Reactive Oxygen Species Enlightened Therapeutic Strategy for Oral and Maxillofacial Diseases—Art of Destruction and Reconstruction
Abstract
1. Introduction
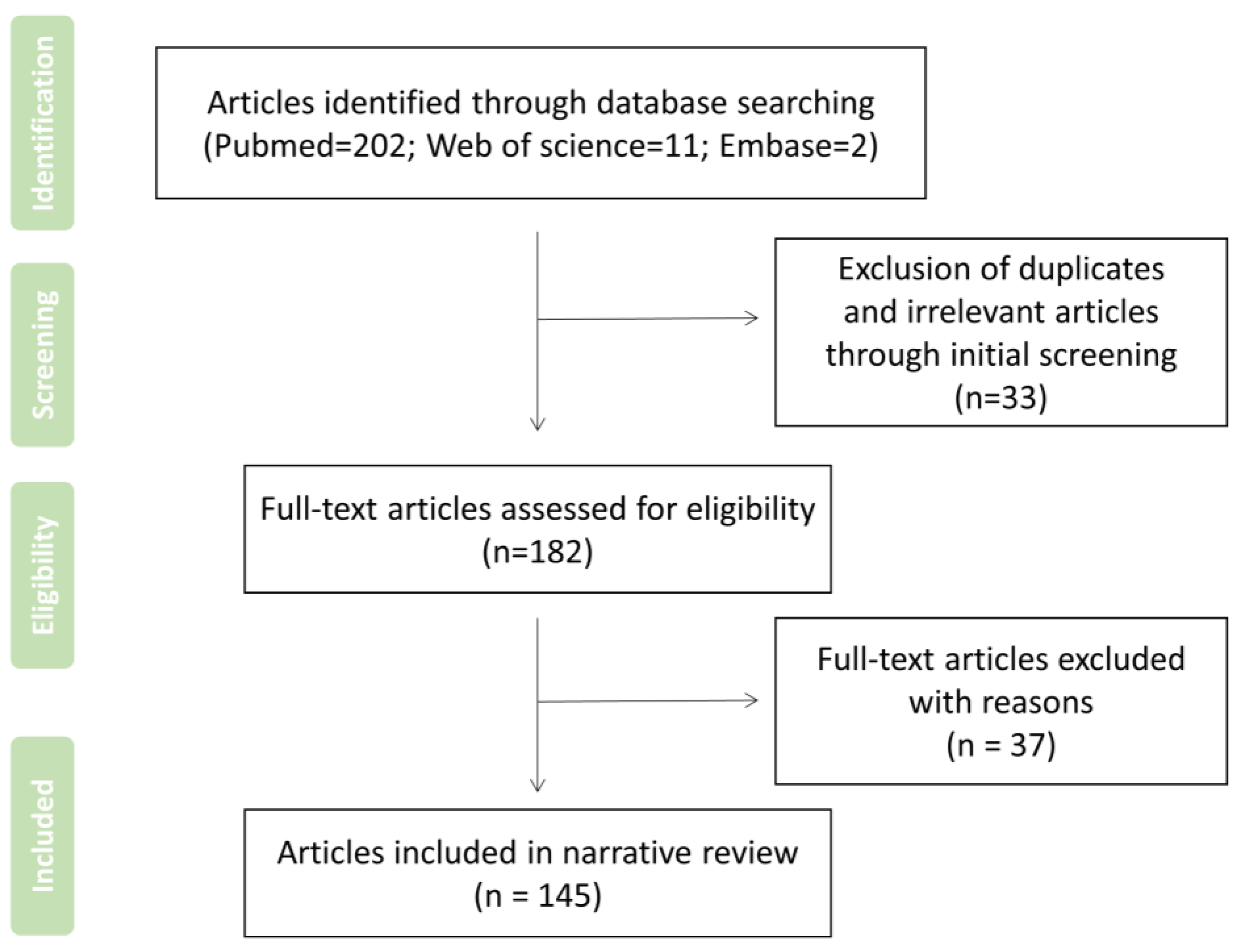
2. Search Strategy and Record Screen
3. ROS and Oral and Maxillofacial Disease-Related Pathological Mechanisms
3.1. Oral Mucosal Diseases
3.2. Periodontitis
3.3. Other Chronic Infectious Oral Diseases
4. Treatment Strategy for Oral Diseases by Encouraging Production or Consumption of ROS
4.1. Antioxidant Defense Systems
4.2. Advanced Antioxidant Drugs and Their Applications
4.3. Emerging ROS-Enlightened Therapeutic Strategy for Oral Disease
5. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kirtonia, A.; Sethi, G.; Garg, M. The multifaceted role of reactive oxygen species in tumorigenesis. Cell. Mol. Life Sci. 2020, 77, 4459–4483. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, P.P.; Gupta, S.; Singh, A.K.; Prajapati, K.S.; Shuaib, M.; Kumar, S. MicroRNA Targeting Nicotinamide Adenine Dinucleotide Phosphate Oxidases in Cancer. Antioxid. Redox Signal. 2020, 32, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.R.; Wang, J.Y.; Li, Y.Y.; Wang, F.; Yang, F.J.; Xu, W.Q. Synthesis and Radioprotective Activity of Mitochondria Targeted Dihydropyridines In Vitro. Int. J. Mol. Sci. 2017, 18, 2233. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Lee, D.H.; Cho, C.K.; Chung, H.Y.; Bae, S.; Jhon, G.J.; Soh, J.W.; Jeoung, D.I.; Lee, S.J.; Lee, Y.S. HSP25 inhibits radiation-induced apoptosis through reduction of PKC delta-mediated ROS production. Oncogene 2005, 24, 3715–3725. [Google Scholar] [CrossRef][Green Version]
- Fedyaeva, A.V.; Stepanov, A.V.; Lyubushkina, I.V.; Pobezhimova, T.P.; Rikhvanov, E.G. Heat shock induces production of reactive oxygen species and increases inner mitochondrial membrane potential in winter wheat cells. Biochemistry 2014, 79, 1202–1210. [Google Scholar] [CrossRef]
- Driedonks, N.; Xu, J.M.; Peters, J.L.; Park, S.; Rieu, I. Multi-Level Interactions between Heat Shock Factors, Heat Shock Proteins, and the Redox System Regulate Acclimation to Heat. Front. Plant Sci. 2015, 6, 999. [Google Scholar] [CrossRef]
- Slimen, I.B.; Najar, T.; Ghram, A.; Dabbebi, H.; Ben Mrad, M.; Abdrabbah, M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int. J. Hyperthermia 2014, 30, 513–523. [Google Scholar] [CrossRef] [PubMed]
- De Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet Light Induced Generation of Reactive Oxygen Species. Adv. Exp. Med. Biol. 2017, 996, 15–23. [Google Scholar]
- El-Hussein, A.; Manoto, S.L.; Ombinda-Lemboumba, S.; Alrowaili, Z.A.; Mthunzi-Kufa, P. A Review of Chemotherapy and Photodynamic Therapy for Lung Cancer Treatment. Anticancer Agents Med. Chem. 2021, 21, 149–161. [Google Scholar] [CrossRef]
- Grande, F.; Tucci, P. Titanium Dioxide Nanoparticles: A Risk for Human Health? Mini Rev. Med. Chem. 2016, 16, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Singh, S.P.; Häder, D.P.; Sinha, R.P. Detection of reactive oxygen species (ROS) by the oxidant-sensing probe 2′,7′-dichlorodihydrofluorescein diacetate in the cyanobacterium Anabaena variabilis PCC 7937. Biochem. Biophys. Res. Commun. 2010, 397, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.O.; Lee, M.H.; Kim, G.H.; Kim, E.H. Quantitation of the ROS production in plasma and radiation treatments of biotargets. Sci. Rep. 2019, 9, 19837. [Google Scholar] [CrossRef] [PubMed]
- He, T.T.; Hatem, E.; Vernis, L.; Huang, M.E. Generation of oxidative stress as an anticancer therapeutic strategy. Free Radic. Biol. Med. 2016, 96, S23. [Google Scholar] [CrossRef]
- Ando, K.; Fujita, T. Metabolic syndrome and oxidative stress. Free Radic. Biol. Med. 2009, 47, 213–218. [Google Scholar] [CrossRef]
- Zimmerman, M.C. Angiotensin II and angiotensin-1-7 redox signaling in the central nervous system. Curr. Opin. Pharmacol. 2011, 11, 138–143. [Google Scholar] [CrossRef]
- Terzi, A.; Suter, D.M. The role of NADPH oxidases in neuronal development. Free Radic. Biol. Med. 2020, 154, 33–47. [Google Scholar] [CrossRef]
- Tepel, M.; Echelmeyer, M.; Orie, N.N.; Zidek, W. Increased intracellular reactive oxygen species in patients with end-stage renal failure: Effect of hemodialysis. Kidney Int. 2000, 58, 867–872. [Google Scholar] [CrossRef]
- Ding, Q.; Tian, Y.; Wang, X.; Li, P.; Su, D.; Wu, C.C.; Zhang, W.; Tang, B. Oxidative Damage of Tryptophan Hydroxylase-2 Mediated by Peroxisomal Superoxide Anion Radical in Brains of Mouse with Depression. J. Am. Chem. Soc. 2020, 142, 20735–20743. [Google Scholar] [CrossRef]
- Fink, M.P. Role of reactive oxygen and nitrogen species in acute respiratory distress syndrome. Curr. Opin. Crit. Care 2002, 8, 6–11. [Google Scholar] [CrossRef]
- Kim, H.R.; Lee, G.H.; Cho, E.Y.; Chae, S.W.; Ahn, T.; Chae, H.J. Bax inhibitor 1 regulates ER-stress-induced ROS accumulation through the regulation of cytochrome P450 2E1. J. Cell Sci. 2009, 122, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, P.; Nagini, S. Cytochrome P450 Structure, Function and Clinical Significance: A Review. Curr. Drug Targets 2018, 19, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.S.; Oh, H.; Rhee, S.G.; Do Yoo, Y. Regulation of reactive oxygen species generation in cell signaling. Mol. Cells 2011, 32, 491–509. [Google Scholar] [CrossRef]
- Zangar, R.C.; Davydov, D.R.; Verma, S. Mechanisms that regulate production of reactive oxygen species by cytochrome P450. Toxicol. Appl. Pharmacol. 2004, 199, 316–331. [Google Scholar] [CrossRef] [PubMed]
- Mironczuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E. Endogenous non-enzymatic antioxidants in the human body. Adv. Med. Sci. 2018, 63, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.H.; Lee, K.W.; Hsu, C.C.; Chen, J.Y.; Wang, Y.H.; Chen, K.K.; Wang, H.M.; Huang, H.W.; Huang, B. Expression of a splice variant of CYP26B1 in betel quid-related oral cancer. Sci. World J. 2014, 2014, 810561. [Google Scholar] [CrossRef] [PubMed]
- Iannitti, T.; Rottigni, V.; Palmieri, B. Role of free radicals and antioxidant defences in oral cavity-related pathologies. J. Oral Pathol. Med. 2012, 41, 649–661. [Google Scholar] [CrossRef]
- Buczko, P.; Zalewska, A.; Szarmach, I. Saliva and oxidative stress in oral cavity and in some systemic disorders. J. Physiol. Pharmacol. 2015, 66, 3–9. [Google Scholar]
- Contini, C.; Olianas, A.; Serrao, S.; Deriu, C.; Iavarone, F.; Boroumand, M.; Bizzarro, A.; Lauria, A.; Faa, G.; Castagnola, M.; et al. Top-Down Proteomics of Human Saliva Highlights Anti-inflammatory, Antioxidant, and Antimicrobial Defense Responses in Alzheimer Disease. Front. Neurosci. 2021, 15, 668852. [Google Scholar] [CrossRef]
- Park, J.J.; Hah, Y.S.; Ryu, S.; Cheon, S.Y.; Won, S.J.; Lee, J.S.; Hwa, J.S.; Seo, J.H.; Chang, H.W.; Kim, S.W.; et al. MDM2-dependent Sirt1 degradation is a prerequisite for Sirt6-mediated cell death in head and neck cancers. Exp. Mol. Med. 2021, 53, 422–431. [Google Scholar] [CrossRef]
- Lin, C.S.; Wang, Y.C.; Huang, J.L.; Hung, C.C.; Chen, J.Y.F. Autophagy and reactive oxygen species modulate cytotoxicity induced by suppression of ATM kinase activity in head and neck cancer cells. Oral Oncol. 2012, 48, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.T.; Hossain, A.; Pin, C.H.; Yahya, N.A. Zinc and Metallothionein in the Development and Progression of Dental Caries. Biol. Trace Elem. Res. 2019, 187, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.K.; Anjana, G.; Zareena, M.A.; Sunil, E.A. Antioxidant Capacity of Saliva: Effect on Onset and Progression of Dental Caries. Oral Maxillofac. Pathol. J. 2017, 8, 19–22. [Google Scholar]
- Zhang, B.; Yang, Y.; Yi, J.R.; Zhao, Z.H.; Ye, R. Hyperglycemia modulates M1/M2 macrophage polarization via reactive oxygen species overproduction in ligature-induced periodontitis. J. Periodontal Res. 2021, 56, 991–1005. [Google Scholar] [CrossRef]
- Da Silva, G.F.Z.; Ming, L.J. Metallo-ROS in Alzheimer’s disease: Oxidation of neurotransmitters by Cu-II-beta-amyloid and neuropathology of the disease. Angew. Chem. Int. Ed. 2007, 46, 3337–3341. [Google Scholar] [CrossRef]
- Geiser, T.; Ishigaki, M.; Van Leer, C.; Matthay, M.A.; Broaddus, V.C. H2O2 inhibits alveolar epithelial wound repair in vitro by induction of apoptosis. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2004, 287, L448–L453. [Google Scholar] [CrossRef]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef]
- Lebert, D.; Squirrell, J.M.; Freisinger, C.; Rindy, J.; Golenberg, N.; Frecentese, G.; Gibson, A.; Eliceiri, K.W.; Huttenlocher, A. Damage-induced reactive oxygen species regulate vimentin and dynamic collagen-based projections to mediate wound repair. eLife 2018, 7, e30703. [Google Scholar] [CrossRef]
- Lee, M.C.; Kawai, Y.; Shoji, H.; Yoshino, F.; Miyazaki, H.; Kato, H.; Suga, M.; Kubota, E. Evidence of reactive oxygen species generation in synovial fluid from patients with temporomandibular disease by electron spin resonance spectroscopy. Redox Rep. 2004, 9, 331–336. [Google Scholar] [CrossRef]
- Kallenborn-Gerhardt, W.; Schroder, K.; Geisslinger, G.; Schmidtko, A. NOXious signaling in pain processing. Pharmacol. Ther. 2013, 137, 309–317. [Google Scholar] [CrossRef]
- Mustafa, M.B.; Porter, S.R.; Smoller, B.R.; Sitaru, C. Oral mucosal manifestations of autoimmune skin diseases. Autoimmun. Rev. 2015, 14, 930–951. [Google Scholar] [CrossRef] [PubMed]
- Sardaro, N.; Della Vella, F.; Incalza, M.A.; DI Stasio, D.; Lucchese, A.; Contaldo, M.; Laudadio, C.; Petruzzi, M. Oxidative Stress and Oral Mucosal Diseases: An Overview. In Vivo 2019, 33, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, G.; Zanoli, P.; Zavatti, M.; Baraldi, M. Further evidence of the antiulcer activity of IAC, a novel free radical scavenger. Pharmacology 2011, 88, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, I.; Kohen, R.; Shalish, M.; Varon, D.; Shai, E.; Koren, E. The Oxidant-Scavenging Abilities in the Oral Cavity May Be Regulated by a Collaboration among Antioxidants in Saliva, Microorganisms, Blood Cells and Polyphenols: A Chemiluminescence-Based Study. PLoS ONE 2013, 8, e63062. [Google Scholar] [CrossRef]
- Yoshino, F.; Yoshida, A.; Nakajima, A.; Wada-Takahashi, S.; Takahashi, S.S.; Lee, M.C. Alteration of the redox state with reactive oxygen species for 5-fluorouracil-induced oral mucositis in hamsters. PLoS ONE 2013, 8, e82834. [Google Scholar] [CrossRef]
- Kim, D.R.; Kim, J.; Oh, J.Y.; Kim, H.Y.; Kim, Y.J.; Chang, M.S. Protective effect of Salvia miltiorrhiza Bunge on 5-fluorouracil-induced oral mucositis. Int. J. Mol. Med. 2017, 40, 39–46. [Google Scholar] [CrossRef][Green Version]
- Scully, C.; Shotts, R. ABC of oral health—Mouth ulcers and other causes of orofacial soreness and pain. Br. Med. J. 2000, 321, 162–165. [Google Scholar] [CrossRef]
- Lake, R.I.E.; Thomas, S.J.; Martin, N.G. Genetic factors in the aetiology of mouth ulcers. Genet. Epidemiol. 1997, 14, 17–33. [Google Scholar] [CrossRef]
- Dudding, T.; Haworth, S.; Lind, P.A.; Sathirapongsasuti, J.F.; Tung, J.Y.; Mitchell, R.; Colodro-Conde, L.; Medland, S.E.; Gordon, S.; Elsworth, B.; et al. Genome wide analysis for mouth ulcers identifies associations at immune regulatory loci. Nat. Commun. 2019, 10, 1052. [Google Scholar] [CrossRef]
- Hayrinenimmonen, R.; Nordstrom, D.; Malmstrom, M.; Hietanen, J.; Konttinen, Y.T. Immune-inflammatory cells in recurrent oral ulcers (ROU). Scand. J. Dent. Res. 1991, 99, 510–518. [Google Scholar]
- Gu, Y.; Huang, Y.; Qiu, Z.; Xu, Z.; Li, D.; Chen, L.; Jiang, J.; Gao, L. Vitamin B2 functionalized iron oxide nanozymes for mouth ulcer healing. Sci. China Life Sci. 2020, 63, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Ishigatsubo, Y.; Samukawa, S. Behcet’s disease from the aspect of autoinflammatory disease. Nihon Rinsho Men’eki Gakkai Kaishi = Jpn. J. Clin. Immunol. 2011, 34, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Tursen, U. Pathophysiology of the Behcet’s Disease. Pathol. Res. Int. 2012, 2012, 493015. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. 1), S74–S84. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89 (Suppl. 1), S159–S172. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.C.; Brock, G.R.; Milward, M.R.; Ling, N.; Matthews, J.B. Compromised GCF total antioxidant capacity in periodontitis: Cause or effect? J. Clin. Periodontol. 2007, 34, 103–110. [Google Scholar] [CrossRef]
- Canakci, C.F.; Cicek, Y.; Canakci, V. Reactive oxygen species and human inflammatory periodontal diseases. Biochemistry 2005, 70, 619–628. [Google Scholar] [CrossRef]
- Sculley, D.V.; Langley-Evans, S.C. Association between salivary antioxidant status and periodontal disease. Proc. Nutr. Soc. 2002, 61, 110A. [Google Scholar] [CrossRef]
- Waddington, R.J.; Moseley, R.; Embery, G. Periodontal disease mechanisms—Reactive oxygen species: A potential role in the pathogenesis of periodontal diseases. Oral Dis. 2000, 6, 138–151. [Google Scholar] [CrossRef]
- Dahiya, P.; Kamal, R.; Gupta, R.; Bhardwaj, R.; Chaudhary, K.; Kaur, S. Reactive oxygen species in periodontitis. J. Indian Soc. Periodontol. 2013, 17, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.C.; Milward, M.R.; Dietrich, T. The prevalence of inflammatory periodontitis is negatively associated with serum antioxidant concentrations. J. Nutr. 2007, 137, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.S.; Patil, V.P.; Gokhale, N.; Acharya, A.; Kangokar, P. Chronic Periodontitis in Type 2 Diabetes Mellitus: Oxidative Stress as a Common Factor in Periodontal Tissue Injury. J. Clin. Diagn. Res. 2016, 10, BC12–BC16. [Google Scholar] [CrossRef]
- Soory, M. Oxidative stress induced mechanisms in the progression of periodontal diseases and cancer: A common approach to redox homeostasis? Cancers 2010, 2, 670–692. [Google Scholar] [CrossRef] [PubMed]
- Bunpeng, N.; Boriboonhirunsarn, D.; Boriboonhirunsarn, C.; Sawangpanyangkura, T.; Tansriratanawong, K. Association between gestational diabetes mellitus and periodontitis via the effect of reactive oxygen species in peripheral blood cells. J. Periodontol. 2021, 93, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Herrera, M.; Abad-Jimenez, Z.; Silvestre, F.J.; Lopez-Domenech, S.; Silvestre-Rangil, J.; Marquez-Arrico, C.F.; Silvestre-Rangil, J.; Víctor, V.M.; Rocha, M. Effect of Non-Surgical Periodontal Treatment on Oxidative Stress Markers in Leukocytes and Their Interaction with the Endothelium in Obese Subjects with Periodontitis: A Pilot Study. J. Clin. Med. 2020, 9, 2117. [Google Scholar] [CrossRef]
- Ekuni, D.; Tomofuji, T.; Endo, Y.; Kasuyama, K.; Irie, K.; Azuma, T.; Tamaki, N.; Mizutani, S.; Kojima, A.; Morita, M. Hydrogen-rich water prevents lipid deposition in the descending aorta in a rat periodontitis model. Arch. Oral Biol. 2012, 57, 1615–1622. [Google Scholar] [CrossRef]
- Bahar, B.; Singhrao, S.K. An evaluation of the molecular mode of action of trans-resveratrol in the Porphyromonas gingivalis lipopolysaccharide challenged neuronal cell model. Mol. Biol. Rep. 2021, 48, 147–156. [Google Scholar] [CrossRef]
- Ower, P.C.; Ciantar, M.; Newman, H.N.; Wilson, M.; Bulman, J.S. The effects on chronic periodontitis of a subgingivally-placed redox agent in a slow-release device. J. Clin. Periodontol. 1995, 22, 494–500. [Google Scholar] [CrossRef]
- Chapple, I.L.C.; Matthews, J.B. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontology 2007, 43, 160–232. [Google Scholar] [CrossRef]
- Bhattarai, G.; Poudel, S.B.; Kook, S.H.; Lee, J.C. Resveratrol prevents alveolar bone loss in an experimental rat model of periodontitis. Acta Biomater. 2016, 29, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Ichinose-Tsuno, A.; Aoki, A.; Takeuchi, Y.; Kirikae, T.; Shimbo, T.; Lee, M.C.I.; Yoshino, F.; Maruoka, Y.; Itoh, T.; Ishikawa, I.; et al. Antimicrobial photodynamic therapy suppresses dental plaque formation in healthy adults: A randomized controlled clinical trial. BMC Oral Health 2014, 14, 152. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, L.; Zhu, Z.; Xiao, A.; Yu, H.; Gan, X. Blockade of cyclophilin D rescues dexamethasone-induced oxidative stress in gingival tissue. PLoS ONE 2017, 12, e0173270. [Google Scholar] [CrossRef]
- Kajfasz, J.K.; Rivera-Ramos, I.; Scott-Anne, K.; Gregoire, S.; Abranches, J.; Lemos, J.A. Transcription of Oxidative Stress Genes Is Directly Activated by SpxA1 and, to a Lesser Extent, by SpxA2 in Streptococcus mutans. J. Bacteriol. 2015, 197, 2160–2170. [Google Scholar] [CrossRef] [PubMed]
- Preza, D.; Olsen, I.; Willumsen, T.; Grinde, B.; Paster, B.J. Diversity and site-specificity of the oral microflora in the elderly. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Munson, M.A.; Banerjee, A.; Watson, T.F.; Wade, W.G. Molecular analysis of the microflora associated with dental caries. In Proceedings of the Abstracts of the General Meeting of the American Society for Microbiology, Washington, DC, USA, 18–22 May 2003. [Google Scholar]
- Shitsuka, C.; Ibuki, F.K.; Nogueira, F.N.; Mendes, F.M.; Bonecker, M. Assessment of oxidative stress in saliva of children with dental erosion. Einstein 2018, 16, eAO4203. [Google Scholar] [CrossRef] [PubMed]
- Pyati, S.A.; Kumar, R.N.; Kumar, V.; Kumar, N.H.P.; Reddy, K.M.P. Salivary Flow Rate, pH, Buffering Capacity, Total Protein, Oxidative Stress and Antioxidant Capacity in Children with and without Dental Caries. J. Clin. Pediatr. Dent. 2018, 42, 445–449. [Google Scholar] [CrossRef]
- Battino, M.; Ferreiro, M.S.; Gallardo-Castillo, I.; Newman, H.N.; Bullon, P. The antioxidant capacity of saliva. J. Clin. Periodontol. 2002, 29, 189–194. [Google Scholar] [CrossRef]
- Rahmani, M.; Ghorchi, V.; Rezaei, F.; Vaisi-Raygani, A. Evaluation of Total Antioxidant Capacity of Saliva in High School Students. Glob. J. Health Sci. 2015, 8, 89–94. [Google Scholar] [CrossRef]
- Mansurova, F.K.; Dzhuraeva, S.F.; Nazarova, O.D. Presence of Helicobacter pylori in the oral mucosa of patients with upper digestive tract diseases. Izvestiya Akademii Nauk Respubliki Tadzhikistan Otdelenie Biologicheskikh i Meditsinskikh Nauk 2001, 144, 74–79. [Google Scholar]
- Rong, J.F. The interaction between ROS and HIF-1 alpha regulates Helicobacter pylori-induced macrophage polarization. J. Gastroenterol. Hepatol. 2019, 34, 142. [Google Scholar]
- Huang, X.W.; Luo, R.H.; Zhao, Q.; Shen, Z.Z.; Huang, L.L.; An, X.Y.; Zhao, L.J.; Wang, J.; Huang, Y.Z. Helicobacter pylori induces mitochondrial DNA mutation and reactive oxygen species level in AGS cells. Int. J. Med. Sci. 2011, 8, 56–67. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wen, X.; Wu, J.M.; Wang, F.T.; Liu, B.; Huang, C.H.; Wei, Y.Q. Deconvoluting the role of reactive oxygen species and autophagy in human diseases. Free Radic. Biol. Med. 2013, 65, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-S.; Henderson, J.P. Emerging concepts of biofilms in infectious diseases. Mo. Med. 2009, 106, 292–296. [Google Scholar] [PubMed]
- Gao, L.; Liu, Y.; Kim, D.; Li, Y.; Hwang, G.; Naha, P.C.; Cormode, D.P.; Koo, H. Nanocatalysts promote Streptococcus mutans biofilm matrix degradation and enhance bacterial killing to suppress dental caries in vivo. Biomaterials 2016, 101, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Mccord, J.M.; Fridovich, I. Utility of superoxide dismutase in studying free radical reactions 2. radicals generated by interaction of sulfite, dimethyl sulfoxide, and oxygen. J. Biol. Chem. 1969, 244, 6056. [Google Scholar] [CrossRef]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef]
- Fan, J.; Yin, J.J.; Ning, B.; Wu, X.C.; Hu, Y.; Ferrari, M.; Anderson, G.J.; Wei, J.; Zhao, Y.; Nie, G. Direct evidence for catalase and peroxidase activities of ferritin-platinum nanoparticles. Biomaterials 2011, 32, 1611–1618. [Google Scholar] [CrossRef]
- Kish, S.J.; Morito, C.; Hornykiewicz, O. Glutathione-peroxidase activity in parkinsons-disease brain. Neurosci. Lett. 1985, 58, 343–346. [Google Scholar] [CrossRef]
- Morinobu, S.; Kumagai, S. Glutathione, glutathione peroxidase (GPx), glutathione S-transferase (GST). Nihon rinsho. Jpn. J. Clin. Med. 2004, 62 (Suppl. 11), 557–559. [Google Scholar]
- Kabir, Y.; Seidel, R.; Mcknight, B.; Moy, R. DNA Repair Enzymes: An Important Role in Skin Cancer Prevention and Reversal of Photodamage—A Review of the Literature. J. Drugs Dermatol. 2015, 14, 297–301. [Google Scholar]
- Miao, L.; St Clair, D.K. Regulation of superoxide dismutase genes: Implications in disease. Free Radic. Biol. Med. 2009, 47, 344–356. [Google Scholar] [CrossRef]
- Glorieux, C.; Calderon, P.B. Catalase, a remarkable enzyme: Targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Biol. Chem. 2017, 398, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Flohé, L. Regulatory Phenomena in the Glutathione Peroxidase Superfamily. Antioxid. Redox Signal. 2020, 33, 498–516. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Bilteanu, L.; Serban, A.I. Antioxidant Determination with the Use of Carbon-Based Electrodes. Chemosensors 2021, 9, 72. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Cronin, M.T. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [PubMed]
- Tokumaru, O.; Shuto, Y.; Ogata, K.; Kamibayashi, M.; Bacal, K.; Takei, H.; Yokoi, I.; Kitano, T. Dose-dependency of multiple free radical-scavenging activity of edaravone. J. Surg. Res. 2018, 228, 147–153. [Google Scholar] [CrossRef]
- Haider, K.; Haider, M.R.; Neha, K.; Yar, M.S. Free radical scavengers: An overview on heterocyclic advances and medicinal prospects. Eur. J. Med. Chem. 2020, 204, 112607. [Google Scholar] [CrossRef]
- Chaudière, J.; Ferrari-Iliou, R. Intracellular antioxidants: From chemical to biochemical mechanisms. Food Chem. Toxicol. 1999, 37, 949–962. [Google Scholar] [CrossRef]
- Hellman, N.E.; Gitlin, J.D. Ceruloplasmin metabolism and function. Annu. Rev. Nutr. 2002, 22, 439–458. [Google Scholar] [CrossRef]
- Nikolic, A.; Cabarkapa, V.; Novakov Mikic, A.; Jakovljević, A.; Stosic, Z. Ceruloplasmin and antioxidative enzymes in pre-eclampsia. J. Matern. Fetal Neonatal Med. 2016, 29, 2987–2993. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, Y.; Yoshida, H.; Kondo, K. Potential Anti-Atherosclerotic Properties of Astaxanthin. Mar. Drugs 2016, 14, 35. [Google Scholar] [CrossRef]
- Sousa, A.; Araújo, P.; Azevedo, J.; Cruz, L.; Fernandes, I.; Mateus, N.; de Freitas, V. Antioxidant and antiproliferative properties of 3-deoxyanthocyanidins. Food Chem. 2016, 192, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef] [PubMed]
- Megna, M.; Lembo, S.; Balato, N.; Monfrecola, G. “Active” photoprotection: Sunscreens with DNA repair enzymes. G. Ital. Dermatol. Venereol. 2017, 152, 302–307. [Google Scholar] [CrossRef]
- Bansal, A.; Simon, M.C. Glutathione metabolism in cancer progression and treatment resistance. J. Cell Biol. 2018, 217, 2291–2298. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Maruhashi, T.; Kihara, Y.; Higashi, Y. Bilirubin and Endothelial Function. J. Atheroscler. Thromb. 2019, 26, 688–696. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea Polyphenols in Promotion of Human Health. Nutrients 2018, 11, 39. [Google Scholar] [CrossRef]
- Das Gupta, S.; Suh, N. Tocopherols in cancer: An update. Mol. Nutr. Food Res. 2016, 60, 1354–1363. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, L.; Aweya, J.J.; Zheng, Z.; Zhong, M.; Chen, J.; Wang, F.; Zhang, Y. Litopenaeus vannamei hemocyanin exhibits antitumor activity in S180 mouse model in vivo. PLoS ONE 2017, 12, e0183783. [Google Scholar] [CrossRef] [PubMed]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Khanna, D.; Kalra, S. Minocycline and Doxycycline: More Than Antibiotics. Curr. Mol. Pharmacol. 2021, 14, 1046–1065. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.G.; Zhang, X.L.; Sun, Y.H.; Lin, W. Antioxidative capacity and enzyme activity in Haematococcus pluvialis cells exposed to superoxide free radicals. Chin. J. Oceanol. Limnol. 2010, 28, 1–9. [Google Scholar] [CrossRef]
- Cunningham, F.X., Jr.; Gantt, E. Genes and enzymes of carotenoid biosynthesis in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 557–583. [Google Scholar] [CrossRef]
- Kang, H.; Kim, H. Astaxanthin and beta-carotene in Helicobacter pylori-induced Gastric Inflammation: A Mini-review on Action Mechanisms. J. Cancer Prev. 2017, 22, 57–61. [Google Scholar] [CrossRef]
- Barreiros, D.; Nelson, P.F.; Paula-Silva, F.W.G.; Oliveira, K.M.H.D.; Lucisano, M.P.; Rossi, A.D.; Silva, L.A.B.; Küchler, E.C.; Silva, R.A.B. MMP2 and MMP9 are Associated with Apical Periodontitis Progression and Might be Modulated by TLR2 and MyD88. Braz. Dent. J. 2018, 29, 43–47. [Google Scholar] [CrossRef]
- Menezes-Silva, R.; Khaliq, S.; Deeley, K.; Letra, A.; Vieira, A.R. Genetic Susceptibility to Periapical Disease: Conditional Contribution of MMP2 and MMP3 Genes to the Development of Periapical Lesions and Healing Response. J. Endod. 2012, 38, 604–607. [Google Scholar] [CrossRef]
- Chen, P.; Xuan, D.Y.; Zhang, J.C. Periodontitis aggravates kidney damage in obese mice by MMP2 regulation. Bratisl. Med. J.-Bratisl. Lek. Listy 2017, 118, 740–745. [Google Scholar] [CrossRef]
- Deveci, B.; Ayna, B.; Tacir, I.H.; Deveci, E.; Tuncer, M.C.; Pala, A. Effects of nicotine administration in rats on MMP2 and VEGF levels in periodontal membrane. Folia Morphol. 2018, 77, 471–477. [Google Scholar] [CrossRef]
- Kim, S.J.; Park, Y.S.; Paik, H.D.; Chang, H.I. Effect of anthocyanins on expression of matrix metalloproteinase-2 in naproxen-induced gastric ulcers. Br. J. Nutr. 2011, 106, 1792–1801. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.H.; Mahmood, K.; Rothstein, S.J. ROS Induces Anthocyanin Production via Late Biosynthetic Genes and Anthocyanin Deficiency Confers the Hypersensitivity to ROS-Generating Stresses in Arabidopsis. Plant. Cell Physiol. 2017, 58, 1364–1377. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Vong, C.T.; Gao, C.; Chen, S.; Wu, X.; Wang, S.; Wang, Y. Bilirubin Nanomedicines for the Treatment of Reactive Oxygen Species (ROS)-Mediated Diseases. Mol. Pharm. 2020, 17, 2260–2274. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Liu, W.; Mu, Y.P.; Zhang, H.; Wang, X.N.; Zhao, C.Q.; Chen, J.M.; Liu, P. Pharmacological Effects of Salvianolic Acid B against Oxidative Damage. Front. Pharmacol. 2020, 11, 572373. [Google Scholar] [CrossRef]
- Yeo, J.; Lee, J.; Yoon, S.; Kim, W.J. Tannic acid-based nanogel as an efficient anti-inflammatory agent. Biomater. Sci. 2020, 8, 1148–1159. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, Y.; Li, X.; Xu, X.; Chen, Y.; Zhu, R.; Yin, L. Macrophage-targeting and reactive oxygen species (ROS)-responsive nanopolyplexes mediate anti-inflammatory siRNA delivery against acute liver failure (ALF). Biomater. Sci. 2018, 6, 1986–1993. [Google Scholar] [CrossRef]
- Sun, H.Y. Analysis of Periodontitis Biomarker Expression in Gingival Crevicular Fluids. J. Dent. Hyg. Sci. 2021, 21, 45–51. [Google Scholar]
- Wang, T.; Li, Y.R.; Cornel, E.J.; Li, C.; Du, J.Z. Combined Antioxidant-Antibiotic Treatment for Effectively Healing Infected Diabetic Wounds Based on Polymer Vesicles. ACS Nano 2021, 15, 9027–9038. [Google Scholar] [CrossRef]
- Casas, A.I.; Nogales, C.; Mucke, H.A.M.; Petraina, A.; Cuadrado, A.; Rojo, A.I.; Ghezzi, P.; Jaquet, V.; Augsburger, F.; Dufrasne, F.; et al. On the Clinical Pharmacology of Reactive Oxygen Species. Pharmacol. Rev. 2020, 72, 801–828. [Google Scholar] [CrossRef]
- Baskaran, R.; Lee, J.; Yang, S.G. Clinical development of photodynamic agents and therapeutic applications. Biomater. Res. 2018, 22, 25. [Google Scholar] [CrossRef]
- Diogo, P.; Gonçalves, T.; Palma, P.; Santos, J.M. Photodynamic Antimicrobial Chemotherapy for Root Canal System Asepsis: A Narrative Literature Review. Int. J. Dent. 2015, 2015, 269205. [Google Scholar] [CrossRef] [PubMed]
- Prażmo, E.J.; Kwaśny, M.; Łapiński, M.; Mielczarek, A. Photodynamic Therapy as a Promising Method Used in the Treatment of Oral Diseases. Adv. Clin. Exp. Med. 2016, 25, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qiu, L.; Wang, C.; Gao, Z.; Zhou, S.; Cui, P.; Jiang, P.; Hu, H.; Ni, X.; Du, X.; et al. Nanodot-doped peptide hydrogels for antibacterial phototherapy and wound healing. Biomater. Sci. 2021, 10, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Mohd Nasir, N.; Lee, B.K.; Yap, S.S.; Thong, K.L.; Yap, S.L. Cold plasma inactivation of chronic wound bacteria. Arch. Biochem. Biophys. 2016, 605, 76–85. [Google Scholar] [CrossRef]
- Kumar, C.S.; Sarada, P.; Reddy, C.S.; Reddy, M.S.; Dsv, N. Plasma torch toothbrush a new insight in fear free dentisry. J. Clin. Diagn. Res. 2014, 8, ZE07–ZE10. [Google Scholar]
- Chen, M.; Zhang, Y.; Sky Driver, M.; Caruso, A.N.; Yu, Q.; Wang, Y. Surface modification of several dental substrates by non-thermal, atmospheric plasma brush. Dent. Mater. 2013, 29, 871–880. [Google Scholar] [CrossRef]
- Saydjari, Y.; Kuypers, T.; Gutknecht, N. Laser Application in Dentistry: Irradiation Effects of Nd:YAG 1064 nm and Diode 810 nm and 980 nm in Infected Root Canals—A Literature Overview. BioMed Res. Int. 2016, 2016, 8421656. [Google Scholar] [CrossRef]
- Deppe, H.; Horch, H.H. Laser applications in oral surgery and implant dentistry. Lasers Med. Sci. 2007, 22, 217–221. [Google Scholar] [CrossRef]
- Farivar, S.; Malekshahabi, T.; Shiari, R. Biological effects of low level laser therapy. J. Lasers Med. Sci. 2014, 5, 58–62. [Google Scholar]
- İslam, A.; Özverel, C.S.; Yilmaz, H.G. Comparative evaluation of low-level laser therapy on proliferation of long-term cryopreserved human dental pulp cells isolated from deciduous and permanent teeth. Lasers Med. Sci. 2021, 36, 421–427. [Google Scholar] [CrossRef]
- Yamakawa, S.; Niwa, T.; Karakida, T.; Kobayashi, K.; Yamamoto, R.; Chiba, R.; Yamakoshi, Y.; Hosoya, N. Effects of Er:YAG and Diode Laser Irradiation on Dental Pulp Cells and Tissues. Int. J. Mol. Sci. 2018, 19, 2429. [Google Scholar] [CrossRef] [PubMed]
- Alkahtani, R.; Stone, S.; German, M.; Waterhouse, P. A review on dental whitening. J. Dent. 2020, 100, 103423. [Google Scholar] [CrossRef] [PubMed]
- Olmedo, D.; Kury, M.; Resende, B.A.; Cavalli, V. Use of antioxidants to restore bond strength after tooth bleaching with peroxides. Eur. J. Oral Sci. 2021, 129, e12773. [Google Scholar] [CrossRef] [PubMed]
- Jha, N.; Ryu, J.J.; Choi, E.H.; Kaushik, N.K. Generation and Role of Reactive Oxygen and Nitrogen Species Induced by Plasma, Lasers, Chemical Agents, and Other Systems in Dentistry. Oxid. Med. Cell. Longev. 2017, 2017, 7542540. [Google Scholar] [CrossRef] [PubMed]


| External Factors | Mechanism | References |
|---|---|---|
| Heat | Atrocious heat exposure induces the damage of mitochondria and causes excessive production of mitochondrial ROS, which can upregulate the transcription of heat shock factors (HSFs) and heat shock proteins (HSPs). | [8] |
| Ultraviolet light | Generally, UV can directly affect cell components or photosensitive mechanisms to promote the production of ROS. To be specific, UV can affect catalase process and upregulate the synthesis of nitric oxide synthase (NOS), which leads to decreased expression of protein kinase C (PKC) and increased production of ROS. | [9] |
| Pharmaceutical products and cosmetics | Some therapeutic drugs or cosmetics, such as the photochemical interaction with light, photosensitizer (PS), and molecular oxygen, produce ROS, contributing to oxidative stress, genotoxicity, inflammation, potentially carcinogenesis, and metabolic change, which eventually brings about the apoptosis of cells. | [10,11] |
| Other radiation | Besides UV-A, UV-B, or thermal radiation, X-ray exposures and other radiation could also generate ROS, which may be due to the observed oxidative stress. | [12,13] |
| PubMed: | Web of Science | Embase |
|---|---|---|
| ((((“Oral diseases”[Mesh] OR “Maxillofacial diseases”[Mesh]) OR “Oral cavity”[Mesh]) OR “Oral and maxillofacial”[Mesh]) OR (((((((oral mucosal diseases[Title/Abstract] OR periodontitis[Title/Abstract]) OR periodontal disease[Title/Abstract]) OR dental caries[Title/Abstract]) OR periodontal therapy[Title/Abstract]) OR Oral antioxidant treatment[Title/Abstract]) OR antioxidant drugs[Title/Abstract]) OR oral diseases therapy [Title/Abstract]) AND ((((“ROS”[Title/Abstract] OR reactive oxygen species[Title/Abstract]) OR anti-ROS[Title/Abstract]) OR oxidative stress[Title/Abstract]) OR ((oxidation treatment[Mesh] OR pathological mechanism[Mesh]) OR “antioxidant ”[Mesh])) | #1:TS = (oral diseases) OR TS = (maxillofacial diseases) OR TS = (periodontal diseases) OR TS = (oral mucosal disease) OR TS = (periodontitis) OR TS = (dental caries) OR TS = (periodontal pocket) OR TS = (oral diseases therapy) #2:TS = (ROS) OR TS = (reactive oxygen species) OR TS = (oxidative stress) OR TS = (antioxidant) OR TS = (antioxidant material) #1 AND #2 | ‘oral diseases’/exp OR ‘maxillofacial diseases’/exp OR ‘periodontal disease’/ exp OR ‘periodontitis’:ab,ti OR ‘dental caries’: ab,ti OR ‘oral mucosal disease’: ab,ti OR ‘periodontal tissue’: ab,ti OR ‘oral diseases therapy’: ab,ti OR ‘periodontal treatment’: ab,ti AND (‘reactive oxygen species’/exp OR ‘ROS’/exp OR ‘oxidative stress’/exp OR ‘leptin’: ab,ti OR ‘antioxidant’: ab,ti OR ‘antioxidant treament’: ab,ti OR ‘antioxidant material’: ab,ti OR ‘oxidation treatment’: ab,ti) |
| Classification Criteria | Classifications | Examples | References |
|---|---|---|---|
| Functional mechanism | Preventative antioxidants | DNA repair enzymes, catalase, superoxide dismutase enzymes, glutathione peroxidase superfamily, e.g., selenium-containing glutathione peroxidases (GPxs). | [92,93,94,95,96,97] |
| Metal ion sequestrators aiming at metal-induced toxicity and carcinogenicity: albumin, lactoferrin, transferrin, haptoglobin, ceruloplasmin, Vitamin E, melatonin, ascorbate, carotenoids, hemopexin, catalase, superoxide dismutase, polyphenolic flavonoids, glutathione peroxidase, uric acid, glutathione reductase. | [96,97] | ||
| Radical scavengers or chain-breaking antioxidants | Carotenoids, such as vitamin A, α-tocopherol (vitamin E), ascorbate, catechol, edaravone, melatonin and tryptophan derivatives and other thiols (free or protein bound), uric acid, polyphenols (flavonoids), albumin, bilirubin, ubiquinone (reduced form), reduced glutathione. | [98,99] | |
| Location | Intracellular | Mainly hydrophilic scavengers, including catalase, DNA repair enzymes, superoxide dismutase enzymes 1 and 2, ascorbate, glutathione peroxidase, ergothioneine. | [100] |
| Extracellular | Ascorbate, selenium-glutathione peroxidase, superoxide dismutase enzyme 3, albumin, lactoferrin, transferrin, uric acid, haptoglobin, ceruloplasmin, carotenoids, e.g., astaxanthin and allopurinol. | [88,101,102,103] | |
| Membrane associated | Hydrophobic scavengers are presented in cell membranes, where they restrain or interrupt chain reactions of lipid peroxidation, such as α-tocopherol (vitamin E), carotenoids, deoxyanthocyanins. | [100,104] | |
| Protected structure | DNA protective antioxidants | DNA-repairing enzymes, including photolyase, endonuclease and 8-oxoguanine glycosylase, reduced glutathione, superoxide dismutase enzymes 1 and 2, cysteine and glutathione peroxidase. | [93,105,106,107] |
| Protein-protective antioxidants | Sequestration of transition metals by preventative antioxidants. Antioxidant enzymes as stated above. Scavenging by the competitive reaction scheme, competing substrates. | [96,97,107,108] | |
| Lipid-protective antioxidants | Ascorbate (vitamin C), α-tocopherol (vitamin E), reduced ubiquinone, carotenoids, reduced glutathione, bilirubin, and glutathione peroxidase. | [100,104,109] | |
| Origin | Exogenous antioxidants (obtained simply by the diet): phytonutrients | Carotenoids (e.g., astaxanthin), ascorbic acid, tocopherols (α, β, γ, δ), polyphenols (resveratrol, theaflavins, thearubigins, flavonoids, catechins), folic acid, cysteine. | [71,110,111] |
| Endogenous antioxidants (synthesized by the body) | Hemocyanin, superoxide dismutase, ferritin, catalase, glutathione peroxidase, reduced glutathione, glutathione S-transferase, ceruloplasmin, proteases, transferrin, peroxisomes, glycosylases. | [96,97,112] | |
| Artificial synthetic | Aspirin, statins, and renin-angiotensin system inhibitors, N-acetylcysteine, penicillin amine, tetracyclines. | [113,114] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, Y.; Mei, Y.; Zou, R.; Niu, L.; Dong, S. Reactive Oxygen Species Enlightened Therapeutic Strategy for Oral and Maxillofacial Diseases—Art of Destruction and Reconstruction. Biomedicines 2022, 10, 2905. https://doi.org/10.3390/biomedicines10112905
Zhang Y, Zhang Y, Mei Y, Zou R, Niu L, Dong S. Reactive Oxygen Species Enlightened Therapeutic Strategy for Oral and Maxillofacial Diseases—Art of Destruction and Reconstruction. Biomedicines. 2022; 10(11):2905. https://doi.org/10.3390/biomedicines10112905
Chicago/Turabian StyleZhang, Yuwei, Yifei Zhang, Yukun Mei, Rui Zou, Lin Niu, and Shaojie Dong. 2022. "Reactive Oxygen Species Enlightened Therapeutic Strategy for Oral and Maxillofacial Diseases—Art of Destruction and Reconstruction" Biomedicines 10, no. 11: 2905. https://doi.org/10.3390/biomedicines10112905
APA StyleZhang, Y., Zhang, Y., Mei, Y., Zou, R., Niu, L., & Dong, S. (2022). Reactive Oxygen Species Enlightened Therapeutic Strategy for Oral and Maxillofacial Diseases—Art of Destruction and Reconstruction. Biomedicines, 10(11), 2905. https://doi.org/10.3390/biomedicines10112905








