Is Restless Legs Syndrome De Facto Thyroid Disease?
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Characteristics of the Study Group
3.2. Characteristics and Epidemiology of Restless Legs Syndrome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tipton, P.W.; Wszolek, Z.K. Restless Legs Syndrome and Nocturnal Leg Cramps: A Review and Guide to Diagnosis and Treatment. Pol. Arch. Intern. Med. 2017, 127, 865–872. [Google Scholar] [CrossRef] [Green Version]
- Ohayon, M.M.; O’Hara, R.; Vitiello, M.V. Epidemiology of Restless Legs Syndrome: A Synthesis of the Literature. Sleep Med. Rev. 2012, 16, 283–295. [Google Scholar] [CrossRef] [Green Version]
- Siemiński, M.; Nyka, W.M.; Siemińska-Nitka, A. Zespół Niespokojnych Nóg w Praktyce Ogólnolekarskiej. Forum Med. Rodz. 2008, 2, 132–138. [Google Scholar]
- Seong-Jin, C.; Jin Pyo, C.; Bong-Jim, H.; Hong Jin, J.; Sung Man, C.; Maeng Je, C.; Hochang, B.L. Restless Legs Syndrome in a Community Sample of Korean Adults: Prevalence, Impact on Quality of Life, and Association with DSM-IV Psychiatric Disorders. Sleep 2009, 32, 1069–1078. [Google Scholar]
- Tsuboi, Y.; Imamura, A.; Sugimura, M.; Nakano, S.; Shirakawa, S.; Yamada, T. Prevalence of Restless Legs Syndrome in a Japanese Elderly Population. Park. Relat. Disord. 2009, 15, 598–601. [Google Scholar] [CrossRef]
- Chen, N.H.; Chuang, L.P.; Yang, C.T.; Kushida, C.A.; Hsu, S.C.; Wang, P.C.; Lin, S.W.; Chou, Y.T.; Chen, R.S.; Li, H.Y.; et al. The Prevalence of Restless Legs Syndrome in Taiwanese Adults. Psychiatry Clin. Neurosci. 2010, 64, 170–178. [Google Scholar] [CrossRef]
- Panda, S.; Taly, A.; Sinha, S.; Gururaj, G.; Girish, N.; Nagaraja, D. Sleep-Related Disorders among a Healthy Population in South India. Neurol. India 2012, 60, 68–74. [Google Scholar] [CrossRef]
- Baker, J.; Hung, A. Movement Disorders in Women. Semin. Neurol. 2017, 37, 653–660. [Google Scholar] [CrossRef]
- Seeman, M.V. Why Are Women Prone to Restless Legs Syndrome? Int. J. Environ. Res. Public Health 2020, 17, 368. [Google Scholar] [CrossRef] [Green Version]
- Wichniak, A. Zaburzenia Snu. In Psychiatria. Podręcznik dla Studentów Medycyny; Jarema, M., Rabe-Jabłońska, J., Eds.; PZWL Wydawnictwo Lekarskie: Warsow, Poland, 2011; pp. 303–328. [Google Scholar]
- Sarayloo, F.; Dion, P.A.; Rouleau, G.A. Meis1 and Restless Legs Syndrome: A Comprehensive Review. Front. Neurol. 2019, 10, 935. [Google Scholar] [CrossRef]
- Pereira, J.C.; Pradella-Hallinan, M.; Pessoa, H.d.L. Imbalance between Thyroid Hormones and the Dopaminergic System Might Be Central to the Pathophysiology of Restless Legs Syndrome: A Hypothesis. Clinics 2010, 65, 547–554. [Google Scholar] [CrossRef] [Green Version]
- Pereira, J.C.; Pradella-Hallinan, M. Willis-Ekbom Disease (Restless Legs Syndrome) Pathophysiology: The Imbalance Between Dopamine and Thyroid Hormone Theory. J. Sleep Disord. Ther. 2013, 2, 2–7. [Google Scholar] [CrossRef]
- Pereira, J.C.; da Silva Neto, J.L.P.; Pradella-Hallinan, M. Restless Legs Syndrome in Subjects with a Knee Prosthesis: Evidence That Symptoms Are Generated in the Periphery. Clinics 2011, 66, 1955–1959. [Google Scholar] [CrossRef] [Green Version]
- Allen, R.P.; Picchietti, D.L.; Garcia-Borreguero, D.; Ondo, W.G.; Walters, A.S.; Winkelman, J.W.; Zucconi, M.; Ferri, R.; Trenkwalder, C.; Lee, H.B. Restless Legs Syndrome/Willis–Ekbom Disease Diagnostic Criteria: Updated International Restless Legs Syndrome Study Group (IRLSSG) Consensus Criteria—History, Rationale, Description, and Significance. Sleep Med. 2014, 15, 860–873. [Google Scholar] [CrossRef]
- Tan, E.K.; Ho, S.C.; Eng, P.; Loh, L.M.; Koh, L.; Lum, S.Y.; Teoh, M.L.; Yih, Y.; Khoo, D. Restless Legs Symptoms in Thyroid Disorders. Park. Relat. Disord. 2004, 10, 149–151. [Google Scholar] [CrossRef]
- Ahmed, N.; Kandil, M.; Elfil, M.; Jamal, A.; Koo, B.B. Hypothyroidism in Restless Legs Syndrome. J. Sleep Res. 2021, 30, e13091. [Google Scholar] [CrossRef]
- Geng, C.; Yang, Z.; Kong, X.; Xu, P.; Zhang, H. Association between Thyroid Function and Disease Severity in Restless Legs Syndrome. Front. Neurol. 2022, 13. [Google Scholar] [CrossRef]
- Pradella-Hallinan, M.; Pereira, J.C.; Martins, J.R.M. Restless Legs Syndrome, and Symptoms of Restless Syndrome in Patients with Graves’ Disease: A Cross-Sectional Survey. Clinics 2020, 75, e2140. [Google Scholar] [CrossRef]
- Weinstock, L.B.; Walters, A.S.; Paueksakon, P. Restless Legs Syndrome—Theoretical Roles of Inflammatory and Immune Mechanisms. Sleep Med. Rev. 2012, 16, 341–354. [Google Scholar] [CrossRef]
- Kucuk, A.; Uslu, A.U.; Yilmaz, R.; Salbas, E.; Solak, Y.; Tunc, R. Relationship between Prevalence and Severity of Restless Legs Syndrome and Anaemia in Patients with Systemic Lupus Erythematosus. Int. J. Rheum. Dis. 2017, 20, 469–473. [Google Scholar] [CrossRef]
- Katz, P.; Pedro, S.; Michaud, K. CS-30 Sleep Disturbances among Women with Systemic Lupus Erythematosus (SLE). In Proceedings of the Clinical Sciences, Fourth Biannual Scientific Meeting of the North and South American and Caribbean Lupus Community, Armonk, NY, USA, 13–15 September 2018; Lupus Foundation of America: New York, NY, USA, 2018; pp. A39.2–A40. [Google Scholar]
- Falup-Pecurariu, C.; Enache, A.; Duca, L.; Fotescu, C.; Falup-Pecurariu, O.; Monescu, V.; Diaconu, Ş.; Sirbu, C. Restless Legs Syndrome in Systemic Lupus Erythematosus: A Case control Study. Exp. Ther. Med. 2021, 22, 802. [Google Scholar] [CrossRef]
- Hassan, N.; Pineau, C.A.; Clarke, A.E.; Vinet, E.; Ng, R.; Bernatsky, S. q Systemic Lupus and Risk of Restless Legs Syndrome. J. Rheumatol. 2011, 38, 874–876. [Google Scholar] [CrossRef]
- Taylor-Gjevre, R.M.; Gjevre, J.A.; Skomro, R.; Nair, B. Restless Legs Syndrome in a Rheumatoid Arthritis Patient Cohort. JCR J. Clin. Rheumatol. 2009, 15, 12–15. [Google Scholar] [CrossRef]
- Tekatas, A.; Pamuk, O.N. Increased Frequency of Restless Leg Syndrome in Patients with Ankylosing Spondylitis. Int. J. Rheum. Dis. 2015, 18, 58–62. [Google Scholar] [CrossRef]
- Leverment, S.; Clarke, E.; Wadeley, A.; Sengupta, R. Prevalence and Factors Associated with Disturbed Sleep in Patients with Ankylosing Spondylitis and Non-Radiographic Axial Spondyloarthritis: A Systematic Review. Rheumatol. Int. 2017, 37, 257–271. [Google Scholar] [CrossRef] [Green Version]
- Schell, C.; Schleich, R.; Walker, F.; Yazdi, A.S.; Lerche, H.; Röcken, M.; Axmann, D.; Ghoreschi, K.; Eberle, F.C. Restless Legs Syndrome in Psoriasis: An Unexpected Comorbidity. Eur. J. Dermatol. 2015, 25, 255–260. [Google Scholar] [CrossRef]
- Sandikci, S.C.; Colak, S.; Aydoğan Baykara, R.; Öktem, A.; Cüre, E.; Omma, A.; Kucuk, A. Evaluation of Restless Legs Syndrome and Sleep Disorders in Patients with Psoriatic Arthritis. Z. Rheumatol. 2019, 78, 987–995. [Google Scholar] [CrossRef]
- Moccia, M.; Pellecchia, M.T.; Erro, R.; Zingone, F.; Marelli, S.; Barone, D.G.; Ciacci, C.; Strambi, L.F.; Barone, P. Restless Legs Syndrome Is a Common Feature of Adult Celiac Disease. Mov. Disord. 2010, 25, 877–881. [Google Scholar] [CrossRef]
- Weinstock, L.B.; Walters, A.S.; Mullin, G.E.; Duntley, S.P. Celiac Disease Is Associated with Restless Legs Syndrome. Dig. Dis. Sci. 2010, 55, 1667–1673. [Google Scholar] [CrossRef]
- Staszewski, J.; Pogoda-Wesołowska, A.; Goryszewska, M.; Antoniak, M. Objawy i Schorzenia Neurologiczne w Chorobach Związanych z Glutenem. Neurol. Po Dyplomie 2020, 11, 41–54. [Google Scholar]
- Onalan, A.; Matu, Z.; Pehlivan, M.; Akman, G. Restless Legs Syndrome in Patients with Behçet’s Disease and Multiple Sclerosis: Prevalence, Associated Conditions and Clinical Features. Noro Psikiyatr. Ars 2017, 57, 3–8. [Google Scholar] [CrossRef]
- Greener, M. Recent Studies Highlight the Burden of Comorbidities in Multiple Sclerosis. Br. J. Neurosci. Nurs. 2020, 16, 203–208. [Google Scholar] [CrossRef]
- Vávrová, J.; Kemlink, D.; Šonka, K.; Havrdová, E.; Horáková, D.; Pardini, B.; Müller-Myhsok, B.; Winkelmann, J. Restless Legs Syndrome in Czech Patients with Multiple Sclerosis: An Epidemiological and Genetic Study. Sleep Med. 2012, 13, 848–851. [Google Scholar] [CrossRef]
- Kulkarni, J.; Butler, S.; Riecher-Rössler, A. Estrogens and SERMS as Adjunctive Treatments for Schizophrenia. Front. Neuroendocr. 2019, 53, 100743. [Google Scholar] [CrossRef]
- Knezevic, J.; Starchl, C.; Tmava Berisha, A.; Amrein, K. Thyroid-Gut-Axis: How Does the Microbiota Influence Thyroid Function? Nutrients 2020, 12, 1769. [Google Scholar] [CrossRef]
- O’Keefee, S.T.; Gavin, K.; Lavan, J.N. Iron Status and Restless Legs Syndrome in the Elderly. Age Ageing 1994, 23, 200–203. [Google Scholar] [CrossRef]
- Allen, R.P. The Prevalence and Impact of Restless Legs Syndrome on Patients with Iron Deficiency Anaemia. Am. J. Hematol. 2013, 88, 261–264. [Google Scholar] [CrossRef]
- Trenkwalder, C.; Winkelmann, J.; Oertel, W.; Virgin, G.; Roubert, B.; Mezzacasa, A. Ferric Carboxymaltose in Patients with Restless Legs Syndrome and Nonanemic Iron Deficiency: A Randomized Trial. Mov. Disord. 2017, 32, 1478–1482. [Google Scholar] [CrossRef] [Green Version]
- Allen, R.P.; Picchietti, D.L.; Auerbach, M.; Cho, Y.W.; Connor, J.R.; Earley, C.J.; Garcia-Borreguero, D.; Kotagal, S.; Manconi, M.; Ondo, W.; et al. Evidence-Based and Consensus Clinical Practice Guidelines for the Iron Treatment of Restless Legs Syndrome/Willis-Ekbom Disease in Adults and Children: An IRLSSG Task Force Report. Sleep Med. 2018, 41, 27–44. [Google Scholar] [CrossRef]
- Mizuno, S.; Mihana, T.; Miyaoka, T.; Inagaki, T.; Horiguchi, J. CSF Iron, Ferritin and Transferrin Levels in Restless Legs Syndrome. J. Sleep Res. 2005, 14, 43–47. [Google Scholar] [CrossRef]
- Rabindrakumar, M.S.K.; Pujitha Wickramasinghe, V.; Gooneratne, L.; Arambepola, C.; Senanayake, H.; Thoradeniya, T. The Role of Haematological Indices in Predicting Early Iron Deficiency among Pregnant Women in an Urban Area of Sri Lanka. BMC Hematol. 2018, 18, 37. [Google Scholar] [CrossRef] [PubMed]
- Szczepanek-Parulska, E.; Hernik, A.; Ruchała, M. Anaemia in Thyroid Diseases. Pol. Arch. Intern. Med. 2017, 127, 352–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, S.S.; Mohammed, A.A. Effects of Thyroid Dysfunction on Hematological Parameters: Case Controlled Study. Ann. Med. Surg. 2020, 57, 52–55. [Google Scholar] [CrossRef]
- Bojdo, P.; Gąsiorkiewicz, B.; Koczurkiewcz-Adamczyk, P.; Pękala, E. Rola Stresu Oksydacyjnego w Etiologii Wybranych Chorób Cywilizacyjnych. Biochem. Farm. 2021, 77, 111–120. [Google Scholar]
- Cikrikcioglu, M.A.; Hursitoglu, M.; Erkal, H.; Kınas, B.E.; Sztajzel, J.; Cakirca, M.; Arslan, A.G.; Erek, A.; Halac, G.; Tukek, T. Oxidative Stress and Autonomic Nervous System Functions in Restless Legs Syndrome. Eur. J. Clin. Invest. 2011, 41, 734–742. [Google Scholar] [CrossRef]
- Vizioli, L.; Muscari, S.; Muscari, A. The Relationship of Mean Platelet Volume with the Risk and Prognosis of CardiovascularDiseases. Int. J. Clin. Pract. 2009, 63, 1509–1515. [Google Scholar] [CrossRef]
- Celik, K.; Cikrikcioglu, M.A.; Halac, G.; Kilic, E.; Ayhan, S.; Ozaras, N.; Yildiz, K.; Yildiz, R.S.; Zorlu, M.; Karatoprak, C.; et al. Serum Endocan Levels in Women with Restless Legs Syndrome. Neuropsychiatr. Dis. Treat. 2015, 11, 2919–2925. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.; Park, J.J.; Ryoo, N.Y.; Kim, S.-H.; Song, C.; Han, I.-T.; Hong, C.-G.; Ha, C.K.; Choi, S.H. Syndrome of Inappropriate Antidiuretic Hormone Secretion Associated with Pramipexole in a Patient with Parkinson’s Disease. J. Mov. Disord. 2010, 3, 54–56. [Google Scholar] [CrossRef]
- Cimsir, M.T.; Savas, H.B. Investigation of Biochemical Data in Pregnant Women Diagnosed with Restless Legs Syndrome. J. Clin. Exp. Investig. 2021, 12, em00767. [Google Scholar] [CrossRef]
- Gupta, R.; Gupta, R.; Kumar, N.; Rawat, V.S.; Ulfberg, J.; Allen, R.P. Restless Legs Syndrome among Subjects Having Chronic Liver Disease: A Systematic Review and Meta-Analysis. Sleep Med. Rev. 2021, 58, 101463. [Google Scholar] [CrossRef]
- Franco, R.A.; Ashwathnarayan, R.; Deshpandee, A.; Knox, J.; Daniel, J.; Eastwood, D.; Franco, J.; Saeian, K. The High Prevalence of Restless Legs Syndrome Symptoms in Liver Disease in an Academic-Based Hepatology Practice. J. Clin. Sleep Med. 2008, 4, 45–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riar, S.K.; Greenbaum, L.A.; Bliwise, D.L.; Leu, R.M. Restless Legs Syndrome in Chronic Kidney Disease: Is Iron or Inflammatory Status to Blame? J. Clin. Sleep Med. 2019, 15, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Morse, A.M.; Kothare, S. Seeking the Cause of Restless Legs Syndrome in Chronic Kidney Disease. J. Clin. Sleep Med. 2019, 15, 1559–1560. [Google Scholar] [CrossRef]
- Zhang, L.-Y.; Ma, X.-Y.; Lin, J.; Liu, W.-H.; Guo, W.; Yin, L.; Wang, S.-X.; Li, X.; Li, J.; Jin, L.-L.; et al. Prevalence and Risk Factors of Restless Legs Syndrome in Hemodialysis Patients. Nat. Sci. Sleep 2020, 12, 19–27. [Google Scholar] [CrossRef]
- Mao, S.; Shen, H.; Huang, S.; Zhang, A. Restless Legs Syndrome in Dialysis Patients: A Meta-Analysis. Sleep Med. 2014, 15, 1532–1538. [Google Scholar] [CrossRef]
- De Souza Martins, S.C.; Romão, L.F.; Faria, J.C.; de Holanda Afonso, R.C.; Murray, S.A.; Pellizzon, C.H.; Mercer, J.A.; Cameron, L.-C.; Moura-Neto, V. Effect of Thyroid Hormone T3 on Myosin-Va Expression in the Central Nervous System. Brain Res. 2009, 1275, 1–9. [Google Scholar] [CrossRef]
- Panaite, P.-A.; Barakat-Walter, I. Thyroid Hormone Enhances Transected Axonal Regeneration and Muscle Reinnervation Following Rat Sciatic Nerve Injury. J. Neurosci. Res. 2010, 88, 1751–1763. [Google Scholar] [CrossRef] [PubMed]
- Dezonne, R.S.; Stipursky, J.; Araujo, A.P.B.; Nones, J.; Pavão, M.S.G.; Porcionatto, M.; Gomes, F.C.A. Thyroid Hormone Treated Astrocytes Induce Maturation of Cerebral Cortical Neurons through Modulation of Proteoglycan Levels. Front. Cell. Neurosci. 2013, 7, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Donangelo, I.; Abe, K.; Scremin, O.; Ke, S.; Li, F.; Milanesi, A.; Liu, Y.-Y.; Brent, G.A. Thyroid Hormone Treatment Activates Protective Pathways in Both in Vivo and in Vitro Models of Neuronal Injury. Mol. Cell. Endocrinol. 2017, 452, 120–130. [Google Scholar] [CrossRef]
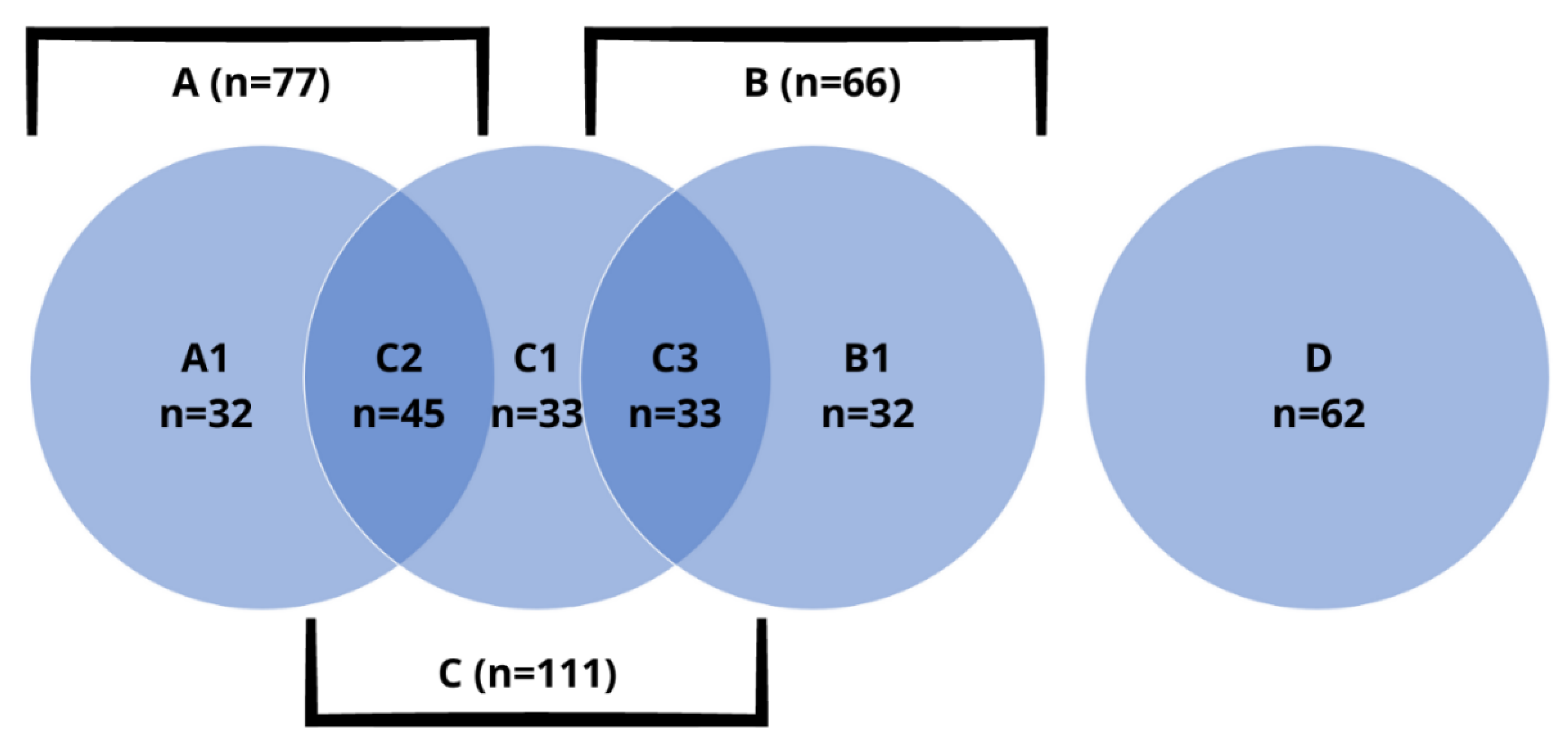
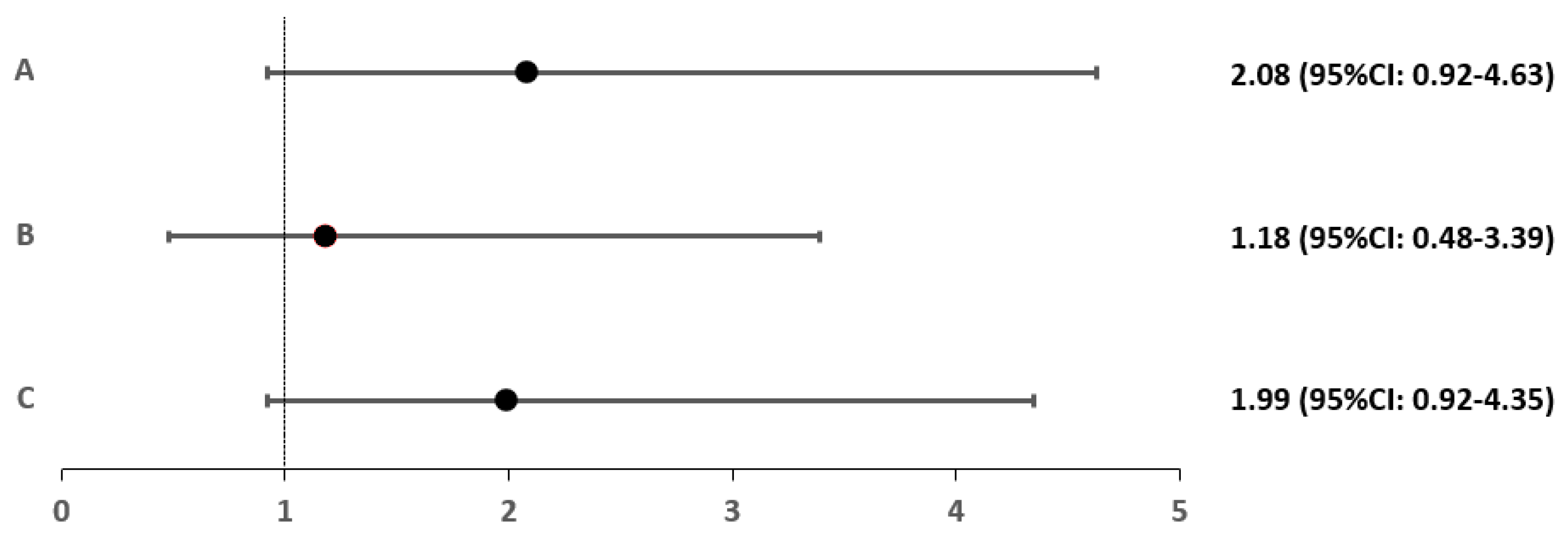

| Subgroup (N) | Sex (Female vs. Male) | Average Age in Years (±SD) | Average BMI in kg/m2 (±SD) |
|---|---|---|---|
| A1 (32) | 81.3% (26) vs. 18.7% (6) | 40.5 ± 10.2 | 29.9 ± 7.1 |
| B1 (45) | 71.1% (32) vs. 28.9% (13) | 38.6 ± 12.8 | 27.6 ± 3.4 |
| C1 (33) | 87.9% (29) vs. 12.1% (4) | 39.3 ± 13.1 | 25.7 ± 5.5 |
| C2 (33) | 93.9% (31) vs. 6.1% (2) | 39.5 ± 12.3 | 25.7 ± 5.7 |
| C3 (32) | 90.6% (29) vs. 9.4% (3) | 36.6 ± 14.4 | 28.3 ± 7.4 |
| D (62) | 41.9% (26) vs. 58.1% (36) | 35.2 ± 14.0 | 25.8 ± 5.7 |
| Parameter | C1 vs. C2 (p Value) | C1 vs. C3 (p Value) | C2 vs. C3 (p Value) |
|---|---|---|---|
| RBC [×106/μL] | ns (p = 0.991) | 4.5 ± 0.3 vs. 4.3 ± 0.3 (p = 0.016) | 4.5 ± 0.5 vs. 4.3 ± 0.3 (p = 0.049) |
| Hematocrit [%] | ns (p = 0.676) | 39.8 ± 3.0 vs. 38.1 ± 2.5 (p = 0.048) | 40.1 ± 3.3 vs. 38.1 ± 2.5 (p = 0.030) |
| PLT [x 103/μL] | ns (p = 0.276) | 274.3 ± 63.34 vs. 236.2 ± 52.5 (p = 0.042) | ns (p = 0.278) |
| RDW-CV [%] | ns (p = 0.836) | 12.8 ± 0.7 vs. 12.3 ± 0.7 (p = 0.040) | 12.9 ± 0.7 vs. 12.3 ± 0.7 (p = 0.015) |
| serum sodium [mmol/L] | ns (p = 0.444) | 143.1 ± 1.1 vs. 141.6 ± 0.7 (p = 0.028) | ns (p = 0.269) |
| TSH [uIU/mL] | 1.5 ± 0.7 vs. 2.1 ± 1.2 (p = 0.018) | ns (p = 0.729) | ns (p = 0.090) |
| Subgroup (N) | Average Thyroid Volume in cm3 (±SD) |
|---|---|
| A1 | 12.0 ± 2.1 |
| B1 | 14.3 ± 6.0 |
| C1 | 16.4 ± 4.5 |
| C2 | 9.3 ± 4.8 |
| C3 | 18.9 ± 9.8 |
| D | 17.6 ± 6.5 |
| Parameter | Patients with RLS | Patients without RLS | p Value |
|---|---|---|---|
| Group A | |||
| thyroid volume [cm3] | 6.1 ± 4.1 | 10.8 ± 4.1 | 0.008 |
| MCHC [g/dL] | 34.4 ± 0.3 | 33.8 ± 0.8 | 0.035 |
| ALT [U/L] | 18.9 ± 8.1 | 13.9 ± 5.4 | 0.041 |
| Group B | |||
| creatinine [mg/dL] | 0.9 ± 0.1 | 0.7 ± 0.2 | 0.002 |
| fT3 [pg/mL] | 2.8 ± 0.2 | 3.3 ± 0.3 | 0.004 |
| Group C | |||
| ALT [U/L] | 18.2 ± 8.8 | 14.4 ± 4.3 | 0.011 |
| AST [U/L] | 25.1 ± 16.9 | 19.5 ± 5.0 | 0.021 |
| calcium [mmol/L] | 2.3 ± 0.1 | 2.2 ± 0.1 | 0.003 |
| ferritin [μg/dL] | 35.6 ± 26.7 | 62.2 ± 49.6 | 0.022 |
| fT3 [pg/mL] | 2.9 ± 0.4 | 3.1 ± 0.3 | 0.009 |
| Group D | |||
| thyroid volume [cm3] | 13.18 ± 4.44 | 18.73 ± 6.37 | 0.026 |
| MCV [fl] | 85.00 ± 3.37 | 88.38 ± 2.83 | 0.006 |
| MCHC [pg] | 28.48 ± 1.48 | 29.81 ± 0.97 | 0.004 |
| PDW [fl] | 14.25 ± 1.36 | 12.53 ± 2.10 | 0.036 |
| magnesium [mmol/L] | 0.69 ± 0.02 | 0.76 ± 0.04 | 0.002 |
| Parameter | Patients with RLS | Patients without RLS | p Value |
|---|---|---|---|
| Subgroup A1 | |||
| ferritin [μg/dL] | 35.4 ± 28.4 | 60.9 ± 50.8 | 0.026 |
| Subgroup B1 | |||
| MCV [fl] | 86.5 ± 3.4 | 88.9 ± 3.9 | 0.023 |
| fT3 [pg/mL] | 2.7 ± 0.3 | 3.2 ± 0.2 | 0.004 |
| Subgroup C1 | |||
| PDW [fl] | 11.9 ± 1.2 | 13.3 ± 1.7 | 0.045 |
| MPV [fl] | 11.0 ± 0.8 | 10.2 ± 0.2 | 0.021 |
| P-LCR [%] | 27.1 ± 3.9 | 33.3 ± 6.3 | 0.019 |
| calcium [mmol/L] | 2.3 ± 0.1 | 2.2 ± 0.1 | 0.001 |
| Subgroup C2 | |||
| thyroid volume [cm3] | 6.1 ± 4.1 | 10.5 ± 4.5 | 0.022 |
| MCHC [g/dL] | 34.4 ± 0.3 | 33.8 ± 0.8 | 0.030 |
| Subgroup C3 | |||
| creatinine [mg/dL] | 0.8 ± 0.1 | 0.6 ± 0.2 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suwała, S.; Rzeszuto, J.; Glonek, R.; Krintus, M.; Junik, R. Is Restless Legs Syndrome De Facto Thyroid Disease? Biomedicines 2022, 10, 2502. https://doi.org/10.3390/biomedicines10102502
Suwała S, Rzeszuto J, Glonek R, Krintus M, Junik R. Is Restless Legs Syndrome De Facto Thyroid Disease? Biomedicines. 2022; 10(10):2502. https://doi.org/10.3390/biomedicines10102502
Chicago/Turabian StyleSuwała, Szymon, Jakub Rzeszuto, Rafał Glonek, Magdalena Krintus, and Roman Junik. 2022. "Is Restless Legs Syndrome De Facto Thyroid Disease?" Biomedicines 10, no. 10: 2502. https://doi.org/10.3390/biomedicines10102502
APA StyleSuwała, S., Rzeszuto, J., Glonek, R., Krintus, M., & Junik, R. (2022). Is Restless Legs Syndrome De Facto Thyroid Disease? Biomedicines, 10(10), 2502. https://doi.org/10.3390/biomedicines10102502






