Real-Life Performance of Mepolizumab in T2-High Severe Refractory Asthma with the Overlapping Eosinophilic-Allergic Phenotype
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Skin Prick Test
2.3. Blood and Serological Analysis
2.4. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics of Subjects at Baseline
3.2. Baseline Blood Eosinophils and Total IgE
3.3. Prevalence, sIgE Reactivity and Individual Molecular Profile in Serum sIgE from Severe Uncontrolled Asthmatic Subjects
3.4. Assessment of Clinical Severity, Asthma Exacerbations, Pulmonary Function, and T2 Inflammation Biomarkers after Therapy with Mepolizumab 100 mg-q4w for 52 Weeks
3.5. Subgroup Analysis of Patients with Nasal Polyposis
3.6. Sub-Analysis of Responders after 52 Weeks Treatment with Mepolizumab
3.7. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pawankar, R.; Canonica, G.W.; Holgate, S.T.; Lockey, R.F. Allergic diseases and asthma: A major global health concern. Curr. Opin. Allergy Clin. Immunol. 2012, 12, 39–41. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, K.; Lesosky, M.; García-Marcos, L.; Asher, M.I.; Pearce, N.; Ellwood, E.; Bissell, K.; El Sony, A.; Ellwood, P.; Marks, G.B.; et al. Global Asthma Network Phase I Study Group. The burden of asthma, hay fever and eczema in adults in 17 countries: GAN Phase I study. Eur. Respir. J. 2022, 60, 2102865. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaian, F.; Ledford, D.K.; Casale, T.B. Biologic and New Therapies in Asthma. Immunol. Allergy Clin. 2017, 37, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Wangberg, H.; Woessner, K. Choice of biologics in asthma endotypes. Curr. Opin. Allergy Clin. Immunol. 2021, 21, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Fahy, J.V. Type 2 inflammation in asthma-present in most, absent in many. Nat. Rev. Immunol. 2015, 15, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Shepard, K., 2nd; Yang, M.; Raut, P.; Pazwash, H.; Holweg, C.T.; Choo, E. Overlap of allergic, eosinophilic and type 2 inflammatory subtypes in moderate-to-severe asthma. Clin. Exp. Allergy 2021, 51, 546–555. [Google Scholar] [CrossRef]
- Frøssing, L.; Silberbrandt, A.; Von Bülow, A.; Backer, V.; Porsbjerg, C. The Prevalence of Subtypes of Type 2 Inflammation in an Unselected Population of Patients with Severe Asthma. J. Allergy Clin. Immunol. Pract. 2021, 9, 1267–1275. [Google Scholar] [CrossRef]
- Jeong, K.Y.; Lee, J.Y.; Son, M.; Yi, M.-H.; Yong, T.-S.; Shin, J.U.; Lee, K.H.; Kim, Y.-J.; Park, K.H.; Park, H.J.; et al. Profiles of IgE sensitization to Der f 1, Der f 2, Der f 6, Der f 8, Der f 10, and Der f 20 in Korean house dust mite allergy patients. Allergy Asthma. Immunol. Res. 2015, 7, 483–488. [Google Scholar] [CrossRef] [Green Version]
- Jacquet, A. Characterization of innate immune responses to house dust mite allergens: Pitfalls and imitations. Front. Allergy 2021, 2, 662378. [Google Scholar] [CrossRef]
- Winna Soleha, W.; Iswanti, F.C. Innate Immune Response to House Dust Mite Allergens in Allergic Asthma. Mol. Cell Biomed. Sci. 2021, 5, 104–114. [Google Scholar] [CrossRef]
- Trivedi, M.; Denton, E. Asthma in Children and Adults-What Are the Differences and What Can They Tell us About Asthma? Front. Pediatr. 2019, 7, 256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, P.D.; O’Byrne, P.M. Epithelial-Derived Cytokines in Asthma. Chest 2017, 151, 1338–1344. [Google Scholar] [CrossRef] [PubMed]
- Svenningsen, S.; Nair, P. Asthma Endotypes and an Overview of Targeted Therapy for Asthma. Front. Med. 2017, 4, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humbert, M.; Albers, F.C.; Bratton, D.J.; Yancey, S.W.; Liu, M.C.; Hozawa, S.; Kwon, N. Effect of mepolizumab in severe eosinophilic asthma according to omalizumab eligibility. Respir. Med. 2019, 154, 69–75. [Google Scholar] [CrossRef]
- Global Initiative for Asthma. 2021 GINA Report, Global Strategy for Asthma Management and Prevention (2021 Update). Available online: https://ginasthma.org/gina-reports (accessed on 24 September 2022).
- Kavanagh, J.E.; Hearn, A.P.; Jackson, D.J. A pragmatic guide to choosing biologic therapies in severe asthma. Breathe 2021, 17, 210144. [Google Scholar] [CrossRef]
- Agencia Española de Medicamentos y Productos Sanitarios. Available online: https://www.aemps.gob.es/medicamentosUsoHumano/informesPublicos/docs/IPT-mepolizumab-Nucala-asma_EPOC.pdf (accessed on 7 September 2022).
- Bermejo, I.; Stevenson, M.; Cooper, K.; Harnan, S.; Hamilton, J.; Clowes, M.; Saha, S. Mepolizumab for Treating Severe Eosinophilic Asthma: An Evidence Review Group Perspective of a NICE Single Technology Appraisal. Pharmacoeconomics 2018, 36, 131–144. [Google Scholar] [CrossRef]
- Farne, H.A.; Wilson, A.; Powell, C.; Bax, L.; Milan, S.J. Anti-IL5 therapies for asthma. Cochrane Database Syst. Rev. 2017, 9, CD010834. [Google Scholar]
- Oppenheimer, J.; Hoyte, F.C.L.; Phipatanakul, W.; Silver, J.; Howarth, P.; Lugogo, N.L. Allergic and eosinophilic asthma in the era of biomarkers and biologics: Similarities, differences and misconceptions. Ann. Allergy Asthma. Immunol. 2022, 129, 169–180. [Google Scholar] [CrossRef]
- Juliá-Serdá, G.; Cabrera-Navarro, P.; Acosta-Fernández, O.; Pérez, P.J.M.; Losada-Cabrera, P.; García-Bello, M.; Carrillo-Díaz, T.; Antó-Boqué, J. High prevalence of asthma and atopy in the Canary Islands, Spain. Int. J. Tuberc. Lung Dis. 2011, 15, 536–541. [Google Scholar] [CrossRef]
- Perez-Garcia, J.; Hernández-Pérez, J.M.; González-Pérez, R.; Sardón, O.; Martin-Gonzalez, E.; Espuela-Ortiz, A.; Mederos-Luis, E.; Callero, A.; Herrera-Luis, E.; Corcuera, P.; et al. The Genomics and Metagenomics of Asthma Severity (GEMAS) Study: Rationale and Design. J. Pers. Med. 2020, 10, 123. [Google Scholar] [CrossRef]
- Casas-Maldonado, F.; Álvarez-Gutiérrez, F.-J.; Blanco-Aparicio, M.; Domingo-Ribas, C.; Cisneros-Serrano, C.; Soto-Campos, G.; Román-Bernal, B.; González-Barcala, F.-J. Monoclonal antibody treatment for severe uncontrolled asthma in Spain: Analytical map. J. Asthma. 2021, 17, 1–11. [Google Scholar]
- González-Pérez, R.; Poza-Guedes, P.; Pineda, F.; Castillo, M.; Sánchez-Machín, I. Storage Mite Precision Allergy Molecular Diagnosis in the Moderate-to-Severe T2-High Asthma Phenotype. Int. J. Mol. Sci. 2022, 23, 4297. [Google Scholar] [CrossRef] [PubMed]
- 2022 GINA Main Report—Global Initiative for Asthma. Available online: https://ginasthma.org/gina-reports/ (accessed on 15 August 2022).
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef] [PubMed]
- American Thoracic Society; European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am. J. Respir. Crit. Care Med. 2005, 171, 912–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinzerling, L.; Mari, A.; Bergmann, K.C.; Bresciani, M.; Burbach, G.; Darsow, U.; Durham, S.; Fokkens, W.; Gjomarkaj, M.; Haahtela, T.; et al. The skin prick test—European standards. Clin. Transl. Allergy 2013, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Bojcukova, J.; Vlas, T.; Forstenlechner, P.; Panzner, P. Comparison of two multiplex arrays in the diagnostics of allergy. Clin. Transl. Allergy 2019, 9, 31. [Google Scholar] [CrossRef]
- Álvarez-Gutiérrez, F.J.; Blanco-Aparicio, M.; Plaza, V.; Cisneros, C.; García-Rivero, J.L.; Padilla, A.; Soto-Campos, G. Documento de consenso en asma grave en adultos: Actualización 2020. Open Respir. Arch. 2020, 2, 158–174. [Google Scholar] [CrossRef]
- Agache, I.; Beltran, J.; Akdis, C.; Akdis, M.; Canelo-Aybar, C.; Canonica, G.W.; Jutel, M. Efficacy and safety of treatment with biologicals (benralizumab, dupilumab, mepolizumab, omalizumab and reslizumab) for severe eosinophilic asthma. A systematic review for the EAACI Guidelines—Recommendations on the use of biologicals in severe asthma. Allergy 2020, 75, 1023–1042. [Google Scholar] [CrossRef] [Green Version]
- Travers, J.; Marsh, S.; Williams, M.; Weatherall, M.; Caldwell, B.; Shirtcliffe, P.; Beasley, R. External validity of randomised controlled trials in asthma: To whom do the results of the trials apply? Thorax 2007, 62, 219–223. [Google Scholar] [CrossRef] [Green Version]
- Haughney, J.; Morice, A.; Blyth, K.G.; Lee, A.J.; Coutts, A.; McKnight, E.; Pavord, I. A retrospective cohort study in severe asthma describing commonly measured biomarkers: Eosinophil count and IgE levels. Respir. Med. 2018, 134, 117–123. [Google Scholar] [CrossRef] [Green Version]
- Chupp, G.L.; Bradford, E.S.; Albers, F.C.; Bratton, D.J.; Wang-Jairaj, J.; Nelsen, L.M.; Ten Brinke, A. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): A randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir. Med. 2017, 5, 390–400. [Google Scholar] [CrossRef]
- Domingo Ribas, C.; Carrillo Díaz, T.; Blanco Aparicio, M.; Martínez Moragón, E.; Banas Conejero, D.; Sánchez Herrero, M.G. REDES Study Group. REal worlD Effectiveness and Safety of Mepolizumab in a Multicentric Spanish Cohort of Asthma Patients Stratified by Eosinophils: The REDES Study. Drugs 2021, 81, 1763–1774. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.F.; Wenzel, S.E.; Brozek, J.L.; Bush, A.; Castro, M.; Sterk, P.J.; Adcock, I.M.; Bateman, E.D.; Bel, E.H.; Bleecker, E.R.; et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J. 2014, 43, 343–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- d’Alessandro, M.; Bergantini, L.; Perrone, A.; Cameli, P.; Beltrami, V.; Alderighi, L.; Saletti, M. House Dust Mite Allergy and the Der p1 Conundrum: A Literature Review and Case Series. Allergies 2021, 1, 108–114. [Google Scholar] [CrossRef]
- Thomas, W.R. Hierarchy and molecular properties of house dust mite allergens. Allergol. Int. 2015, 64, 304–311. [Google Scholar]
- Tsai, J.J.; Liu, S.-H.; Yin, S.C.; Yang, C.N.; Hsu, H.S.; Chen, W.B.; Liao, E.C.; Lee, W.J.; Pan, H.C.; Sheu, M.L. Mite allergen Der-p2 triggers human B lymphocyte activation and Toll-like receptor-4 induction. PLoS ONE 2011, 6, e23249. [Google Scholar] [CrossRef]
- Prazma, C.M.; Idzko, M.; Douglass, J.A.; Bourdin, A.; Mallett, S.; Albers, F.C.; Yancey, S.W. Response to Mepolizumab Treatment in Patients with Severe Eosinophilic Asthma and Atopic Phenotypes. J. Asthma. Allergy 2021, 14, 675–683. [Google Scholar] [CrossRef]
- Lemiere, C.; Taillé, C.; Lee, J.K.; Smith, S.G.; Mallett, S.; Albers, F.C.; Bradford, E.S.; Yancey, S.W.; Liu, M.C. Impact of baseline clinical asthma characteristics on the response to mepolizumab: A post hoc meta-analysis of two Phase III trials. Respir. Res. 2021, 22, 184. [Google Scholar] [CrossRef]
- Schleich, F.; Graff, S.; Nekoee, H.; Moermans, C.; Henket, M.; Sanchez, C.; Louis, R. Real-word experience with mepolizumab: Does it deliver what it has promised? Clin. Exp. Allergy 2020, 50, 687–695. [Google Scholar] [CrossRef]
- Schatz, M.; Kosinski, M.; Yarlas, A.S.; Hanlon, J.; Watson, M.E.; Jhingran, P. The minimally important difference of the Asthma Control Test. J. Allergy Clin. Immunol. 2009, 124, 719–723.e1. [Google Scholar] [CrossRef]
- Charles, D.; Shanley, J.; Temple, S.N.; Rattu, A.; Khaleva, E.; Roberts, G. Real-world efficacy of treatment with benralizumab, dupilumab, mepolizumab and reslizumab for severe asthma: A systematic review and meta-analysis. Clin. Exp. Allergy 2022, 52, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Ortega, H.G.; Liu, M.C.; Pavord, I.D.; Brusselle, G.G.; Fitzgerald, J.M.; Chetta, A.; Humbert, M.; Katz, L.E.; Keene, O.N.; Yancey, S.W.; et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N. Engl. J. Med. 2014, 371, 1198–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bel, E.H.; Wenzel, S.E.; Thompson, P.J.; Prazma, C.M.; Keene, O.N.; Yancey, S.W.; Ortega, H.G.; Pavord, I.D. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N. Engl. J. Med. 2014, 371, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Haldar, P.; Brightling, C.E.; Hargadon, B.; Gupta, S.; Monteiro, W.; Sousa, A.; Pavord, I.D. Mepolizumab and exacerbations of refractory eosinophilic asthma. N. Engl. J. Med. 2009, 360, 973–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kardas, G.; Kuna, P.; Panek, M. Biological Therapies of Severe Asthma and Their Possible Effects on Airway Remodeling. Front. Immunol. 2020, 11, 1134. [Google Scholar] [CrossRef] [PubMed]
- Harvey, E.S.; Langton, D.; Katelaris, C.; Stevens, S.; Farah, C.S.; Gillman, A.; Gibson, P.G. Mepolizumab effectiveness and identification of super-responders in severe asthma. Eur. Respir. J. 2020, 55, 1902420. [Google Scholar] [CrossRef] [PubMed]
- Alt, J.A.; Thomas, A.J.; Curtin, K.; Wong, J.; Rudmik, L.; Orlandi, R.R. Mortality risk in patients with chronic rhinosinusitis and its association to asthma. Int. Forum. Allergy Rhinol. 2017, 7, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Han, J.K.; Bachert, C.; Fokkens, W.; Desrosiers, M.; Wagenmann, M.; Lee, S.E.; Smith, S.G.; Martin, N.; Mayer, B.; Yancey, S.W.; et al. Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2021, 9, 1141–1153. [Google Scholar] [CrossRef]




| Variable | Severe Uncontrolled Asthma |
|---|---|
| n = 61 (%) | 61 (100) |
| Age (y.o.) mean (SD) | 45.98 (38.89) |
| <20 y.o. (%) | 5 (8.19) |
| >20 y.o. (%) | 56 (91.81) |
| Sex (Female%/Male%) | 60.65/39.35 |
| BMI mean (SD) | 29.10 (4.04) |
| Smoking | |
| Never smoker | 41 (67.21) |
| Former smoker | 17 (27.86) |
| Smoker | 3 (4.91) |
| Bronchiectasis (Chest CT Scanner) | 3 (4.91) |
| SPT+ HDM and/or SM | 51 (83.6) |
| Allergic Rhinitis (%) | 52 (85.24) |
| Atopic Dermatitis (%) | 9 (14.75) |
| Nasal Polyposis (%) | 22 (36.06) |
| NERD (%) | 11 (18.03) |
| Chronic Rinosinusitis (%) | 17 (27.86) |
| Eosinophilic Esophagitis (%) | 2 (3.2) |
| Food Allergy (%) | 2 (3.2) |
| Asthma Onset at Childhood (%) | 42 (68.85) |
| Family History of Atopy (%) | 49 (80.32) |
| Variables | Baseline | After 52-Weeks of Mepolizumab | p |
|---|---|---|---|
| ACT * | 13.8 ± 4.61 | 18.91 ± 4.79 | <0.0001 |
| Number of annual AEs * | 3.37 ± 1.34 | 0.97 ± 1.19 | <0.0001 |
| Use of OCS (mg/day) * | 11.3 ± 9.6 | 6.5 ± 4.2 | <0.0001 |
| FVC (mL) * | 3015.21 ± 829.1 | 3315.17 ± 828.29 | 0.0018 |
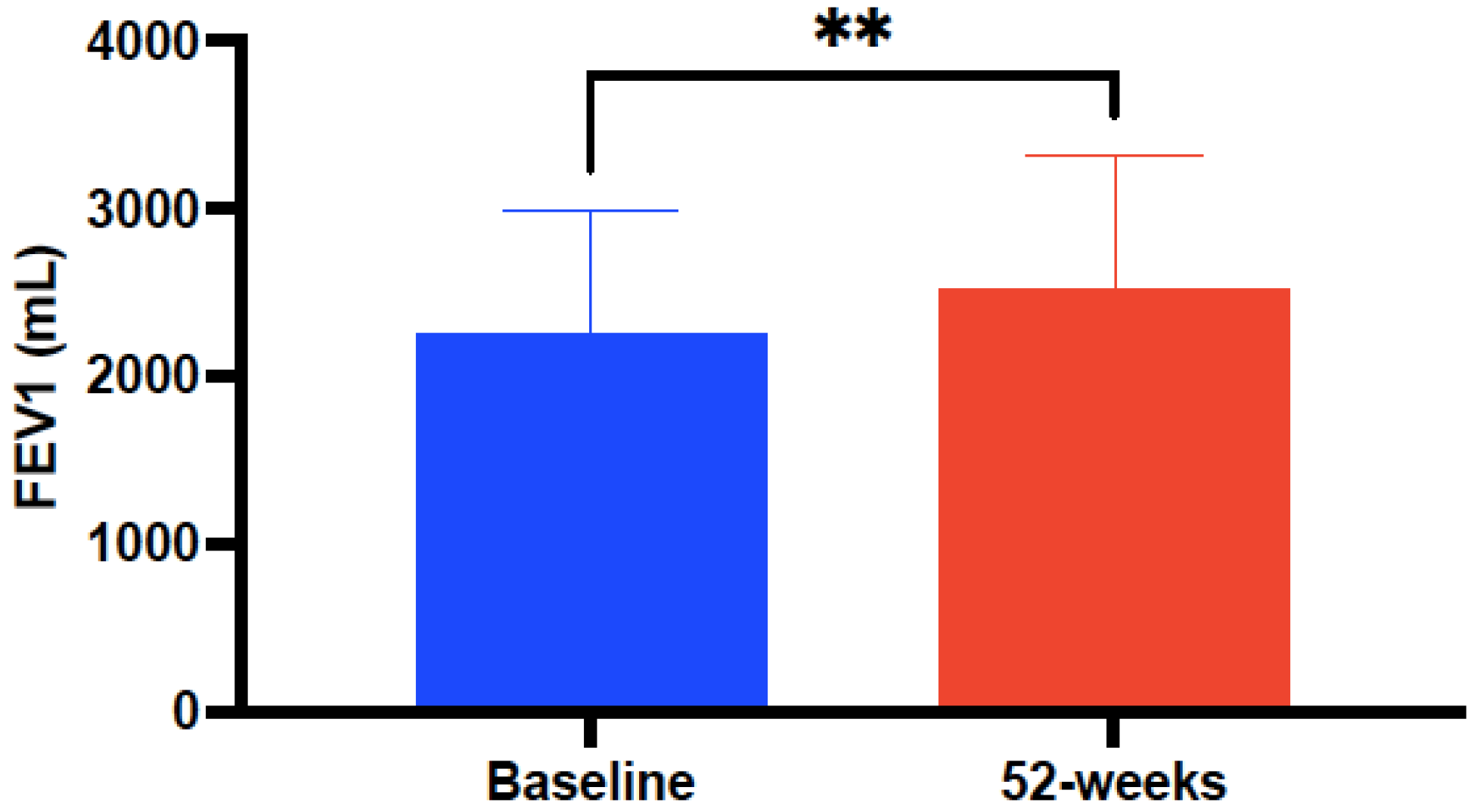
| FEV1 (mL) * | 2283.27 ± 721.5 | 2602.04 ± 701.12 | <0.0001 |
| FEV1% * | 72.75 ± 17.85 | 80.95 ± 18.17 | 0.0134 |
| SNOT-22 * | 53.42 ± 22.66 | 39.39 ± 23.13 | 0.0003 |
| FENO (ppb) | 53.35 ± 33.46 | 57.51 ± 47.59 | 0.3410 |
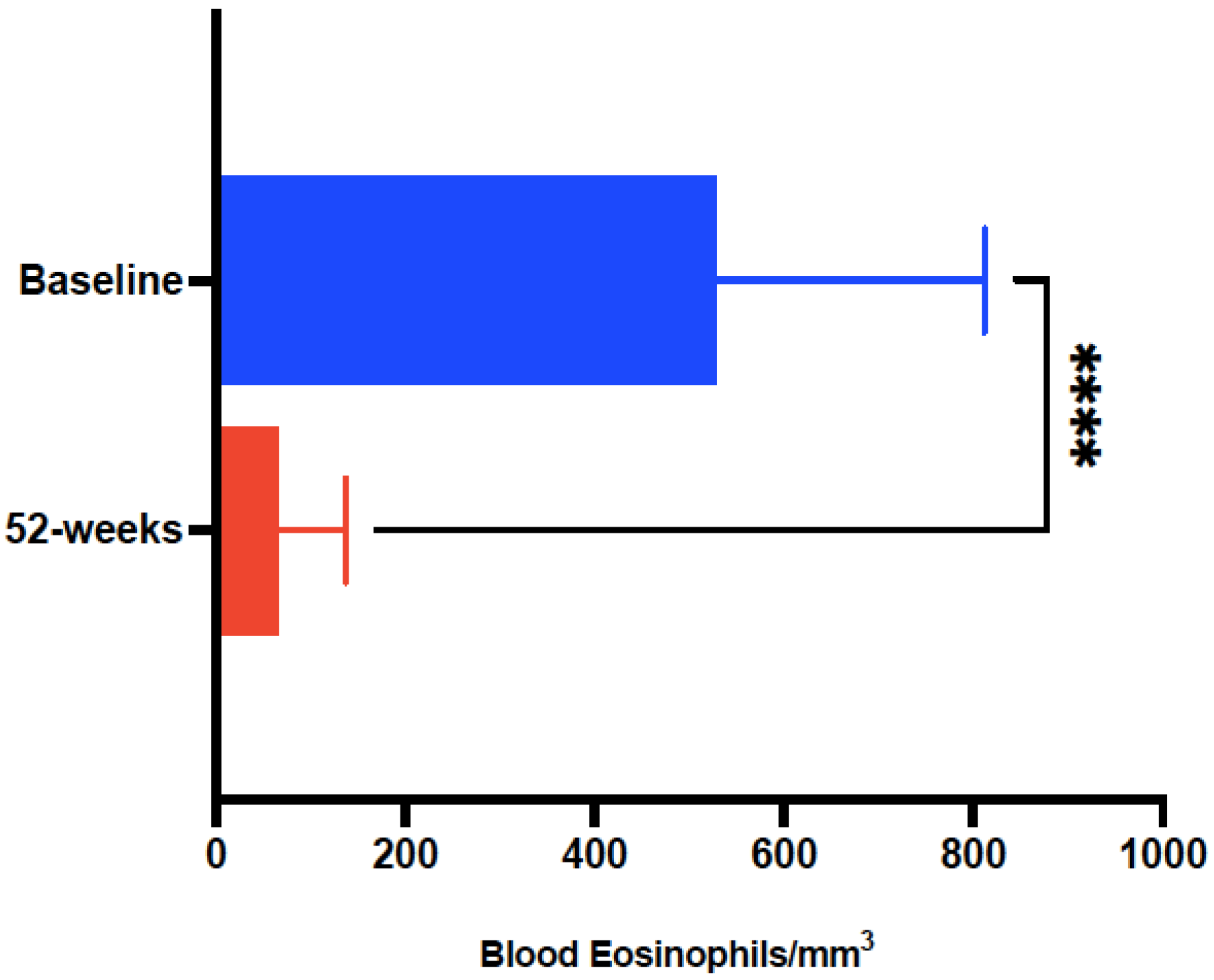
| Eosinophils/μL peripheral blood * | 541 ± 283 | 59.19 ± 48.95 | <0.0001 |
| Total IgE (IU/mL) | 614.53 ± 1196 | 1016.26 ± 2477.84 | 0.8520 |
| sIgE D. pteronyssinus (kUA/L) | 49 ± 49.67 | 43.93 ± 48.94 | 0.37 |
| sIgE D. farinae (kUA/L) | 40.17 ± 46.52 | 41.47 ± 48.07 | 0.2010 |
| sIgE Blomia tropicalis (kUA/L) | 3.67 ± 15.98 | 2.88 ± 9.37 | 0.2691 |
| Variables | Baseline | After 52-Weeks of Mepolizumab | p |
|---|---|---|---|
| ACT * (<600) | 12.38 ± 4.66 | 18.03 ± 5.27 | <0.0001 |
| ACT * (≥600) | 14.63 ± 4.19 | 20.41 ± 3.43 | <0.0001 |
| Annual AEs * (<600) | 3.33 ± 1.49 | 1.06 ± 1.38 | <0.0001 |
| Annual AEs * (≥600) | 3.47 ± 0.96 | 0.8 ± 0.73 | <0.0001 |
| FEV1 * (<600) | 2236.19 ± 764.3 | 2400.64 ± 730.59 | 0.002 |
| FEV1 * (≥600) | 2387.36 ± 622 | 2933.76 ± 476.09 | 0.0002 |
| FENO (<600) | 47.6 ± 20.81 | 41.42 ± 28.75 | 0.4 |
| FENO (≥600) | 67.75 ± 53.72 | 79.21 ± 60.88 | 0.84 |
| Variables | Baseline | After 52-Weeks of Mepolizumab | p |
|---|---|---|---|
| ACT * | 15 ± 4.08 | 22.33 ± 4.08 | <0.0001 |
| Number of annual AEs * | 3.04 ± 1.13 | 0.53 ± 0.7 | <0.0001 |
| Use of OCS (mg/day) * | 10.21 ± 8.2 | 4.6 ± 3.9 | <0.0001 |
| Number of nasal polyp surgeries * | 1.4 ± 0.9 | 0.22 ± 0.42 | <0.0001 |
| FVC (mL) * | 3473.54 ± 866.5 | 3815.66 ± 948.45 | 0.0082 |
| FEV1 (mL) * | 2608.18 ± 746.7 | 2965.46 ± 708.79 | 0.0004 |
| FEV1% | 77.77 ± 15.53 | 85.28 ± 17.63 | 0.073 |
| SNOT-22 * | 63.75 ± 20.31 | 33.5 ± 20.92 | <0.0001 |
| FENO (ppb) | 58.9 ± 22.61 | 70.8 ± 44.77 | 0.711 |
| Eosinophils/μL peripheral blood * | 591 ± 322 | 62 ± 47.14 | <0.0001 |
| Total IgE (IU/mL) | 295.44 ± 344.05 | 325.09 ± 366.65 | 0.124 |
| sIgE D. pteronyssinus (kUA/L) | 1.71 ± 3.77 | 5.78 ± 11.59 | 0.224 |
| sIgE D. farinae (kUA/L) | 1.03 ± 2.62 | 3.8 ± 7.18 | 0.776 |
| sIgE Blomia tropicalis (kUA/L) | 0 | 0 | 0.385 |
| Variable | n (%) |
|---|---|
| Number of patients with adverse events related to mepolizumab | 3 (4.91) |
| Musculoskeletal disorders | 2 (3.27) |
| Arthromyalgia | 2 (3.27) |
| Drug administration site disorders | 1 (1.63) |
| Local pain after subcutaneous injection | 1 (1.63) |
| Nervous system disorders | 1 (1.63) |
| Headache | 1 (1.63) |
| Gastrointestinal disorders | 1 (1.63) |
| Dypepsia | 1 (1.63) |
| Patients with adverse events leading to treatment discontinuation | 2 (3.27) |
| Serious adverse events | 0 (0%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Pérez, R.; Poza-Guedes, P.; Mederos-Luis, E.; Sánchez-Machín, I. Real-Life Performance of Mepolizumab in T2-High Severe Refractory Asthma with the Overlapping Eosinophilic-Allergic Phenotype. Biomedicines 2022, 10, 2635. https://doi.org/10.3390/biomedicines10102635
González-Pérez R, Poza-Guedes P, Mederos-Luis E, Sánchez-Machín I. Real-Life Performance of Mepolizumab in T2-High Severe Refractory Asthma with the Overlapping Eosinophilic-Allergic Phenotype. Biomedicines. 2022; 10(10):2635. https://doi.org/10.3390/biomedicines10102635
Chicago/Turabian StyleGonzález-Pérez, Ruperto, Paloma Poza-Guedes, Elena Mederos-Luis, and Inmaculada Sánchez-Machín. 2022. "Real-Life Performance of Mepolizumab in T2-High Severe Refractory Asthma with the Overlapping Eosinophilic-Allergic Phenotype" Biomedicines 10, no. 10: 2635. https://doi.org/10.3390/biomedicines10102635
APA StyleGonzález-Pérez, R., Poza-Guedes, P., Mederos-Luis, E., & Sánchez-Machín, I. (2022). Real-Life Performance of Mepolizumab in T2-High Severe Refractory Asthma with the Overlapping Eosinophilic-Allergic Phenotype. Biomedicines, 10(10), 2635. https://doi.org/10.3390/biomedicines10102635







