas, G.; Kelemen, D.; Lasztity, N.; Morvay, Z.; Olah, A.; Pap, A.; Parniczky, A.; et al. Acute pancreatitis. Evidence-based practice guidelines, prepared by the Hungarian Pancreatic Study Group. Orv. Hetil. 2015, 156, 244–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marta, K.; Farkas, N.; Szabo, I.; Illes, A.; Vincze, A.; Par, G.; Sarlos, P.; Bajor, J.; Szucs, A.; Czimmer, J.; et al. Meta-Analysis of Early Nutrition: The Benefits of Enteral Feeding Compared to a Nil Per Os Diet Not Only in Severe, but Also in Mild and Moderate Acute Pancreatitis. Int. J. Mol. Sci. 2016, 17, 1691. [Google Scholar] [CrossRef] [Green Version]
- Patai, A.; Solymosi, N.; Mohacsi, L.; Patai, A.V. Indomethacin and diclofenac in the prevention of post-ERCP pancreatitis: A systematic review and meta-analysis of prospective controlled trials. Gastrointest. Endosc. 2017, 85, 1144–1156.e1. [Google Scholar] [CrossRef]
- Escobar, J.; Pereda, J.; Arduini, A.; Sandoval, J.; Moreno, M.L.; Perez, S.; Sabater, L.; Aparisi, L.; Cassinello, N.; Hidalgo, J.; et al. Oxidative and nitrosative stress in acute pancreatitis. Modulation by pentoxifylline and oxypurinol. Biochem. Pharmacol. 2012, 83, 122–130. [Google Scholar] [CrossRef]
- Waldron, R.T.; Chen, Y.; Pham, H.; Go, A.; Su, H.Y.; Hu, C.; Wen, L.; Husain, S.Z.; Sugar, C.A.; Roos, J.; et al. The Orai Ca(2+) channel inhibitor CM4620 targets both parenchymal and immune cells to reduce inflammation in experimental acute pancreatitis. J. Physiol. 2019, 597, 3085–3105. [Google Scholar] [CrossRef]
- Ahmad, A.; Haas De Mello, A.; Szczesny, B.; Toro, G.; Marcatti, M.; Druzhyna, N.; Liaudet, L.; Tarantini, S.; Salomao, R.; Garcia Soriano, F.; et al. Effects of the Poly(ADP-Ribose) Polymerase Inhibitor Olaparib in Cerulein-Induced Pancreatitis. Shock 2020, 53, 653–665. [Google Scholar] [CrossRef]
- Mota, R.A.; Sanchez-Bueno, F.; Saenz, L.; Hernandez-Espinosa, D.; Jimeno, J.; Tornel, P.L.; Martinez-Torrano, A.; Ramirez, P.; Parrilla, P.; Yelamos, J. Inhibition of poly(ADP-ribose) polymerase attenuates the severity of acute pancreatitis and associated lung injury. Lab. Investig. 2005, 85, 1250–1262. [Google Scholar] [CrossRef] [Green Version]
- Mabley, J.G.; Pacher, P.; Deb, A.; Wallace, R.; Elder, R.H.; Szabo, C. Potential role for 8-oxoguanine DNA glycosylase in regulating inflammation. FASEB J. 2005, 19, 290–292. [Google Scholar] [CrossRef]
- Sandrasegaran, K.; Tahir, B.; Barad, U.; Fogel, E.; Akisik, F.; Tirkes, T.; Sherman, S. The Value of Secretin-Enhanced MRCP in Patients With Recurrent Acute Pancreatitis. AJR Am. J. Roentgenol. 2017, 208, 315–321. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Liu, Y.; Zhou, S.F.; Qiu, P.; Xu, L.; Wen, P.; Wen, J.; Xiao, X. Effect of Somatostatin, Ulinastatin and Gabexate on the Treatment of Severe Acute Pancreatitis. Am. J. Med. Sci. 2016, 351, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Nakamichi, I.; Habtezion, A.; Zhong, B.; Contag, C.H.; Butcher, E.C.; Omary, M.B. Hemin-activated macrophages home to the pancreas and protect from acute pancreatitis via heme oxygenase-1 induction. J. Clin. Investig. 2005, 115, 3007–3014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, L.; Jabrailova, B.; Soliman, H.; Hofer, S.; Strobel, O.; Hackert, T.; Buchler, M.W.; Werner, J. Pharmacological cholinergic stimulation as a therapeutic tool in experimental necrotizing pancreatitis. Pancreas 2014, 43, 41–46. [Google Scholar] [CrossRef]
- Tekin, S.O.; Teksoz, S.; Terzioglu, D.; Arikan, A.E.; Ozcevik, H.; Uslu, E. Use of infliximab in treatment of acute pancreatitis. Bratisl. Lek. Listy 2015, 116, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Abu-El-Haija, M.; Gukovskaya, A.S.; Andersen, D.K.; Gardner, T.B.; Hegyi, P.; Pandol, S.J.; Papachristou, G.I.; Saluja, A.K.; Singh, V.K.; Uc, A.; et al. Accelerating the Drug Delivery Pipeline for Acute and Chronic Pancreatitis: Summary of the Working Group on Drug Development and Trials in Acute Pancreatitis at the National Institute of Diabetes and Digestive and Kidney Diseases Workshop. Pancreas 2018, 47, 1185–1192. [Google Scholar] [CrossRef]
- Bakondi, E.; Singh, S.B.; Hajnady, Z.; Nagy-Penzes, M.; Regdon, Z.; Kovacs, K.; Hegedus, C.; Madacsy, T.; Maleth, J.; Hegyi, P.; et al. Spilanthol Inhibits Inflammatory Transcription Factors and iNOS Expression in Macrophages and Exerts Anti-inflammatory Effects in Dermatitis and Pancreatitis. Int. J. Mol. Sci. 2019, 20, 4308. [Google Scholar] [CrossRef] [Green Version]
- Foitzik, T.; Stufler, M.; Hotz, H.G.; Klinnert, J.; Wagner, J.; Warshaw, A.L.; Schulzke, J.D.; Fromm, M.; Buhr, H.J. Glutamine stabilizes intestinal permeability and reduces pancreatic infection in acute experimental pancreatitis. J. Gastrointest. Surg. 1997, 1, 40–46. [Google Scholar] [CrossRef]
- Kim, N.; Park, J.M.; Lee, S.H.; Kim, B.H.; Son, J.H.; Ryu, J.K.; Kim, Y.T.; Lee, W. Effect of Combinatory Treatment with Resveratrol and Guggulsterone on Mild Acute Pancreatitis in Mice. Pancreas 2017, 46, 366–371. [Google Scholar] [CrossRef]
- Tsang, S.W.; Guan, Y.F.; Wang, J.; Bian, Z.X.; Zhang, H.J. Inhibition of pancreatic oxidative damage by stilbene derivative dihydro-resveratrol: Implication for treatment of acute pancreatitis. Sci. Rep. 2016, 6, 22859. [Google Scholar] [CrossRef] [Green Version]
- Gasic, U.; Ciric, I.; Pejcic, T.; Radenkovic, D.; Djordjevic, V.; Radulovic, S.; Tesic, Z. Polyphenols as Possible Agents for Pancreatic Diseases. Antioxidants 2020, 9, 547. [Google Scholar] [CrossRef]
- Xia, S.; Wang, J.; Kalionis, B.; Zhang, W.; Zhao, Y. Genistein protects against acute pancreatitis via activation of an apoptotic pathway mediated through endoplasmic reticulum stress in rats. Biochem. Biophys. Res. Commun. 2019, 509, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Sheng, B.; Zhao, L.; Zang, X.; Zhen, J.; Liu, Y.; Bian, W.; Chen, W. Quercetin inhibits caerulein-induced acute pancreatitis through regulating miR-216b by targeting MAP2K6 and NEAT1. Inflammopharmacology 2021, 29, 549–559. [Google Scholar] [CrossRef]
- Li, Y.; Pan, Y.; Gao, L.; Zhang, J.; Xie, X.; Tong, Z.; Li, B.; Li, G.; Lu, G.; Li, W. Naringenin Protects against Acute Pancreatitis in Two Experimental Models in Mice by NLRP3 and Nrf2/HO-1 Pathways. Mediators Inflamm. 2018, 2018, 3232491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mrazek, A.A.; Bhatia, V.; Falzon, M.; Spratt, H.; Chao, C.; Hellmich, M.R. Apigenin Decreases Acinar Cell Damage in Pancreatitis. Pancreas 2019, 48, 711–718. [Google Scholar] [CrossRef]
- Jia, R.; Ma, J.; Meng, W.; Wang, N. Dihydromyricetin inhibits caerulin-induced TRAF3-p38 signaling activation and acute pancreatitis response. Biochem. Biophys. Res. Commun. 2018, 503, 1696–1702. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Wang, K.; Yuan, C.; Xing, R.; Ni, J.; Hu, G.; Chen, F.; Wang, X. Luteolin protects mice from severe acute pancreatitis by exerting HO-1-mediated anti-inflammatory and antioxidant effects. Int. J. Mol. Med. 2017, 39, 113–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, I.J.; Bae, G.S.; Choi, S.B.; Kim, D.G.; Shin, J.Y.; Seo, S.H.; Choi, M.O.; Kim, T.H.; Song, H.J.; Park, S.J. Fisetin attenuates cerulein-induced acute pancreatitis through down regulation of JNK and NF-kappaB signaling pathways. Eur. J. Pharmacol. 2014, 737, 149–158. [Google Scholar] [CrossRef]
- Gaman, L.; Dragos, D.; Vlad, A.; Robu, G.C.; Radoi, M.P.; Stroica, L.; Badea, M.; Gilca, M. Phytoceuticals in Acute Pancreatitis: Targeting the Balance between Apoptosis and Necrosis. Evid.-Based Complement. Altern. Med. 2018, 2018, 5264592. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, H.; Singer, P.; Halpern, Z.; Bruck, R. Polyphenols in the treatment of inflammatory bowel disease and acute pancreatitis. Gut 2007, 56, 426–435. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Yuan, L.; Wang, W.; Zhang, M.; Wang, Q.; Li, S.; Zhang, L.; Hu, K. Tricetin protects against 6-OHDA-induced neurotoxicity in Parkinson’s disease model by activating Nrf2/HO-1 signaling pathway and preventing mitochondria-dependent apoptosis pathway. Toxicol. Appl. Pharmacol. 2019, 378, 114617. [Google Scholar] [CrossRef]
- Ge, Q.; Chen, L.; Tang, M.; Zhang, S.; Liu, L.; Gao, L.; Ma, S.; Kong, M.; Yao, Q.; Feng, F.; et al. Analysis of mulberry leaf components in the treatment of diabetes using network pharmacology. Eur. J. Pharmacol. 2018, 833, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhu, F.; Shen, M.; Qiu, L.; Tang, M.; Xia, H.; Chen, L.; Yuan, Y.; Ma, S.; Chen, K. Network pharmacology-based analysis on bioactive anti-diabetic compounds in Potentilla discolor bunge. J. Ethnopharmacol. 2019, 241, 111905. [Google Scholar] [CrossRef] [PubMed]
- Pistelli, L.; Bertoli, A.; Noccioli, C.; Mendez, J.; Musmanno, R.A.; Di Maggio, T.; Coratza, G. Antimicrobial activity of Inga fendleriana extracts and isolated flavonoids. Nat. Prod. Commun. 2009, 4, 1679–1683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, P.Y.; Hsieh, M.J.; Hsieh, Y.S.; Chen, P.N.; Yang, J.S.; Lo, F.C.; Yang, S.F.; Lu, K.H. Tricetin inhibits human osteosarcoma cells metastasis by transcriptionally repressing MMP-9 via p38 and Akt pathways. Environ. Toxicol. 2017, 32, 2032–2040. [Google Scholar] [CrossRef] [PubMed]
- Chao, R.; Chow, J.M.; Hsieh, Y.H.; Chen, C.K.; Lee, W.J.; Hsieh, F.K.; Yu, N.Y.; Chou, M.C.; Cheng, C.W.; Yang, S.F.; et al. Tricetin suppresses the migration/invasion of human glioblastoma multiforme cells by inhibiting matrix metalloproteinase-2 through modulation of the expression and transcriptional activity of specificity protein 1. Expert Opin. Ther. Targets 2015, 19, 1293–1306. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.Y.; Chang, W.A.; Tsai, Y.M.; Hsu, Y.L.; Chiang, H.H.; Chou, S.H.; Huang, M.S.; Kuo, P.L. Tricetin, a dietary flavonoid, suppresses benzo(a)pyreneinduced human nonsmall cell lung cancer bone metastasis. Int. J. Oncol. 2015, 46, 1985–1993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, L.; Zhang, X.; Hou, M.; Gao, F. Natural flavone tricetin suppresses oxidized LDL-induced endothelial inflammation mediated by Egr-1. Int. Immunopharmacol. 2020, 80, 106224. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.F.; Hu, P.F.; Xiong, Y.; Bao, J.P.; Qian, J.; Wu, L.D. Tricetin Protects Rat Chondrocytes against IL-1beta-Induced Inflammation and Apoptosis. Oxid. Med. Cell Longev. 2019, 2019, 4695381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Wang, Z.; Sidhu, P.S.; Desai, U.R.; Zhou, Q. 6-Hydroxyflavone and derivatives exhibit potent anti-inflammatory activity among mono-, di- and polyhydroxylated flavones in kidney mesangial cells. PLoS ONE 2015, 10, e0116409. [Google Scholar] [CrossRef] [PubMed]
- Gout, J.; Pommier, R.M.; Vincent, D.F.; Kaniewski, B.; Martel, S.; Valcourt, U.; Bartholin, L. Isolation and culture of mouse primary pancreatic acinar cells. J. Vis. Exp. 2013, 78, 50514. [Google Scholar] [CrossRef] [Green Version]
- Hegedus, C.; Lakatos, P.; Kiss-Szikszai, A.; Patonay, T.; Gergely, S.; Gregus, A.; Bai, P.; Hasko, G.; Szabo, E.; Virag, L. Cytoprotective dibenzoylmethane derivatives protect cells from oxidative stress-induced necrotic cell death. Pharmacol. Res. 2013, 72, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Regdon, Z.; Robaszkiewicz, A.; Kovacs, K.; Rygielska, Z.; Hegedus, C.; Bodoor, K.; Szabo, E.; Virag, L. LPS protects macrophages from AIF-independent parthanatos by downregulation of PARP1 expression, induction of SOD2 expression, and a metabolic shift to aerobic glycolysis. Free Radic. Biol. Med. 2019, 131, 184–196. [Google Scholar] [CrossRef]
- Hegyi, P.; Venglovecz, V.; Pallagi, P.; Maleth, J.; Takacs, T.; Rakonczay, Z., Jr. Galanin, a potent inhibitor of pancreatic bicarbonate secretion, is involved in the induction and progression of cerulein-induced experimental acute pancreatitis. Pancreas 2011, 40, 155–156, author reply 156–157. [Google Scholar] [CrossRef]
- Kim, D.U.; Bae, G.S.; Kim, M.J.; Choi, J.W.; Kim, D.G.; Song, H.J.; Park, S.J. Icariin attenuates the severity of ceruleininduced acute pancreatitis by inhibiting p38 activation in mice. Int. J. Mol. Med. 2019, 44, 1563–1573. [Google Scholar] [CrossRef]
- Kiernan, J.A. Histological and Histochemical Methods: Theory and Practice, 5th ed.; Scion Publishing: Banbury, UK, 2015; pp. 454–490. [Google Scholar]
- Ceranowicz, P.; Warzecha, Z.; Dembinski, A.; Cieszkowski, J.; Dembinski, M.; Sendur, R.; Kusnierz-Cabala, B.; Tomaszewska, R.; Kuwahara, A.; Kato, I. Pretreatment with obestatin inhibits the development of cerulein-induced pancreatitis. J. Physiol. Pharmacol. 2009, 60, 95–101. [Google Scholar] [PubMed]
- Regdon, Z.; Demeny, M.A.; Kovacs, K.; Hajnady, Z.; Nagy-Penzes, M.; Bakondi, E.; Kiss, A.; Hegedus, C.; Virag, L. High-content screening identifies inhibitors of oxidative stress-induced parthanatos: Cytoprotective and anti-inflammatory effects of ciclopirox. Br. J. Pharmacol. 2021, 178, 1095–1113. [Google Scholar] [CrossRef]
- El-Hamoly, T.; Hajnady, Z.; Nagy-Penzes, M.; Bakondi, E.; Regdon, Z.; Demeny, M.A.; Kovacs, K.; Hegedus, C.; Abd El-Rahman, S.S.; Szabo, E.; et al. Poly(ADP-Ribose) Polymerase 1 Promotes Inflammation and Fibrosis in a Mouse Model of Chronic Pancreatitis. Int. J. Mol. Sci. 2021, 22, 3593. [Google Scholar] [CrossRef]
- Martinez-Bosch, N.; Fernandez-Zapico, M.E.; Navarro, P.; Yelamos, J. Poly(ADP-Ribose) Polymerases: New Players in the Pathogenesis of Exocrine Pancreatic Diseases. Am. J. Pathol. 2016, 186, 234–241. [Google Scholar] [CrossRef] [Green Version]
- Tasci, S.; Senocak, R.; Lapsekili, E.; Kaymak, S.; Yigit, T. The effects of a TNF-alpha inhibitor and HBO combination on the severity of pancreatitis and oxidative response in an experimental model of acute pancreatitis. Bratisl. Lek. Listy 2019, 120, 417–422. [Google Scholar] [CrossRef]
- Kim, H. Cerulein pancreatitis: Oxidative stress, inflammation, and apoptosis. Gut Liver 2008, 2, 74–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Wang, H.; Lu, M.; Qiao, X.; Sun, B.; Zhang, W.; Xue, D. Pancreatic Acinar Cells Employ miRNAs as Mediators of Intercellular Communication to Participate in the Regulation of Pancreatitis-Associated Macrophage Activation. Mediat. Inflamm. 2016, 2016, 6340457. [Google Scholar] [CrossRef] [Green Version]
- Gwozdz, G.P.; Steinberg, W.M.; Werner, M.; Henry, J.P.; Pauley, C. Comparative evaluation of the diagnosis of acute pancreatitis based on serum and urine enzyme assays. Clin. Chim. Acta 1990, 187, 243–254. [Google Scholar] [CrossRef]
- Dabrowski, A.; Konturek, S.J.; Konturek, J.W.; Gabryelewicz, A. Role of oxidative stress in the pathogenesis of caerulein-induced acute pancreatitis. Eur. J. Pharmacol. 1999, 377, 1–11. [Google Scholar] [CrossRef]
- Yu, J.H.; Kim, H. Oxidative stress and inflammatory signaling in cerulein pancreatitis. World J. Gastroenterol. 2014, 20, 17324–17329. [Google Scholar] [CrossRef] [PubMed]
- Virag, L.; Robaszkiewicz, A.; Rodriguez-Vargas, J.M.; Oliver, F.J. Poly(ADP-ribose) signaling in cell death. Mol. Asp. Med. 2013, 34, 1153–1167. [Google Scholar] [CrossRef]
- Geraets, L.; Moonen, H.J.; Brauers, K.; Wouters, E.F.; Bast, A.; Hageman, G.J. Dietary flavones and flavonoles are inhibitors of poly(ADP-ribose)polymerase-1 in pulmonary epithelial cells. J. Nutr. 2007, 137, 2190–2195. [Google Scholar] [CrossRef] [Green Version]
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Davies, K.J.; Ursini, F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Malleo, G.; Mazzon, E.; Siriwardena, A.K.; Cuzzocrea, S. Role of tumor necrosis factor-alpha in acute pancreatitis: From biological basis to clinical evidence. Shock 2007, 28, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Gea-Sorli, S.; Closa, D. Role of macrophages in the progression of acute pancreatitis. World J. Gastrointest. Pharmacol. Ther. 2010, 1, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Bai, P.; Virag, L. Role of poly(ADP-ribose) polymerases in the regulation of inflammatory processes. FEBS Lett. 2012, 586, 3771–3777. [Google Scholar] [CrossRef] [Green Version]
- Demeny, M.A.; Virag, L. The PARP Enzyme Family and the Hallmarks of Cancer Part 2: Hallmarks Related to Cancer Host Interactions. Cancers 2021, 13, 2057. [Google Scholar] [CrossRef]
- Mayerle, J.; Sendler, M.; Hegyi, E.; Beyer, G.; Lerch, M.M.; Sahin-Toth, M. Genetics, Cell Biology, and Pathophysiology of Pancreatitis. Gastroenterology 2019, 156, 1951–1968 e1951. [Google Scholar] [CrossRef] [Green Version]
- Gullo, L.; Cavicchi, L.; Tomassetti, P.; Spagnolo, C.; Freyrie, A.; D’Addato, M. Effects of ischemia on the human pancreas. Gastroenterology 1996, 111, 1033–1038. [Google Scholar] [CrossRef]
- Lonardo, A.; Grisendi, A.; Bonilauri, S.; Rambaldi, M.; Selmi, I.; Tondelli, E. Ischaemic necrotizing pancreatitis after cardiac surgery. A case report and review of the literature. Ital. J. Gastroenterol. Hepatol. 1999, 31, 872–875. [Google Scholar]
- Dembinski, A.; Warzecha, Z.; Ceranowicz, P.; Stachura, J.; Tomaszewska, R.; Konturek, S.J.; Sendur, R.; Dembinski, M.; Pawlik, W.W. Pancreatic damage and regeneration in the course of ischemia-reperfusion induced pancreatitis in rats. J. Physiol. Pharmacol. 2001, 52, 221–235. [Google Scholar]
- Furukawa, M.; Kimura, T.; Sumii, T.; Yamaguchi, H.; Nawata, H. Role of local pancreatic blood flow in development of hemorrhagic pancreatitis induced by stress in rats. Pancreas 1993, 8, 499–505. [Google Scholar] [CrossRef]
- Warzecha, Z.; Dembinski, A.; Ceranowicz, P.; Dembinski, M.; Cieszkowski, J.; Kusnierz-Cabala, B.; Naskalski, J.W.; Jaworek, J.; Konturek, S.J.; Pawlik, W.W.; et al. Influence of ischemic preconditioning on blood coagulation, fibrinolytic activity and pancreatic repair in the course of caerulein-induced acute pancreatitis in rats. J. Physiol. Pharmacol. 2007, 58, 303–319. [Google Scholar] [PubMed]
- Maduzia, D.; Ceranowicz, P.; Cieszkowski, J.; Chmura, A.; Galazka, K.; Kusnierz-Cabala, B.; Warzecha, Z. Administration of warfarin accelerates the recovery in ischemia/reperfusion-induced acute pancreatitis. J. Physiol. Pharmacol. 2020, 71, 417–427. [Google Scholar] [CrossRef]
- Vovkun, T.V.; Yanchuk, P.I.; Shtanova, L.Y.; Shalamay, A.S. Tissue Blood Flow in the Digestive Organs of Rats with Acute Pancreatitis after Corvitin Administration. Fiziol. Zhurnal 2015, 61, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Datsyuk, O.I. Impact of Quercetin on Systemic and Splanchnic Blood Circulation in a Complex of Preoperative Preparation in Patients, Suffering an Acute Pancreatitis. Klin Khir 2016, 13–15. [Google Scholar] [PubMed]


| Gene | Forward Primers (5→3) | Reverse Primers (5→3) |
|---|---|---|
| IL1β | CAACCAACAAGTGATATTCTCCATG | GATCCACACTCTCCAGCTGCA |
| IL6 | GAGGATACCACTCCCAACAGACC | AAGTGCATCATCGTTGTTCATACA |
| IL10 | GGCGCTGTCATCGATTTCTC | ATGGCCTTGTAGACACTTTGG |
| TNFα | CATCTTCTCAAAATTCGAGTGACAA | TGGGAGTAGACAAGGTACAACCC |
| TGFβ | CCGCAACAACGCCATCTATG | GTTCCACATGTTGCTCCACAC |
| IFNγ | GGAACTGGCAAAAGGATGGTG | ATGTTGTTGCTGATGGCCTG |
| CCL5 | TGCAGTCGTGTTTGTCACTC | AGAGCAAGCAATGACAGGGA |
| CXCL10 | GATGACGGGCCAGTGAGAAT | CGTGGCAATGATCTCAACAC |
| MMP2 | AACGGTCGGGAATACAGCAG | GTAAACAACGCTTCATGGGGG |
| 36B4 | GGACCCGAGAAGACCTCCTT | GCACATCACTCAGAATTCAATCC |
| RPS26 | CCACAATTCAGACCTGCTG | GGGTAATTTTCCTTCCGTCCT |
| 0 | 1 | 2 | 3 | |
|---|---|---|---|---|
| Edema | no edema | interlobular edema | interlobular and moderate intralobular edema | interlobular and severe intralobular edema |
| Infiltration | absent | low perivascular infiltration | moderate perivascular and low diffuse infiltration | severe diffuse infiltration |
| Necrosis | none or negligible | observed in one third of the cells | observed in half of the cells | observed in most cells |
| TUNEL | 0–5 average spot number/400× field | 6–15 average spot number/400× field | 16–35 average spot number/400× field | >5 average spot number/400× field |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagy-Pénzes, M.; Hajnády, Z.; Regdon, Z.; Demény, M.Á.; Kovács, K.; El-Hamoly, T.; Maléth, J.; Hegyi, P.; Hegedűs, C.; Virág, L. Tricetin Reduces Inflammation and Acinar Cell Injury in Cerulein-Induced Acute Pancreatitis: The Role of Oxidative Stress-Induced DNA Damage Signaling. Biomedicines 2022, 10, 1371. https://doi.org/10.3390/biomedicines10061371
Nagy-Pénzes M, Hajnády Z, Regdon Z, Demény MÁ, Kovács K, El-Hamoly T, Maléth J, Hegyi P, Hegedűs C, Virág L. Tricetin Reduces Inflammation and Acinar Cell Injury in Cerulein-Induced Acute Pancreatitis: The Role of Oxidative Stress-Induced DNA Damage Signaling. Biomedicines. 2022; 10(6):1371. https://doi.org/10.3390/biomedicines10061371
Chicago/Turabian StyleNagy-Pénzes, Máté, Zoltán Hajnády, Zsolt Regdon, Máté Á. Demény, Katalin Kovács, Tarek El-Hamoly, József Maléth, Péter Hegyi, Csaba Hegedűs, and László Virág. 2022. "Tricetin Reduces Inflammation and Acinar Cell Injury in Cerulein-Induced Acute Pancreatitis: The Role of Oxidative Stress-Induced DNA Damage Signaling" Biomedicines 10, no. 6: 1371. https://doi.org/10.3390/biomedicines10061371
APA StyleNagy-Pénzes, M., Hajnády, Z., Regdon, Z., Demény, M. Á., Kovács, K., El-Hamoly, T., Maléth, J., Hegyi, P., Hegedűs, C., & Virág, L. (2022). Tricetin Reduces Inflammation and Acinar Cell Injury in Cerulein-Induced Acute Pancreatitis: The Role of Oxidative Stress-Induced DNA Damage Signaling. Biomedicines, 10(6), 1371. https://doi.org/10.3390/biomedicines10061371








