Inhibition of TRIF-Dependent Inflammation Decelerates Afterload-Induced Myocardial Remodeling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Transaortic Constriction
2.2. Echocardiography
2.3. Langendorff-Working Heart Preparation
2.4. Cell Isolation and Culture
2.5. siRNA-Transfection
2.6. Histology
2.7. Hydroxoproline Assay
2.8. Immunohistochemistry
2.9. Detection of Apoptosis
2.10. RNA Extraction and Real-Time PCR for Gene Expression
2.11. Western Blot Analysis
2.12. Cell Proliferation ELISA
2.13. Statistical Analysis
3. Results
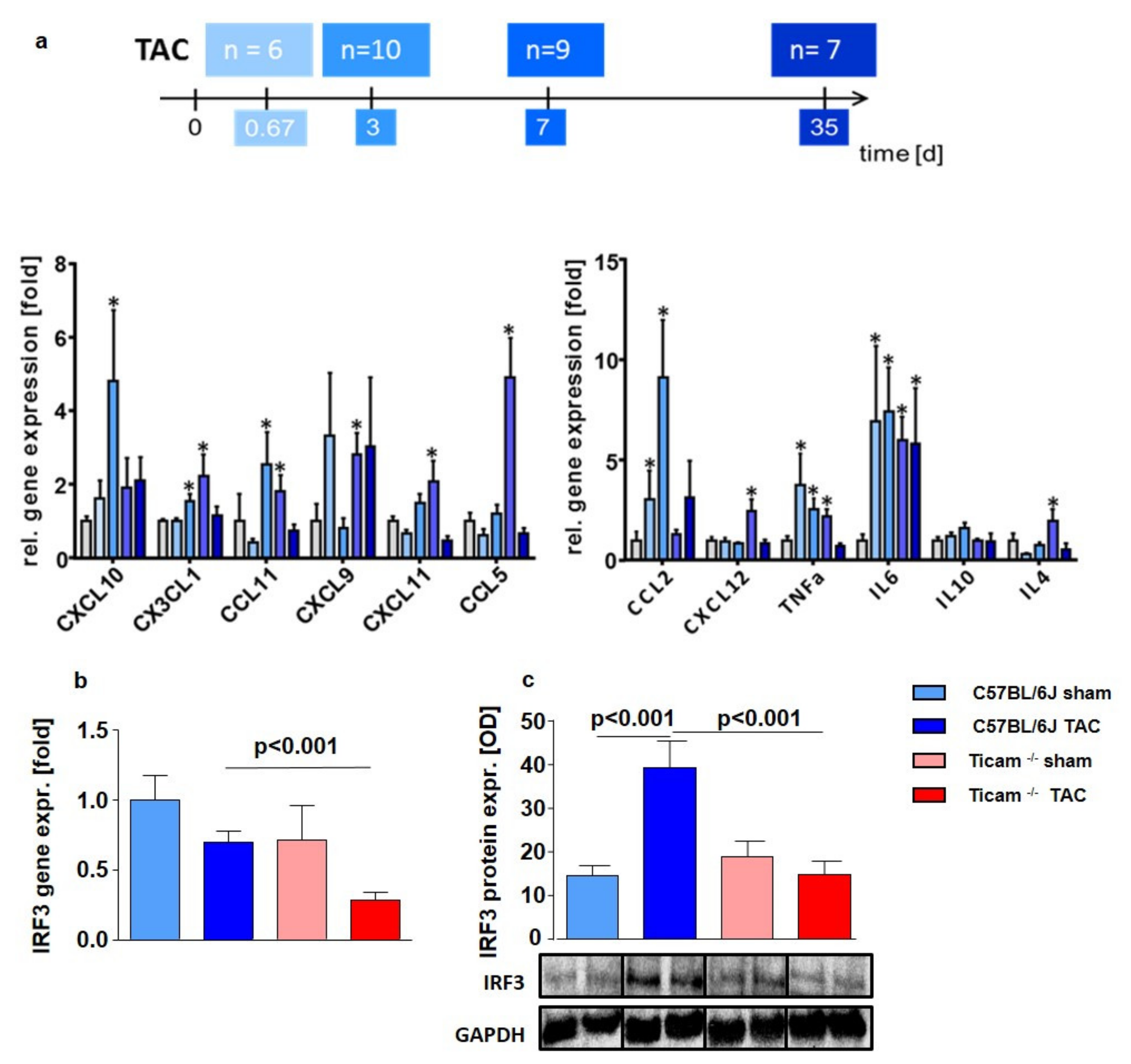
3.1. Time Course of Cytokines Expression
3.2. TRIF-Dependent Cytokine Expression in Heart and LPS Stimulated Monocytes
3.3. Echocardiography
3.4. Working Heart Preparation
3.5. Cardiomyocyte Hypertrophy
3.6. Accumulation of Inflammatory Cell
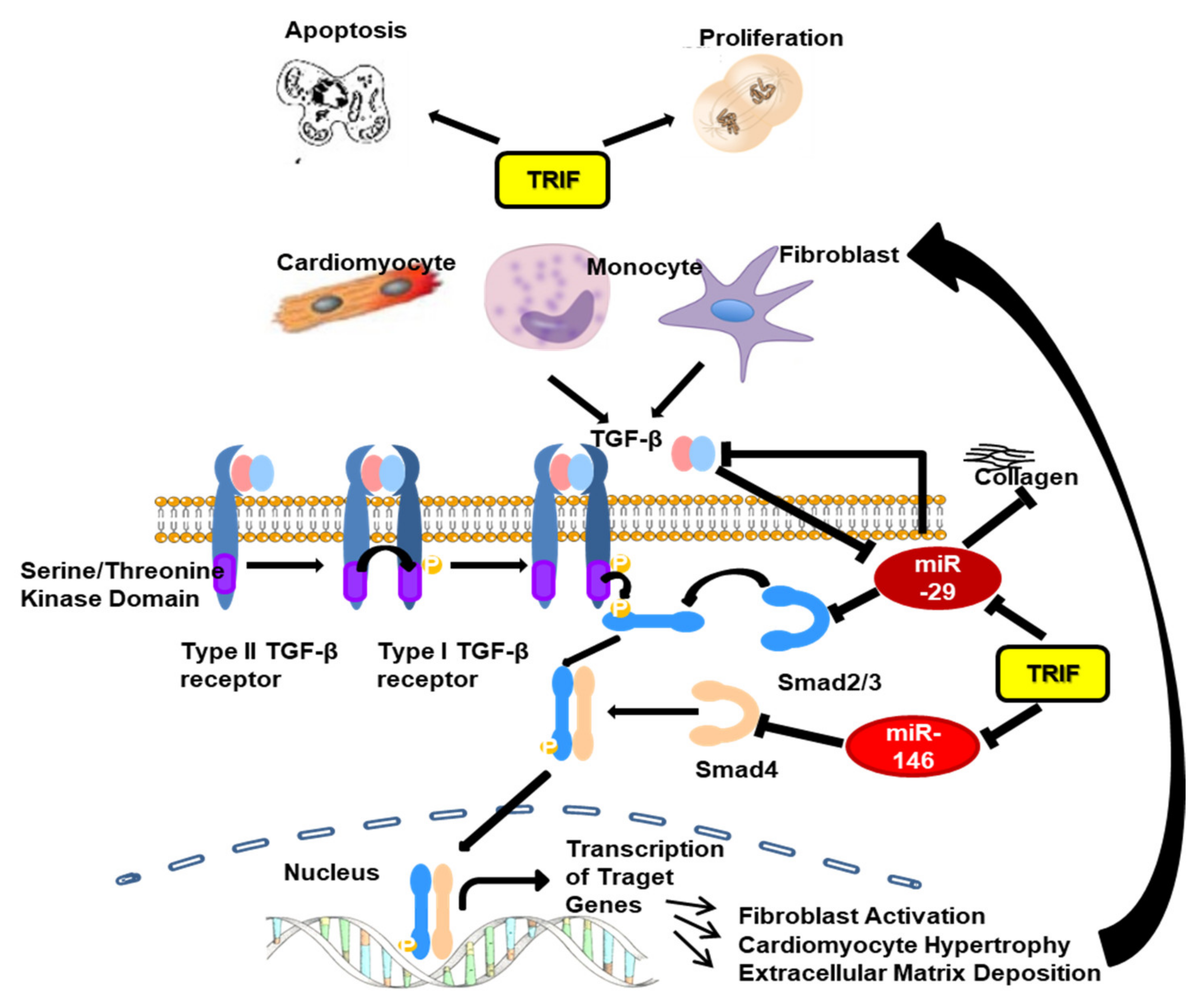
3.7. Fibrosis and TGFβ Signal Pathway
3.8. Fibrosis Associated miRNAs
3.9. Proliferation and Apoptosis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dorn, G.W.; Robbins, J.; Sugden, P.H. Phenotyping Hypertrophy: Eschew Obfuscation. Circ. Res. 2003, 92, 1171–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunter, J.J.; Chien, K.R. Signaling pathways for cardiac hypertrophy and failure. N. Engl. J. Med. 1999, 341, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.; Garrison, R.J.; Savage, D.D.; Kannel, W.B.; Castelli, W.P. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N. Engl. J. Med. 1990, 322, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Rapacciuolo, A.; Naga Prasad, S.V.; Takaoka, H.; Thomas, S.A.; Koch, W.J.; Rockman, H.A. Genetic alterations that inhibit in vivo pressure-overload hypertrophy prevent cardiac dysfunction despite increased wall stress. Circulation 2002, 105, 85–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diwan, A.; Dorn, G.W. Decompensation of cardiac hypertrophy: Cellular mechanisms and novel therapeutic targets. Physiology 2007, 22, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.W.; Shaw, J.A.; Kirshenbaum, L.A. Multiple Facets of NF-κB in the Heart. Circ. Res. 2011, 108, 1122–1132. [Google Scholar] [CrossRef] [Green Version]
- Mann, D.L. Inflammatory mediators and the failing heart: Past, present, and the foreseeable future. Circ. Res. 2002, 91, 988–998. [Google Scholar] [CrossRef]
- Damas, J.K.; Eiken, H.G.; Oie, E.; Bjerkeli, V.; Yndestad, A.; Ueland, T.; Tonnessen, T.; Geiran, O.R.; Aass, H.; Simonsen, S.; et al. Myocardial expression of CC- and CXC-chemokines and their receptors in human end-stage heart failure. Cardiovasc. Res. 2000, 47, 778–787. [Google Scholar] [CrossRef] [Green Version]
- Gullestad, L.; Aukrust, P. Review of trials in chronic heart failure showing broad-spectrum anti-inflammatory approaches. Am. J. Cardiol. 2005, 95, 17C–23C, discussion 38C–40C. [Google Scholar] [CrossRef]
- Everett, B.M.; Cornel, J.; Lainscak, M.; Anker, S.D.; Abbate, A.; Thuren, T.; Libby, P.; Glynn, R.J.; Ridker, P.M. Anti-Inflammatory Therapy with Canakinumab for the Prevention of Hospitalization for Heart Failure. Circulation 2019, 139, 1289–1299. [Google Scholar] [CrossRef]
- Chen, C.; Feng, Y.; Zou, L.; Wang, L.; Chen, H.H.; Cai, J.Y.; Xu, J.M.; Sosnovik, D.E.; Chao, W. Role of extracellular RNA and TLR3-Trif signaling in myocardial ischemia-reperfusion injury. J. Am. Heart Assoc. 2014, 3, e000683. [Google Scholar] [CrossRef] [Green Version]
- Hardarson, H.S.; Baker, J.S.; Yang, Z.; Purevjav, E.; Huang, C.H.; Alexopoulou, L.; Li, N.; Flavell, R.A.; Bowles, N.E.; Vallejo, J.G. Toll-like receptor 3 is an essential component of the innate stress response in virus-induced cardiac injury. American journal of physiology. Heart Circ. Physiol. 2007, 292, H251–H258. [Google Scholar] [CrossRef] [Green Version]
- Riad, A.; Westermann, D.; Zietsch, C.; Savvatis, K.; Becher, P.M.; Bereswill, S.; Heimesaat, M.M.; Lettau, O.; Lassner, D.; Dorner, A.; et al. TRIF is a critical survival factor in viral cardiomyopathy. J. Immunol. 2011, 186, 2561–2570. [Google Scholar] [CrossRef] [Green Version]
- Negishi, H.; Osawa, T.; Ogami, K.; Ouyang, X.; Sakaguchi, S.; Koshiba, R.; Yanai, H.; Seko, Y.; Shitara, H.; Bishop, K.; et al. A critical link between Toll-like receptor 3 and type II interferon signaling pathways in antiviral innate immunity. Proc. Natl. Acad. Sci. USA 2008, 105, 20446–20451. [Google Scholar] [CrossRef] [Green Version]
- Kaczorowski, D.J.; Nakao, A.; Vallabhaneni, R.; Mollen, K.P.; Sugimoto, R.; Kohmoto, J.; Zuckerbraun, B.S.; McCurry, K.R.; Billiar, T.R. Mechanisms of Toll-like receptor 4 (TLR4)-mediated inflammation after cold ischemia/reperfusion in the heart. Transplantation 2009, 87, 1455–1463. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.H.; Ha, T.Z.; Chen, Q.; Li, C.F. Role of MyD88-dependent nuclear factor-kappaB signaling pathway in the development of cardiac hypertrophy in vivo. Zhonghua Yi Xue Za Zhi 2005, 85, 267–272. [Google Scholar]
- Ha, T.; Li, Y.; Hua, F.; Ma, J.; Gao, X.; Kelley, J.; Zhao, A.; Haddad, G.E.; Williams, D.L.; William Browder, I.; et al. Reduced cardiac hypertrophy in toll-like receptor 4-deficient mice following pressure overload. Cardiovasc. Res. 2005, 68, 224–234. [Google Scholar] [CrossRef]
- Hoebe, K.; Du, X.; Georgel, P.; Janssen, E.; Tabeta, K.; Kim, S.; Goode, J.; Lin, P.; Mann, N.; Mudd, S. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature 2003, 424, 743–748. [Google Scholar] [CrossRef]
- Custodis, F.; Eberl, M.; Kilter, H.; Bohm, M.; Laufs, U. Association of RhoGDIalpha with Rac1 GTPase mediates free radical production during myocardial hypertrophy. Cardiovasc. Res. 2006, 71, 342–351. [Google Scholar] [CrossRef]
- Reil, J.C.; Hohl, M.; Reil, G.H.; Granzier, H.L.; Kratz, M.T.; Kazakov, A.; Fries, P.; Muller, A.; Lenski, M.; Custodis, F.; et al. Heart rate reduction by If-inhibition improves vascular stiffness and left ventricular systolic and diastolic function in a mouse model of heart failure with preserved ejection fraction. Eur. Heart J. 2013, 34, 2839–2849. [Google Scholar] [CrossRef]
- Kaczorowski, D.J.; Nakao, A.; McCurry, K.R.; Billiar, T.R. Toll-like receptors and myocardial ischemia/reperfusion, inflammation, and injury. Curr. Cardiol. Rev. 2009, 5, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Kaczorowski, D.J.; Tsung, A.; Billiar, T.R. Innate immune mechanisms in ischemia/reperfusion. Front. Biosci. 2009, 1, 91–98. [Google Scholar]
- Jiang, D.S.; Zhang, X.F.; Gao, L.; Zong, J.; Zhou, H.; Liu, Y.; Zhang, Y.; Bian, Z.Y.; Zhu, L.H.; Fan, G.C.; et al. Signal regulatory protein-alpha protects against cardiac hypertrophy via the disruption of toll-like receptor 4 signaling. Hypertension 2014, 63, 96–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, M.V.; Swaminathan, P.D.; Luczak, E.D.; Kutschke, W.; Weiss, R.M.; Anderson, M.E. MyD88 mediated inflammatory signaling leads to CaMKII oxidation, cardiac hypertrophy and death after myocardial infarction. J. Mol. Cell Cardiol. 2012, 52, 1135–1144. [Google Scholar] [CrossRef] [Green Version]
- Ha, T.; Hua, F.; Li, Y.; Ma, J.; Gao, X.; Kelley, J.; Zhao, A.; Haddad, G.E.; Williams, D.L.; Browder, I.W.; et al. Blockade of MyD88 attenuates cardiac hypertrophy and decreases cardiac myocyte apoptosis in pressure overload-induced cardiac hypertrophy in vivo. American journal of physiology. Heart Circ. Physiol. 2006, 290, H985–H994. [Google Scholar] [CrossRef] [Green Version]
- Richards, M.R.; Black, A.S.; Bonnet, D.J.; Barish, G.D.; Woo, C.W.; Tabas, I.; Curtiss, L.K.; Tobias, P.S. The LPS2 mutation in TRIF is atheroprotective in hyperlipidemic low density lipoprotein receptor knockout mice. Innate Immun. 2013, 19, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.E.; Strieter, R.M.; Zhang, K.; Phan, S.H.; Standiford, T.J.; Lukacs, N.W.; Kunkel, S.L. A role for C-C chemokines in fibrotic lung disease. J. Leukoc. Biol. 1995, 57, 782–787. [Google Scholar] [CrossRef] [Green Version]
- Tesch, G.H. MCP-1/CCL2: A new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. American journal of physiology. Ren. Physiol. 2008, 294, F697–F701. [Google Scholar] [CrossRef]
- Kershenobich Stalnikowitz, D.; Weissbrod, A.B. Liver fibrosis and inflammation. A review. Ann. Hepatol. 2003, 2, 159–163. [Google Scholar] [CrossRef]
- Hintermann, E.; Bayer, M.; Pfeilschifter, J.M.; Luster, A.D.; Christen, U. CXCL10 promotes liver fibrosis by prevention of NK cell mediated hepatic stellate cell inactivation. J. Autoimmun. 2010, 35, 424–435. [Google Scholar] [CrossRef] [Green Version]
- Dobaczewski, M.; Chen, W.; Frangogiannis, N.G. Transforming growth factor (TGF)-beta signaling in cardiac remodeling. J. Mol. Cell Cardiol. 2011, 51, 600–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Komers, R.; Carew, R.; Winbanks, C.E.; Xu, B.; Herman-Edelstein, M.; Koh, P.; Thomas, M.; Jandeleit-Dahm, K.; Gregorevic, P.; et al. Suppression of microRNA-29 Expression by TGF-β1 Promotes Collagen Expression and Renal Fibrosis. J. Am. Soc. Nephrol. 2012, 23, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Kriegel, A.J.; Liu, Y.; Fang, Y.; Ding, X.; Liang, M. The miR-29 family: Genomics, cell biology, and relevance to renal and cardiovascular injury. Physiol. Genom. 2012, 44, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Kumarswamy, R.; Thum, T. Non-coding RNAs in Cardiac Remodeling and Heart Failure. Circ. Res. 2013, 113, 676–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayawardena, T.M.; Egemnazarov, B.; Finch, E.A.; Zhang, L.; Payne, J.A.; Pandya, K.; Zhang, Z.; Rosenberg, P.; Mirotsou, M.; Dzau, V.J. MicroRNA-Mediated In Vitro and In Vivo Direct Reprogramming of Cardiac Fibroblasts to Cardiomyocytes. Circ. Res. 2012, 110, 1465–1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroen, B.; Heymans, S. Small but smart—microRNAs in the center of inflammatory processes during cardiovascular diseases, the metabolic syndrome and aging. Cardiovasc. Res. 2011, 93, 605–613. [Google Scholar] [CrossRef] [Green Version]
- Creemers, E.E.; Pinto, Y.M. Molecular mechanisms that control interstitial fibrosis in the pressure-overloaded heart. Cardiovasc. Res. 2011, 89, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Van Rooij, E.; Sutherland, L.B.; Thatcher, J.E.; DiMaio, J.M.; Naseem, R.H.; Marshall, W.S.; Hill, J.A.; Olson, E.N. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc. Natl. Acad. Sci. USA 2008, 105, 13027–13032. [Google Scholar] [CrossRef] [Green Version]
- Lan, H.Y. Diverse roles of TGF-beta/Smads in renal fibrosis and inflammation. Int. J. Biol. Sci. 2011, 7, 1056–1067. [Google Scholar] [CrossRef] [Green Version]
- Heggermont, W.A.; Heymans, S. MicroRNAs are involved in end-organ damage during hypertension. Hypertension 2012, 60, 1088–1093. [Google Scholar] [CrossRef] [Green Version]
- Labbaye, C.; Testa, U. The emerging role of MIR-146A in the control of hematopoiesis, immune function and cancer. J. Hematol. Oncol. 2012, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Huang, C.; Sun, X.; Long, X.R.; Lv, X.W.; Li, J. MicroRNA-146a modulates TGF-beta1-induced hepatic stellate cell proliferation by targeting SMAD4. Cell Signal 2012, 24, 1923–1930. [Google Scholar] [CrossRef]










| Parameter | WT | TRIF−/− | ||||
|---|---|---|---|---|---|---|
| Sham | TAC | p-Value | Sham | TAC | p-Value | |
| d7: LVPW; d [mm] | 0.64 ± 0.02 | 0.79 ± 0.06 | <0.05 | 0.64 ± 0.03 | 0.65 ± 0.04 | ns |
| d7: LVM [fold] | 1.00 ± 0.04 | 1.45 ± 0.07 | <0.001 | 1.00 ± 0.02 | 1.16 ± 0.08 | ns |
| d35: LVPW; d [mm] | 0.64 ± 0.02 | 0.74 ± 0.05 | <0.001 | 0.65 ± 0.02 | 0.73 ± 0.03 | <0.001 |
| d35: LVM [fold] | 1.20 ± 0.05 | 1.62 ± 0.14 | <0.001 | 1.19 ± 0.05 | 1.50 ± 0.08 | <0.001 |
| d7: EF [%] | 40.33 ± 1.44 | 39.34 ± 1.69 | ns | 42.31 ± 1.51 | 42.76 ± 1.97 | ns |
| d35 EF [%] | 40.80 ± 2.59 | 38.03 ± 1.57 | ns | 41.54 ± 2.37 | 36.64 ± 2.42 | ns |
| Parameter | WT | TRIF−/− | ||||
|---|---|---|---|---|---|---|
| Sham | TAC | p-Value | Sham | TAC | p-Value | |
| d35: EF [%] | 46.74 ± 5.67 | 39.67 ± 6.05 | ns | 45.15 ± 7.02 | 39.03 ± 6.66 | ns |
| d70: EF [%] | 44.81 ± 7.56 | 35.66 ± 7.19 | ns | 49.32 ± 3.12 | 44.21 ± 4.36 | ns |
| d35: CO [ml/min] | 6.60 ± 0.44 | 6.63 ± 1.21 | ns | 5.15 ± 0.60 | 6.43 ± 0.69 | ns |
| d70: CO [ml/min] | 8.43 ± 0.86 | 5.22 ± 077 | ns | 10.41 ± 0.57 | 6.63 ± 0.64 | <0.01 |
| d35: SV [µl] | 16.94 ± 0.56 | 17.14 ± 3.12 | ns | 13.34 ± 0.89 | 14.49 ± 1.56 | ns |
| d70: SV [µl] | 30.31 ± 1.76 | 12.79 ± 2.10 | <0.05 | 24.27 ± 1.41 | 15.81 ± 1.51 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bettink, S.I.; Reil, J.-C.; Kazakov, A.; Körbel, C.; Millenaar, D.; Laufs, U.; Scheller, B.; Böhm, M.; Schirmer, S.H. Inhibition of TRIF-Dependent Inflammation Decelerates Afterload-Induced Myocardial Remodeling. Biomedicines 2022, 10, 2636. https://doi.org/10.3390/biomedicines10102636
Bettink SI, Reil J-C, Kazakov A, Körbel C, Millenaar D, Laufs U, Scheller B, Böhm M, Schirmer SH. Inhibition of TRIF-Dependent Inflammation Decelerates Afterload-Induced Myocardial Remodeling. Biomedicines. 2022; 10(10):2636. https://doi.org/10.3390/biomedicines10102636
Chicago/Turabian StyleBettink, Stephanie I., Jan-Christian Reil, Andrey Kazakov, Christina Körbel, Dominic Millenaar, Ulrich Laufs, Bruno Scheller, Michael Böhm, and Stephan H. Schirmer. 2022. "Inhibition of TRIF-Dependent Inflammation Decelerates Afterload-Induced Myocardial Remodeling" Biomedicines 10, no. 10: 2636. https://doi.org/10.3390/biomedicines10102636
APA StyleBettink, S. I., Reil, J.-C., Kazakov, A., Körbel, C., Millenaar, D., Laufs, U., Scheller, B., Böhm, M., & Schirmer, S. H. (2022). Inhibition of TRIF-Dependent Inflammation Decelerates Afterload-Induced Myocardial Remodeling. Biomedicines, 10(10), 2636. https://doi.org/10.3390/biomedicines10102636





