Alpha-Lipoic Acid Supplementation Restores Early Age-Related Sensory and Endothelial Dysfunction in the Skin
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.1.1. Animal Experiments
2.1.2. LPA Treatment
2.2. General State
2.3. Microvascular Experiments
2.3.1. Animal Preparation
2.3.2. Skin Resistance to Low-Pressure Application (PIV)
2.3.3. Endothelium Dependent or Independent Vasodilation
2.3.4. Contribution of NOS and COX Pathways in Ach-Mediated Vasodilation
2.4. Skin Sensory Nerve Fiber Sensitivity Analysis
2.4.1. Tail Flick Test
2.4.2. Von Frey Test
2.4.3. Randall–Selitto Test (Mechanical Noxious Stimuli)
2.5. Statistical Analyses
3. Results
3.1. LPA Limited Weight Gain and Improved Glycemia Regulation of “Healthy Aging” BN strain and “Poorly Aging” Wistar Strain
3.2. LPA Tended to Improve the Age-Related Alterations of Skin Ability to Resist to Low Pressures in Old BN but Not in Wistar Strain
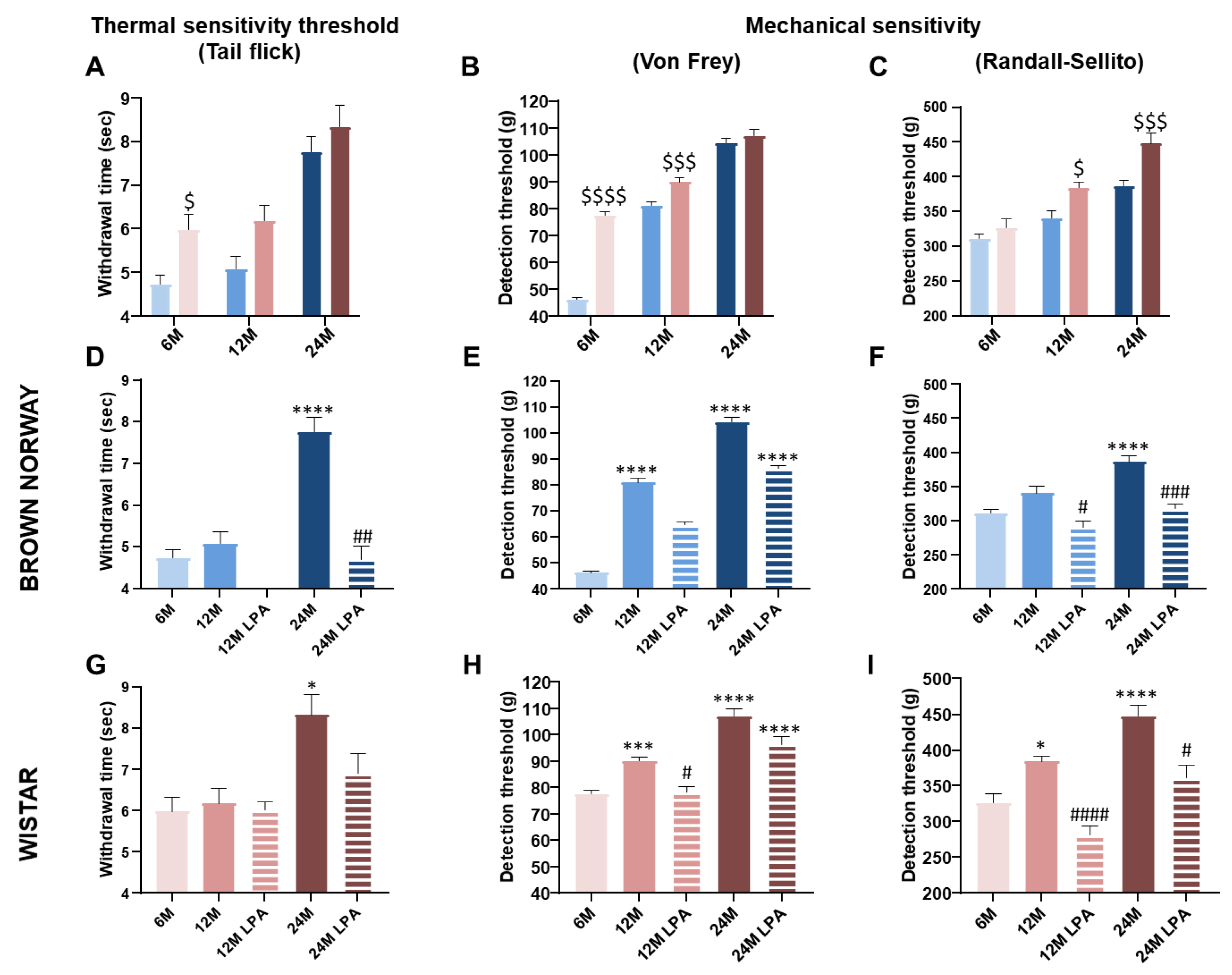
3.3. LPA Improved Skin Sensory Nerve Sensitivity in Both Strains
3.4. LPA Restored the NO Pathway Involved in Endothelial Function in the Wistar Strain
3.4.1. Effect of Aging on Endothelium-Dependent and -Independent Vasodilation
3.4.2. Contribution of NOS and COX Pathways over Time
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fromy, B.; Sigaudo-Roussel, D.; Gaubert-Dahan, M.-L.; Rousseau, P.; Abraham, P.; Benzoni, D.; Berrut, G.; Saumet, J.L. Aging-Associated Sensory Neuropathy Alters Pressure-Induced Vasodilation in Humans. J. Invest. Dermatol. 2010, 130, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Holowatz, L.A.; Houghton, B.L.; Wong, B.J.; Wilkins, B.W.; Harding, A.W.; Kenney, W.L.; Minson, C.T. Nitric Oxide and Attenuated Reflex Cutaneous Vasodilation in Aged Skin. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H1662–H1667. [Google Scholar] [CrossRef] [PubMed]
- Holowatz, L.A.; Thompson-Torgerson, C.; Kenney, W.L. Aging and the Control of Human Skin Blood Flow. Front. Biosci. J. Virtual Libr. 2010, 15, 718–739. [Google Scholar] [CrossRef] [PubMed]
- Gaubert, M.L.; Sigaudo-Roussel, D.; Tartas, M.; Berrut, G.; Saumet, J.L.; Fromy, B. Endothelium-Derived Hyperpolarizing Factor as an in Vivo Back-up Mechanism in the Cutaneous Microcirculation in Old Mice. J. Physiol. 2007, 585, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Bentov, I.; Reed, M.J. The Effect of Aging on the Cutaneous Microvasculature. Microvasc. Res. 2015, 100, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Kenney, W.L.; Edward, F. Adolph Distinguished Lecture: Skin-Deep Insights into Vascular Aging. J. Appl. Physiol. 2017, 123, 1024–1038. [Google Scholar] [CrossRef] [PubMed]
- De Bengy, A.-F.; Lamartine, J.; Sigaudo-Roussel, D.; Fromy, B. Newborn and Elderly Skin: Two Fragile Skins at Higher Risk of Pressure Injury. Biol. Rev. Camb. Philos. Soc. 2022, 97, 874–895. [Google Scholar] [CrossRef] [PubMed]
- Gaubert-Dahan, M.-L.; Castro-Lionard, K.; Blanchon, M.-A.; Fromy, B. Severe Sensory Neuropathy Increases Risk of Heel Pressure Ulcer in Older Adults. J. Am. Geriatr. Soc. 2013, 61, 2050–2052. [Google Scholar] [CrossRef] [PubMed]
- Labeau, S.O.; Afonso, E.; Benbenishty, J.; Blackwood, B.; Boulanger, C.; Brett, S.J.; Calvino-Gunther, S.; Chaboyer, W.; Coyer, F.; Deschepper, M.; et al. Prevalence, Associated Factors and Outcomes of Pressure Injuries in Adult Intensive Care Unit Patients: The DecubICUs Study. Intensive Care Med. 2021, 47, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Khalil, Z.; Ralevic, V.; Bassirat, M.; Dusting, G.J.; Helme, R.D. Effects of Ageing on Sensory Nerve Function in Rat Skin. Brain Res. 1994, 641, 265–272. [Google Scholar] [CrossRef]
- Fromy, B.; Abraham, P.; Saumet, J.L. Non-Nociceptive Capsaicin-Sensitive Nerve Terminal Stimulation Allows for an Original Vasodilatory Reflex in the Human Skin. Brain Res. 1998, 811, 166–168. [Google Scholar] [CrossRef]
- Fouchard, M.; Misery, L.; Le Garrec, R.; Sigaudo-Roussel, D.; Fromy, B. Alteration of Pressure-Induced Vasodilation in Aging and Diabetes, a Neuro-Vascular Damage. Front. Physiol. 2019, 10, 862. [Google Scholar] [CrossRef] [PubMed]
- Fromy, B.; Lingueglia, E.; Sigaudo-Roussel, D.; Saumet, J.L.; Lazdunski, M. Asic3 Is a Neuronal Mechanosensor for Pressure-Induced Vasodilation That Protects against Pressure Ulcers. Nat. Med. 2012, 18, 1205–1207. [Google Scholar] [CrossRef] [PubMed]
- Puca, A.A.; Carrizzo, A.; Ferrario, A.; Villa, F.; Vecchione, C. Endothelial Nitric Oxide Synthase, Vascular Integrity and Human Exceptional Longevity. Immun. Ageing A 2012, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Rossman, M.J.; LaRocca, T.J.; Martens, C.R.; Seals, D.R. Healthy Lifestyle-Based Approaches for Successful Vascular Aging. J. Appl. Physiol. 2018, 125, 1888–1900. [Google Scholar] [CrossRef] [PubMed]
- Seals, D.R.; Nagy, E.E.; Moreau, K.L. Aerobic Exercise Training and Vascular Function with Ageing in Healthy Men and Women. J. Physiol. 2019, 597, 4901–4914. [Google Scholar] [CrossRef]
- Beitner, H. Randomized, Placebo-Controlled, Double Blind Study on the Clinical Efficacy of a Cream Containing 5% Alpha-Lipoic Acid Related to Photoageing of Facial Skin. Br. J. Dermatol. 2003, 149, 841–849. [Google Scholar] [CrossRef]
- El-Komy, M.; Shalaby, S.; Hegazy, R.; Abdel Hay, R.; Sherif, S.; Bendas, E. Assessment of Cubosomal Alpha Lipoic Acid Gel Efficacy for the Aging Face: A Single-Blinded, Placebo-Controlled, Right-Left Comparative Clinical Study. J. Cosmet. Dermatol. 2017, 16, 358–363. [Google Scholar] [CrossRef]
- Kubota, Y.; Musashi, M.; Nagasawa, T.; Shimura, N.; Igarashi, R.; Yamaguchi, Y. Novel Nanocapsule of α-Lipoic Acid Reveals Pigmentation Improvement: α-Lipoic Acid Stimulates the Proliferation and Differentiation of Keratinocyte in Murine Skin by Topical Application. Exp. Dermatol. 2019, 28 Suppl 1, 55–63. [Google Scholar] [CrossRef]
- Külkamp-Guerreiro, I.C.; Souza, M.N.; Bianchin, M.D.; Isoppo, M.; Freitas, J.S.; Alves, J.A.; Piovezan, A.P.; Pohlmann, A.R.; Guterres, S.S. Evaluation of Lipoic Acid Topical Application on Rats Skin Wound Healing. Acta Cir. Bras. 2013, 28, 708–715. [Google Scholar] [CrossRef][Green Version]
- Zhou, Z.; Liu, C.; Wan, X.; Fang, L. Development of a w/o Emulsion Using Ionic Liquid Strategy for Transdermal Delivery of Anti—Aging Component α—Lipoic Acid: Mechanism of Different Ionic Liquids on Skin Retention and Efficacy Evaluation. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2020, 141, 105042. [Google Scholar] [CrossRef] [PubMed]
- Bilska, A.; Włodek, L. Lipoic Acid-the Drug of the Future? Pharmacol. Rep. 2005, 57, 570–577. [Google Scholar] [PubMed]
- Ho, Y.-S.; Lai, C.-S.; Liu, H.-I.; Ho, S.-Y.; Tai, C.; Pan, M.-H.; Wang, Y.-J. Dihydrolipoic Acid Inhibits Skin Tumor Promotion through Anti-Inflammation and Anti-Oxidation. Biochem. Pharmacol. 2007, 73, 1786–1795. [Google Scholar] [CrossRef] [PubMed]
- Demiot, C.; Fromy, B.; Saumet, J.L.; Sigaudo-Roussel, D. Preservation of Pressure-Induced Cutaneous Vasodilation by Limiting Oxidative Stress in Short-Term Diabetic Mice. Cardiovasc. Res. 2006, 69, 245–252. [Google Scholar] [CrossRef][Green Version]
- Lipman, R.D.; Chrisp, C.E.; Hazzard, D.G.; Bronson, R.T. Pathologic Characterization of Brown Norway, Brown Norway x Fischer 344, and Fischer 344 x Brown Norway Rats with Relation to Age. J. Gerontol. A Biol. Sci. Med. Sci. 1996, 51, B54–B59. [Google Scholar] [CrossRef] [PubMed]
- Harraz, O.F.; Jensen, L.J. Aging, Calcium Channel Signaling and Vascular Tone. Mech. Ageing Dev. 2020, 191, 111336. [Google Scholar] [CrossRef] [PubMed]
- Fromy, B.; Merzeau, S.; Abraham, P.; Saumet, J.-L. Mechanisms of the Cutaneous Vasodilator Response to Local External Pressure Application in Rats: Involvement of CGRP, Neurokinins, Prostaglandins and NO. Br. J. Pharmacol. 2000, 131, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Fizanne, L.; Fromy, B.; Preckel, M.-P.; Sigaudo-Roussel, D.; Saumet, J.L. Effect of Isoflurane on Skin-Pressure-Induced Vasodilation. J. Vasc. Res. 2003, 40, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Garry, A.; Sigaudo-Roussel, D.; Merzeau, S.; Dumont, O.; Saumet, J.L.; Fromy, B. Cellular Mechanisms Underlying Cutaneous Pressure-Induced Vasodilation: In Vivo Involvement of Potassium Channels. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H174–H180. [Google Scholar] [CrossRef] [PubMed]
- Deuis, J.R.; Dvorakova, L.S.; Vetter, I. Methods Used to Evaluate Pain Behaviors in Rodents. Front. Mol. Neurosci. 2017, 10, 284. [Google Scholar] [CrossRef]
- Yam, M.F.; Loh, Y.C.; Oo, C.W.; Basir, R. Overview of Neurological Mechanism of Pain Profile Used for Animal “Pain-Like” Behavioral Study with Proposed Analgesic Pathways. Int. J. Mol. Sci. 2020, 21, 4355. [Google Scholar] [CrossRef] [PubMed]
- Sigaudo-Roussel, D.; Demiot, C.; Fromy, B.; Koïtka, A.; Lefthériotis, G.; Abraham, P.; Saumet, J.L. Early Endothelial Dysfunction Severely Impairs Skin Blood Flow Response to Local Pressure Application in Streptozotocin-Induced Diabetic Mice. Diabetes 2004, 53, 1564–1569. [Google Scholar] [CrossRef]
- Vivancos, G.G.; Verri, W.A.; Cunha, T.M.; Schivo, I.R.S.; Parada, C.A.; Cunha, F.Q.; Ferreira, S.H. An Electronic Pressure-Meter Nociception Paw Test for Rats. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Medicas E Biol. 2004, 37, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Yeomans, D.C.; Proudfit, H.K. Nociceptive Responses to High and Low Rates of Noxious Cutaneous Heating Are Mediated by Different Nociceptors in the Rat: Electrophysiological Evidence. Pain 1996, 68, 141–150. [Google Scholar] [CrossRef]
- Nagy, J.I.; Vincent, S.R.; Staines, W.A.; Fibiger, H.C.; Reisine, T.D.; Yamamura, H.I. Neurotoxic Action of Capsaicin on Spinal Substance P Neurons. Brain Res. 1980, 186, 435–444. [Google Scholar] [CrossRef]
- Nakamura, K.; Kuntzman, R.; Maggio, A.C.; Augulis, V.; Conney, A.H. Influence of 6-Hydroxydopamine on the Effect of Morphine on the Tail-Flick Latency. Psychopharmacologia 1973, 31, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Le Bars, D.; Gozariu, M.; Cadden, S.W. Animal Models of Nociception. Pharmacol. Rev. 2001, 53, 597–652. [Google Scholar] [PubMed]
- Bowden, J.L.; McNulty, P.A. Age-Related Changes in Cutaneous Sensation in the Healthy Human Hand. Age 2013, 35, 1077–1089. [Google Scholar] [CrossRef]
- Viseux, F.J.F. The Sensory Role of the Sole of the Foot: Review and Update on Clinical Perspectives. Neurophysiol. Clin. Clin. Neurophysiol. 2020, 50, 55–68. [Google Scholar] [CrossRef]
- Taguchi, T.; Ota, H.; Matsuda, T.; Murase, S.; Mizumura, K. Cutaneous C-Fiber Nociceptor Responses and Nociceptive Behaviors in Aged Sprague-Dawley Rats. Pain 2010, 151, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.J.; Shore, A.C. Skin Blood Flow Responses to the Iontophoresis of Acetylcholine and Sodium Nitroprusside in Man: Possible Mechanisms. J. Physiol. 1996, 496, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Melik, Z.; Zaletel, P.; Virtic, T.; Cankar, K. L-Arginine as Dietary Supplement for Improving Microvascular Function. Clin. Hemorheol. Microcirc. 2017, 65, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Algotsson, A.; Nordberg, A.; Winblad, B. Influence of Age and Gender on Skin Vessel Reactivity to Endothelium-Dependent and Endothelium-Independent Vasodilators Tested with Iontophoresis and a Laser Doppler Perfusion Imager. J. Gerontol. A Biol. Sci. Med. Sci. 1995, 50, M121–M127. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Cupisti, A.; Mariani, S.; Santoro, G.; Pentimone, F. Endothelium-Dependent and Endothelium-Independent Skin Vasoreactivity in the Elderly. Aging Clin. Exp. Res. 2002, 14, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Jin, Y.; Yang, Z.; Wang, L.; Gao, X.; Lui, L.; Ma, H. Reduced Arterial Elasticity Is Associated with Endothelial Dysfunction in Persons of Advancing Age: Comparative Study of Noninvasive Pulse Wave Analysis and Laser Doppler Blood Flow Measurement. Am. J. Hypertens. 2004, 17, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Seals, D.R.; Jablonski, K.L.; Donato, A.J. Aging and Vascular Endothelial Function in Humans. Clin. Sci. 2011, 120, 357–375. [Google Scholar] [CrossRef]
- Muller-Delp, J.M.; Spier, S.A.; Ramsey, M.W.; Delp, M.D. Aging Impairs Endothelium-Dependent Vasodilation in Rat Skeletal Muscle Arterioles. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H1662–H1672. [Google Scholar] [CrossRef] [PubMed]
- Woodman, C.R.; Price, E.M.; Laughlin, M.H. Selected Contribution: Aging Impairs Nitric Oxide and Prostacyclin Mediation of Endothelium-Dependent Dilation in Soleus Feed Arteries. J. Appl. Physiol. 2003, 95, 2164–2170. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gendron, M.-E.; Thorin-Trescases, N.; Villeneuve, L.; Thorin, E. Aging Associated with Mild Dyslipidemia Reveals That COX-2 Preserves Dilation despite Endothelial Dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H451–H458. [Google Scholar] [CrossRef] [PubMed]
- James, M.A.; Tullett, J.; Hemsley, A.G.; Shore, A.C. Effects of Aging and Hypertension on the Microcirculation. Hypertension 2006, 47, 968–974. [Google Scholar] [CrossRef]
- Labinskyy, N.; Csiszar, A.; Orosz, Z.; Smith, K.; Rivera, A.; Buffenstein, R.; Ungvari, Z. Comparison of Endothelial Function, O2-* and H2O2 Production, and Vascular Oxidative Stress Resistance between the Longest-Living Rodent, the Naked Mole Rat, and Mice. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H2698–H2704. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nisoli, E.; Tonello, C.; Cardile, A.; Cozzi, V.; Bracale, R.; Tedesco, L.; Falcone, S.; Valerio, A.; Cantoni, O.; Clementi, E.; et al. Calorie Restriction Promotes Mitochondrial Biogenesis by Inducing the Expression of ENOS. Science 2005, 310, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Rexhaj, E.; Paoloni-Giacobino, A.; Rimoldi, S.F.; Fuster, D.G.; Anderegg, M.; Somm, E.; Bouillet, E.; Allemann, Y.; Sartori, C.; Scherrer, U. Mice Generated by in Vitro Fertilization Exhibit Vascular Dysfunction and Shortened Life Span. J. Clin. Invest. 2013, 123, 5052–5060. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Mital, S.; Ojaimi, C.; Csiszar, A.; Kaley, G.; Hintze, T.H. Premature Death and Age-Related Cardiac Dysfunction in Male ENOS-Knockout Mice. J. Mol. Cell. Cardiol. 2004, 37, 671–680. [Google Scholar] [CrossRef]
- Muller-Delp, J.M.; Gurovich, A.N.; Christou, D.D.; Leeuwenburgh, C. Redox Balance in the Aging Microcirculation: New Friends, New Foes, and New Clinical Directions. Microcirculation 2012, 19, 19–28. [Google Scholar] [CrossRef]
- Donato, A.J.; Machin, D.R.; Lesniewski, L.A. Mechanisms of Dysfunction in the Aging Vasculature and Role in Age-Related Disease. Circ. Res. 2018, 123, 825–848. [Google Scholar] [CrossRef]
- Rodríguez-Mañas, L.; El-Assar, M.; Vallejo, S.; López-Dóriga, P.; Solís, J.; Petidier, R.; Montes, M.; Nevado, J.; Castro, M.; Gómez-Guerrero, C.; et al. Endothelial Dysfunction in Aged Humans Is Related with Oxidative Stress and Vascular Inflammation. Aging Cell 2009, 8, 226–238. [Google Scholar] [CrossRef]
- Ungvari, Z.; Kaley, G.; de Cabo, R.; Sonntag, W.E.; Csiszar, A. Mechanisms of Vascular Aging: New Perspectives. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 1028–1041. [Google Scholar] [CrossRef]
- Van der Loo, B.; Labugger, R.; Skepper, J.N.; Bachschmid, M.; Kilo, J.; Powell, J.M.; Palacios-Callender, M.; Erusalimsky, J.D.; Quaschning, T.; Malinski, T.; et al. Enhanced Peroxynitrite Formation Is Associated with Vascular Aging. J. Exp. Med. 2000, 192, 1731–1744. [Google Scholar] [CrossRef]
- Decorps, J.; Saumet, J.L.; Sommer, P.; Sigaudo-Roussel, D.; Fromy, B. Effect of Ageing on Tactile Transduction Processes. Ageing Res. Rev. 2014, 13, 90–99. [Google Scholar] [CrossRef]
- Skedung, L.; El Rawadi, C.; Arvidsson, M.; Farcet, C.; Luengo, G.S.; Breton, L.; Rutland, M.W. Mechanisms of Tactile Sensory Deterioration amongst the Elderly. Sci. Rep. 2018, 8, 5303. [Google Scholar] [CrossRef] [PubMed]
- Guergova, S.; Dufour, A. Thermal Sensitivity in the Elderly: A Review. Ageing Res. Rev. 2011, 10, 80–92. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, S.; Nagi, S.S.; McGlone, F.; Olausson, H. The Effects of Ageing on Tactile Function in Humans. Neuroscience 2021, 464, 53–58. [Google Scholar] [CrossRef]
- Namer, B. Age Related Changes in Human C-Fiber Function. Neurosci. Lett. 2010, 470, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.; Evangelopoulos, A.; Koutalas, P. Alpha-Lipoic Acid and Diabetic Neuropathy. Rev. Diabet. Stud. RDS 2009, 6, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.; Hanefeld, M.; Ruhnau, K.J.; Meissner, H.P.; Lobisch, M.; Schütte, K.; Gries, F.A. Treatment of Symptomatic Diabetic Peripheral Neuropathy with the Anti-Oxidant Alpha-Lipoic Acid. A 3-Week Multicentre Randomized Controlled Trial (ALADIN Study). Diabetologia 1995, 38, 1425–1433. [Google Scholar] [CrossRef]
- Garrett, N.E.; Malcangio, M.; Dewhurst, M.; Tomlinson, D.R. Alpha-Lipoic Acid Corrects Neuropeptide Deficits in Diabetic Rats via Induction of Trophic Support. Neurosci. Lett. 1997, 222, 191–194. [Google Scholar] [CrossRef]
- Nickander, K.K.; McPhee, B.R.; Low, P.A.; Tritschler, H. Alpha-Lipoic Acid: Antioxidant Potency against Lipid Peroxidation of Neural Tissues in Vitro and Implications for Diabetic Neuropathy. Free Radic. Biol. Med. 1996, 21, 631–639. [Google Scholar] [CrossRef]
- Koïtka, A.; Abraham, P.; Bouhanick, B.; Sigaudo-Roussel, D.; Demiot, C.; Saumet, J.L. Impaired Pressure-Induced Vasodilation at the Foot in Young Adults with Type 1 Diabetes. Diabetes 2004, 53, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Fromy, B.; Josset-Lamaugarny, A.; Aimond, G.; Pagnon-Minot, A.; Marics, I.; Tattersall, G.J.; Moqrich, A.; Sigaudo-Roussel, D. Disruption of TRPV3 Impairs Heat-Evoked Vasodilation and Thermoregulation: A Critical Role of CGRP. J. Invest. Dermatol. 2018, 138, 688–696. [Google Scholar] [CrossRef]
- Molz, P.; de Freitas, B.S.; Uberti, V.H.; da Costa, K.M.; Kist, L.W.; Bogo, M.R.; Schröder, N. Effects of Lipoic Acid Supplementation on Age- and Iron-Induced Memory Impairment, Mitochondrial DNA Damage and Antioxidant Responses. Eur. J. Nutr. 2021, 60, 3679–3690. [Google Scholar] [CrossRef] [PubMed]






| BN Rats | Wistar Rats | |
|---|---|---|
| Skin sensory nerve fiber sensitivity | Alteration of mechanical sensitivity (at 12 and 24 months) and thermal sensitivity (at 24 months only) Partially or fully restored by LPA | Alteration of mechanical sensitivity (at 12 and 24 months) and thermal sensitivity (at 24 months only) Partially or fully restored by LPA |
| Endothelial function Endothelial factor contribution | Preservation over time NOS pathway over time LPA: no effect | Decrease from 12 months Fully restored by LPA at 12 months only NOS pathway at 6 months COX pathway at 12 months Beneficial effect on the NOS and COX pathways at 12 months |
| Skin resistance to low pressure | Gradual decrease over time Tended to be improved by LPA at 12 months only | Drastic reduction from 12 months Not improved by LPA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Bengy, A.-F.; Decorps, J.; Martin, L.S.; Pagnon, A.; Chevalier, F.P.; Sigaudo-Roussel, D.; Fromy, B. Alpha-Lipoic Acid Supplementation Restores Early Age-Related Sensory and Endothelial Dysfunction in the Skin. Biomedicines 2022, 10, 2887. https://doi.org/10.3390/biomedicines10112887
de Bengy A-F, Decorps J, Martin LS, Pagnon A, Chevalier FP, Sigaudo-Roussel D, Fromy B. Alpha-Lipoic Acid Supplementation Restores Early Age-Related Sensory and Endothelial Dysfunction in the Skin. Biomedicines. 2022; 10(11):2887. https://doi.org/10.3390/biomedicines10112887
Chicago/Turabian Stylede Bengy, Anne-France, Johanna Decorps, Lisa S. Martin, Aurélie Pagnon, Fabien P. Chevalier, Dominique Sigaudo-Roussel, and Bérengère Fromy. 2022. "Alpha-Lipoic Acid Supplementation Restores Early Age-Related Sensory and Endothelial Dysfunction in the Skin" Biomedicines 10, no. 11: 2887. https://doi.org/10.3390/biomedicines10112887
APA Stylede Bengy, A.-F., Decorps, J., Martin, L. S., Pagnon, A., Chevalier, F. P., Sigaudo-Roussel, D., & Fromy, B. (2022). Alpha-Lipoic Acid Supplementation Restores Early Age-Related Sensory and Endothelial Dysfunction in the Skin. Biomedicines, 10(11), 2887. https://doi.org/10.3390/biomedicines10112887






