Abstract
Huntington’s disease (HD) is distinguished by a triple repeat of CAG in exon 1, an increase in poly Q in the Htt gene, and a loss of GABAergic medium spiny neurons (MSN) in the striatum and white matter of the cortex. Mitochondrial ETC-complex dysfunctions are involved in the pathogenesis of HD, including neuronal energy loss, synaptic neurotrophic decline, neuronal inflammation, apoptosis, and grey and white matter destruction. A previous study has demonstrated that beta Boswellic acid (β-BA), a naturally occurring phytochemical, has several neuroprotective properties that can reduce pathogenic factors associated with various neurological disorders. The current investigation aimed to investigate the neuroprotective potential of β-BA at oral doses of 5, 10, and 15 mg/kg alone, as well as in conjunction with the potent antioxidant vitamin E (8 mg/kg, orally) in 3-NP-induced experimental HD rats. Adult Wistar rats were separated into seven groups, and 3-NP, at a dose of 10 mg/kg, was orally administered to each group of adult Wistar rats beginning on day 1 and continuing through day 14. The neurotoxin 3-NP induces neurodegenerative, g, neurochemical, and pathological alterations in experimental animals. Continuous injection of 3-NP, according to our results, aggravated HD symptoms by suppressing ETC-complex-II, succinate dehydrogenase activity, and neurochemical alterations. β-BA, when taken with vitamin E, improved behavioural dysfunctions such as neuromuscular and motor impairments, as well as memory and cognitive abnormalities. Pharmacological treatments with β-BA improved and restored ETC complexes enzymes I, II, and V levels in brain homogenates. β-BA treatment also restored neurotransmitter levels in the brain while lowering inflammatory cytokines and oxidative stress biomarkers. β-BA’s neuroprotective potential in reducing neuronal death was supported by histopathological findings in the striatum and cortex. As a result, the findings of this research contributed to a better understanding of the potential role of natural phytochemicals β-BA in preventing neurological illnesses such as HD.
1. Introduction
Huntington’s disease (HD) is a fatal neurological disease that is autosomal, dominantly inherited, fully penetrant, and characterized by progressive chorea, motor and mental issues, cognitive impairment, aggression, and weight loss [1]. It is believed that the Htt genes, which are responsible for HD, have spread worldwide [2]. According to the global prevalence rate in 2017, 14 people per 100,000 are affected by HD each year, with a gender-related prevalence ratio of 13 women to 14 men. The prevalence rate was determined to be 66,787 after research was conducted in 2020 in eight different countries [3,4].
Huntington’s disease (HD) is characterised by an abnormally high amount of polyglutamine (poly Q) in the huntingtin (Htt) protein due to a triple repeat of cytosine, adenine, and guanine (CAG). CAG is typically repeated 35 times; however, in HD patients, the frequency rises from 36 to 39 times. CAG repetition is caused by a huntingtin (mHtt) gene mutation [5]. HD is a multisystem disorder because it affects the striatum, basal ganglia, cerebral cortex, and hippocampus [6]. The striatum and the cortex both suffer from the degeneration of neurons, followed by HD [7]. The brain’s striatum loses GABAergic medium spiny neurons (MSN), and the cortex experiences neurodegeneration, which alters mitochondrial function, inflammatory markers, oxidative stress, and neurotransmitters [8]. The specific mechanism by which neurons die in HD is unknown. HD causes damage to the brain’s thalamus, hypothalamus, subthalamic nucleus, substantia nigra (SN), and cerebellar nuclei [9,10]. Patients with HD may encounter cognitive problems; behavioural changes are frequently the most unpleasant feature of the disorder for patients and relatives [11]. Motor symptoms like “restlessness” and chorea, an irregular involuntary movement originating from the Greek word “dance,” are the most obvious HD symptoms [12]. HD is distinguished by quick, abrupt, irregular, unpredictable, and non-stereotypical movements [13]. The mitochondrial neurotoxin 3-NP inhibits succinate dehydrogenase, a TCA cycle enzyme, and the electron transport chain enzyme irreversibly [14,15]. Endogenously produced superoxide and hydroxyl free radicals can damage lipids, proteins, and DNA, altering brain structure, function, neurotransmitter, and inflammatory levels [16]. Reactive oxygen species (ROS) have been identified as potential mediators of neuronal cell death in HD [17]. Systemic administration of 3-NP results in CNS lesions that selectively target MSN in the striatum, reflecting HD’s spatial and neuronal selectivity [18].
The neurotoxin 3-NP impairs cognition and induces motor abnormalities such as dystonia, involuntary jerky movements, torsion spasms, and facial grimaces [19]; it is useful for determining the neuronal susceptibility and motor impairments associated with HD [20]. Striatum and brain lesions induced by 3-NP, together with increasing lactate levels, increased NMDA-receptor activation in rats, and this effect can be mitigated by employing NMDA-receptor antagonists [21]. Several investigations have linked 3-NP toxicity to increases in striatal hydroxyl (OH-) and superoxide (O2•-) free radical generation and oxidative damage indicators in the CNS [22]. Oxidative stress and NMDA enhance neuroinflammation and raise or activate proinflammatory markers (TNF-α and IL-1β) [23]. Increased DNA fragmentation and decreased apurinic or apyrimidinic endonuclease synthesis [24,25] indicate that older rats are more vulnerable to the oxidative stress generated by 3-NP. Following 3-NP injection, striatal glutathione (GSH), catalase (CAT), and superoxide dismutase (SOD) levels were reduced, indicating deficiencies in antioxidant mechanisms [26].
The bark of the Boswelliaserrata tree, usually found in the Burseraceae family, is used to make the gummy dry oleo-resin exudate. β-BA has been shown to decrease 5-lipoxygenase (5-LO), topoisomerases, angiogenesis, leukotrienes, and arachidonic acid, all known to inhibit proinflammatory and inflammatory cytokines [27]. β-BA potentially inhibits ERK and MAPK to protect the suprachiasmatic nucleus (SCN) following excessive glutamate release [28] or catalyse oxidation via the cytochrome p450 enzyme [27]. BAs and their derivatives have also exhibited anticarcinogenic, anti-hyperlipidemic, anti-atherosclerotic, anti-ulcer, wound-healing, analgesic, and antihyperglycemic activities [29,30]. According to the research, β-BA can modulate mitochondrial complex dysregulation by decreasing ROS production in stroke [31], Parkinson’s [32], and cancer [33], which is a major component in the formation of oxidative stress. In Alzheimer’s disease (AD), β-BA also participates in neurogenesis by balancing Ach and AchE [34]. Multiple sclerosis (MS) [35] and traumatic brain injury (TBI) [36] are two conditions wherein BA lowers glutamate levels, which results in excitotoxicity and oxidative damage to neuronal cells. Memory is also enhanced by β-BA because it increases the number of pyramidal cells in the striatum and the cortex [37].
Beta-tocopherol, also known as vitamin E, is an antioxidant that protects against damage to lipids. Vitamin E has been shown to reduce reactive oxygen species (ROS), inflammation, and oxidative damage, all of which may contribute to neuronal and neurochemical function recovery [38]. Vitamin E is used as a supplement, therapy, and standard medicine in various interventions due to its neuroprotective effect on CNS illnesses such as Alzheimer’s, Parkinson’s, ALS, and stroke [39]. A limited range of medications is available for managing HD symptoms. All of these findings, together with the lack of pharmacological intervention, urge the investigator to conduct an experiment to evaluate the efficacy of β-BA against 3-NP-induced HD in rats.
2. Material and Methods
2.1. Experimental Animals
The study used male Wistar rats that were 8–9 months old and weighed 220–250 g. The animals utilised in the experiment were obtained from the Institute’s Central Animal House. Polycyclic cages with wire mesh tops and soft bedding were used to house the animals. The animals were kept under controlled conditions with a 12 h light–dark cycle, controlled temperature (22 ± 2 °C), controlled humidity (65–70%), and free access to food and water. The experimental protocol was approved by Institutional Animal Ethics Committee (IAEC) as per the guidelines of the committee for the purpose of control and supervision of experiments on animals (CPCSEA), Government of India (573/PO/Re/S/02/CPCSEA). Animals were acclimatized to laboratory conditions prior to research.
2.2. Drugs and Chemicals
The 3-nitropropionic acid (3-NP) was purchased from Sigma-Aldrich (St. Louis, MO, USA). β-BA was provided as an ex gratia sample from BAPEX, Rajasthan. Analytical-grade versions of all other compounds were also employed in the investigation. Drug and chemical solutions were freshly prepared before administration to the rats. The recommended dosage of 3-NP is 10 mg/kg intraperitoneally (i.p.), given for 15 days. The saline solution contained 5% DMSO and had a pH of 7.4. For oral use, β-BA was dissolved in water (with 2% ethanol) (p.o.). As a standard drug, vitamin E (Evion® 200 marketed formulation, manufactured by Merck Limited, New York City, NY, USA, manufacturing date: May 2018 and expiry date: July 2020) was dissolved in water (with ethanol) and administered by the oral route (p.o.).
2.3. Experimental Grouping of Animals
The total number of days of the experiment was 15. A total of 42 Wistar male rats aged 8–9 months with a body weight of 220–250 g were required for conducting the protocol schedule. Rats were placed in polycyclic cages with wire mesh tops along with the soft, sterilized and residue-free wood saving with free food and water access. The animals were kept under controlled conditions with a 12 h light–dark cycle, controlled temperature (22.2 °C), controlled humidity (65–70%), and free access to food and water. The effect of the circadian rhythm was minimized by performing experiments from 9:00 a.m. to 1:00 p.m. The researcher performed an unblinded preclinical study on rats. Animals were randomly divided into seven groups as follows: Group 1: normal control; Group 2: Βeta-BOSWELLIC ACID (15 mg/kg/day, p.o.) perse; Group 3: 3-NP control (10 mg/kg/day, i.p.); Group 4: Βeta-Boswellic ACID (5 mg/kg/day, p.o.) + 3-NP (10 mg/kg/day, i.p.); Group 5: Βeta-Boswellic ACID (10 mg/kg/day, p.o.) + 3-NP (10 mg/kg/day, i.p.); Group 6: Βeta-Boswellic ACID (15 mg/kg/day, p.o.) + 3-NP (10 mg/kg/day, i.p.); Group 7: Vitamin E (8 mg/kg, p.o.) + 3-NP (10 mg/kg/day, i.p.) (Figure 1).
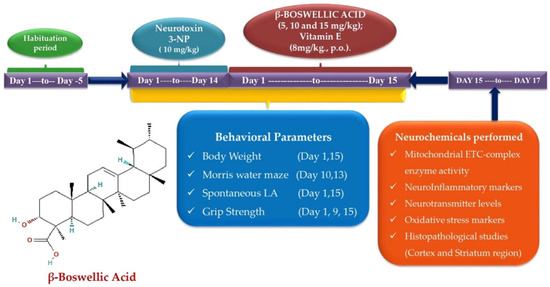
Figure 1.
Behavioural and neurochemical data estimation protocol schedule.
2.4. Measurement of Body Weight
Each rat’s body weight was measured on the first and last days of the protocol schedule. Body weight was calculated to evaluate changes in food and water intake in rats following the administration of 3-NP, along with various dosages of β-BA and conventional medication (vitamin E). Body weight was calculated as the percentage of change in the body weight of rats from the 1st day of the protocol to the last day, i.e., the 15th day of the treatment [40].
2.5. Parameters of Behavioral Assessment
2.5.1. Morris Water Maze Test (MWM)
The Morris water maze apparatus was used to assess spatial learning and memory with cognition by observing rats’ performance efficiency. ELT and TSTQ tasks were conducted on the 10th, 13th, and last days of the protocol schedule. ELT refers to finding the platform in an MWM within a certain amount of time (in seconds) by rats. On the last day of the protocol, a hidden platform was put in a certain quadrant, and the rats were free to swim in the MWM for 120 s so that the TSTQ task could test how well their memories had been consolidated [41].
2.5.2. Spontaneous Locomotor Activity (LA)
The actophotometer was used to assess spontaneous locomotor movements and motor coordination. The LA of each rat was analysed on the 1st and 15th days of the protocol schedule. The LA was measured for 5 min after the animal was placed in the actophotometer, and the value was stated as the number of beams crossed by the animal in 5 min [42].
2.5.3. String Test for Grip Strength
A grip strength test was used to assess the ability of the forepaws to grasp and hold a wire (Hague Ave., Columbus, OH, USA). The grip strength test was performed on the 1st and 15th days of the procedure schedule. The latency of the rats’ paws to hold the wire against the researcher’s force was measured in terms of fall time, and the retaining latency on the wire was used to measure the rats’ gripping strength. Kilogram-force (KgF) is the force measurement unit used to quantify grip strength [43].
2.6. Biochemical Assessment of Parameters
2.6.1. Biological Samples Preparation
The animal’s brain was removed with the decapitation method on the sixteenth day of the protocol schedule. The rat’s brain was cleaned with an ice-cold isotonic saline solution. Brain slices were homogenized and then put into a cold phosphate buffer solution of 0.1 M (7.4 pH). The homogeneous mixture was centrifuged at 10,500× g for 15 min to separate the supernatant. The biochemical changes (oxidative stress, neurotransmitter, neurochemical, cellular, and molecular) that happened in the brain after treatment with 3-NP and different doses of β-BA, in addition to the standard drug, were studied using a brain sample (supernatant) [44].
2.6.2. Analyse the Enzyme Activity of Mitochondrial ETC Complexes in Rat Brain Homogenate
Post-Mitochondrial Supernatant (PMS) Preparation
The activity and concentration of the mitochondrial ETC-complex enzymes in rat brain homogenate were assessed by PMS. To prepare PMS, homogenate the entire brain and centrifuge at 5000 rpm for 20 min at 4 degrees Celsius to extract the supernatant. Pellets produced during PMS synthesis were combined in a 1:10 ratio with 0.1 M sodium phosphate buffer (pH 7.4) and gently stirred for 60 min at 4 °C. After centrifuging the mixture at 16,000× g for 30 min at 0 °C, it was resuspended in the same buffer. Add 250 mmol/L of additional sucrose to the resuspended pellet. After three rounds of centrifugation, enough buffer sucrose solution was produced to extract the mitochondrial fraction for further analysis [45].
Analyse the Enzyme Activity of Mitochondrial Complex-I (NADPH Dehydrogenase) Protein in Rat Brain Homogenate
The activity of the complex I enzyme was determined by oxidising NADH in an assay medium at 340 nm for 3 min at 37 °C. Rotenone exhibited sensitivity due to the complex I enzyme when 2 mM rotenone molecule reacted with complex I, and its activity was expressed in nM/mg protein [46].
Analyse the Enzyme Activity of Mitochondrial Complex-II (Succinate Dehydrogenase/SDH) Protein in Rat Brain Homogenate
The activity of the complex II enzyme was determined by combining homogenate gradient fraction (50 L) with sodium succinate solution (0.3 mL) and spectrophotometrically quantifying the absorbance at 490 nm (Shimadzu, UV-1700, Kyoto, Japan). The results were calculated using the chromophore molar extinction coefficient (1.36104 M−1 cm−1) and given as reduced INT. The values were given in nM/mg protein [47].
Analyse the Enzyme Activity of Mitochondrial ETC Complex-V (ATP Synthase) Protein in Rat Brain Homogenate
Sonication processes were conducted with the help of ice-cold perchloric acid (0.1 N) utilised on the aliquots of the homogenate aliquots to quickly inactivate the ATPase. Sonicate the homogenate, and then centrifuge it at 14,000× g for 5 min at 4 °C. The supernatant was collected, then neutralised with 1 N NaOH and preserved at −80 °C for further analysis. The amount of ATP in the supernatant was measured by reverse-phase HPLC (Perkin Elmer). The standard was dissolved, and the absorbance at 254 nm was used to make a standard ATP reference solution [48].
2.6.3. Analysis of Neuroinflammatory Biomarkers
TNF-α and IL-1β Protein Concentration in the Brain
Inflammatory markers such as TNF-α and IL-1β levels in rat brain homogenate were determined using the ELISA kit from E-EL-R0019/TNF-α and E-EL-R0012/IL-1β Elabscienes (Wuhan, China). The activity of inflammatory indicators was represented as pg/mg protein [49,50].
2.6.4. Analysis of Neurotransmitters
Dopamine Concentration in Rat’s Brain Homogenate
Striatal tissue samples were used to determine the dopamine levels in brain homogenates. Assess the DA level by using an electrochemical detector (ECD) and high-performance liquid chromatography (HPLC) (ECD Waters, Milford, MA, USA) in rat brain homogenates. The mobile phase was buffered with the help of sodium citrate (pH 4.5) and acetonitrile (87:13, v/v). The preparation of sodium citrate buffer was performed by the addition of citric acid (10 mM), NaH2PO4 (25 mM), EDTA (25 mM), and 1-heptane sulfonic acid (2 mM). A voltage of 0.75 V was used to conduct the electrochemical condition of the experiment and maintain its range from 5 to 50 nA. The separation was performed by using a 0.89 mL/min flow rate. A sample (20 mL) was manually injected into the injector. Homogenize the rat brain sample with centrifugation at 12,000× g for 5 min after adding 0.2 M perchloric acid to the homogenising solution. Dopamine levels were expressed in ng/mg [51].
GABA and Glutamate Concentration in Rat’s Brain Homogenate
The GABA and glutamate concentrations were determined using HPLC connected to an ECD. A Waters standard system, which includes a high-pressure isocratic pump, a 20 L manual sample injector valve, a C18 re-versed-phase column, and a UV detector, was used in conjunction with an ECD. The mobile phase was composed of 22% methanol, 25 mM EDTA, and 100 mM anhydrous disodium hydrogen phosphate (pH: 6.5). Experiments were performed with an electrochemical potential of +0.65 V and a range of sensitivity from 5 to 50 nA. The separation was conducted at a 1.2 mL/min flow rate with a 40 °C column temperature. Manual sample injections (20 L) were performed using a rheodyne valve injector. The frozen brain samples were thawed and mixed together with 0.2 M perchloric acid on the day of the experiment. Afterwards, the samples were subjected to 12,000 g of anteroposterior force for 15 min. When the supernatant was filtered through 0.22 m nylon filters and derivatized with OPA/-ME (o-pthalaldehyde/-mercaptoethanol), it was ready to be injected into the HPLC sample injector. Waters HPLC provided Breeze, version 3.2, which was used to record and analyse the data. We were able to calculate the concentrations of the amino acids of interest by extrapolating a standard curve with a concentration standard of between 10 and 100 ng/mL. Values were given in terms of ng/mg of protein, and a normal control group was used for comparison [52].
2.6.5. Analysis of Oxidative Stress Parameters
Acetylcholinesterase (AChE) Concentration in Rat’s Brain Homogenate
A test was performed to figure out how much acetylcholine esterase was in the brain homogenate. A total of 3 mL of phosphate buffer (pH-8) and 0.05 mL of supernatant were mixed with 0.10 mL of DTNB and 0.10 mL of acetylthiocholine iodide. Spectrophotometry was used to measure the instantaneous changes at 420 nm, and the amount of AChE in the supernatant was calculated in terms of M/mg protein [53].
Malondialdehyde (MDA) Concentration in Rat’s Brain Homogenate
The Wills method was used to assess the end product of lipid peroxidation, MDA. Thiobarbituric acid was used to process the brain homogenate supernatant before 532 nm spectrophotometric measurement. MDA levels were expressed as nm/mg of protein [54].
Glutathione (GSH) Concentration in Rat’s Brain Homogenate
This assay was used to quantify the reduced glutathione level in rats’ brains homogenate after I.P. administration of 3-NP. The cold digestion process was performed with 1 ml supernatant in 4% sulfosalicylic acid (1 mL) at 4 °C for one hour, and then the collected sample was centrifuged at 1200 rpm for 15 min. In 1 mL supernatant, add 0.2 mL of DTNB and 2.7 mL phosphate buffer of 0.1 M concentration at pH 8. After the process, spectrophotometric measurements generated a yellow colour at the immediate level of 412 nm. The level of reduced GSH was expressed as M/mg protein [55].
Superoxide Dismutase (SOD) Concentration in Rat’s Brain Homogenate
The same method was used for epinephrine auto-oxidation at pH 10.4, spectrophotometrically assessing the level of SOD. The procedure began with the addition of 0.8 mL of pH 10.4 50 mM glycine buffer and 0.02 mL of epinephrine to the 0.2 mL of supernatant. After 5 min of incubation, the absorbance was taken at 480 nm. The SOD activity was reported at nM/mg of protein [56].
Catalase (CAT) Concentration in Rat’s Brain Homogenate
The concentration of CAT in rat brain homogenate can be measured by mixing 0.1 mL of supernatant with 1.9 mL of 50 mM phosphate buffer in a cuvette while keeping the pH at 7. After adding 1.0 mL of a freshly made 30 mM H2O2 solution, the reactions were started. Spectrophotometric analysis at 240 nm was used to determine the rate of H2O2 decomposition. The data were presented as micromoles of µM/ H2O2 decomposition per minute [57].
2.7. Histopathology
Following the end of the experimental procedure, the rats were anaesthetized with a 270 mg/kg i.p. dosage of sodium barbiturates. Decapitate the rats, then carefully separate their whole brains and wash them in PBS. Whole brains were preserved by being immersed in PBS containing 4% paraformaldehyde at a pH of 7.4. Afterwards, a microtome was used to cut sections of brain wax block that were between 4 and 5 mm in thickness. Hematoxylin and eosin staining and fluorescence microscopy (MOTICAM-Ba310 image plus 2.0, Schertz, TA, USA) at 100× magnification were used for a morphological study of brain sections (Cortex and striatum). At 100× magnification, different cell characteristics were seen in a Moticam, such as the size of the cell, apoptotic cells, inflammatory cells, and vacuolization in the cortex and striatum [58].
2.8. Statistical Analysis
Using one- and two-way ANOVA with post-hoc tests, the data was analysed and displayed through the analysed intervention. The data from the several experimental groups were separated and compared using Tukey’s multi-comparison test and Bonferroni’s test, respectively. Two-way ANOVA with Bonferroni’s posthoc test was performed to examine the behavioural changes (body weight, ELT, spontaneous LA, and grip strength) that occurred in rats after treatment. While neurochemical (estimation of mitochondrial complex activity, inflammatory biomarkers, neurotransmitter levels, and oxidative stress indicators) and behavioural alterations were evaluated using one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test for multiple comparisons (TSTQ time). At a p-value of 0.01, the findings are considered statistically significant. The Kolmogorov–Smirnov test was utilised to ascertain the normal distribution of the sample size. The statistical analysis and visualization of the data were performed in the form of mean and standard deviation (SD) using GraphPad Prism version 5.03 for Windows (GraphPad Software, San Diego, CA, USA).
3. Results
The study aimed to investigate the possible protective effect of β-B Ain HD. The therapeutic effects were evaluated using initial and final weight differences between animals in the control, inducing agent, and test control groups. Behavioural parameters like memory and learning were tested by a Morris water maze (MWM); for motor coordination grip strength (Rota-rod) model was used; for locomotor activity, an actophotometer was used; by histopathological examination of the brain tissue, tissue necrosis in the striatum and cortex was compared.
3.1. β-Boswellic Acid Ameliorates the Decreased Body Weight in 3-NP-Treated Rats
Body weight was measured on the 1st and 15th days of the procedure schedule. Rats had significantly decreased body weight following chronic 3-NP treatment compared with the normal and perse groups. Normal and β-BA15 perse groups did not show any significant changes. Different doses of β-BA (5, 10, and 15 mg/kg) were shown to be more effective in treating rats than the 3-NP-induced HD group [two-way ANOVA:F (6, 70) = 123.13, p < 0.01] (Figure 2). In conventional medicine, vitamin E was shown to significantly improve body weight regulation compared to different doses of β-BA.
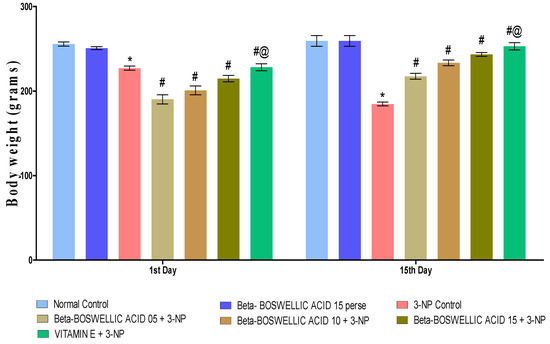
Figure 2.
β-Boswellic acid ameliorates the decreased body weight in 3-NP-induced HD in rats. The Bonferroni two-way ANOVA post-hoc test was used to express significant statistical data as mean ± SD (n = 6) and * 3-NP (p < 0.01) versus normal and β-BA perse; # β-BA5 + 3NP, β-BA10 + 3NP, and β-BA15 + 3NP (p < 0.01) versus 3-NP; #@ Vitamin E + 3-NP (p < 0.01) versus β-BA5 + 3NP, β-BA10 + 3NP, and β-BA15 + 3NP.
3.2. Behavioural Parameters
These behavioural (MWM, grip strength, locomotor activity) parameters were used to explore the neuroprotective effects of β-BA at the doses of 5, 10 & 15 mg/kg, p.o. compared with vitamin E.
3.2.1. β-Boswellic Acid Ameliorates Spatial Navigation Task in 3-NP Treated Rats
Spatial navigation tasks were conducted on the 10th and 13th days of the trial, according to the protocol. These two tasks, the escape latency task and time spent in the targeted quadrant (TSTQ), were performed by rats for the spatial navigation task. TSTQ was performed on the 13th day of the protocol schedule, while ELT was performed on the 10th and 13th days. There were no significant differences observed between the normal and perse groups. Chronic administration of 3-NP significantly increased ELT time and decreased TSTQ compared with the normal and β-BA15 perse group. Treatment with different doses of β-BA (5, 10, and 15 mg/kg) resulted in a substantial improvement in ELT [two-way ANOVA:F (6,70) = 31.88, p < 0.01] (Figure 3A) and TSTQ [one-way ANOVA:F (6, 30) = 1.548, p < 0.01] (Figure 3B) in rats compared with the 3-NP-induced HD. Furthermore, compared with the varying doses of β-BA and the standard medicine, vitamin E showed a considerable role in reducing ELT duration and elevating TSTQ.
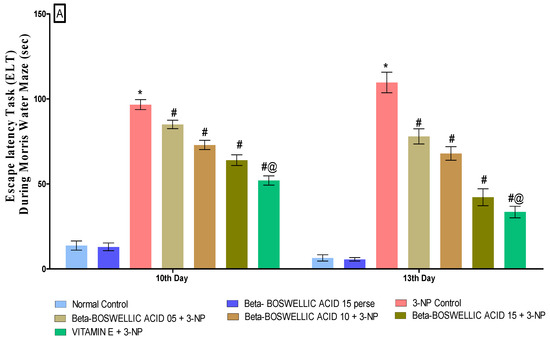
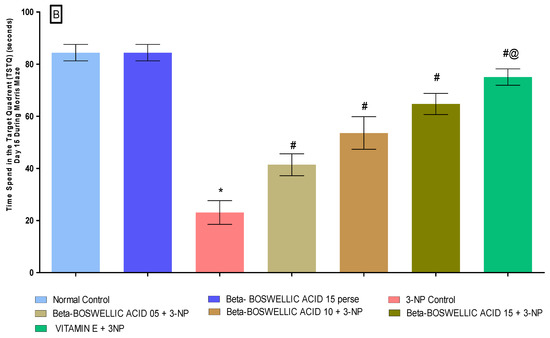
Figure 3.
(A): β-Boswellic acid ameliorates ELT on Morris water maze in 3-NP-induced HD in rats. (B): β-Boswellic acid ameliorates TSTQ on Morris water maze in 3-NP-induced HD in rats. The Bonferroni Two-way ANOVA and Tukey’s one-way ANOVA post-hoc test was used to express significant statistical data as mean ± SD (n = 6); * 3-NP (p < 0.01) versus normal and β-BA perse; # β-BA5 + 3NP, β-BA10 + 3NP, and β-BA15 + 3NP (p < 0.01) versus 3-NP; #@ vitamin E + 3NP (p < 0.01) versus β-BA5 + 3NP, β-BA10 + 3NP, and β-BA15 + 3NP.
3.2.2. β-Boswellic Acid Ameliorates Locomotor Activity in 3-NP-Treated Rats
On the 1st and 15th days of the experimentation schedule, locomotor activity (LA) was carried out to quantify the effect on the movement of β-BA alone and in combination with the standard drug, i.e., vitamin E. Neither the first nor fifteenth day showed any significant difference between the normal and β-BA15 perse groups. When 3-NP was administered to rats for the first time on day 1, no major changes were noticed when compared with the normal and perse groups. However, substantial differences were observed when 3-NP was chronically given over the course of 15 days. A series of β-BA (5, 10, and 15 mg/kg doses) showed a significant improvement in the locomotor activity against 3-NP-induced HD in rats [Two-way ANOVA:F (6, 70) = 97.34, p < 0.01] (Figure 4). On the 15th day of the trial, conventional medication, i.e., vitamin E, showed a considerable restoration in locomotion compared to different dosages of β-BA on the 15th day of the trial.
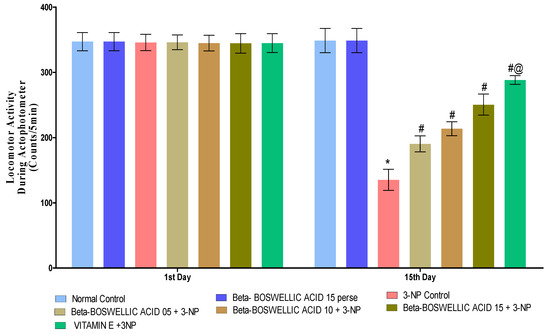
Figure 4.
β-Boswellic acid ameliorates locomotion activity on actophotometer in3-NP-induced HD in rats. The Bonferroni two-way ANOVA and Tukey’s one-way ANOVA post-hoc test was used to express significant statistical data as mean ± SD (n = 6); * 3-NP (p < 0.01) versus normal and β-BA perse; # β-BA5 + 3NP, β-BA10 + 3NP, and β-BA15 + 3NP (p < 0.01) versus 3-NP; #@ vitamin E + 3NP (p < 0.01) versus β-BA5 + 3NP, β-BA10 + 3NP, and β-BA15 + 3NP.
3.2.3. β-Boswellic Acid Ameliorates Grip Strength in 3-NP-Treated Rats
The grip strength task was completed three days after the protocol schedule (1st and 15th). We found no statistically significant differences between the control and β-BA15 perse groups. On the first day following the administration of 3-NP, no significant differences were seen when compared with normal and perse. However, during the chronic treatment of 3-NP, the HD rat’s grip strength significantly decreased on day 15. Grip strength was significantly enhanced in HD rats after they were treated with a series of doses of β-BA [two-way ANOVA:F (12, 105) = 686.96, p < 0.01] (Figure 5). When we compared different doses of β-BA and conventional vitamin E, we found that they improved grip strength.
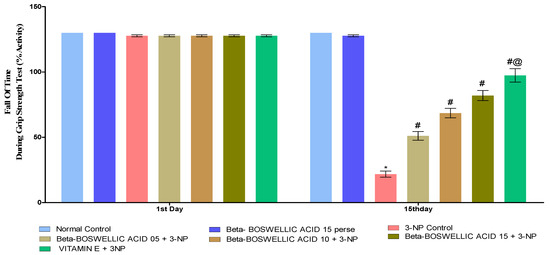
Figure 5.
β-Boswellic acid ameliorates grip strength during grip strength task 3-NP-induced HD in rats. The Bonferroni two-way ANOVA and Tukey’s one-way ANOVA post-hoc test was used to express significant statistical data as mean ± SD (n = 6); * 3-NP (p < 0.01) versus normal and β-BA perse; # β-BA5 + 3NP, β-BA10 + 3NP, and β-BA15 + 3NP (p < 0.01) versus 3-NP; #@ vitamin E+ 3NP (p < 0.01) versus β-BA5 + 3NP, β-BA10 + 3NP, and β-BA15 + 3NP.
3.3. Biochemical Parameters
3.3.1. β Boswellic Acid Ameliorates Mitochondrial ETC Complexes Activity in 3-NP Treated Rats
The activity of mitochondrial enzyme complexes (Complex I, II, and V) in rat brain homogenates was quantified at the end of the protocol schedule. No discernible differences were seen between the normal and β-BA15 perse groups. Mitochondrial enzyme activity was shown to significantly decrease following repeated 3-NP treatment. A substantial increase in mitochondrial enzyme complex I [One-way ANOVA:F (6, 30) = 0.5016, p < 0.01] (Figure 6A), complex II [One-way ANOVA:F (6, 30) = 0.7639, p < 0.01] (Figure 6B) and complex V [One-way ANOVA:F (6, 30) = 0.4812, p < 0.01] (Figure 6C) activity was seen in HD rats after treatment with varying dosages (5, 10, and 15 mg/kg) of β-BA. Treatment with a standard drug, such as vitamin E, has been shown to boost mitochondrial enzyme activity and stop mitochondria from becoming dysfunctional.
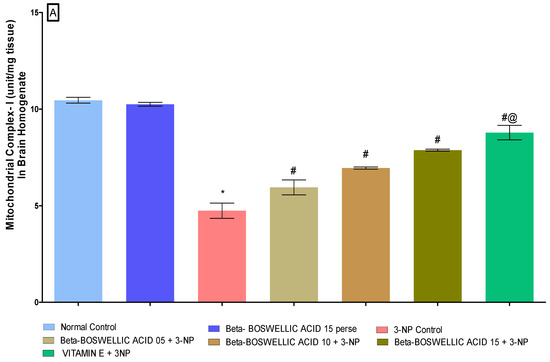
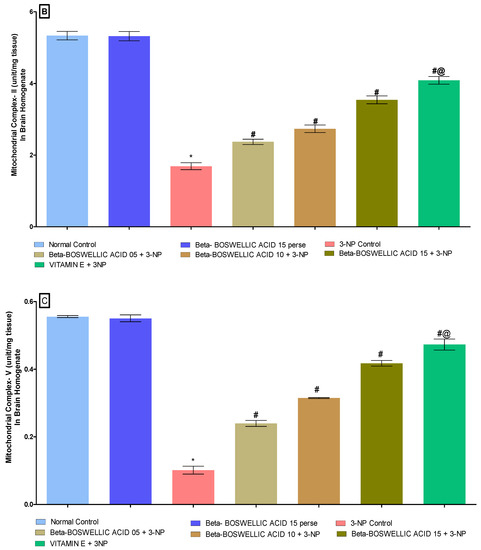
Figure 6.
(A–C): β-Boswellic acid ameliorates mitochondrial ETC complexes activity in brain homogenates of 3-NP-induced HD in rats. The Bonferroni two-way ANOVA and Tukey’s one-way ANOVA post-hoc test was used to express significant statistical data as mean ± SD (n = 6); * 3-NP (p < 0.01) versus normal and β-BA perse; # β-BA5 + 3NP, β-BA10 + 3NP, and β-BA15 + 3NP (p < 0.01) versus 3-NP; #@ vitamin E + 3NP (p < 0.01) versus β-BA5 + 3NP, β-BA10 + 3NP, and β-BA15 + 3NP.
3.3.2. β-Boswellic Acid Ameliorates Inflammatory Markers (TNF-α,& IL-1β) Level in 3-NP Treated Rats
We estimated the level of inflammatory markers (TNF-α and IL-1β) in the rat brain homogenates at the end of the treatment schedule. No statistically significant differences were found in either the normal or the perse group. When rats were given 3-NP for an extended period of time, inflammatory markers increased in comparison to the control and perse groups; however, when the animals were given β-BA, TNF-α [one-way ANOVA:F (6, 30) = 0.6244, p < 0.01] (Figure 7A) and IL-1β [one-way ANOVA:F (6, 30) = 0.1916, p < 0.01] (Figure 7B) levels were significantly reduced and improved in a dose-dependent manner. In rats with 3-NP-induced HD, standard drugs (like vitamin E) helped restore signs of inflammation.
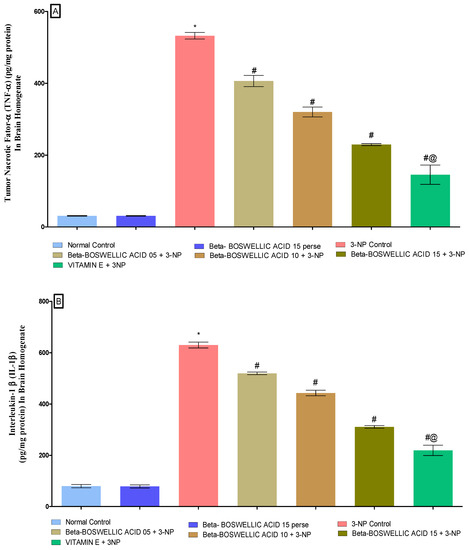
Figure 7.
(A,B): β-Boswellic acid ameliorates inflammatory markers level in brain homogenates of 3-NP-induced HD in rats. The Tukey’s one-way ANOVA post-hoc test was used to express significant statistical data as mean ± SD (n = 6); * 3-NP (p < 0.01) versus normal and β-BA perse; # β-BA5 + 3NP, β-BA10 + 3NP, and β-BA15 + 3NP (p < 0.01) versus 3-NP; #@ vitamin E + 3NP (p < 0.01) versus β-BA5 + 3NP, β-BA10 + 3NP, and β-BA15 + 3NP.
3.3.3. β-Boswellic Acid Ameliorates Neurotransmitter Levels (Dopamine, Glutamate, and GABA) in 3-NP Treated Rats
According to the protocol schedule, neurotransmitter levels were quantified in the brain homogenates of rats at the end of the protocol. There was no difference observed in the normal and β-BA15 perse groups. On the other hand, the chronic administration of 3-NP showed a significant decrease in dopamine (DA) and GABA levels while increasing glutamate levels in HD rats. However, a dose-dependent treatment of β-BA significantly alleviates DA [One-way ANOVA:F (6, 30) = 0.2963, p < 0.01] (Figure 8A) and GABA levels [One-way ANOVA:F (6, 30) = 1.016, p < 0.01] (Figure 8C), whereas it decreases glutamate levels [One-way ANOVA:F (6, 30) = 1.327, p < 0.01] (Figure 8B) of 3-NP treated rats. Standard treatment played a key role in restoring the level of neurotransmitters in rats that had been affected by 3-NP.
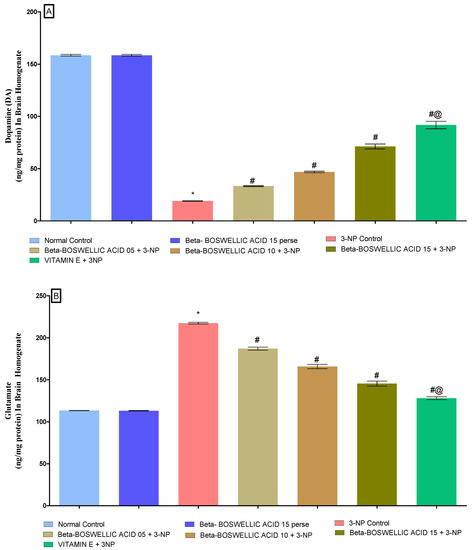
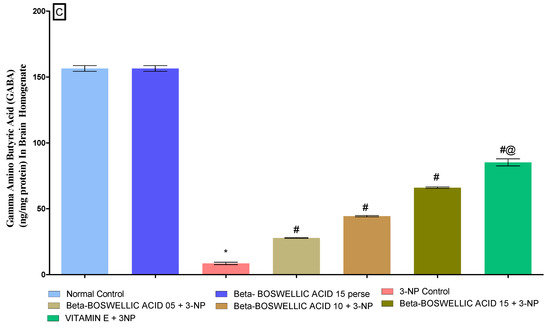
Figure 8.
(A–C): β-Boswellic acid ameliorated neurotransmitter levels in brain homogenates of 3-NP-induced HD in rats. The Tukey’s one-way ANOVA post-hoc test was used to express significant statistical data as mean ± SD (n = 6); * 3-NP (p < 0.01) versus normal and β-BA perse; # β-BA5 + 3NP, β-BA10 + 3NP, and β-BA15 + 3NP (p < 0.01) versus 3-NP; #@ vitamin E + 3NP (p < 0.01) versus β-BA5 + 3NP, β-BA10 + 3NP, and β-BA15 + 3NP.
3.3.4. β-Boswellic Acid Amelioratesoxidative Stress (AChE, MDA, Reduced GSH, SOD, and CAT) Parameters in 3-NP Treated Rats
At the end of the protocol, the levels of oxidative indicators in rat brain homogenates were measured; these included the enzyme acetylcholinesterase (AChE), as well as malondialdehyde (MDA), glutathione (GSH), superoxide dismutase (SOD), and catalase (CAT). No significant differences were found between the normal and β-BA 15 perse groups. However, significant differences were seen after the administration of 3-NP. β-BA also reduced AChE [one-way ANOVA:F (6, 30) = 1.175, p < 0.01] (Figure 9A), MDA [one-way ANOVA: F (6, 30) = 0.5999, p < 0.01] (Figure 9B), GSH [one-way ANOVA: F (6, 30) = 1.003, p < 0.01] (Figure 9C), SOD [One-way ANOVA:F (6, 30) = 1.195, p < 0.01] (Figure 9D), CAT [one-way ANOVA:F (6, 30) = 1.274, p < 0.01] (Figure 9E) level in a dose-dependent manner in 3-NP-induced HD in rats. Compared with the various doses of β-BA, the standard medicine vitamin E efficiently regulated the modulated level of oxidative markers.
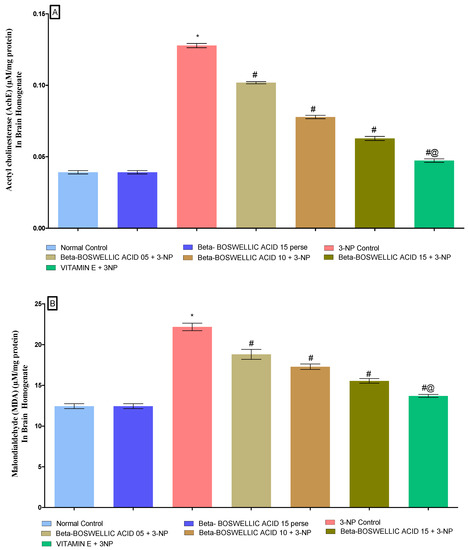
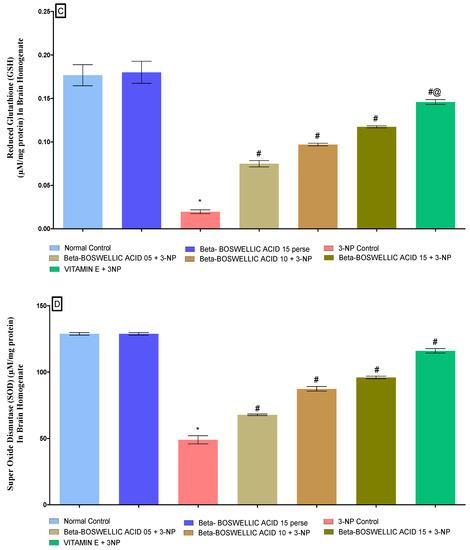
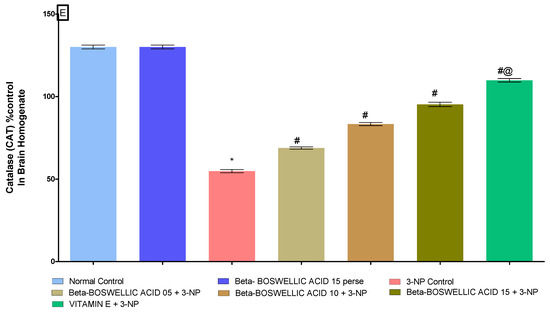
Figure 9.
(A–E): β-Boswellic acid ameliorates the oxidative stress parameters in brain homogenates of 3-NP-induced HD in rats. The Tukey’s one-way ANOVA post-hoc test was used to express significant statistical data as mean ± SD (n = 6); * 3-NP (p < 0.01) versus normal and β-BA perse; # β-BA5 + 3NP, β-BA10 + 3NP, and β-BA15 + 3NP (p < 0.01) versus 3-NP; #@ vitamin E + 3NP (p < 0.01) versus β-BA5 + 3NP, β-BA10 + 3NP, and β-BA15 + 3NP.
3.4. Histopathological Analysis
Effect of β-Boswellic Acid on 3-NP Induced Histopathological Changes in Rat Brain
Histological sections of the cortex and striatum from normal, β-BA- and vitamin E-treated rats revealed properly sized, intact pyramidal-shaped neuronal cells with a clearly visible cell nucleus without vacuolization and a continuous cell membrane without haemorrhage. After a prolonged i.p. injection of 3-NP to develop HD in rats, histopathological alterations were detected. The histological examination of a brain segment treated with 3-nitropropionic acid (cortex and striatum) revealed many neuronal spaces with fuzzy borders and haemorrhages in cerebral tissues. In contrast, the putamen and caudate revealed localized neurodegeneration and necrosis, cell feature loss, and pyramidal shape loss.
β-BA15 mg/kg, p.o. supplementation significantly attenuated 3-NP-induced histological alteration as compared to β-BA5 mg/kg, p.o. and β-BA10 mg/kg, p.o. supplementation. When treated with 3-NP 10 mg/kg + β-BA5 mg/kg, p.o., caudate, and putamen showed focal neurodegeneration and necrosis with a loss of cell details, shape, and size. The caudate and putamen showed focal mild degenerative changes in treatment with 3-NP 10 mg/kg + β-BA10 mg/kg, p.o. The caudate and putamen showed mild focal gliosis in treatment with 3-NP 10 mg/kg + β-BA15 mg/kg, p.o. (Figure 10).
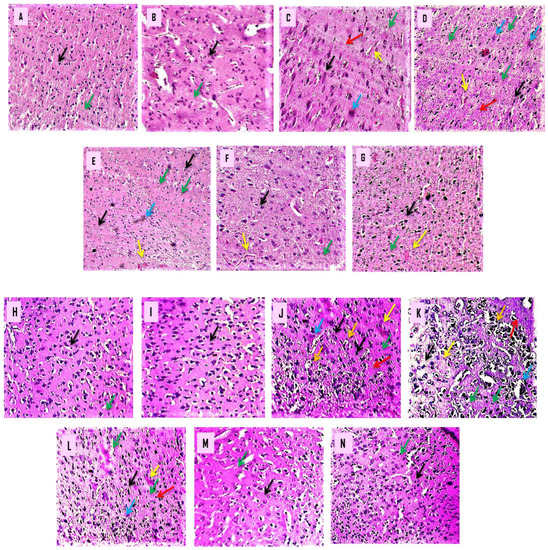
Figure 10.
(A–N): β-Boswellic acid ameliorates the histopathological changes in the brain’s striatum and cortex region after administration of 3-NP in rats to induce HD. The goal of this experiment was to analyse histopathological information of the striatum and cortex sections by using H- and E-stain. After 3-NP delivery into a rat’s brain, striatal and cortical sections revealed the characteristic morphological alterations associated with HD: uneven, flattened cells; cells devoid of nucleus and membrane; bleeding; apoptosis; degeneration; gliosis; and vacuolization of cells. The red arrow represents neuronal cell degeneration, and apoptotic cells are shown with the blue arrow; cortical bleeding is shown with the yellow arrow; gliosis is shown with the green arrow; cell vacuolization is shown with the orange arrow, especially in the cortical section of the brain, and the black arrow displays the form of the cell. (A) This diagram depicts a typical, randomly assigned group of six rats characterized by a high neuronal cell density and a pyramidal shape. (B) It did not show a big difference between the β-BA perse group and the normal group in shape or cell density. (C) Schematic representation of the 3-NP-treated group, which had uneven and flattened cells, cells lacking a nucleus and membrane, haemorrhage, apoptosis, gliosis and vacuolization of the cell (cortical region). (D–F) Diagrams demonstrated that, when compared with the 3-NP-treated group, β-BA 5, β-BA 10, and β-BA 15 significantly reduced irregular and flattened cells, cells without nucleus and membrane, bleeding, apoptotic degeneration, gliosis and vacuolization of a cell (cortical region). (G) As shown in the diagram, the β-BA 5, β-BA 10, and β-BA 15 groups had a lower rate of restoring neurons than the vitamin E. (A magnification scale of 100× was used for morphological studies of brain sections such as the cortex and striatum).
4. Discussion
Our hypothesis stated that -BA has a neuroprotective effect as a CAG triplet repeat inhibitor in 3-NP-induced HD rats by lowering mHtt gene expression and preventing neurodegeneration in medium spiny neurons (MSN). Neurochemical and behavioural alterations were seen after i.p. treatment of 3-NP to rats. We determined that this was due to the neurodegeneration of GABAergic medium spiny neurons (MSN) and CAG repetition in the striatum and cortex.
β-BA is a terpenoid composed of a pentacyclic chain of carbon chains [59]. -BA was synthesised using the herb Boswellia serrate [46]. β-BA has previously been found to be neuroprotective against streptozotocin-induced Alzheimer’s disease, to aid in mitochondrial malfunction recovery, and to regulate behavioural and neurochemical abnormalities [60]. In ALS rats, acetyl-11-keto-beta Boswellic acid improved body weight, memory and cognition, locomotion, and muscle incoordination [61,62]. Clinical data and research revealed that frequent weight loss occurs in the later stages of HD, resulting in chronic fatigue syndrome [63]. In juveniles and adults, 3-NP mimics and produces HD-like behaviour (weight loss, muscular wasting, and skeletal muscle atrophy) [64]. β -BA protects neurochemicals from damage by regulating inflammatory cytokines, neurotransmitters, and oxidative stress [65,66]. Moreover, in current investigations, vitamin E was used as a therapeutic intervention because of its antioxidative effects, and it also helps with neuroprotection [67]. According to our research findings, long-term treatment of 3-NP-induced HD resulted in decreased rat body weight. The return to a normal weight was assisted by both β-BA and vitamin E.
CAG repetition may cause muscle incoordination, muscle wasting, and skeletal muscle atrophy in HD patients [68]. Degeneration and lesion formation in rat cortical and striatal neurons delayed muscle movement and impaired coordination [69]. Chronic 3-NP injection promoted mitochondrial dysfunction, disrupting neurotransmitters that influence motor and muscular activity [21,70]. In our experiment, HD-induced rats treated with β-BA and 3-NP improved their locomotor activity and gripping muscle strength in a dose-dependent manner.
3-NP is a mitochondrial toxin [71,72] that inhibits succinate dehydrogenase (ETC complex II) function, leading to mitochondrial failure and an increase in the CGA repeating cycle, which results in the putamen and caudate neurodegeneration [73,74,75]. Neurodegeneration [76] and mitochondrial failure [77] affect morphology, behaviour, and neurochemistry.
The mitochondrial deficit may result in ROS, which increases oxidative stress [78,79] and lowers antioxidants, resulting in the progression of brain inflammation and proinflammatory cytokines, as well as the deterioration of HD [80]. The neurotoxin 3-NP changes rats’ neurochemistry, behaviour, and morphology after intraperitoneal administration. The neurotoxin 3-NP investigates HD using an animal model [81]. In this work, rats were fed 3-NP daily for 14 days to induce HD-like symptoms. Cognitive and motor impairment are clinical symptoms of Huntington’s disease [82]. Frontostriatal loop neurodegeneration increases glutamate levels, decreasing short-term working memory and causing abnormalities in learning behaviour [83]. Mitochondrial toxin 3-NP produces neurodegeneration in the frontostriatal loop, resulting in cognitive and memory deficits [84]. Our findings suggest that β-BA may benefit rats suffering from memory and cognitive impairments caused by long-term exposure to 3-NP.
Mitochondria are essential in the control of ROS generation and neuroprotective properties [85]. Mitochondrial malfunction increases ROS production and causes neuronal injury [86]. The neurotoxin 3-NP inhibits the activity of the ETC complexes I, II, and V [87,88]. The neurotoxin 3-NP may promote inefficient energy generation and accelerate disease progression. In the process of our investigation, we found that 3-NP caused HD to be produced in the brains of rats. Additionally, the mitochondrial ETC complexes I, II, and V enzyme levels were also determined in rat brain homogenates. Whereas β-BA and vitamin E significantly restored the ETC-complex enzyme level in brain homogenate samples. Proinflammatory cytokines (TNF-α, IL-1β) were activated in response to neurodegeneration and neurotoxicity, increasing the likelihood of disease [89]. Damage to GABAergic MSN caused by 3-NP (as reported in [19,90]) led to increased proinflammatory cytokines. We revealed that a high dose of β-BA significantly reduced the level of proinflammatory cytokines in 3-NP-exposed rats.
Patients with HD may experience changes in their levels of DA, glutamate, and GABA due to neurodegeneration caused by MSN [91]. The neurotoxin 3-NP changes neurotransmitter levels in rats and individuals with Parkinson’s disease [92]. We found that giving rats 3-NP generates neurotransmitter alterations comparable with those observed in HD patients, where a current investigation found that β-BA and vitamin E contributed to restoring the neurotransmitter levels.
Clinical findings show that HD patients have increased oxidative stress (AChEs and MDA) due to reduced antioxidant levels (GSH, SOD, and CAT) related to excessive ROS production [93]. Mitochondrial dysfunction is closely connected to aberrant ROS generation [94]. As previously stated, the mitochondrial toxin 3-NP modulates the amount of oxidative stress [95]. In our investigation, i.p. injections of 3-NP affected the levels of oxidative biomarkers in the striatum, and the cortex was restored by β-BA.
Degeneration of MSN neurons could be seen in the striatum and cortex of HD patients. In HD-induced rats’ brains, H- and E-staining revealed morphological abnormalities, apoptotic cells, cells losing nucleus and membrane, degenerative neurons, and gliosis [96]. The neurotoxin 3-NP caused neurodegeneration and was found in HD patients [97,98].
According to the findings of our research, administering high dosages of β-BA could reverse the histopathological change in 3-NP-toxicated HD rats. Based on the results of our research and the statistics, we believe that high-dose β-BA may be a more effective treatment for HD in further studies. However, the underlying technique must be confirmed using knock-in and knock-out procedures. Cellular and molecular marker studies such as Western blot and immunohistochemistry will almost definitely confirm these findings. According to our findings, conventional medications worked better when paired with beta Boswellic acid and could be used to treat Huntington’s disease. We also proposed an additional study to better understand the relationship between prescribed drugs and HD treatments.
5. Conclusions
In this investigation, we revealed that β-BA exhibited a dose-dependent neuroprotective effect compared with the conventional treatment of vitamin E after 3-NP i.p. injection-induced lesions and neurodegeneration in rats’ brains. Based on our histological findings, we concluded that a dose-dependent administration of β-BA acid could slow the progression of neurodegeneration as well as morphological change in the striatum and cortex. The neurotoxin 3-NP caused neurobehavioral dysfunctions, including motor and cognitive abnormalities, which could be reversed with β-BA therapy, providing additional protection against developing HD-like symptoms. Our findings strongly suggested that β-BA could reduce cytokine production while restoring mitochondrial ETC-complex enzymes, neurotransmitter imbalances, and antioxidant potential. In addition, β-BA has a role in restoring neurobehavioral, neurochemical, cellular, and disease-causing processes regulated by the striatum and the cortex region of the brain. All of these findings suggest that β-BA has clinical potential as a treatment for the pathogenesis of HD.
Author Contributions
Investigation, original draft, writing review, A.K., S.B., S.M. and M.S.; data curation, validation, editing, A.G., T.H.A., A.A., M.A. and A.F.A.; formal analysis, A.S.N. and R.K.; resources, conceptualization, supervision, S.B. and S.M. All data were generated in-house, and no paper mill was used. All authors agree to be accountable for all aspects of this work, ensuring integrity and accuracy. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported and funded by institutional grants from the Institutional Animal Ethics Committee (IAEC) as per the guidelines of the committee for the purpose of control and supervision of experiments on animals (CPCSEA) and the Government of India with registration number 573/PO/Re/S/02/CPCSEA. The authors are thankful to the Researchers Supporting Project number (RSP2022R491), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Institutional Animal Ethics Committee (IAEC) as per the guidelines of the committee for the purpose of control and supervision of experiments on animals (CPCSEA) and the Government of India with registration number 573/PO/Re/S/02/CPCSEA.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analysed during this study are included in this article. There are no separate or additional files.
Acknowledgments
The authors express their gratitude to Sanjeev Thacker, Seth G.L. Bihani S.D. College of Technical Education, Institute of Pharmaceutical sciences and Drug Research, Sri Ganganagar-335001 (Raj.), India, for their incredible vision and support. Authors also express gratitude to Parveen Garg and G.D. Gupta, ISF College of Pharmacy (an Autonomous College), Moga (Punjab), India. The authors are thankful to the Researchers Supporting Project number (RSP2022R491), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ACh | Acetylcholine |
| AChE | Acetyl cholinesterase |
| AD | Alzheimer disease |
| ALS | Amyotrophic lateral sclerosis |
| ANOVA | Analysis of variance |
| ATP | Adenosine triphosphate |
| β-BA | Beta Boswellic acid |
| BG | Basal Ganglia |
| CAG | Cytosine, adenine, guanine |
| CAT | Catalase |
| CNS | Central nervous system |
| DA | Dopamine |
| DNA | Deoxyribonucleic acid |
| DTNB | 5,5′-dithiobis-(2-nitrobenzoic acid) |
| ECD | Electron capture detector |
| EDTA | Ethylenediaminetetraacetic acid |
| ELISA | Enzyme-linked immunosorbent assay |
| ELT | Escape latency test |
| ETC | Electron transport chain |
| GABA | Gamma-amino butyric acid |
| GP | Globus pallidus |
| GSH | Glutathione |
| HD | Huntington diseases |
| H2O2 | Hydrogen peroxide |
| HPLC | High-Performance Liquid Chromatography |
| Htt | Huntigtin gene |
| LA | Locomotor activity |
| 5-LO | 5-lipoxygenase |
| MDA | Malondialdehyde |
| MAPK | Mitogen-activated protein kinase |
| mHtt | Mutated huntigtin gene |
| MSN | Medium spiny neurons |
| MWM | Morris water maze |
| NMDA | N-methyl-D-aspartate |
| 3-NP | 3-Nitropropionic acid |
| PD | Parkinson’s disases |
| Poly Q | Polyglutamine |
| O2• | Superoxide |
| OH | Hydroxyl ion |
| ROS | Reactive oxygen species |
| SLA | Spontaneous locomotor activity |
| SN | Substantianigra |
| SOD | Superoxide dismutase |
| TCA | Trichloroacetic acid |
| TH | Tyrosine hydroxylase |
| TL | Transfer latency |
| TNF-α | Tumour necrosis factor-α |
| TSTQ | Time spent in the target quadrant zone |
References
- Ajitkumar, A.; De Jesus, O. Huntington Disease. In StatPearls [Internet]; StatPearls Publishing: Tampa, FL, USA, 2021. [Google Scholar]
- Warby, S.C.; Visscher, H.; Collins, J.A.; Doty, C.N.; Carter, C.; Butland, S.L.; Hayden, A.R.; Kanazawa, I.; Ross, C.J.; Hayden, M.R. HTT haplotypes contribute to differences in Huntington disease prevalence between Europe and East Asia. Eur. J. Hum. Genet. 2011, 19, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Exuzides, A.; Reddy, S.R.; Chang, E.; Paydar, C.; Yohrling, G. Epidemiology of Huntington’s disease in the United States Medicare and Medicaid Populations. Neuroepidemiology 2022, 3, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Crowell, V.; Houghton, R.; Tomar, A.; Fernandes, T.; Squitieri, F. Modeling Manifest Huntington’s Disease Prevalence Using Diagnosed Incidence and Survival Time. Neuroepidemiology 2021, 55, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.E.; Collins, J.A.; Kay, C.; McDonald, C.; Dolzhenko, E.; Xia, Q.; Bečanović, K.; Drögemöller, B.I.; Semaka, A.; Nguyen, C.M.; et al. Length of uninterrupted CAG, independent of polyglutamine size, results in increased somatic instability, hastening onset of Huntington disease. Am. J. Hum. Genet. 2019, 104, 1116–1126. [Google Scholar] [CrossRef]
- Mehrabi, N.F.; Singh-Bains, M.K.; Waldvogel, H.J.; Faull, R.L. Cortico-Basal Ganglia Interactions in Huntington’s. Ann. Neurodegener. Disord. 2016, 1, 1007. [Google Scholar]
- Mehrabi, N.F.; Waldvogel, H.J.; Tippett, L.J.; Hogg, V.M.; Synek, B.J.; Faull, R.L. Symptom heterogeneity in Huntington’s disease correlates with neuronal degeneration in the cerebral cortex. Neurobiol. Dis. 2016, 96, 67–74. [Google Scholar] [CrossRef]
- Ehrlich, M.E. Huntington’s disease and the striatal medium spiny neuron: Cell-autonomous and non-cell-autonomous mechanisms of disease. Neurotherapeutics 2012, 9, 270–284. [Google Scholar] [CrossRef]
- Waldvogel, H.J.; Kim, E.H.; Tippett, L.J.; Vonsattel, J.P.; Faull, R.L. The neuropathology of Huntington’s disease. Behavioral neurobiology of Huntington’s disease and Parkinson’s disease. Curr. Top. Behav. Neurosci. 2015, 22, 33–80. [Google Scholar]
- Rüb, U.; Seidel, K.; Heinsen, H.; Vonsattel, J.P.; Den Dunnen, W.F.; Korf, H.W. Huntington’s disease (HD): The Neuropathology of a Multisystem Neurodegen-erative Disorder of the Human Brain. Brain Pathol. 2016, 26, 726–740. [Google Scholar] [CrossRef]
- Rosenblatt, A. Neuropsychiatry of Huntington’s disease. Dialogues Clin. Neurosci. 2022, 9, 191–197. [Google Scholar] [CrossRef]
- Testa, C.M.; Jankovic, J. Huntington disease: A quarter century of progress since the gene discovery. J. Neurol. Sci. 2019, 396, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Roth, J. Clinical symptomatology of Huntington’s disease. In Pathology, Pre-Vention and Therapeutics of Neurodegenerative Disease; Springer: Berlin/Heidelberg, Germany, 2019; pp. 117–131. [Google Scholar]
- Beal, M.F.; Brouillet, E.; Jenkins, B.; Henshaw, R.; Rosen, B.; Hyman, B.T. Age-dependent striatal excitotoxic lesions produced by the endogenous mitochondrial inhibitor malonate. J. Neurochem. 1993, 61, 1147–1150. [Google Scholar] [CrossRef] [PubMed]
- Naseri, N.N.; Bonica, J.; Xu, H.; Park, L.C.; Arjomand, J.; Chen, Z.; Gibson, G.E. Novel metabolic abnormalities in the tricarboxylic acid cycle in peripheral cells from Huntington’s disease patients. PLoS ONE 2016, 11, e0160384. [Google Scholar] [CrossRef] [PubMed]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Fukui, K. Reactive oxygen species induce neurite degeneration before induction of cell death. J. Clin. Biochem. Nutr. 2016, 3, 155–159. [Google Scholar] [CrossRef]
- Cirillo, G.; Maggio, N.; Bianco, M.R.; Vollono, C.; Sellitti, S.; Papa, M. Discriminative behavioral assessment unveils remarkable reactive astrocytosis and early molecular correlates in basal ganglia of 3-nitropropionic acid subchronic treated rats. Neurochem. Int. 2010, 56, 152–160. [Google Scholar] [CrossRef]
- Kaur, N.; Jamwal, S.; Gill, H.K.; Bansal, P.K. Animal models of Huntington’s disease. In Animal Models of Neurological Disorders; Springer: Singapore, 2017; pp. 43–57. [Google Scholar]
- Han, I.; You, Y.; Kordower, J.H.; Brady, S.T.; Morfini, G.A. Differential vulnerability of neurons in Huntington’s disease: The role of cell type-specific features. J. Neurochem. 2010, 113, 1073–1091. [Google Scholar] [CrossRef]
- Kumar, P.; Kalonia, H.; Kumar, A. Huntington’s disease: Pathogenesis to animal models. Pharmacol. Rep. 2010, 62, 1–14. [Google Scholar] [CrossRef]
- Johri, A.; Beal, M.F. Antioxidants in Huntington’s disease. Biochim. Bio Phys. (BBA) Mol. Basis Dis. 2012, 1822, 664–674. [Google Scholar] [CrossRef]
- González-Reyes, R.E.; Nava-Mesa, M.O.; Vargas-Sánchez, K.; Ariza-Salamanca, D.; Mora-Muñoz, L. Involvement of astrocytes in Alzheimer’s disease from a neuroinflammatory and oxidative stress perspective. Front. Mol. Neurosci. 2017, 10, 427. [Google Scholar] [CrossRef]
- Silva-Palacios, A.; Colín-González, A.L.; López-Cervantes, S.P.; Zazueta, C.; Lu-na-López, A.; Santamaría, A.; Königsberg, M. Tert-buthylhydroquinone pre-conditioning exerts dual effects in old female rats exposed to 3-nitropropionic acid. Redox Biol. 2017, 12, 610–624. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Mulgund, A.; Alshahrani, S.; Assidi, M.; Abuzenadah, A.M.; Sharma, R.; Sabanegh, E. Reactive oxygen species and sperm DNA damage in infertile men presenting with low level leukocytospermia. Reprod. Biol. Endocrinol. 2014, 12, 126. [Google Scholar] [CrossRef] [PubMed]
- Thangarajan, S.; Ramachandran, S.; Krishnamurthy, P. Chrysin exerts neuro-protective effects against 3-Nitropropionic acid induced behavioral des-pair—Mitochondrial dysfunction and striatal apoptosis via upregulating Bcl-2 gene and downregulating Bax—Bad genes in male wistar rats. Biomed. Pharmacother. 2016, 84, 514–525. [Google Scholar] [PubMed]
- Iram, F.; Khan, S.A.; Husain, A. Phytochemistry and potential therapeutic actions of Boswellic acids: A mini-review. Asian Pac. J. Trop. Biomed. 2017, 7, 513–523. [Google Scholar] [CrossRef]
- Karmarkar, S.W.; Bottum, K.M.; Krager, S.L.; Tischkau, S.A. ERK/MAPK is essential for endogenous neuroprotection in SCN2. 2 cells. PLoS ONE 2011, 6, e23493. [Google Scholar] [CrossRef]
- Al-Yasiry, A.R.; Kiczorowska, B. Frankincense-therapeutic properties. Adv. Hyg. Exp. Med. Postep. Hig. I Med. Dosw. 2016, 70, 380–391. [Google Scholar] [CrossRef]
- Roy, N.K.; Parama, D.; Banik, K.; Bordoloi, D.; Devi, A.K.; Thakur, K.K.; Padmavathi, G.; Shakibaei, M.; Fan, L.; Sethi, G.; et al. An update on pharma-cological potential of boswellic acids against chronic diseases. Int. J. Mol. Sci. 2019, 20, 4101. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, W.; Zhang, Y.; Yu, Q.; Zeng, M.; Gan, J.; Zhang, W.; Jiang, X.; Li, H. Focus on the role of mitochondria in NLRP3 inflammasome activation: A pro-spective target for the treatment of ischemic stroke. Int. J. Mol. Med. 2022, 49, 74. [Google Scholar] [CrossRef]
- Kheradmand, H.; Babaloo, H.; Vojgani, Y.; Mirzakhanlouei, S.; Bayat, N. PCL/gelatin scaffolds and beta-boswellic acid synergistically increase the efficiency of CGR8 stem cells differentiation into dopaminergic neuron: A new paradigm of Parkinson’s disease cell therapy. J. Biomed. Mater. Res. Part A 2021, 109, 562–571. [Google Scholar] [CrossRef]
- Li, C.; He, Q.; Xu, Y.; Lou, H.; Fan, P. Synthesis of 3-O-acetyl-11-keto-β-boswellic acid (AKBA)-derived amides and their mitochondria-targeted antitumor activities. ACS Omega 2022, 7, 9853–9866. [Google Scholar] [CrossRef]
- Haghaei, H.; Soltani, S.; Hosseini, S.A.; Rashidi, M.R.; Karima, S. Boswellic acids as promising leads in drug development against Alzheimer’s disease. Pharm. Sci. 2020, 27, 14–31. [Google Scholar] [CrossRef]
- Stürner, K.H.; Stellmann, J.P.; Dörr, J.; Paul, F.; Friede, T.; Schammler, S.; Reinhardt, S.; Gellissen, S.; Weissflog, G.; Faizy, T.D.; et al. standardised frankincense extract reduces disease activity in relapsing-remitting multiple sclerosis (the SABA phase IIa trial). J. Neurol. Neurosurg. Psychiatry 2018, 89, 330–338. [Google Scholar] [CrossRef]
- Wang, M.; Yu, J.; Yang, Q.; Guo, C.; Zhang, W.; Li, W.; Weng, Y.; Ding, Y.; Wang, J. Beta-Boswellic Acid Protects Against Cerebral Ischemia/Reperfusion Injury via the Protein Kinase C Epsilon/Nuclear Factor Erythroid 2-like 2/Heme Ox-ygenase-1 Pathway. Mol. Neurobiol. 2022, 59, 4242–4256. [Google Scholar] [CrossRef] [PubMed]
- Rajabian, A.; Sadeghnia, H.; Fanoudi, S.; Hosseini, A. Genus Boswellia as a new candidate for neurodegenerative disorders. Iran. J. Basic Med. Sci. 2020, 23, 277. [Google Scholar] [PubMed]
- Ambrogini, P.; Torquato, P.; Bartolini, D.; Albertini, M.C.; Lattanzi, D.; Di Palma, M.; Marinelli, R.; Betti, M.; Minelli, A.; Cuppini, R.; et al. Excitotoxicity, neuroinflammation and oxidant stress as molecular bases of epileptogenesis and epilepsy-derived neurodegeneration: The role of vitamin E. Biochim. Bio Phys. (BBA) Mol. Basis Dis. 2019, 1865, 1098–1112. [Google Scholar] [CrossRef]
- Tarawneh, R.; Galvin, J.E. Potential future neuroprotective therapies for neurodegenerative disorders and stroke. Clin. Geriatr. Med. 2010, 26, 125–147. [Google Scholar] [CrossRef]
- Gupta, R.; Mehan, S.; Sethi, P.; Prajapati, A.; Alshammari, A.; Alharbi, M.; Al-Mazroua, H.A.; Narula, A.S. Smo-Shh agonist Purmorphamine prevents neurobehavioral and neurochemical defects in 8-OH-DPAT-induced experimental model of obsessive-compulsive disorder. Brain Sci. 2022, 12, 342. [Google Scholar] [CrossRef]
- Duggal, P.; Mehan, S. Neuroprotective approach of anti-cancer microtubule stabilizers against tauopathy associated dementia: Current status of clinical and preclinical findings. J. Alzheimer’s Dis. Rep. 2019, 3, 179–218. [Google Scholar] [CrossRef]
- Sharma, A.; Mehan, S. Targeting PI3K-AKT/mTOR signaling in the prevention of autism. Neurochem. Int. 2021, 147, 105067. [Google Scholar] [CrossRef]
- Dudi, R.; Mehan, S. Neuroprotection of brain permeable Forskolin ameliorates behavioral, biochemical and histopatho-logical alterations in rat model of intracerebral hemorrhage. Pharmaspire 2018, 10, 68–86. [Google Scholar]
- Alam, M.M.; Minj, E.; Yadav, R.K.; Mehan, S. Neuroprotective potential of ade-nylcyclase/cAMP/CREB and mitochondrial CoQ10 activator in amyotrophic lateral sclerosis rats. Curr. Bioact. Compd. 2021, 17, 53–69. [Google Scholar] [CrossRef]
- Mehan, S.; Rahi, S.; Tiwari, A.; Kapoor, T.; Rajdev, K.; Sharma, R.; Khera, H.; Kosey, S.; Kukkar, U.; Dudi, R. Adenylatecyclase activator forskolin alleviates intracerebroventricular propionic acid-induced mitochondrial dysfunction of autistic rats. Neural Regen. Res. 2020, 15, 1140. [Google Scholar] [CrossRef] [PubMed]
- Rajdev, K.; Siddiqui, E.M.; Jadaun, K.S.; Mehan, S. Neuroprotective potential of solanesol in a combined model of intracerebral and intraventricular hemor-rhage in rats. IBRO Rep. 2020, 8, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Mehan, S.; Khera, H.; Sharma, R. Neuroprotective Strategies of Blood-Brain Barrier Penetrant “Forskolin” (AC/cAMP/PKA/CREB Activator) to Ameliorate Mitochondrial Dysfunctioning in Neurotoxic Experimental Model of Autism. In Recent Advances in Neurodegeneration; IntechOpen: London, UK, 2019. [Google Scholar]
- Sharma, R.; Rahi, S.; Mehan, S. Neuroprotective potential of solanesol in intracerebroventricular propionic acid induced experimental model of autism: Insights from behavioral and biochemical evidence. Toxicol. Rep. 2019, 6, 1164–1175. [Google Scholar] [CrossRef]
- Siddiqui, E.M.; Mehan, S.; Upadhayay, S.; Khan, A.; Halawi, M.; Halawi, A.A.; Alsaffar, R.M. Neuroprotective efficacy of 4-Hydroxyisoleucine in experimentally induced intracerebral hemorrhage. Saudi J. Biol. Sci. 2021, 28, 6417–6431. [Google Scholar] [CrossRef]
- Shandilya, A.; Mehan, S.; Kumar, S.; Sethi, P.; Narula, A.S.; Alshammari, A.; Alharbi, M.; Alasmari, A.F. Activation of IGF-1/GLP-1 Signalling via 4-Hydroxyisoleucine Prevents Motor Neuron Impairments in Experimental ALS-Rats Exposed to Methylmercury-Induced Neurotoxicity. Molecules 2022, 27, 3878. [Google Scholar] [CrossRef]
- Kaur, R.; Parveen, S.; Mehan, S.; Khanna, D.; Kalra, S. Neuroprotective effect of ellagic acid against chronically scopolamine induced Alzheimer’s type memory and cognitive dysfunctions: Possible behavioural and biochemical evidences. Int. J. Preven. Med. Res. 2015, 1, 45–64. [Google Scholar]
- Rahi, S.; Gupta, R.; Sharma, A.; Mehan, S. Smo-Shh signaling activator purmor-phamine ameliorates neurobehavioral, molecular, and morphological alterations in an intracerebroventricular propionic acid-induced experimental model of autism. Hum. Exp. Toxicol. 2021, 40, 1880–1898. [Google Scholar] [CrossRef]
- Bala, R.; Khanna, D.; Mehan, S.; Kalra, S. Experimental evidence for the potential of lycopene in the management of scopolamine induced amnesia. RSC Adv. 2015, 5, 72881–72892. [Google Scholar] [CrossRef]
- Sahu, R.; Mehan, S.; Kumar, S.; Prajapati, A.; Alshammari, A.; Alharbi, M.; Assiri, M.A.; Narula, A.S. Effect of alpha-mangostin in the prevention of behavioural and neurochemical defects in methylmercury-induced neurotoxicity in ex-perimental rats. Toxicol. Rep. 2022, 9, 977–998. [Google Scholar] [CrossRef] [PubMed]
- Han, X.R.; Wen, X.; Wang, Y.J.; Wang, S.; Shen, M.; Zhang, Z.F.; Fan, S.H.; Shan, Q.; Wang, L.; Li, M.Q.; et al. Effects of CREB1 gene silencing on cognitive dysfunc-tion by mediating PKA-CREB signaling pathway in mice with vascular de-mentia. Mol. Med. 2018, 24, 18. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, R.; Sharma, V.; Mehan, S.; Sharma, N.; Bedi, K.L. Amelioration of in-tracerebroventricularstreptozotocin induced cognitive dysfunction and oxi-dative stress by vinpocetine—A PDE1 inhibitor. Eur. J. Pharmacol. 2009, 620, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Mehan, S.; Parveen, S.; Kalra, S. Adenylcyclase activator forskolin protects against Huntington’s disease-like neurodegenerative disorders. Neural Re-Gener. Res. 2017, 12, 290. [Google Scholar] [CrossRef]
- Khera, R.; Mehan, S.; Bhalla, S.; Kumar, S.; Alshammari, A.; Alharbi, M.; Sadhu, S.S. Guggulsterone mediated JAK/STAT and PPAR-gamma modulation prevents neurobehavioral and neurochemical abnormalities in propionic acid-induced experimental model of autism. Molecules 2022, 27, 889. [Google Scholar] [CrossRef] [PubMed]
- Safayhi, H.; Mack, T.; Sabieraj, J.; Anazodo, M.I.; Subramanian, L.R.; Ammon, H.P. Boswellic acids: Novel, specific, nonredox inhibitors of 5-lipoxygenase. J. Pharmacol. Exp. Ther. 1992, 261, 1143–1146. [Google Scholar]
- Túnez, I.; Tasset, I.; Pérez-De La Cruz, V.; Santamaría, A. 3-Nitropropionic acid as a tool to study the mechanisms involved in Huntington’s disease: Past, present and future. Molecules 2010, 15, 878–916. [Google Scholar] [CrossRef] [PubMed]
- Jinu, A. Elucidation of Role of 3-O-Acetyl-11-Keto-β-Boswellic Acid (AKBA) on Peroxisome Proliferator-Activated Receptor-γ in Scopolamine Induced Cognitive Impairment Model in Rats. Ph.D. Thesis, KMCH College Of Pharmacy, Coimbatore, India, 2020. [Google Scholar]
- Minj, E.; Upadhayay, S.; Mehan, S. Nrf2/HO-1 signaling activator ace-tyl-11-keto-beta Boswellic acid (AKBA)-Mediated Neuroprotection in Methyl Mercury-induced experimental model of ALS. Neurochem. Res. 2021, 46, 2867–2884. [Google Scholar] [CrossRef]
- Aziz, N.A.; Roos, R.A. Characteristics, pathophysiology and clinical manage-ment of weight loss in Huntington’s disease. Neurodegener. Dis. Manag. 2013, 3, 253–266. [Google Scholar] [CrossRef]
- Kim, H.S.; Song, J. Cell therapy in Huntington’s disease. In Progress in Stem Cell Transplantation; IntechOpen: London, UK, 2015; pp. 77–109. [Google Scholar]
- Stürner, K.H.; Verse, N.; Yousef, S.; Martin, R.; Sospedra, M. Boswellic acids reduce T h17 differentiation via blockade of IL-1β-mediated IRAK 1 signaling. Eur. J. Immunol. 2014, 44, 1200–1212. [Google Scholar] [CrossRef]
- Beghelli, D.; Isani, G.; Roncada, P.; Andreani, G.; Bistoni, O.; Bertocchi, M.; Lupidi, G.; Alunnoet, A. Antioxidant and ex vivo immune systemregulatory properties of Boswelliaserrata extracts. Oxidative Med. Cell. Longev. 2017, 2017, 7468064. [Google Scholar] [CrossRef]
- Joshi, Y.B.; Praticò, D. Vitamin E in aging, dementia, and Alzheimer’s disease. Biofactors 2012, 38, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Waters, C.W.; Varuzhanyan, G.; Talmadge, R.J.; Voss, A.A. Huntington disease skeletal muscle is hyperexcitable owing to chloride and potassium channel dysfunction. Proc. Natl. Acad. Sci. USA 2013, 110, 9160–9165. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Levy, S.; Raval, A.P.; Perez-Pinzon, M.A.; Defazio, R.A. Forebrain ischemia triggers GABAergic system degeneration in substantia nigra at chronic stages in rats. Cardiovasc. Psychiatry Neurol. 2010, 2010, 506952. [Google Scholar] [CrossRef] [PubMed]
- Christopherson, K.S.; Bredt, D.S. Nitric oxide in excitable tissues: Physiological roles and disease. J. Clin. Investig. 1997, 100, 2424–2429. [Google Scholar] [CrossRef]
- Beal, M.F.; Brouillet, E.; Jenkins, B.G.; Ferrante, R.J.; Kowall, N.W.; Miller, J.M.; Storey, E.; Srivastava, R.; Rosen, B.R.; Hyman, B.T. Neurochemical and histologic characterization of striatal excitotoxiclesions produced by the mitochondrial toxin 3-nitropropionic acid. J. Neurosci. 1993, 13, 4181–4192. [Google Scholar] [CrossRef] [PubMed]
- Bossi, S.R.; Simpson, J.R.; Isacson, O. Age dependence of striatalneuronal death caused by mitochondrial dysfunction. Neuroreport 1993, 4, 73–76. [Google Scholar] [CrossRef]
- De Moura, M.B.; Dos Santos, L.S.; Van Houten, B. Mitochondrial dysfunction in neurodegenerative diseases and cancer. Environ. Mol. Mutagen. 2010, 51, 391–405. [Google Scholar] [CrossRef]
- Rohlena, J.; Dong, L.F.; Neuzil, J. Targeting the mitochondrial electron transport chain complexes for the induction of apoptosis and cancer treatment. Curr. Pharm. Biotechnol. 2013, 14, 377–389. [Google Scholar] [CrossRef]
- Shasaltaneh, M.D.; Naghdi, N.; Ramezani, S.; Alizadeh, L.; Riazi, G.H. Protection of Beta Boswellic Acid against Streptozotocin-induced Alzheimerʼs Model by Reduction of Tau Phosphorylation Level and Enhancement of Reelin Expression. Planta Med. 2022, 88, 367–379. [Google Scholar] [CrossRef]
- Ahuja, M.; Bishnoi, M.; Copra, K. Protective effect of minocycline, a semisynthetic second generation tetracycline against 3-nitropropionic acid (3-NP)-induced neurotoxicity. Toxicology 2008, 244, 111–122. [Google Scholar] [CrossRef]
- Golpich, M.; Amini, E.; Mohamed, Z.; Azman Ali, R.; Mohamed Ibrahim, N.; Ah-madiani, A. Mitochondrial dysfunction and biogenesis in neurodegenerative diseases: Pathogenesis and treatment. CNS Neurosci. Ther. 2017, 23, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kalonia, H.; Kumar, A. Role of LOX/COX pathways in 3-nitropropionicacid-induced Huntington’s disease-like symptoms in rats: Protective effect of licofenole. Br. J. Pharmacol. 2011, 162, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Montilla, P.; Túnez, I.; Munoz, M.C.; Salcedo, M.; Feijóo, M.; Muñoz-Castañeda, J.R.; Bujalance, I. Effect of glucocorticoids on 3-nitropropionic acid-induced oxidativestressing synaptosomes. Eur. J. Pharmacol. 2004, 488, 19–25. [Google Scholar] [CrossRef]
- Reus, G.Z.; Titus, S.E.; Abelaira, H.M.; Freitas, S.M.; Tuon, T.; Quevedo, J.; Budni, J. Neurochemical correlation between major depressive disorder and neuro-degenerative diseases. Life Sci. 2016, 158, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative stress in neuro-degenerative diseases: From molecular mechanisms to clinical applications. Oxidative Med. Cell Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef]
- Ross, C.A.; Pantelyat, A.; Kogan, J.; Brandt, J. Determinants of functional disability in Huntington’s disease: Role of cognitive and motor dysfunction. Mov. Disord. 2014, 29, 1351–1358. [Google Scholar] [CrossRef]
- Uddin, L.Q. Cognitive and behavioural flexibility: Neural mechanisms and clinical considerations. Nat. Rev. Neurosci. 2021, 22, 167–179. [Google Scholar] [CrossRef]
- Wang, E.A.; Cepeda, C.; Levine, M.S. Cognitive deficits in Huntington’s disease: Insights from animal models. Curr. Transl. Geriatr. Exp. Gerontol. Rep. 2012, 1, 29–38. [Google Scholar] [CrossRef][Green Version]
- Sas, K.; Szabó, E.; Vécsei, L. Mitochondria, oxidative stress and the kynurenine system, with a focus on ageing and neuroprotection. Molecules 2018, 23, 191. [Google Scholar] [CrossRef]
- Jha, S.K.; Jha, N.K.; Kumar, D.; Ambasta, R.K.; Kumar, P. Linking mitochondrial dysfunction, metabolic syndrome and stress signaling in Neuro-degeneration. Biochim. Bio Phys. (BBA) Mol. Basis Dis. 2017, 1863, 1132–1146. [Google Scholar] [CrossRef]
- Chaturvedi, R.K.; Beal, M.F. Mitochondrial approaches for neuroprotection. Ann. N. Y. Acad. Sci. 2008, 1147, 395–412. [Google Scholar] [CrossRef] [PubMed]
- Sandhir, R.; Sood, A.; Mehrotra, A.; Kamboj, S.S. N-Acetylcysteine reverses mito-chondrial dysfunctions and behavioral abnormalities in 3-nitropropionic acid-induced Huntington’s disease. Neurodegener. Dis. 2012, 9, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Pal, A.; Das, S. Terpenoids in Treatment of Neurodegenerative Disease. In Terpenoids Against Human Diseases; CRC Press: Boca Raton, FL, USA, 2019; pp. 95–117. [Google Scholar]
- Cepeda, C.; Murphy, K.P.; Parent, M.; Levine, M.S. The role of dopamine in Huntington’s disease. Prog. Brain Res. 2014, 211, 235–254. [Google Scholar] [PubMed]
- Khan, H.; Ullah, H.; Tundis, R.; Belwal, T.; Devkota, H.P.; Daglia, M.; Cetin, Z.; Saygili, E.I.; Campos, M.D.; Capanoglu, E.; et al. Dietary flavonoids in the management of huntington’s disease: Mechanism and clinical perspective. EFood 2020, 1, 38–52. [Google Scholar] [CrossRef]
- Tabassum, R.; Jeong, N.Y. Potential for therapeutic use of hydrogen sulfide in oxidative stress-induced neurodegenerative diseases. Int. J. Med. Sci. 2019, 16, 1386. [Google Scholar] [CrossRef] [PubMed]
- Cha, M.Y.; Kim, D.K.; Mook-Jung, I. The role of mitochondrial DNA mutation on neurodegenerative diseases. Exp. Mol. Med. 2015, 47, e150. [Google Scholar] [CrossRef]
- Colle, D.; Hartwig, J.M.; Soares, F.A.; Farina, M. Probucol modulates oxidative stress and excitotoxicity in Huntington’s disease models in vitro. Brain Res. Bull. 2012, 87, 397–405. [Google Scholar] [CrossRef]
- Saad, M.A.; Ahmed, M.A.; Elbadawy, N.N.; Abdelkader, N.F. Nano-ivabradine averts behavioral anomalies in Huntington’s disease rat model via modulat-ingRhes/m-tor pathway. Prog. Neuro-Psychopharmacol. Bio-Log. Psychiatry 2021, 111, 110368. [Google Scholar] [CrossRef]
- Elbaz, E.M.; Helmy, H.S.; El-Sahar, A.E.; Saad, M.A.; Sayed, R.H. Lercanidipine boosts the efficacy of mesenchymal stem cell therapy in 3-NP-induced Huntington’s disease model rats via modulation of the calcium/calcineurin/NFATc4 and Wnt/β-catenin signalling pathways. Neuro-Chem. Int. 2019, 131, 104548. [Google Scholar]
- El-Sahar, A.E.; Rastanawi, A.A.; El-Yamany, M.F.; Saad, M.A. Dapagliflozinim-proves behavioral dysfunction of Huntington’s disease in rats via inhibiting apoptosis-related glycolysis. Life Sci. 2020, 257, 118076. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).