Combined DiI and Antibody Labeling Reveals Complex Dysgenesis of Hippocampal Dendritic Spines in a Mouse Model of Fragile X Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. DiIC18 Staining
2.3. Immunofluorescence
2.4. Antibodies
2.5. Microscopy and Image Analysis
2.6. Statistical Analysis
3. Results
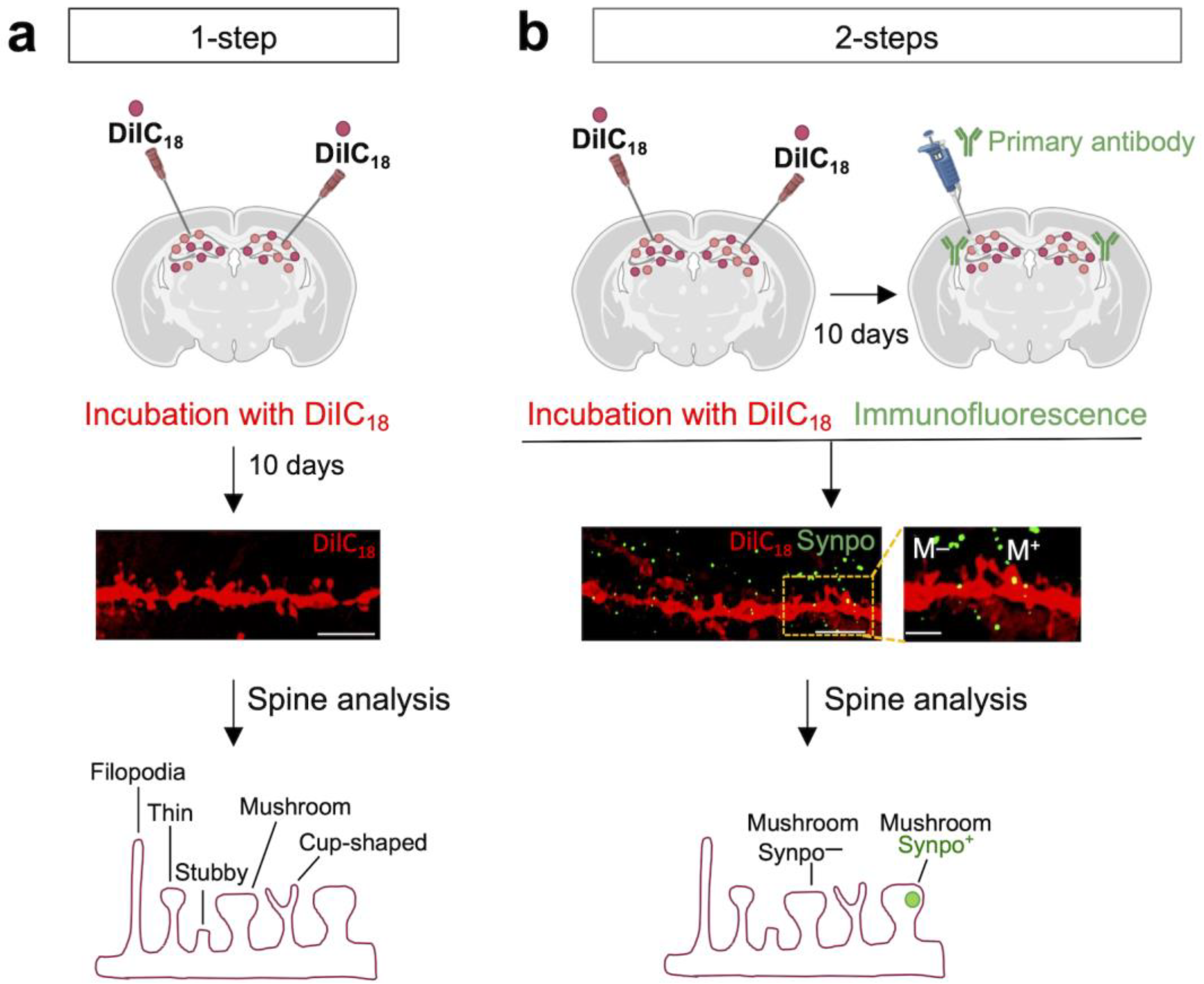
3.1. DiIC18 Combined with Immunolabeling Enables Morphological and Molecular Characterization of Individual Spines
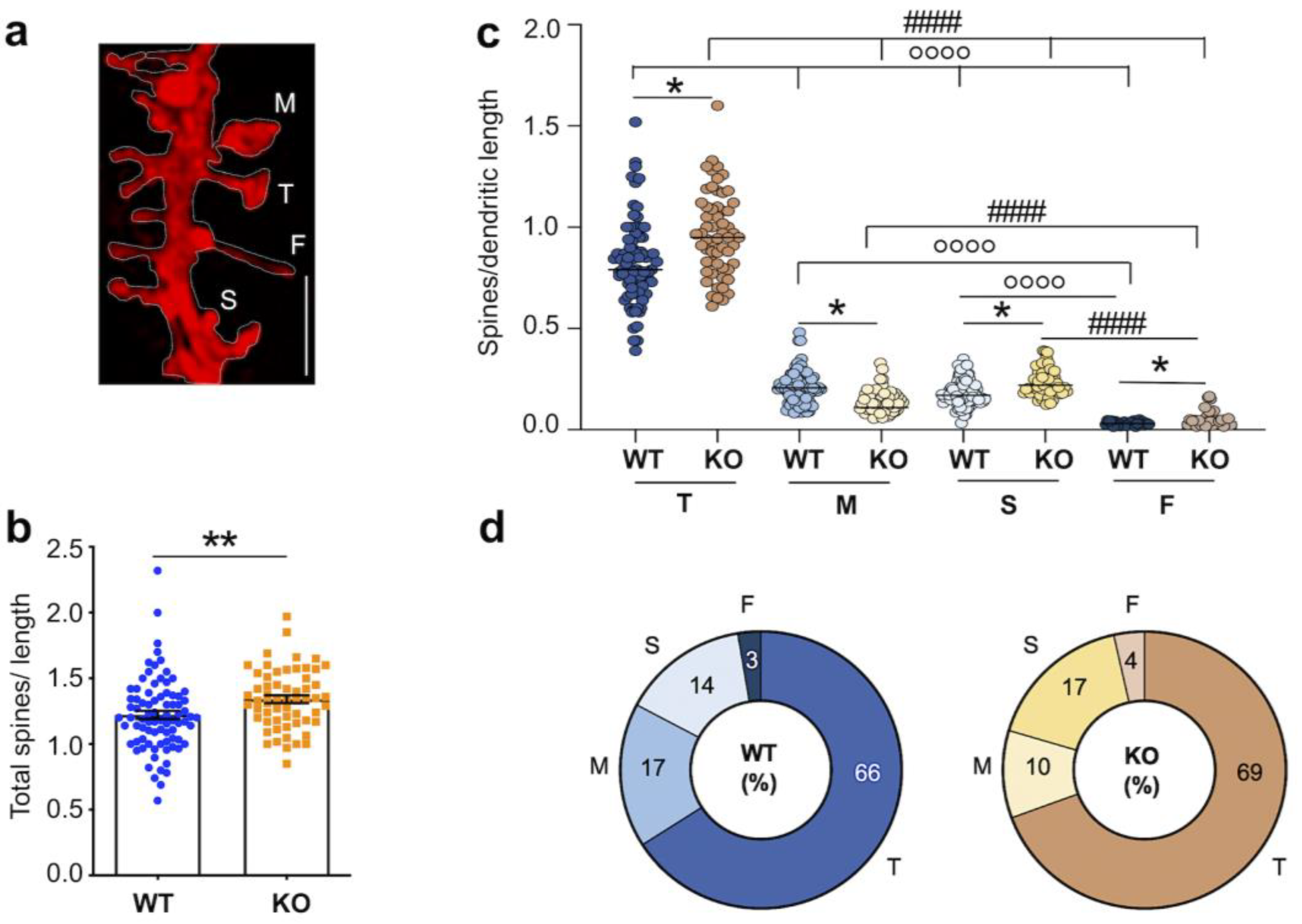
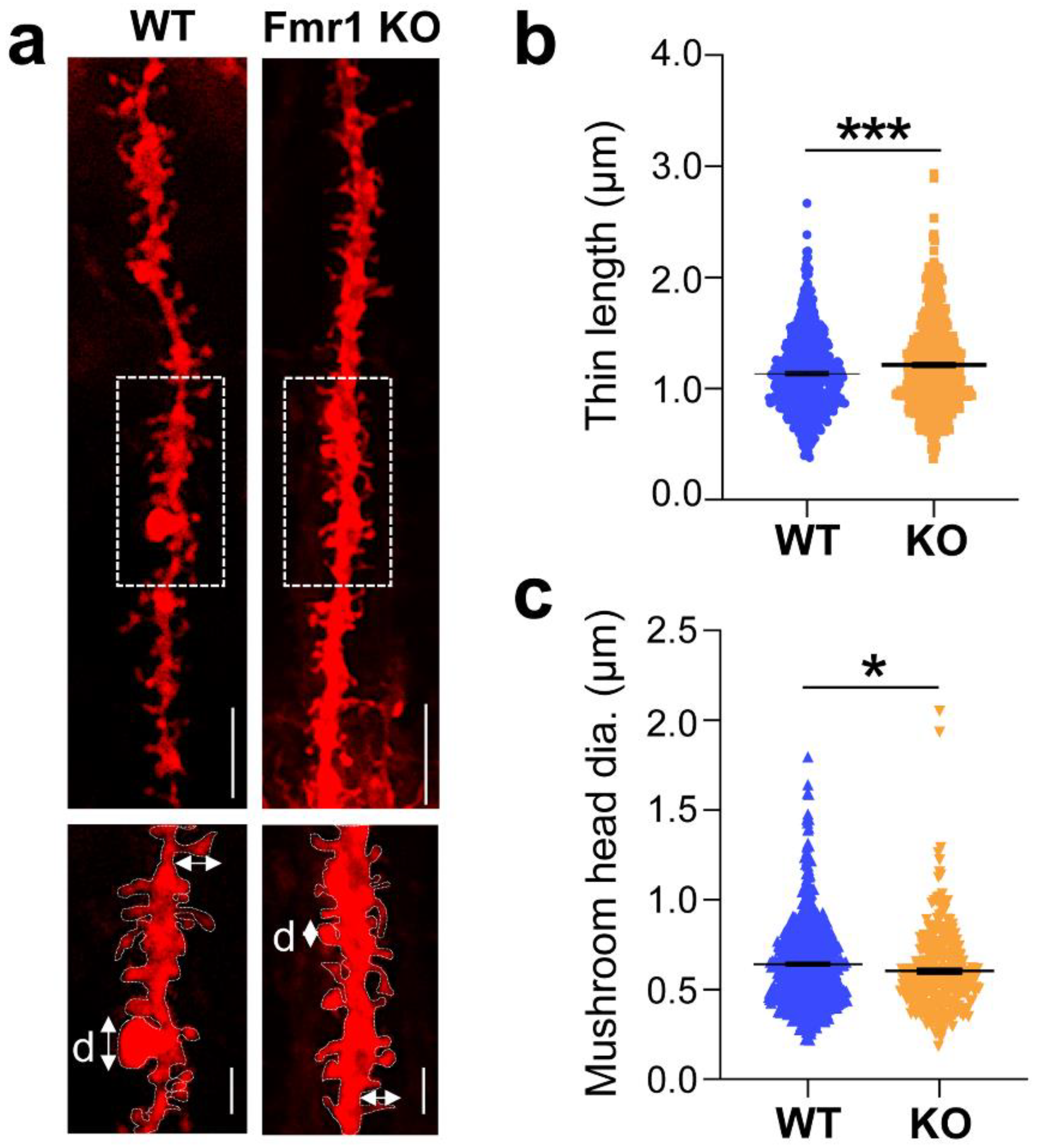
3.2. Dysgenesis of Dendritic Spines in the Hippocampus of Juvenile Fmr1 KO Mice
3.3. Surplus of Mushroom Spines Expressing Synpo in the Hippocampus of Fmr1 KO Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bourne, J.; Harris, K.M. Do Thin Spines Learn to Be Mushroom Spines That Remember? Curr. Opin. Neurobiol. 2007, 17, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Segal, M. Dendritic Spines: Morphological Building Blocks of Memory. Neurobiol. Learn. Mem. 2017, 138, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Penzes, P.; Cahill, M.E.; Jones, K.A.; VanLeeuwen, J.-E.; Woolfrey, K.M. Dendritic Spine Pathology in Neuropsychiatric Disorders. Nat. Neurosci. 2011, 14, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Penzes, P.; Buonanno, A.; Passafaro, M.; Sala, C.; Sweet, R.A. Developmental Vulnerability of Synapses and Circuits Associated with Neuropsychiatric Disorders. J. Neurochem. 2013, 126, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Berry, K.P.; Nedivi, E. Spine Dynamics: Are They All the Same? Neuron 2017, 96, 43–55. [Google Scholar] [CrossRef]
- Grant, S.G.N.; Fransén, E. The Synapse Diversity Dilemma: Molecular Heterogeneity Confounds Studies of Synapse Function. Front. Synaptic Neurosci. 2020, 12, 590403. [Google Scholar] [CrossRef]
- Helm, M.S.; Dankovich, T.M.; Mandad, S.; Rammner, B.; Jähne, S.; Salimi, V.; Koerbs, C.; Leibrandt, R.; Urlaub, H.; Schikorski, T.; et al. A Large-Scale Nanoscopy and Biochemistry Analysis of Postsynaptic Dendritic Spines. Nat. Neurosci. 2021, 24, 1151–1162. [Google Scholar] [CrossRef]
- Cizeron, M.; Qiu, Z.; Koniaris, B.; Gokhale, R.; Komiyama, N.H.; Fransén, E.; Grant, S.G.N. A Brainwide Atlas of Synapses across the Mouse Life Span. Science 2020, 369, 270–275. [Google Scholar] [CrossRef]
- Danielson, E.; Perez de Arce, K.; Cimini, B.; Wamhoff, E.-C.; Singh, S.; Cottrell, J.R.; Carpenter, A.E.; Bathe, M. Molecular Diversity of Glutamatergic and GABAergic Synapses from Multiplexed Fluorescence Imaging. eNeuro 2021, 8, ENEURO.0286-20.2020. [Google Scholar] [CrossRef]
- Venkatesan, S.; Subramaniam, S.; Rajeev, P.; Chopra, Y.; Jose, M.; Nair, D. Differential Scaling of Synaptic Molecules within Functional Zones of an Excitatory Synapse during Homeostatic Plasticity. eNeuro 2020, 7, ENEURO.0407-19.2020. [Google Scholar] [CrossRef]
- Ma, L.; Qiao, Q.; Tsai, J.-W.; Yang, G.; Li, W.; Gan, W.-B. Experience-Dependent Plasticity of Dendritic Spines of Layer 2/3 Pyramidal Neurons in the Mouse Cortex: Experience-Dependent Dendritic Spine Remodeling. Dev. Neurobiol. 2016, 76, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Pignataro, A.; Ammassari-Teule, M. Post-Extinction Selective Persistence of Large Dendritic Spines in Fear Remodeled Circuits May Serve to Reactivate Fear. Curr. Opin. Neurobiol. 2015, 35, 1–5. [Google Scholar] [CrossRef]
- Gisabella, B.; Scammell, T.; Bandaru, S.S.; Saper, C.B. Regulation of Hippocampal Dendritic Spines Following Sleep Deprivation. J. Comp. Neurol. 2020, 528, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lai, C.S.W.; Bai, Y.; Li, W.; Zhao, R.; Yang, G.; Frank, M.G.; Gan, W.-B. REM Sleep Promotes Experience-Dependent Dendritic Spine Elimination in the Mouse Cortex. Nat. Commun. 2020, 11, 4819. [Google Scholar] [CrossRef] [PubMed]
- De Vivo, L.; Bellesi, M.; Marshall, W.; Bushong, E.A.; Ellisman, M.H.; Tononi, G.; Cirelli, C. Ultrastructural Evidence for Synaptic Scaling across the Wake/Sleep Cycle. Science 2017, 355, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.G.N. Synapse Diversity and Synaptome Architecture in Human Genetic Disorders. Hum. Mol. Genet. 2019, 28, R219–R225. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Pozzo-Miller, L. Dendritic Spine Dysgenesis in Autism Related Disorders. Neurosci. Lett. 2015, 601, 30–40. [Google Scholar] [CrossRef]
- Hoover, B.R.; Reed, M.N.; Su, J.; Penrod, R.D.; Kotilinek, L.A.; Grant, M.K.; Pitstick, R.; Carlson, G.A.; Lanier, L.M.; Yuan, L.-L.; et al. Tau Mislocalization to Dendritic Spines Mediates Synaptic Dysfunction Independently of Neurodegeneration. Neuron 2010, 68, 1067–1081. [Google Scholar] [CrossRef]
- Pekala, M.; Doliwa, M.; Kalita, K. Impact of Maternal Immune Activation on Dendritic Spine Development. Dev. Neurobiol. 2021, 81, 524–545. [Google Scholar] [CrossRef]
- Dorostkar, M.M.; Zou, C.; Blazquez-Llorca, L.; Herms, J. Analyzing Dendritic Spine Pathology in Alzheimer’s Disease: Problems and Opportunities. Acta Neuropathol. 2015, 130, 1–19. [Google Scholar] [CrossRef]
- Wang, G.X.; Smith, S.J.; Mourrain, P. Fmr1 KO and Fenobam Treatment Differentially Impact Distinct Synapse Populations of Mouse Neocortex. Neuron 2014, 84, 1273–1286. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shao, L.-X.; Liao, C.; Gregg, I.; Davoudian, P.A.; Savalia, N.K.; Delagarza, K.; Kwan, A.C. Psilocybin Induces Rapid and Persistent Growth of Dendritic Spines in Frontal Cortex In Vivo. Neuron 2021, 109, 2535–2544.e4. [Google Scholar] [CrossRef] [PubMed]
- Phoumthipphavong, V.; Barthas, F.; Hassett, S.; Kwan, A.C. Longitudinal Effects of Ketamine on Dendritic Architecture In Vivo in the Mouse Medial Frontal Cortex. eNeuro 2016, 3, ENEURO.0133-15.2016. [Google Scholar] [CrossRef] [PubMed]
- Staffend, N.A.; Meisel, R.L. DiOlistic Labeling of Neurons in Tissue Slices: A Qualitative and Quantitative Analysis of Methodological Variations. Front. Neuroanat. 2011, 5, 14. [Google Scholar] [CrossRef]
- Cheng, C.; Trzcinski, O.; Doering, L.C. Fluorescent Labeling of Dendritic Spines in Cell Cultures with the Carbocyanine Dye “DiI”. Front. Neuroanat. 2014, 8, 30. [Google Scholar] [CrossRef]
- Matsubayashi, Y.; Iwai, L.; Kawasaki, H. Fluorescent Double-Labeling with Carbocyanine Neuronal Tracing and Immunohistochemistry Using a Cholesterol-Specific Detergent Digitonin. J. Neurosci. Methods 2008, 174, 71–81. [Google Scholar] [CrossRef]
- Trivino-Paredes, J.S.; Nahirney, P.C.; Pinar, C.; Grandes, P.; Christie, B.R. Acute Slice Preparation for Electrophysiology Increases Spine Numbers Equivalently in the Male and Female Juvenile Hippocampus: A DiI Labeling Study. J. Neurophysiol. 2019, 122, 958–969. [Google Scholar] [CrossRef]
- Viggiano, D.; Speranza, L.; Crispino, M.; Bellenchi, G.C.; di Porzio, U.; Iemolo, A.; De Leonibus, E.; Volpicelli, F.; Perrone-Capano, C. Information Content of Dendritic Spines after Motor Learning. Behav. Brain Res. 2018, 336, 256–260. [Google Scholar] [CrossRef]
- Elberger, A.J.; Honig, M.G. Double-Labeling of Tissue Containing the Carbocyanine Dye DiI for Immunocytochemistry. J. Histochem. Cytochem. 1990, 38, 735–739. [Google Scholar] [CrossRef]
- Speranza, L.; Inglebert, Y.; De Sanctis, C.; Wu, P.Y.; Kalinowska, M.; McKinney, R.A.; Francesconi, A. Stabilization of Spine Synaptopodin by MGluR1 Is Required for MGluR-LTD. J. Neurosci. 2022, 42, 1666–1678. [Google Scholar] [CrossRef]
- Hagerman, R.J.; Berry-Kravis, E.; Hazlett, H.C.; Bailey, D.B.; Moine, H.; Kooy, R.F.; Tassone, F.; Gantois, I.; Sonenberg, N.; Mandel, J.L.; et al. Fragile X Syndrome. Nat. Rev. Dis. Prim. 2017, 3, 17065. [Google Scholar] [CrossRef] [PubMed]
- Nobile, V.; Pucci, C.; Chiurazzi, P.; Neri, G.; Tabolacci, E. DNA Methylation, Mechanisms of FMR1 Inactivation and Therapeutic Perspectives for Fragile X Syndrome. Biomolecules 2021, 11, 296. [Google Scholar] [CrossRef]
- Yap, K.; Drakew, A.; Smilovic, D.; Rietsche, M.; Paul, M.H.; Vuksic, M.; Del Turco, D.; Deller, T. The Actin-Modulating Protein Synaptopodin Mediates Long-Term Survival of Dendritic Spines. eLife 2020, 9, e62944. [Google Scholar] [CrossRef] [PubMed]
- Deller, T.; Korte, M.; Chabanis, S.; Drakew, A.; Schwegler, H.; Stefani, G.G.; Zuniga, A.; Schwarz, K.; Bonhoeffer, T.; Zeller, R.; et al. Synaptopodin-Deficient Mice Lack a Spine Apparatus and Show Deficits in Synaptic Plasticity. Proc. Natl. Acad. Sci. USA 2003, 100, 10494–10499. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Honig, M.G.; Hume, R.I. Fluorescent Carbocyanine Dyes Allow Living Neurons of Identified Origin to Be Studied in Long-Term Cultures. J. Cell Biol. 1986, 103, 171–187. [Google Scholar] [CrossRef] [PubMed]
- Honig, M.G.; Hume, R.I. Dil and DiO: Versatile Fluorescent Dyes for Neuronal Labelling and Pathway Tracing. Trends Neurosci. 1989, 12, 333–335, 340–341. [Google Scholar] [CrossRef]
- Sparks, D.L.; Lue, L.-F.; Martin, T.A.; Rogers, J. Neural Tract Tracing Using Di-I: A Review and a New Method to Make Fast Di-I Faster in Human Brain. J. Neurosci. Methods 2000, 103, 3–10. [Google Scholar] [CrossRef]
- Vercelli, A.; Repici, M.; Garbossa, D.; Grimaldi, A. Recent Techniques for Tracing Pathways in the Central Nervous System of Developing and Adult Mammals. Brain Res. Bull. 2000, 51, 11–28. [Google Scholar] [CrossRef]
- Woelfle, S.; Boeckers, T.M. Layer-Specific Vesicular Glutamate Transporter 1 Immunofluorescence Levels Delineate All Layers of the Human Hippocampus Including the Stratum Lucidum. Front. Cell. Neurosci. 2021, 15, 789903. [Google Scholar] [CrossRef]
- Martínez-Cerdeño, V. Dendrite and Spine Modifications in Autism and Related Neurodevelopmental Disorders in Patients and Animal Models: Dendrite and Spine in Autism. Dev. Neurobiol. 2017, 77, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Bagni, C.; Zukin, R.S. A Synaptic Perspective of Fragile X Syndrome and Autism Spectrum Disorders. Neuron 2019, 101, 1070–1088. [Google Scholar] [CrossRef] [PubMed]
- Kasai, H.; Ziv, N.E.; Okazaki, H.; Yagishita, S.; Toyoizumi, T. Spine Dynamics in the Brain, Mental Disorders and Artificial Neural Networks. Nat. Rev. Neurosci. 2021, 22, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Irwin, S.A. Dendritic Spine Structural Anomalies in Fragile-X Mental Retardation Syndrome. Cereb. Cortex 2000, 10, 1038–1044. [Google Scholar] [CrossRef]
- He, C.X.; Portera-Cailliau, C. The Trouble with Spines in Fragile X Syndrome: Density, Maturity and Plasticity. Neuroscience 2013, 251, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Jawaid, S.; Kidd, G.J.; Wang, J.; Swetlik, C.; Dutta, R.; Trapp, B.D. Alterations in CA1 Hippocampal Synapses in a Mouse Model of Fragile X Syndrome. Glia 2018, 66, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Wang, Y.; Liu, D.; Lei, H.; Yang, Z.; Zhang, Z.; Han, M.; Cheng, K.; Chen, Y.; Li, J.; et al. ICAM5 as a Novel Target for Treating Cognitive Impairment in Fragile X Syndrome. J. Neurosci. 2020, 40, 1355–1365. [Google Scholar] [CrossRef]
- Grossman, A.W.; Elisseou, N.M.; McKinney, B.C.; Greenough, W.T. Hippocampal Pyramidal Cells in Adult Fmr1 Knockout Mice Exhibit an Immature-Appearing Profile of Dendritic Spines. Brain Res. 2006, 1084, 158–164. [Google Scholar] [CrossRef]
- Levenga, J.; de Vrij, F.M.S.; Buijsen, R.A.M.; Li, T.; Nieuwenhuizen, I.M.; Pop, A.; Oostra, B.A.; Willemsen, R. Subregion-Specific Dendritic Spine Abnormalities in the Hippocampus of Fmr1 KO Mice. Neurobiol. Learn. Mem. 2011, 95, 467–472. [Google Scholar] [CrossRef]
- Zeiss, C.J. Comparative Milestones in Rodent and Human Postnatal Central Nervous System Development. Toxicol. Pathol. 2021, 49, 1368–1373. [Google Scholar] [CrossRef]
- Chen, H.; Qiao, D.; Wang, C.; Zhang, B.; Wang, Z.; Tang, L.; Wang, Y.; Zhang, R.; Zhang, Y.; Song, L.; et al. Fragile X Mental Retardation Protein Mediates the Effects of Androgen on Hippocampal PSD95 Expression and Dendritic Spines Density/Morphology and Autism-Like Behaviors Through MiR-125a. Front. Cell. Neurosci. 2022, 16, 872347. [Google Scholar] [CrossRef]
- Comery, T.A.; Harris, J.B.; Willems, P.J.; Oostra, B.A.; Irwin, S.A.; Weiler, I.J.; Greenough, W.T. Abnormal Dendritic Spines in Fragile X Knockout Mice: Maturation and Pruning Deficits. Proc. Natl. Acad. Sci. USA 1997, 94, 5401–5404. [Google Scholar] [CrossRef] [PubMed]
- Hering, H.; Sheng, M. Dentritic Spines: Structure, Dynamics and Regulation. Nat. Rev. Neurosci. 2001, 2, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Mundel, P.; Heid, H.W.; Mundel, T.M.; Krüger, M.; Reiser, J.; Kriz, W. Synaptopodin: An Actin-Associated Protein in Telencephalic Dendrites and Renal Podocytes. J. Cell Biol. 1997, 139, 193–204. [Google Scholar] [CrossRef]
- Czarnecki, K.; Haas, C.A.; Bas Orth, C.; Deller, T.; Frotscher, M. Postnatal Development of Synaptopodin Expression in the Rodent Hippocampus. J. Comp. Neurol. 2005, 490, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Holbro, N.; Grunditz, Å.; Oertner, T.G. Differential Distribution of Endoplasmic Reticulum Controls Metabotropic Signaling and Plasticity at Hippocampal Synapses. Proc. Natl. Acad. Sci. USA 2009, 106, 15055–15060. [Google Scholar] [CrossRef]
- Basnayake, K.; Mazaud, D.; Kushnireva, L.; Bemelmans, A.; Rouach, N.; Korkotian, E.; Holcman, D. Nanoscale Molecular Architecture Controls Calcium Diffusion and ER Replenishment in Dendritic Spines. Sci. Adv. 2021, 7, eabh1376. [Google Scholar] [CrossRef]
- Huber, K.M.; Gallagher, S.M.; Warren, S.T.; Bear, M.F. Altered Synaptic Plasticity in a Mouse Model of Fragile X Mental Retardation. Proc. Natl. Acad. Sci. USA 2002, 99, 7746–7750. [Google Scholar] [CrossRef]
- Catania, M.V.; D’Antoni, S.; Bonaccorso, C.M.; Aronica, E.; Bear, M.F.; Nicoletti, F. Group I Metabotropic Glutamate Receptors: A Role in Neurodevelopmental Disorders? Mol. Neurobiol. 2007, 35, 298–307. [Google Scholar] [CrossRef]
- Crispino, M.; Volpicelli, F.; Perrone-Capano, C. Role of the Serotonin Receptor 7 in Brain Plasticity: From Development to Disease. IJMS 2020, 21, 505. [Google Scholar] [CrossRef]
- Bakker, C.E.; Verheij, C.; Willemsen, R.; van der Helm, R.; Oerlemans, F.; Vermey, M.; Bygrave, A.; Hoogeveen, A.; Oostra, B.A.; Reyniers, E.; et al. Fmr1 Knockout Mice: A Model to Study Fragile X Mental Retardation. The Dutch-Belgian Fragile X Consortium. Cell 1994, 78, 23–33. [Google Scholar]
- Bagni, C.; Tassone, F.; Neri, G.; Hagerman, R. Fragile X Syndrome: Causes, Diagnosis, Mechanisms, and Therapeutics. J. Clin. Investig. 2012, 122, 4314–4322. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.G. Synaptopathies: Diseases of the Synaptome. Curr. Opin. Neurobiol. 2012, 22, 522–529. [Google Scholar] [CrossRef]
- Imbriani, P.; Schirinzi, T.; Meringolo, M.; Mercuri, N.B.; Pisani, A. Centrality of Early Synaptopathy in Parkinson’s Disease. Front. Neurol. 2018, 9, 103. [Google Scholar] [CrossRef]
- Shentu, Y.-P.; Huo, Y.; Feng, X.-L.; Gilbert, J.; Zhang, Q.; Liuyang, Z.-Y.; Wang, X.-L.; Wang, G.; Zhou, H.; Wang, X.-C.; et al. CIP2A Causes Tau/APP Phosphorylation, Synaptopathy, and Memory Deficits in Alzheimer’s Disease. Cell Rep. 2018, 24, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Iannuzzi, F.; Frisardi, V.; Annunziato, L.; Matrone, C. Might Fibroblasts from Patients with Alzheimer’s Disease Reflect the Brain Pathology? A Focus on the Increased Phosphorylation of Amyloid Precursor Protein Tyr682 Residue. Brain Sci. 2021, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- Bear, M.F.; Dölen, G.; Osterweil, E.; Nagarajan, N. Fragile X: Translation in Action. Neuropsychopharmacology 2008, 33, 84–87. [Google Scholar] [CrossRef]
- Bagni, C.; Greenough, W.T. From MRNP Trafficking to Spine Dysmorphogenesis: The Roots of Fragile X Syndrome. Nat. Rev. Neurosci. 2005, 6, 376–387. [Google Scholar] [CrossRef]
- Thomazeau, A.; Bosch, M.; Essayan-Perez, S.; Barnes, S.A.; De Jesus-Cortes, H.; Bear, M.F. Dissociation of Functional and Structural Plasticity of Dendritic Spines during NMDAR and MGluR-Dependent Long-Term Synaptic Depression in Wild-Type and Fragile X Model Mice. Mol. Psychiatry 2021, 26, 4652–4669. [Google Scholar] [CrossRef]
- Alberini, C.M.; Travaglia, A. Infantile Amnesia: A Critical Period of Learning to Learn and Remember. J. Neurosci. 2017, 37, 5783–5795. [Google Scholar] [CrossRef]
- Harris, K.M.; Jensen, F.E.; Tsao, B. Three-Dimensional Structure of Dendritic Spines and Synapses in Rat Hippocampus (CA1) at Postnatal Day 15 and Adult Ages: Implications for the Maturation of Synaptic Physiology and Long-Term Potentiation. J. Neurosci. 1992, 12, 2685–2705. [Google Scholar] [CrossRef]
- Sorra, K.E.; Fiala, J.C.; Harris, K.M. Critical Assessment of the Involvement of Perforations, Spinules, and Spine Branching in Hippocampal Synapse Formation. J. Comp. Neurol. 1998, 398, 225–240. [Google Scholar] [CrossRef]
- Sorra, K.E.; Harris, K.M. Overview on the Structure, Composition, Function, Development, and Plasticity of Hippocampal Dendritic Spines. Hippocampus 2000, 10, 501–511. [Google Scholar] [CrossRef]
- Von Bohlen Und Halbach, O. Structure and Function of Dendritic Spines within the Hippocampus. Ann. Anat. 2009, 191, 518–531. [Google Scholar] [CrossRef] [PubMed]
- Berning, S.; Willig, K.I.; Steffens, H.; Dibaj, P.; Hell, S.W. Nanoscopy in a Living Mouse Brain. Science 2012, 335, 551. [Google Scholar] [CrossRef]
- Chazeau, A.; Mehidi, A.; Nair, D.; Gautier, J.J.; Leduc, C.; Chamma, I.; Kage, F.; Kechkar, A.; Thoumine, O.; Rottner, K.; et al. Nanoscale Segregation of Actin Nucleation and Elongation Factors Determines Dendritic Spine Protrusion. EMBO J. 2014, 33, 2745–2764. [Google Scholar] [CrossRef]
- Maiti, P.; Manna, J.; Ilavazhagan, G.; Rossignol, J.; Dunbar, G.L. Molecular Regulation of Dendritic Spine Dynamics and Their Potential Impact on Synaptic Plasticity and Neurological Diseases. Neurosci. Biobehav. Rev. 2015, 59, 208–237. [Google Scholar] [CrossRef]
- Booker, S.A.; Domanski, A.P.F.; Dando, O.R.; Jackson, A.D.; Isaac, J.T.R.; Hardingham, G.E.; Wyllie, D.J.A.; Kind, P.C. Altered Dendritic Spine Function and Integration in a Mouse Model of Fragile X Syndrome. Nat. Commun. 2019, 10, 4813. [Google Scholar] [CrossRef]
- Liu, X.; Kumar, V.; Tsai, N.-P.; Auerbach, B.D. Hyperexcitability and Homeostasis in Fragile X Syndrome. Front. Mol. Neurosci. 2021, 14, 805929. [Google Scholar] [CrossRef]
- Scharkowski, F.; Frotscher, M.; Lutz, D.; Korte, M.; Michaelsen-Preusse, K. Altered Connectivity and Synapse Maturation of the Hippocampal Mossy Fiber Pathway in a Mouse Model of the Fragile X Syndrome. Cereb. Cortex 2018, 28, 852–867. [Google Scholar] [CrossRef]
- Runge, K.; Cardoso, C.; de Chevigny, A. Dendritic Spine Plasticity: Function and Mechanisms. Front. Synaptic Neurosci. 2020, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Bear, M.F.; Huber, K.M.; Warren, S.T. The MGluR Theory of Fragile X Mental Retardation. Trends Neurosci. 2004, 27, 370–377. [Google Scholar] [CrossRef] [PubMed]
- D’Antoni, S.; Spatuzza, M.; Bonaccorso, C.M.; Musumeci, S.A.; Ciranna, L.; Nicoletti, F.; Huber, K.M.; Catania, M.V. Dysregulation of Group-I Metabotropic Glutamate (MGlu) Receptor Mediated Signalling in Disorders Associated with Intellectual Disability and Autism. Neurosci. Biobehav. Rev. 2014, 46 Pt 2, 228–241. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Speranza, L.; Filiz, K.D.; Goebel, S.; Perrone-Capano, C.; Pulcrano, S.; Volpicelli, F.; Francesconi, A. Combined DiI and Antibody Labeling Reveals Complex Dysgenesis of Hippocampal Dendritic Spines in a Mouse Model of Fragile X Syndrome. Biomedicines 2022, 10, 2692. https://doi.org/10.3390/biomedicines10112692
Speranza L, Filiz KD, Goebel S, Perrone-Capano C, Pulcrano S, Volpicelli F, Francesconi A. Combined DiI and Antibody Labeling Reveals Complex Dysgenesis of Hippocampal Dendritic Spines in a Mouse Model of Fragile X Syndrome. Biomedicines. 2022; 10(11):2692. https://doi.org/10.3390/biomedicines10112692
Chicago/Turabian StyleSperanza, Luisa, Kardelen Dalım Filiz, Sarah Goebel, Carla Perrone-Capano, Salvatore Pulcrano, Floriana Volpicelli, and Anna Francesconi. 2022. "Combined DiI and Antibody Labeling Reveals Complex Dysgenesis of Hippocampal Dendritic Spines in a Mouse Model of Fragile X Syndrome" Biomedicines 10, no. 11: 2692. https://doi.org/10.3390/biomedicines10112692
APA StyleSperanza, L., Filiz, K. D., Goebel, S., Perrone-Capano, C., Pulcrano, S., Volpicelli, F., & Francesconi, A. (2022). Combined DiI and Antibody Labeling Reveals Complex Dysgenesis of Hippocampal Dendritic Spines in a Mouse Model of Fragile X Syndrome. Biomedicines, 10(11), 2692. https://doi.org/10.3390/biomedicines10112692




