The Lipid Profile and Biochemical Parameters of COPD Patients in Relation to Smoking Status
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Patient Selection
2.3. Data Collection and Management
2.4. Biochemical Assay
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Labaki, W.W.; Rosenberg, S.R. Chronic Obstructive Pulmonary Disease. Ann. Intern. Med. 2020, 173, ITC17–ITC32. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, R.; De Berardis, B.; Paoletti, L.; Guastadisegni, C. Inflammatory mediators induced by coarse (PM2.5–10) and fine (PM2.5) urban air particles in RAW 264.7 cells. Toxicology 2003, 183, 243–254. [Google Scholar] [CrossRef]
- Huang, Q.; Hu, D.; Wang, X.; Chen, Y.; Wu, Y.; Pan, L.; Li, H.; Zhang, J.; Deng, F.; Guo, X.; et al. The modification of indoor PM2.5 exposure to chronic obstructive pulmonary disease in Chinese elderly people: A meet-in-metabolite analysis. Environ. Int. 2018, 121 (Pt 2), 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Trofor, A.; Petris, O.; Trofor, L.; Man, M.A.; Filipeanu, D.; Miron, R. Biochemistry in Assessing Tobacco Exposure-Smokers versus Non-smokers Correlations with clinical practice. Rev. Chim. 2017, 68, 1002–1006. [Google Scholar] [CrossRef]
- Ames, B.N.; Cathcart, R.; Schwiers, E.; Hochstein, P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proc. Natl. Acad. Sci. USA 1981, 78, 6858–6862. [Google Scholar] [CrossRef]
- Mouhamed, D.H.; Ezzaher, A.; Neffati, F.; Douki, W.; Gaha, L.; Najjar, M.F. Effect of cigarette smoking on plasma uric acid concentrations. Environ. Health Prev. Med. 2011, 16, 307–312. [Google Scholar] [CrossRef]
- Li, X.X.; Zhao, Y.; Huang, L.X.; Xu, H.X.; Liu, X.Y.; Yang, J.J.; Zhang, P.J.; Zhang, Y.H. Effects of smoking and alcohol con-sumption on lipid profile in male adults in northwest rural China. Public Health 2018, 157, 7–13. [Google Scholar] [CrossRef]
- Lee, S.-S.; Seo, J.-S.; Kim, S.-R.; Jeong, J.-E.; Nam, B.-W.; Lee, J.-Y.; Lee, H.-J.; Lee, C.; Lee, C.-U.; Paik, I.-H.; et al. The Changes of Blood Glucose Control and Lipid Profiles after Short-Term Smoking Cessation in Healthy Males. Psychiatry Investig. 2011, 8, 149–154. [Google Scholar] [CrossRef]
- Khojah, H.M.; Ahmed, S.A. Comparative assessment of individual RONS in serum of smokers compared with non-smokers and their correlation with the lipid profile and antioxidant status. J. Int. Med. Res. 2019, 47, 6223–6234. [Google Scholar] [CrossRef]
- Rao, C.S.; Subash, Y.E. The effect of chronic tobacco smoking and chewing on the lipid profile. J. Clin. Diagn. Res. 2013, 7, 31–34. [Google Scholar]
- Freeman, D.J.; Caslake, M.J.; Griffin, B.A.; Hinnie, J.; Tan, C.E.; Watson, T.D.; Packard, C.J.; Shepherd, J. The effect of smoking on post-heparin lipoprotein and hepatic lipase, cholesteryl ester transfer protein and lecithin: Cholesterol acyl transferase activities in human plasma. Eur. J. Clin. Investig. 1998, 28, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Dullaart, R.P.; Hoogenberg, K.; Dikkeschei, B.D.; van Tol, A. Higher plasma lipid transfer protein activities and unfavorable lipoprotein changes in cigarette-smoking men. Arter. Thromb. J. Vasc. Biol. 1994, 14, 1581–1585. [Google Scholar] [CrossRef] [PubMed]
- Chimura, Y.; Daimon, T.; Wakabayashi, I. Proneness to high blood lipid-related indices in female smokers. Lipids Health Dis. 2019, 18, 113. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease-2020 Report. 2020. Available online: https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf (accessed on 15 April 2020).
- Covaci, A.; Voorspoels, S.; Thomsen, C.; van Bavel, B.; Neels, H. Evaluation of total lipids using enzymatic methods for the normalization of persistent organic pollutant levels in serum. Sci. Total Environ. 2006, 366, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Rumora, L.; Hlapčić, I.; Popović-Grle, S.; Rako, I.; Rogić, D.; Čepelak, I. Uric acid and uric acid to creatinine ratio in the as-sessment of chronic obstructive pulmonary disease: Potential biomarkers in multicomponent models comprising IL-1beta. PLoS ONE 2020, 15, e0234363. [Google Scholar] [CrossRef]
- Zaga, V.; Gatta-vecchia, E. Radicali liberi e fumo di sigaretta (Free radicals and cigarette smoke). G. Ital. Mal. Torace 2002, 56, 375–391. [Google Scholar]
- Hanna, B.E.; Hamed, J.M.; Touhala, L.M. Serum uric Acid in smokers. Oman Med. J. 2008, 23, 269–274. [Google Scholar] [CrossRef]
- Trofor, L.; Crisan-Dabija, R.; Cioroiu, M.E.; Man, M.A.; Cioroiu, M.E.; Buculei, I.; Cernat, R.-I.; Stefanescu, C.; Trofor, A.C. Evaluation of oxidative stress in smoking and non-smoking patients diagnosed with anxious-depressive disorder. Farmacia 2020, 68, 82–89. [Google Scholar] [CrossRef]
- Vujic, T.; Nagorni, O.; Maric, G.; Popovic, L.; Jankovic, J. Metabolic syndrome in patients with chronic obstructive pulmonary disease: Frequency and relationship with systemic inflammation. Hippokratia 2017, 20, 110–114. [Google Scholar]
- Minas, M.; Kostikas, K.; Papaioannou, A.I.; Mystridou, P.; Karetsi, E.; Georgoulias, P.; Liakos, N.; Pournaras, S.; Gourgoulianis, K.I. The Association of Metabolic Syndrome with Adipose Tissue Hormones and Insulin Resistance in Patients with COPD without Co-morbidities. COPD 2011, 8, 414–420. [Google Scholar] [CrossRef]
- Itoh, M.; Tsuji, T.; Nakamura, H.; Yamaguchi, K.; Fuchikami, J.-I.; Takahashi, M.; Morozumi, Y.; Aoshiba, K. Systemic effects of acute cigarette smoke exposure in mice. Inhal. Toxicol. 2014, 26, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Medvedev, A.V.; Daniel, K.W.; Collins, S. β-Adrenergic activation of p38 MAP kinase in adipocytes: cAMP induction of the uncoupling protein 1 (UCP1) gene requires p38 MAP kinase. J. Biol. Chem. 2001, 276, 27077–27082. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Yamaguchi, K.; Lee, S.-H.; Tithof, P.K.; Sayler, G.S.; Yoon, J.-H.; Baek, S.J. Evaluation of Polycyclic Aromatic Hydrocarbons in the Activation of Early Growth Response-1 and Peroxisome Proliferator Activated Receptors. Toxicol. Sci. 2005, 85, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Bays, H.E. Adiposopathy is sick fat a cardiovascular disease? J. Am. Coll. Cardiol. 2011, 57, 2461–2473. [Google Scholar] [CrossRef]
- Langin, D. Adipose tissue lipolysis as a metabolic pathway to define pharmacological strategies against obesity and the metabolic syndrome. Pharmacol. Res. 2006, 53, 482–491. [Google Scholar] [CrossRef]
- Ettinger, W.H.; Klinefelter, H.F.; Kwiterovitch, P.O. Effect of short-term, low-dose corticosteroids on plasma lipoprotein lipids. Atherosclerosis 1987, 63, 167–172. [Google Scholar] [CrossRef]
- Esteve, E.; Ricart, W.; Fernández-Real, J.M. Dyslipidemia and inflammation: An evolutionary conserved mechanism. Clin. Nutr. 2005, 24, 16–31. [Google Scholar] [CrossRef]
- Park, S.K.; Larson, J.L. The Relationship Between Physical Activity and Metabolic Syndrome in People With Chronic Obstructive Pulmonary Disease. J. Cardiovasc. Nurs. 2014, 29, 499–507. [Google Scholar] [CrossRef]
- Lam, K.H.; Jordan, R.E.; Jiang, C.Q.; Thomas, G.N.; Miller, M.R.; Zhang, W.S.; Lam, T.H.; Cheng, K.K.; Adab, P. Airflow obstruction and metabolic syn-drome: The Guangzhou Biobank Cohort Study. Eur. Respir. J. 2010, 35, 317–323. [Google Scholar] [CrossRef]
- Śliwińska-Mossoń, M.; Mihułka, E.; Milnerowicz, H. Ocena profilu lipidowego u niepalących i palących młodych zdrowych osób Assessment of lipid profile in non-smoking and smoking young health persons. Prz. Lek. 2014, 71, 585–587. [Google Scholar]
- Szkup, M.; Jurczak, A.; Karakiewicz, B.; Kotwas, A.; Kopeć, J.; Grochans, E. Influence of cigarette smoking on hormone and lipid metabolism in women in late reproductive stage. Clin. Interv. Aging 2018, 13, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-N.; Ha, B.; Seog, W.; Hwang, I.-U. Long-term exposure to air pollution and the blood lipid levels of healthy young men. Environ. Int. 2022, 161, 107119. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.-Y.; Bloom, M.; Markevych, I.; Qian, Z.; Vaughn, M.G.; Cummings-Vaughn, L.A.; Li, S.; Chen, G.; Bowatte, G.; Perret, J.L.; et al. Exposure to ambient air pollution and blood lipids in adults: The 33 Communities Chinese Health Study. Environ. Int. 2018, 119, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Frati, A.C.; Iniestra, F.; Ariza, C.R. Acute Effect of Cigarette Smoking on Glucose Tolerance and Other Cardiovascular Risk Factors. Diabetes Care 1996, 19, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Bornemisza, P.; Suciu, I. Effect of cigarette smoking on the blood glucose level in normals and diabetics. Med. Interne 1980, 18, 353–356. [Google Scholar] [PubMed]
- Rogliani, P.; Lucà, G.; Lauro, D. Chronic obstructive pulmonary disease and diabetes. COPD Res. Pract. 2015, 1, 3. [Google Scholar] [CrossRef]
- Slatore, C.G.; Bryson, C.L.; Au, D.H. The Association of Inhaled Corticosteroid Use with Serum Glucose Concentration in a Large Cohort. Am. J. Med. 2009, 122, 472–478. [Google Scholar] [CrossRef][Green Version]
- Dülger, H.; Dönder, A.; Şekeroğlu, M.R.; Erkoç, R.; Özbay, B. Investigation of the Relationship between Serum Levels of Cotinine and the Renal Function in Active and Passive Smokers. Ren. Fail. 2011, 33, 475–479. [Google Scholar] [CrossRef]
- Orth, S.R.; Ritz, E. The renal risks of smoking: An update. Curr. Opin. Nephrol. Hypertens. 2002, 11, 483–488. [Google Scholar] [CrossRef]
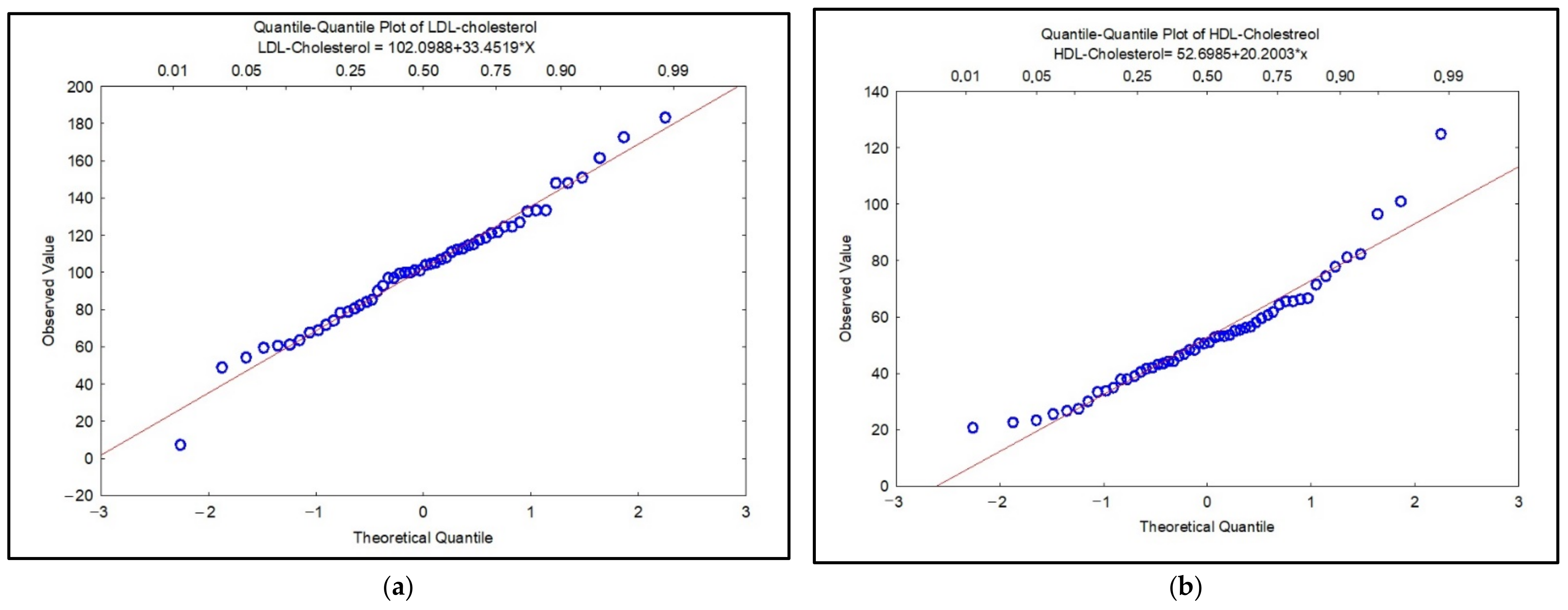

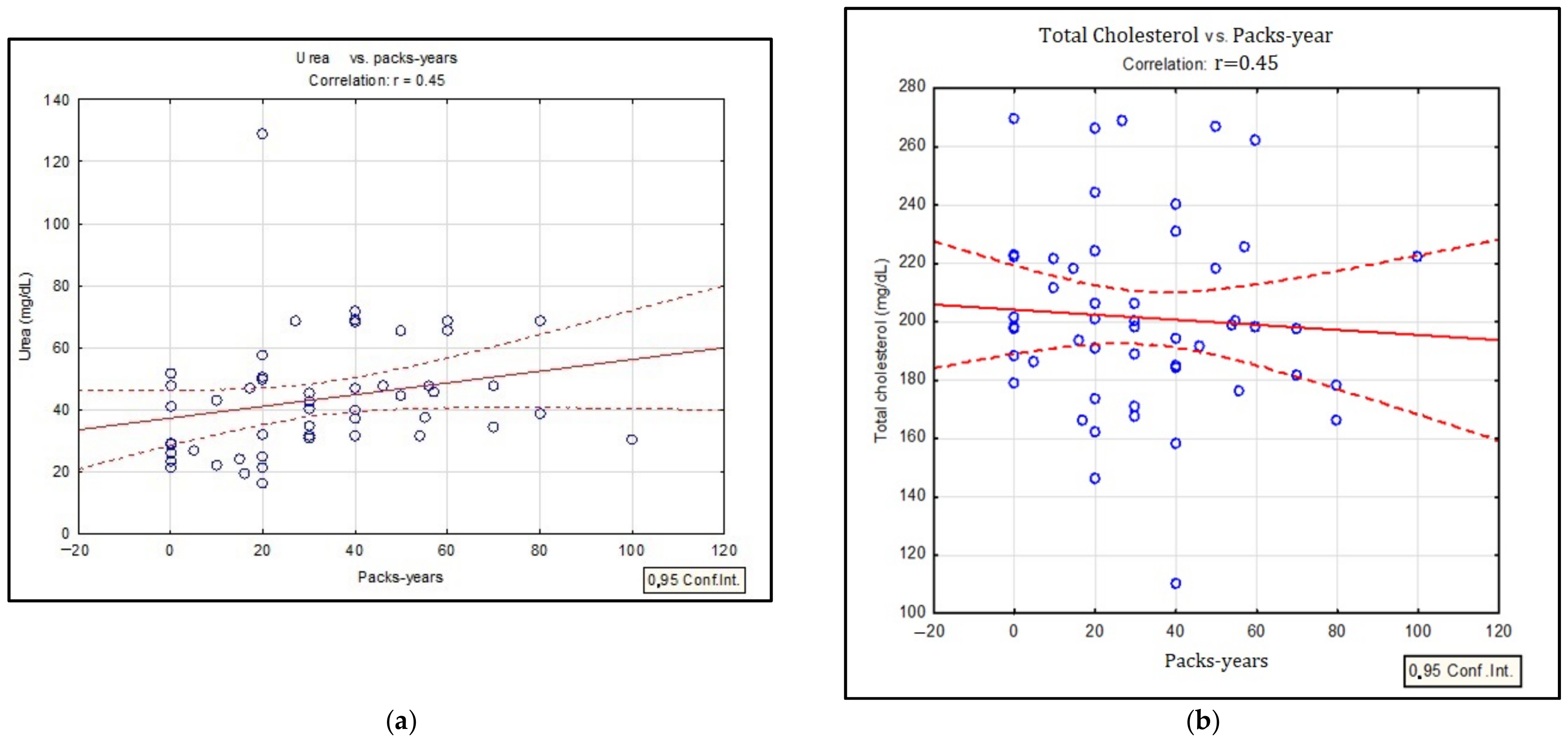
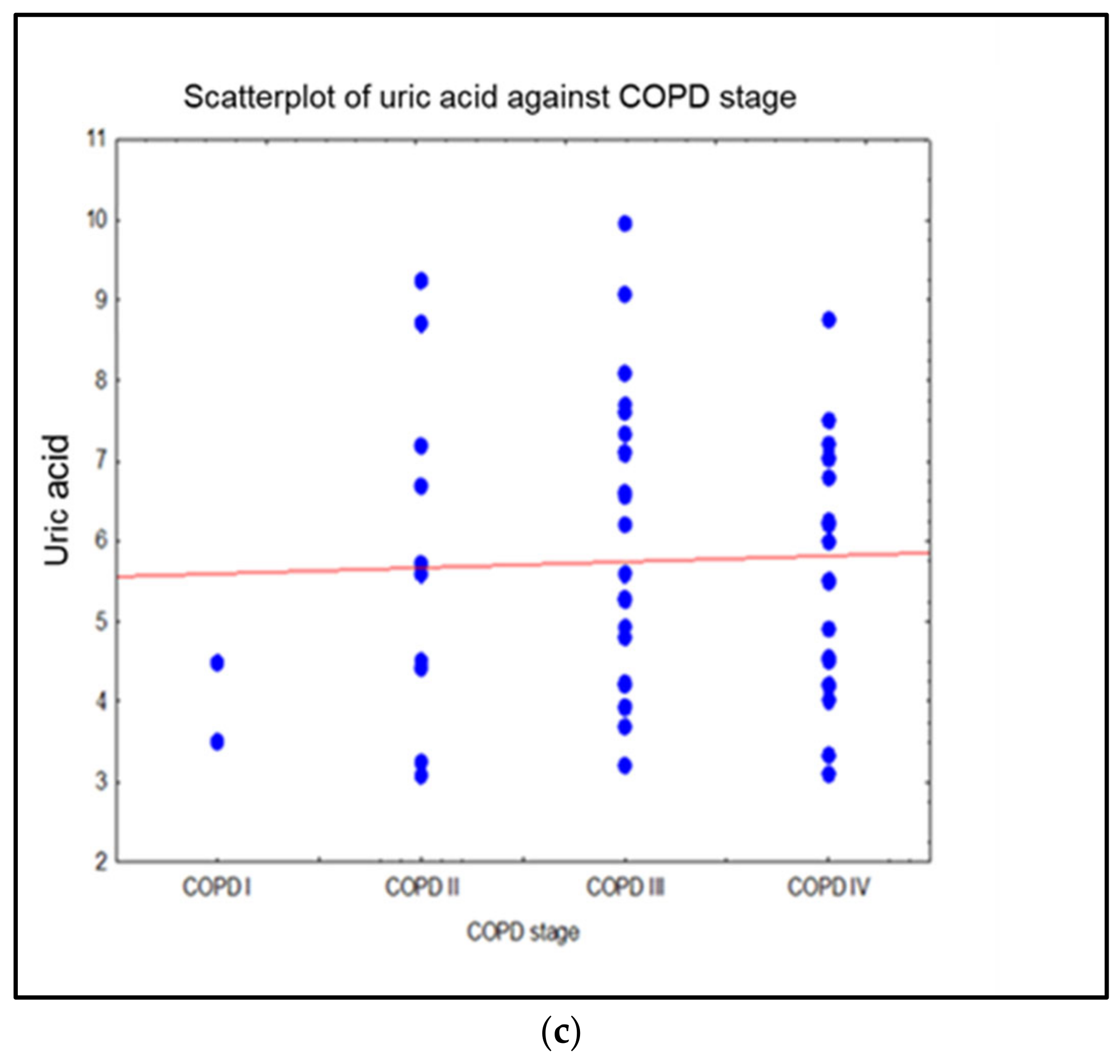
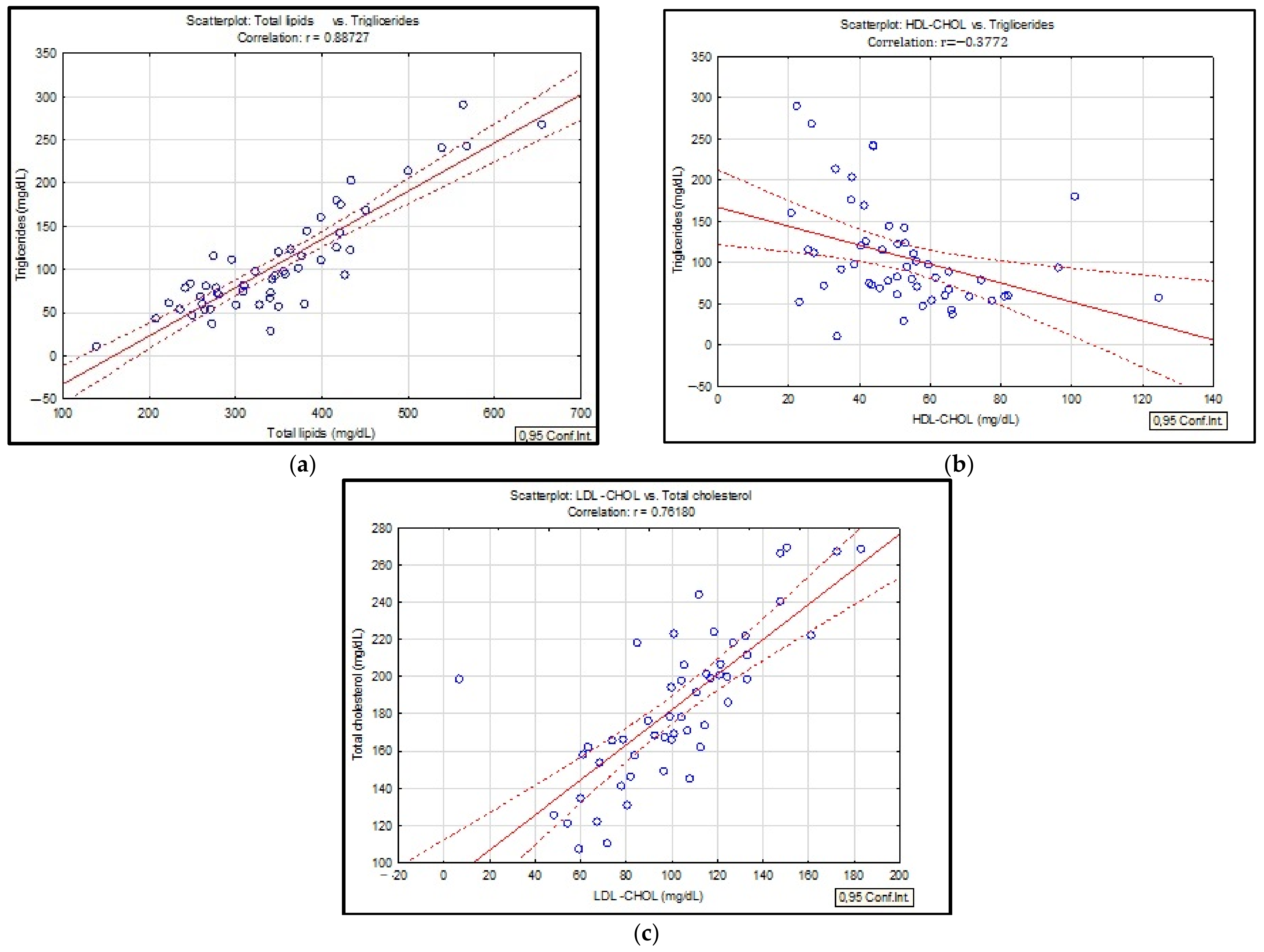

| Characteristic | Smokers | Non-Smokers | Ex-Smokers | p-Value |
|---|---|---|---|---|
| (N = 52) | N = 16 | N = 8 | N = 28 | |
| Median (± SDEV)-age-years | 65 ± 8.82 | 69 ± 9.35 | 68 ± 8.91 | |
| Male N/% | 14/88 | 7/88 | 26/93 | 0.07 |
| Female N/% | 2/12 | 1/12 | 2/7 | 0.69 |
| Urban/Rural area %/% | 50/50 | 33/67 | 46/54 | 0.46 |
| COPD I N/% | 2/12 | - | - | 0.06 |
| COPD II N/% | 3/19 | - | 7/26 | 0.05 |
| COPD III N/% | 5/29 | 5/62 | 12/42 | |
| COPD IV N/% | 6/40 | 3/38 | 9/32 | |
| The “ABCD” assessment tool | ||||
| A N/% | 0 | 0 | 1/4 | |
| B N/% | 4/25 | 3/38 | 10/36 | |
| C N/% | 0 | 0 | 2/7 | |
| D N/% | 12/75 | 5/63 | 15/54 | |
| FEV1 (mean/median) %/% | 37.10/34.55 | 42.02/37.35 | 48.53/43.80 | |
| PY (mean/median) | 50.81/43.0 | - | 30.18/28.5 | |
| Comorbidities | ||||
| Hypertension N/% | 5/56 | 5/62 | 15/56 | |
| Diabetes mellitus N/% | 3/19 | 1/13 | 3/11 | |
| Obesity N/% | 1/6 | 2/25 | 4/14 | |
| Parameters * | Smokers | Non-Smokers | Ex-Smokers | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COPD Patients | Average | SD | Median | Range | Average | SD | Median | Range | Average | SD | Median | Range |
| (N = 16) | (N = 8) | (N = 28) | ||||||||||
| Uric acid | 5.28 | 1.80 | 5. 22 | 3.21–9.08 | 5.93 | 1.77 | 5.42 | 3.10–7.70 | 5.86 | 1.78 | 5.60 | 3.09–9.97 |
| Urea | 49.23 | 14.54 | 47.60 | 30.40–68.9 | 33.66 | 11.49 | 29.10 | 21.50–51.60 | 42.97 | 22.39 | 40.10 | 16.20–129 |
| Creatinine | 0.81 | 0.23 | 0.78 | 0.50–1.16 | 0.74 | 0.28 | 0.70 | 0.35–1.23 | 0.79 | 82.07 | 0.72 | 0.35–2.40 |
| Glucose | 104.26 | 20.16 | 96.00 | 83.20–140.40 | 105.90 | 30.33 | 109.60 | 77.70–134.00 | 109.34 | 24.09 | 106.40 | 68.80–162.50 |
| Triglycerides | 110.32 | 62.10 | 90.26 | 36.82–240.81 | 105.03 | 32.37 | 104.25 | 67.00–168.50 | 104.95 | 69.03 | 83.82 | 10.76–290.00 |
| Total Cholesterol | 200.2 | 29.03 | 194.05 | 121.11–222.07 | 183.49 | 52.55 | 188.52 | 107.30–269.40 | 196.34 | 39.83 | 199.34 | 110.20–268.60 |
| Total lipides | 370.98 | 98.74 | 288.57 | 207.93–538.9 | 346.68 | 71.54 | 345.37 | 241.71–450.82 | 360.96 | 107.60 | 348.41 | 139.21–655.42 |
| HDL-CHOL * | 45.82 | 11.49 | 50.61 | 27.18–66.36 | 53.15 | 21.37 | 47.30 | 25.58–96.19 | 52.48 | 24.76 | 52.60 | 20.73–124.64 |
| LDL-CHOL ** | 106.34 | 41.29 | 99.89 | 6.84–161.14 | 98.26 | 47.57 | 100.045 | 59.13–150.73 | 110.53 | 30.47 | 113.34 | 61.17–183.26 |
| UA | Urea | Creatinine | Glucose | Triglicerides | CHOL | LipT | HDL-CHOL | LDL-CHOL | COPD Stage | Status of Smoking | PY | VEMS | LABA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UA | 1 | |||||||||||||
| Urea | 0.281 | 1 | ||||||||||||
| Creatinine | 0.546 | 0.500 | 1 | |||||||||||
| Glucose | 0.316 | 0.007 | 0.456 | 1 | ||||||||||
| Triglicerides | 0.125 | 0.357 | 0.037 | 0.004 | 1 | |||||||||
| CHOL | 0.048 | 0.105 | 0.014 | 0.044 | 0.089 | 1 | ||||||||
| LipT | 0.031 | 0.308 | 0.046 | 0.060 | 0.884 | 0.945 | 1 | |||||||
| HDL-CHOL | 0.007 | 0.144 | 0.113 | 0.006 | 0.377 | 0.220 | 0.234 | 1 | ||||||
| LDL -CHOL | 0.078 | 0.154 | 0.018 | 0.089 | 0.209 | 0.809 | 0.945 | 0.043 | 1 | |||||
| COPD stage | 0.398 | 0.028 | 0.231 | 0.273 | 0.057 | 0.056 | 0.061 | 0.046 | 0.059 | 1 | ||||
| Status of smoking | 0.025 | 0.182 | 0.144 | 0.067 | 0.140 | 0.453 | 0.120 | 0.09 | 0.297 | 0.204 | 1 | |||
| PY | 0.238 | 0.451 | 0.139 | 0.170 | 0.046 | 0.134 | 0.030 | 0.069 | 0.141 | 0.211 | 0.215 | 1 | ||
| VEMS | 0.180 | 0.044 | 0.072 | 0.039 | 0.227 | 0.117 | 0.100 | 0.188 | 0.068 | 0.142 | 0.252 | 0.068 | 1 | |
| LABA | 0.188 | 0.073 | 0.086 | 0.191 | 0.138 | 0.124 | 0.105 | 0.093 | 0.134 | 0.070 | 0.042 | 0.180 | 0.047 | 1 |
| Mann-Whitney U Test by Variable Obesity; Marked Tests are Significant at p < 0.05000 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rank Sum | Rank Sum | U | Z | p-Value | Z | p-Value | Valid N | Valid N | 2*1sided | |
| Total cholesterol | 1224.50 | 153.50 | 125.50 | 0.84 | 0.40 | 0.84 | 0.40 | 45.00 | 7.00 | 0.40 |
| LipT | 1287.00 | 91.00 | 63.00 | 2.52 | 0.01 | 2.52 | 0.01 | 45.00 | 7.00 | 0.01 |
| HDL-CHOL | 1245.00 | 133.00 | 105.00 | 1.39 | 0.16 | 1.39 | 0.16 | 45.00 | 7.00 | 0.17 |
| LDL -CHOL | 1211.00 | 167.00 | 139.00 | 0.48 | 0.63 | 0.48 | 0.63 | 45.00 | 7.00 | 0.64 |
| Triglicerides | 1217.00 | 161.00 | 133.00 | 0.64 | 0.52 | 0.64 | 0.52 | 45.00 | 7.00 | 0.53 |
| Mann-Whitney U Test by variable Diabetes mellitus; Marked tests are significant at p < 0.05000 | ||||||||||
| Rank Sum | Rank Sum | U | Z | p-value | Z | p-value | Valid N | Valid N | 2*1sided | |
| Total cholesterol | 1152.00 | 226.00 | 117.00 | −1.07 | 0.28 | −1.07 | 0.28 | 45.00 | 7.00 | 0.29 |
| LipT | 1157.00 | 221.00 | 122.00 | −0.94 | 0.35 | −0.94 | 0.35 | 45.00 | 7.00 | 0.36 |
| HDL-CHOL | 1209.00 | 169.00 | 141.00 | 0.43 | 0.67 | 0.43 | 0.67 | 45.00 | 7.00 | 0.67 |
| LDL -CHOL | 1207.00 | 171.00 | 143.00 | 0.38 | 0.71 | 0.38 | 0.71 | 45.00 | 7.00 | 0.71 |
| Triglicerides | 1252.00 | 126.00 | 98.00 | 1.58 | 0.11 | 1.58 | 0.11 | 45.00 | 7.00 | 0.12 |
| Mann-Whitney U Test by variable hypertension; Marked tests are significant at p < 0.05000 | ||||||||||
| Rank Sum | Rank Sum | U | Z | p-value | Z | p-value | Valid N | Valid N | 2*1sided | |
| Total cholesterol | 580.00 | 798.00 | 304.00 | −0.53 | 0.59 | -0.53 | 0.59 | 23.00 | 29.00 | 0.60 |
| LipT | 527.00 | 851.00 | 251.00 | −1.51 | 0.13 | −1.51 | 0.13 | 23.00 | 29.00 | 0.13 |
| HDL-CHOL | 558.00 | 820.00 | 282.00 | −0.94 | 0.35 | −0.94 | 0.35 | 23.00 | 29.00 | 0.35 |
| LDL-CHOL | 526.50 | 851.50 | 250.50 | −1.52 | 0.13 | −1.52 | 0.13 | 23.00 | 29.00 | 0.13 |
| Triglicerides | 607.50 | 770.50 | 331.50 | −0.03 | 0.98 | −0.03 | 0.98 | 23.00 | 29.00 | 0.97 |
| Mann-Whitney U Test by variable urban/rural area; Marked tests are significant at p < 0.05000 | ||||||||||
| Rank Sum | Rank Sum | U | Z | p-value | Z | p-value | Valid N | Valid N | 2*1sided | |
| Triglicerides | 780.50 | 597.50 | 246.50 | 1.67 | 0.10 | 1.67 | 0.10 | 26.00 | 26.00 | 0.09 |
| Total cholesterol | 718.00 | 660.00 | 309.00 | 0.52 | 0.60 | 0.52 | 0.60 | 26.00 | 26.00 | 0.60 |
| HDL-CHOL | 663.00 | 715.00 | 312.00 | −0.47 | 0.64 | −0.47 | 0.64 | 26.00 | 26.00 | 0.64 |
| LDL-CHOL | 786.00 | 592.00 | 241.00 | 1.77 | 0.08 | 1.77 | 0.08 | 26.00 | 26.00 | 0.08 |
| LipT | 607.50 | 670.50 | 331.50 | −0.03 | 0.98 | −0.03 | 0.98 | 26.00 | 26.00 | 0.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicol, C.; Buculei, I.; Melinte, O.E.; Dobrin, M.E.; Stavarache, E.I.; Gavrilescu, C.-M.; Postolache, P.; Matei, D.; Trofor, A. The Lipid Profile and Biochemical Parameters of COPD Patients in Relation to Smoking Status. Biomedicines 2022, 10, 2936. https://doi.org/10.3390/biomedicines10112936
Vicol C, Buculei I, Melinte OE, Dobrin ME, Stavarache EI, Gavrilescu C-M, Postolache P, Matei D, Trofor A. The Lipid Profile and Biochemical Parameters of COPD Patients in Relation to Smoking Status. Biomedicines. 2022; 10(11):2936. https://doi.org/10.3390/biomedicines10112936
Chicago/Turabian StyleVicol, Cristina, Ioana Buculei, Oana Elena Melinte, Mona Elisabeta Dobrin, Emanuel Ioan Stavarache, Cristina-Maria Gavrilescu, Paraschiva Postolache, Daniela Matei, and Antigona Trofor. 2022. "The Lipid Profile and Biochemical Parameters of COPD Patients in Relation to Smoking Status" Biomedicines 10, no. 11: 2936. https://doi.org/10.3390/biomedicines10112936
APA StyleVicol, C., Buculei, I., Melinte, O. E., Dobrin, M. E., Stavarache, E. I., Gavrilescu, C.-M., Postolache, P., Matei, D., & Trofor, A. (2022). The Lipid Profile and Biochemical Parameters of COPD Patients in Relation to Smoking Status. Biomedicines, 10(11), 2936. https://doi.org/10.3390/biomedicines10112936







