Limited Role of Endogenous Vasopressin/Copeptin in Stimulation of ACTH–Cortisol Secretion during Glucagon Stimulation Test in Humans
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
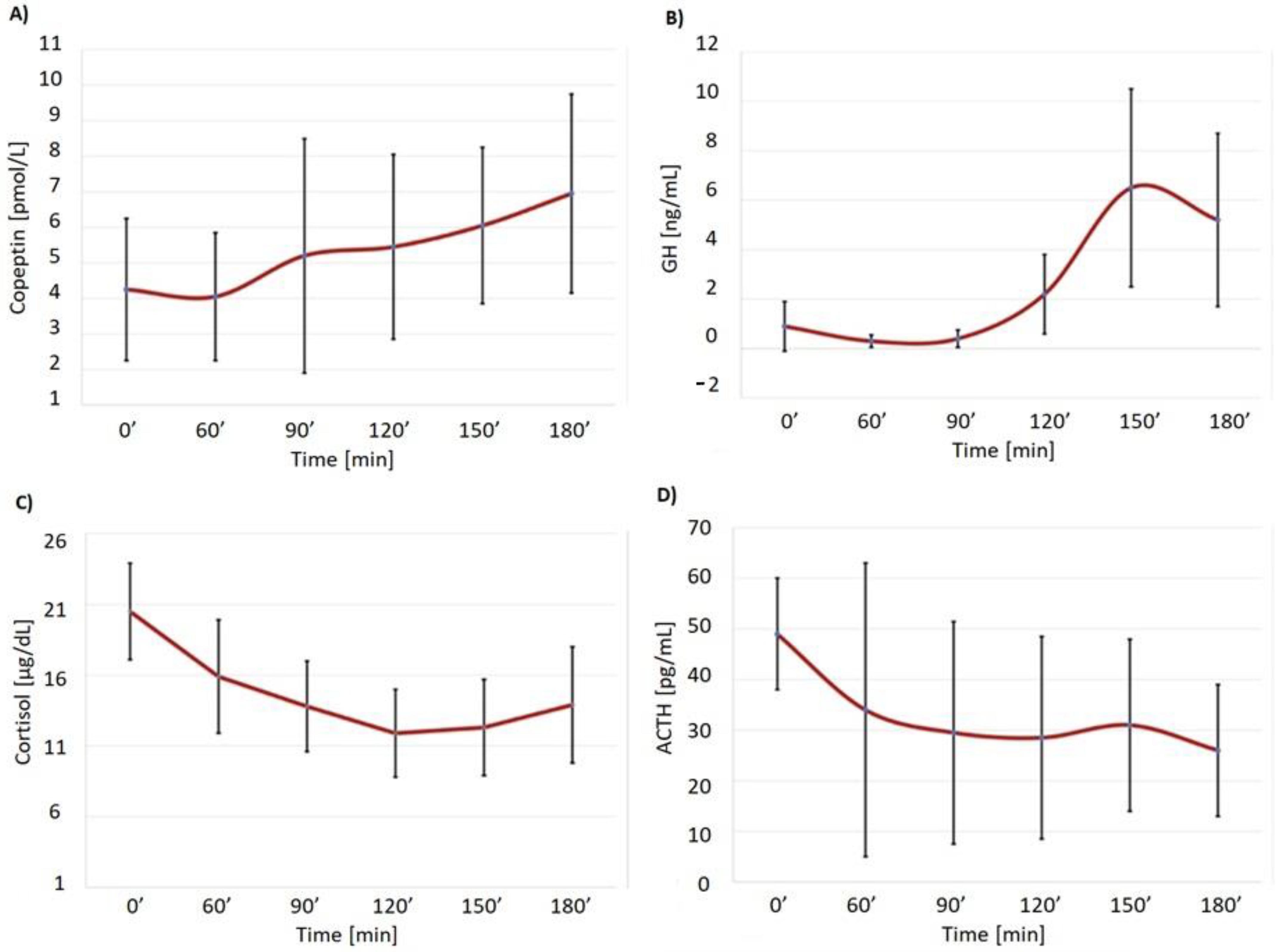
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baylis, P.H.; Zerbe, R.L.; Robertson, G.L. Arginine vasopressin response to insulin-induced hypoglycemia in man. J. Clin. Endocrinol. Metab. 1981, 53, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Morgenthaler, N.G.; Struck, J.; Alonso, C.; Bergmann, A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin. Chem. 2006, 52, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Fenske, W.; Quinkler, M.; Lorenz, D.; Zopf, K.; Haagen, U.; Papassotiriou, J.; Pfeiffer, A.F.; Fassnacht, M.; Störk, S.; Allolio, B. Copeptin in the differential diagnosis of the polydipsia-polyuria syndrome—Revisiting the direct and indirect water deprivation tests. J. Clin. Endocrinol. Metab. 2011, 95, 1506–1515. [Google Scholar] [CrossRef] [PubMed]
- Sailer, C.O.; Refardt, J.; Blum, C.A.; Schnyder, I.; Molina-Tijeras, J.A.; Fenske, W.; Christ-Crain, M. Validity of different copeptin assays in the differential diagnosis of the polyuria-polydipsia syndrome. Sci. Rep. 2021, 11, 10104. [Google Scholar] [CrossRef] [PubMed]
- Katan, M.; Morgenthaler, N.G.; Dixit, K.C.; Rutishauser, J.; Brabant, G.E.; Müller, B.; Christ-Crain, M. Anterior and posterior pituitary function testing with simultaneous insulin tolerance test and a novel copeptin assay. J. Clin. Endocrinol. Metab. 2007, 92, 2640–2643. [Google Scholar] [CrossRef] [PubMed]
- Kacheva, S.; Kolk, K.; Morgenthaler, N.G.; Brabant, G.; Karges, W. Gender-specific co-activation of arginine vasopressin and the hypothalamic-pituitary-adrenal axis during stress. Clin. Endocrinol. 2015, 82, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, K.C.; Lewiński, A.; Skowrońska-Jóźwiak, E.; Stasiak, M.; Horzelski, W.; Brabant, G. Copeptin under glucagon stimulation. Endocrine 2016, 52, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Atila, C.; Gaisl, O.; Voght, D.R.; Werlen, L.; Szinnai, G.; Christ-Crain, M. Glucagon-stimulated copeptin measurements in the differential diagnosis of diabetes insipidus: A double-blind, randomized, placebo-controlled study. Eur. J. Endocrinol. 2022, 187, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, K.C.; Lewiński, A.; Skowrońska-Jóźwiak, E.; Malicka, K.; Horzelski, W.; Brabant, G. Copeptin as a marker of an altered CRH axis in pituitary disease. Endocrine 2017, 57, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.S.; Walker, A.B.; Martin, I.; Wile, D.; Wilding, J.; MacFarlane, I.A. An audit of 500 subcutaneous glucagon stimulation tests to assess growth hormone and ACTH secretion in patients with hypothalamic-pituitary disease. Clin. Endocrinol. 2001, 54, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Yo, W.S.; Toh, L.M.; Brown, S.J.; Howe, W.D.; Henley, D.E.; Lim, E.M. How good is a morning cortisol in predicting an adequate response to intramuscular synacthen stimulation? Clin. Endocrinol. 2014, 81, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Orme, S.M.; Price, A.; Weetman, A.P.; Ross, R.J. Comparison of the diagnostic utility of the simplified and standard i.m. glucagon stimulation test (IMGST). Clin. Endocrinol. 1998, 49, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Childs, G.V.; Westlund, K.N.; Unabia, G. Characterization of anterior pituitary target cells for arginine vasopressin: Including cells that store adrenocorticotropin, thyrotropin-beta and both hormones. Endocrinology 1989, 125, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Sbardella, E.; Isidori, A.M.; Woods, C.P.; Argese, N.; Tomlinson, J.W.; Shine, B.; Jafar-Mohammadi, B.; Grossman, A.B. Baseline morning cortisol as predictor of pituitary-adrenal reserve: A comparison across three assays. Clin. Endocrinol. 2017, 86, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Hamrahian, A.H.; Yuen, K.C.J.; Gordon, M.B.; Pulaski-Liebert, K.J.; Bena, J.; Biller, B.M.K. Revised GH and cortisol cut-points for the glucagon stimulation test in the evaluation of GH and hypothalamic-pituitary-adrenal axes in adults: Results from a prospective randomized multicenter study. Pituitary 2016, 19, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Streeten, D.H.; Andreson, G.H.; Bonaventura, M.M. The potential for serious consequences from misinterpreting normal responses to the rapid adrenocorticotropin test. J. Clin. Endocrinol. Metab. 1996, 81, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Littley, M.D.; Gibson, S.; Shalet, S.M. Comparison of the ACTH and cortisol responses to provocative testing with glucagon and insulin hypoglycaemia in normal subjects. Clin. Endocrinol. 1989, 31, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Yoshida, M.; Kobayashi, Y.; Onodera, M.; Kogusa, K.; Obara, Y. Responses induced by arginine-vasopressin injection in the plasma concentrations of adrenocorticotropic hormone, cortisol, growth hormone and metabolites around weaning time in goats. J. Endocrinol. 2005, 187, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Arvat, E.; Maccagno, B.; Ramunni, J.; Giordano, R.; Broglio, F.; Gianotti, L.; Maccario, M.; Camanni, F.; Ghigo, E. Interaction between glucagon and hexarelin, a peptidyl GH secretagogue, on somatotroph and corticotroph secretion in humans. Eur. J. Endocrinol. 2000, 143, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Chiodera, P.; Gnudi, A.; Bianconi, L.; Camellini, L.; Rossi, G.; Muzzetto, P.; Fagnoni, F.; Schianchi, L.; Volpi, R.; Coiro, V. The infusion of somatostatin reduces the arginine-vasopressin response to insulin-induced hypoglycemia in man. J. Endocrinol. Investig. 1989, 12, 349–353. [Google Scholar] [CrossRef] [PubMed]
| Time (min) | Copeptin (pmol/L) * | GH (ng/mL) ** | ACTH (pg/mL) # | Cortisol (µg/dL) *** | Glucose (mg/dL) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| 0 | 4.35 | 2.62 | 0.87 | 1.47 | 42.34 | 13.67 | 20.48 | 4.9 | 84.2 | 9.14 |
| 60 | 4.13 | 2.32 | 0.34 | 0.34 | 32.13 | 25.86 | 15.79 | 5.53 | 132.3 | 45.4 |
| 90 | 5.29 | 4.49 | 0.46 | 0.53 | 27.43 | 19.32 | 13.64 | 4.46 | 115.2 | 49.1 |
| 120 | 5.42 | 3.56 | 2.19 | 2.10 | 27.59 | 17.85 | 11.82 | 3.97 | 96.0 | 33.3 |
| 150 | 6.13 | 3.09 | 6.42 | 5.72 | 28.96 | 15.49 | 12.50 | 5.10 | 81.5 | 22.9 |
| 180 | 6.93 | 3.80 | 5.10 | 4.86 | 25.60 | 12.60 | 13.75 | 5.89 | 84.1 | 36.7 |
| Patient | Copeptin 0′ (pmol/L) | Copeptin Max (pmol/L) | Increase (%) | Cortisol 0′ (µg/dL) | Cortisol Max 120–180′ (µg/dL) | Increase (%) | GH 0′ (ng/mL) | GH Max 120–180′ (ng/mL) | Increase (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.7 | 6.4 | 137 | 19.63 | 16.81 | −14.4 | 0.15 | 7.44 | 4857 |
| 2 | 5.1 | 8.7 | 70.6 | 26.81 | 17.60 | −34.4 | 3.05 | 9.21 | 202 |
| 3 | 2.0 | 2.2 | 10.0 | 18.15 | 9.36 | −48.4 | 0.09 | 1.02 | 1033 |
| 4 | 2.8 | 3.3 | 17.6 | 18.60 | 7.09 | −61.9 | 0.21 | 4.96 | 2261 |
| 5 | 2.9 | 4.2 | 44.8 | 14.81 | 14.86 | 0.3 | 0.21 | 7.27 | 3361 |
| 6 | 6.2 | 9.0 | 45.2 | 18.75 | 15.34 | −18.2 | 0.12 | 7.18 | 5883 |
| 7 | 6.4 | 16.7 | 160.9 | 19.02 | 17.15 | −9.8 | 0.49 | 5.60 | 1043 |
| 8 | 2.3 | 5.9 | 156.5 | 14.80 | 10.94 | −26.1 | 4.16 | 20.60 | 395 |
| 9 | 10.2 | 11.8 | 15.7 | 25.00 | 17.17 | −31.3 | 0.07 | 8.66 | 12,271 |
| 10 | 2.9 | 12.2 | 320.7 | 29.24 | 28.26 | −3.4 | 0.13 | 2.37 | 1723 |
| Patient | 1 | 2 |
|---|---|---|
| Copeptin 0′ (pmol/L) | 2.5 | 2.3 |
| Copeptin max 30-120′ (pmol/L) | 6.9 | 3.5 |
| Increase (%) | 176 | 52.2 |
| Cortisol 0′ (µg/dL) | 20.45 | 4.23 |
| Cortisol max 30-120′ (µg/dL) | 24.26 | 29.29 |
| Increase (%) | 18.6 | 592 |
| GH 0′ (ng/mL) | 5.15 | 0.05 |
| GH max 30–120′ (ng/mL) | 40.0 | 9.14 |
| Increase (%) | 677 | 18,180 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malicka, K.; Horzelski, W.; Lewiński, A.; Lewandowski, K.C. Limited Role of Endogenous Vasopressin/Copeptin in Stimulation of ACTH–Cortisol Secretion during Glucagon Stimulation Test in Humans. Biomedicines 2022, 10, 2857. https://doi.org/10.3390/biomedicines10112857
Malicka K, Horzelski W, Lewiński A, Lewandowski KC. Limited Role of Endogenous Vasopressin/Copeptin in Stimulation of ACTH–Cortisol Secretion during Glucagon Stimulation Test in Humans. Biomedicines. 2022; 10(11):2857. https://doi.org/10.3390/biomedicines10112857
Chicago/Turabian StyleMalicka, Katarzyna, Wojciech Horzelski, Andrzej Lewiński, and Krzysztof C. Lewandowski. 2022. "Limited Role of Endogenous Vasopressin/Copeptin in Stimulation of ACTH–Cortisol Secretion during Glucagon Stimulation Test in Humans" Biomedicines 10, no. 11: 2857. https://doi.org/10.3390/biomedicines10112857
APA StyleMalicka, K., Horzelski, W., Lewiński, A., & Lewandowski, K. C. (2022). Limited Role of Endogenous Vasopressin/Copeptin in Stimulation of ACTH–Cortisol Secretion during Glucagon Stimulation Test in Humans. Biomedicines, 10(11), 2857. https://doi.org/10.3390/biomedicines10112857






