Atherosclerosis by Virus Infection—A Short Review
Abstract
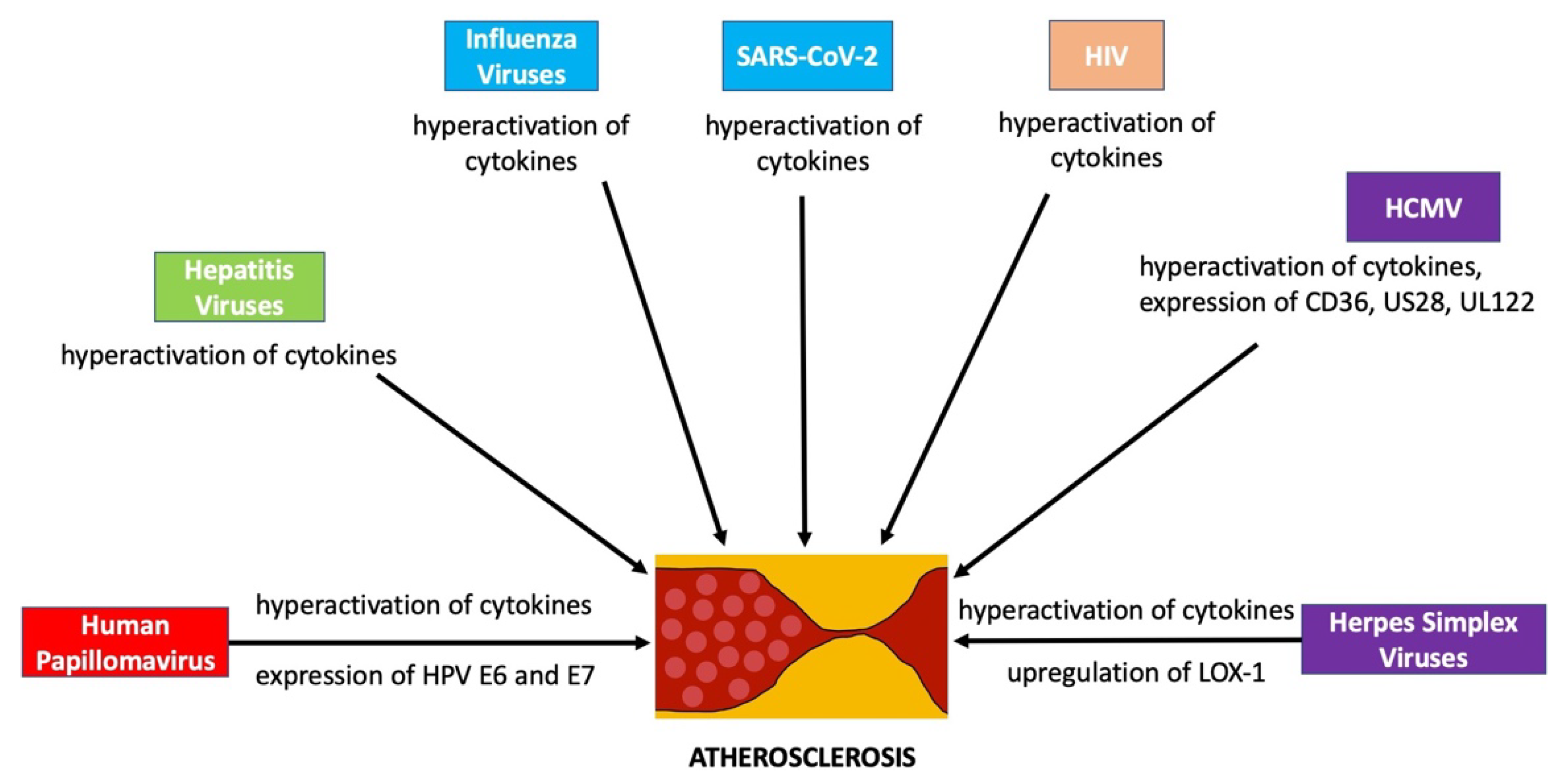
:1. Introduction
2. Influenza Viruses
3. Severe Acute Respiratory Syndrome Coronavirus-2 (SARS CoV-2)
4. Hepatitis Viruses
5. Herpes Simplex Virus (Human Herpesvirus 1 and 2)
6. Human Papillomavirus
7. Human Cytomegalovirus (Human Herpesvirus 5)
8. Human Immunodeficiency Virus
9. Discussion
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Prim. 2019, 5, 56. [Google Scholar] [CrossRef]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Flak, E. Mechanisms of Plaque Formation and Rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef] [PubMed]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef]
- Libby, P.; Loscalzo, J.; Ridker, P.M.; Farkouh, M.E.; Hsue, P.Y.; Fuster, V.; Hasan, A.A.; Amar, S. Inflammation, Immunity, and Infection in Atherothrombosis: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2018, 72, 2071–2081. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.A.; Rosenfeld, M.E. Infection and Atherosclerosis Development. Arch. Med Res. 2015, 46, 339–350. [Google Scholar] [CrossRef] [Green Version]
- Naga Venkata, K.; Pothineni, N.V.K.; Subramany, S.; Kuriakose, K.; Shirazi, L.F.; Romeo, F.; Shah, P.K.; Mehta, J.L. Infections, atherosclerosis, and coronary heart disease. Eur. Hear. J. 2017, 38, 3195–3201. [Google Scholar] [CrossRef]
- Kadosh, B.S.; Garshick, M.S.; Gaztanaga, J.; Moore, K.J.; Newman, J.D.; Pillinger, M.; Ramasamy, R.; Reynolds, H.R.; Shah, B.; Hochman, J.; et al. COVID-19 and the Heart and Vasculature: Novel Approaches to Reduce Virus-Induced Inflammation in Patients with Cardiovascular Disease. Arter. Thromb. Vasc. Biol. 2020, 40, 2045–2053. [Google Scholar] [CrossRef]
- Vinciguerra, M.; Romiti, S.; Fattouch, K.; Bellis, A.D.; Greco, E. Atherosclerosis as Pathogenetic Substrate for Sars-Cov2 Cytokine Storm. J. Clin. Med. 2020, 9, 2095. [Google Scholar] [CrossRef] [PubMed]
- Peretz, A.; Azrad, M.; Blum, A. Influenza virus and atherosclerosis. QJM 2019, 112, 749–755. [Google Scholar] [CrossRef]
- Bunce, P.E.; High, S.M.; Nadjafi, M.; Stanley, K.; Liles, W.C.; Christian, M.D. Pandemic H1N1 Influenza Infection and Vascular Thrombosis. Clin. Infect. Dis. 2011, 52, e14–e17. [Google Scholar] [CrossRef] [Green Version]
- Warren-Gash, C.; Hayward, A.C.; Hemingway, H.; Denaxas, S.; Thomas, S.L.; Timmis, A.D.; Whitaker, H.; Smeeth, L. Influenza infection and risk of acute myocardial infarction in England and Wales: A CALIBER self-controlled case series study. J. Infect. Dis. 2012, 206, 1652–1659. [Google Scholar] [CrossRef] [Green Version]
- Indalao, I.L.; Sawabuchi, T.; Takahashi, E.; Kido, H. IL-1β is a key cytokine that induces trypsin upregulation in the influenza virus-cytokine-trypsin cycle. Arch. Virol. 2017, 162, 201–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julkunen, I.; Melén, K.; Nyqvist, M.; Pirhonen, J.; Sareneva, T.; Matikainen, S. Inflammatory responses in influenza A virus infection. Vaccine 2000, 19, S32–S37. [Google Scholar] [CrossRef]
- Narasaraju, T.; Yang, E.; Samy, R.P.; Ng, H.H.; Poh, W.P.; Liew, A.; Phoon, M.C.; van Rooijen, N.; Chow, V.T. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am. J. Pathol. 2011, 179, 199–210. [Google Scholar] [CrossRef]
- Zeng, H.; Pappas, C.; Belser, J.A.; Houser, K.V.; Zhong, W.; Wadford, D.A.; Stevens, T.; Balczon, R.; Katz, J.M.; Tumpey, T.M. Human pulmonary microvascular endothelial cells support productive replication of highly pathogenic avian influenza viruses: Possible involvement in the pathogenesis of human H5N1 virus infection. J. Virol. 2012, 86, 667–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Le, T.Q.; Kurihara, N.; Chida, J.; Cisse, Y.; Yano, M.; Kido, H. Influenza virus-cytokine-protease cycle in the pathogenesis of vascular hyperpermeability in severe influenza. J. Infect. Dis. 2010, 202, 991–1001. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, Z.; Xu, S.; Rahman, A.; Bragazzi, N.L.; Corrales-Medina, V.F.; Lee, J.; Seet, B.T.; Neame, D.; Thommes, E.; Heffernan, J.; et al. Modelling the linkage between influenza infection and cardiovascular events via thrombosis. Sci. Rep. 2020, 10, 14264. [Google Scholar] [CrossRef]
- Kwong, J.C.; Schwartz, K.L.; Campitelli, M.A.; Chung, H.; Crowcroft, N.S.; Karnauchow, T.; Katz, K.; Ko, D.T.; McGeer, A.J.; McNally, D.; et al. Acute Myocardial Infarction after Laboratory-Confirmed Influenza Infection. N. Engl. J. Med. 2018, 378, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Demina, E.P.; Smutova, V.; Pan, X.; Fougerat, A.; Guo, T.; Zou, C.; Chakraberty, R.; Snarr, B.D.; Shiao, T.C.; Roy, R.; et al. Neuraminidases 1 and 3 Trigger Atherosclerosis by Desialylating Low-Density Lipoproteins and Increasing Their Uptake by Macrophages. J. Am. Hear. Assoc. 2021, 10, e018756. [Google Scholar] [CrossRef]
- Mezentsev, A.; Bezsonov, E.; Kashirskikh, D.; Baig, M.S.; Eid, A.H.; Orekhov, A. Proatherogenic Sialidases and Desialylated Lipoproteins: 35 Years of Research and Current State from Bench to Bedside. Biomedicines 2021, 9, 600. [Google Scholar] [CrossRef]
- Nichol, M.L.; Nordin, J.; Mullooly, J.; Lask, R.; Fillbrandt, K.; Iwane, M. Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly. N. Engl. J. Med. 2003, 348, 1322–1332. [Google Scholar] [CrossRef]
- Naghavi, M.; Barlas, Z.; Siadaty, S.; Naguib, S.; Madjid, M.; Casscells, W. Association of influenza vaccination and reduced risk of recurrent myocardial infarction. Circulation 2000, 102, 3039–3045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majumder, J.; Minko, T. Recent Developments on Therapeutic and Diagnostic Approaches for COVID-19. AAPS J. 2021, 23, 14. [Google Scholar] [CrossRef] [PubMed]
- Kirtipal, N.; Bharadwaj, S.; Kang, S.G. From SARS to SARS-CoV-2, insights on structure, pathogenicity and immunity aspects of pandemic human coronaviruses. Infect. Genet. Evol. 2020, 85, 104502. [Google Scholar] [CrossRef] [PubMed]
- Worobey, M. Dissecting the early COVID-19 cases in Wuhan. Science 2021, 374, 1202–1204. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.V.; Bezsonov, E.E.; Eid, A.H.; Popkova, T.V.; Nedosugova, L.V.; Starodubova, A.V.; Orekhov, A.N. ACE2 Is an Adjacent Element of Atherosclerosis and COVID-19 Pathogenesis. Int. J. Mol. Sci. 2021, 22, 4691. [Google Scholar] [CrossRef] [PubMed]
- Risitano, A.M.; Mastellos, D.C.; Huber-Lang, M.; Yancopoulou, D.; Garlanda, C.; Ciceri, F.; Lambris, J.D. Complement as a target in COVID-19? Nat. Rev. Immunol. 2020, 20, 343–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bangalore, S.; Sharma, A.; Slotwiner, A.; Yatskar, L.; Harari, R.; Shah, B.; Ibrahim, H.; Friedman, G.H.; Thompson, C.; Alviar, C.L.; et al. ST-Segment Elevation in Patients with COVID-19–A Case Series. N. Engl. J. Med. 2020, 382, 2478–2480. [Google Scholar] [CrossRef]
- Doyen, D.; Moceri, P.; Ducreux, D.; Dellamonica, J. Myocarditis in a patient with COVID-19: A cause of raised troponin and ECG changes. Lancet 2020, 395, 1516. [Google Scholar] [CrossRef]
- Vangeel, L.; Chiu, W.; De Jonghe, S.; Maes, P.; Slechten, B.; Raymenants, J.; André, E.; Leyssen, P.; Neyts, J.; Jochmans, D. Remdesivir, Molnupiravir and Nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern. Antivir. Res. 2022, 198, 105252. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. COVID-19: Pfizer’s paxlovid is 89% effective in patients at risk of serious illness, company reports. BMJ 2021, 375, n2713. [Google Scholar] [CrossRef]
- The National Institutes of Health. Antiviral Drugs That Are Approved, Authorized, or Under Evaluation for the Treatment of COVID-19. Available online: https://www.covid19treatmentguidelines.nih.gov/therapies/antiviral-therapy/summary-recommendations/ (accessed on 24 August 2022).
- Loader, M.; Moravek, R.; Witowski, S.E.; Driscoll, L.M. A clinical review of viral hepatitis. JAAPA 2019, 32, 15–20. [Google Scholar] [CrossRef]
- Castaneda, D.; Gonzalez, A.J.; Alomari, M.; Tandon, K.; Zervos, X.B. From hepatitis A to E: A critical review of viral hepatitis. World J. Gastroenterol. 2021, 27, 1691–1715. [Google Scholar] [CrossRef]
- Zhu, J.; Quyyumi, A.A.; Norman, J.E.; Costello, R.; Csako, G.; Epstein, S.E. The possible role of hepatitis A virus in the pathogenesis of atherosclerosis. J. Infect. Dis. 2000, 182, 1583–1587. [Google Scholar] [CrossRef]
- Sung, J.; Song, Y.M.; Choi, Y.H.; Ebrahim, S.; Smith, D.G. Hepatitis B virus seropositivity and the risk of stroke and myocardial infarction. Stroke 2007, 38, 1436–1441. [Google Scholar] [CrossRef] [Green Version]
- Adinolfi, L.E.; Restivo, L.; Zampino, R.; Guerrera, B.; Lonardo, A.; Ruggiero, L.; Riello, F.; Loria, P.; Florio, A. Chronic HCV infection is a risk of atherosclerosis. Role of HCV and HCV-related steatosis. Atherosclerosis 2012, 221, 496–502. [Google Scholar] [CrossRef]
- Oliveira, C.P.; Kappel, C.R.; Siqueira, E.R.; Lima, V.M.; Stefano, J.T.; Michalczuk, M.T.; Marini, S.S.; Barbeiro, H.V.; Soriano, F.G.; Carrilho, F.J.; et al. Effects of hepatitis C virus on cardiovascular risk in infected patients: A comparative study. Int. J. Cardiol. 2013, 164, 221–226. [Google Scholar] [CrossRef] [Green Version]
- Pothineni, N.V.; Delongchamp, R.; Vallurupalli, S.; Ding, Z.; Dai, Y.; Hagedorn, C.H.; Mehta, J.L. Impact of hepatitis C seropositivity on the risk of coronary heart disease events. Am. J. Cardiol. 2014, 114, 1841–1845. [Google Scholar] [CrossRef] [Green Version]
- Migueres, M.; Lhomme, S.; Izopet, J. Hepatitis A: Epidemiology, High-Risk Groups, Prevention and Research on Antiviral Treatment. Viruses 2021, 13, 1900. [Google Scholar] [CrossRef]
- Lang-Meli, J.; Neumann-Haefelin, C.; Thimme, R. Immunotherapy and therapeutic vaccines for chronic HBV infection. Curr. Opin. Virol. 2021, 51, 149–157. [Google Scholar] [CrossRef]
- Looker, K.J.; Magaret, A.S.; May, M.T.; Turner, K.M.; Vickerman, P.; Gottlieb, S.L.; Newman, L.M. Global and Regional Estimates of Prevalent and Incident Herpes Simplex Virus Type 1 Infections in 2012. PLoS ONE 2015, 10, e0140765. [Google Scholar] [CrossRef] [Green Version]
- Looker, K.J.; Magaret, A.S.; Turner, K.M.; Vickerman, P.; Gottlieb, S.L.; Newman, L.M. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PLoS ONE 2015, 10, e114989. [Google Scholar] [CrossRef] [Green Version]
- Kotronias, D.; Kapranos, N. Herpes simplex virus as a determinant risk factor for coronary artery atherosclerosis and myocardial infarction. Vivo 2005, 19, 351–357. [Google Scholar]
- Ibrahim, A.I.; Obeid, M.T.; Jouma, M.J.; Moasis, G.A.; Al-Richane, W.L.; Kindermann, I.; Boehm, M.; Roemer, K.; Mueller-Lantzsch, N.; Gärtner, B.C. Detection of herpes simplex virus, cytomegalovirus and Epstein-Barr virus DNA in atherosclerotic plaques and in unaffected bypass grafts. J. Clin. Virol. 2005, 32, 29–32. [Google Scholar] [CrossRef]
- Wu, Y.P.; Sun, D.D.; Wang, Y.; Liu, W.; Yang, J. Herpes Simplex Virus Type 1 and Type 2 Infection Increases Atherosclerosis Risk: Evidence Based on a Meta-Analysis. BioMed Res. Int. 2016, 2016, 2630865. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Tokunaga, O. Herpesvirus (HSV-1, EBV and CMV) infections in atherosclerotic compared with non-atherosclerotic aortic tissue. Pathol. Int. 2002, 52, 31–39. [Google Scholar] [CrossRef]
- Chirathaworn, C.; Pongpanich, A.; Poovorawan, Y. Herpes simplex virus 1 induced LOX-1 expression in an endothelial cell line, ECV 304. Viral Immunol. 2004, 17, 308–314. [Google Scholar] [CrossRef]
- Hajjar, D.P.; Pomerantz, K.B.; Falcone, D.J.; Weksler, B.B.; Grant, A.J. Herpes simplex virus infection in human arterial cells. Implications in arteriosclerosis. J. Clin. Investig. 1987, 80, 1317–1321. [Google Scholar] [CrossRef]
- Sutherland, M.R.; Raynor, C.M.; Leenknegt, H.; Wright, J.F.; Pryzdial, E.L. Coagulation initiated on herpesviruses. Proc. Natl. Acad. Sci. USA 1997, 94, 13510–13514. [Google Scholar] [CrossRef] [Green Version]
- Hechter, R.C.; Budoff, M.; Hodis, H.N.; Rinaldo, C.R.; Jenkins, F.J.; Jacobson, L.P.; Kingsley, L.A.; Taiwo, B.; Post, W.S.; Margolick, J.B.; et al. Herpes simplex virus type 2 (HSV-2) as a coronary atherosclerosis risk factor in HIV-infected men: Multicenter AIDS cohort study. Atherosclerosis 2012, 223, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Stanfield, B.A.; Kousoulas, K.G.; Fernandez, A.; Gershburg, E. Rational Design of Live-Attenuated Vaccines against Herpes Simplex Viruses. Viruses 2021, 13, 1637. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, L.A.; Upadhyay, R.; Greeley, Z.W.; Margulies, B.J. Current Drugs to Treat Infections with Herpes Simplex Viruses-1 and -2. Viruses 2021, 13, 1228. [Google Scholar] [CrossRef]
- Cubie, H.A. Diseases associated with human papillomavirus infection. Virology 2013, 445, 21–34. [Google Scholar] [CrossRef] [Green Version]
- de Sanjose, S.; Quint, W.G.; Alemany, L.; Geraets, D.T.; Klaustermeier, J.E.; Lloveras, B.; Tous, S.; Felix, A.; Bravo, L.E.; Shin, H.R.; et al. Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol. 2010, 11, 1048–1056. [Google Scholar] [CrossRef]
- Monsonego, J.; Cox, J.T.; Behrens, C.; Sandri, M.; Franco, E.L.; Yap, P.S.; Huh, W. Prevalence of high-risk human papilloma virus genotypes and associated risk of cervical precancerous lesions in a large U.S. screening population: Data from the ATHENA trial. Gynecol. Oncol. 2015, 137, 47–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawson, J.S.; Glenn, W.K.; Tran, D.D.; Ngan, C.C.; Duflou, J.A.; Whitaker, N.J. Identification of Human Papilloma Viruses in Atheromatous Coronary Artery Disease. Front. Cardiovasc. Med. 2015, 2, 17. [Google Scholar] [CrossRef] [Green Version]
- Kuo, H.K.; Fujise, K. Human papillomavirus and cardiovascular disease among U.S. women in the National Health and Nutrition Examination Survey, 2003 to 2006. J. Am. Coll. Cardiol. 2011, 58, 2001–2006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathore, A.S.; Gulati, N.; Shetty, D.C.; Jain, A. To analyze the concomitant expression of human papillomavirus-16 in the pathogenetic model of p53-dependant pathway in oral squamous cell carcinoma. J. Oral Maxillofac. Pathol. 2016, 20, 342–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonin, L.R.; Madden, K.; Shera, K.; Ihle, J.; Matthews, C.; Aziz, S.; Perez-Reyes, N.; McDougall, J.K.; Conroy, S.C. Generation and characterization of human smooth muscle cell lines derived from atherosclerotic plaque. Arter. Thromb. Vasc. Biol. 1999, 19, 575–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guevara, N.V.; Kim, H.S.; Antonova, E.I.; Chan, L. The absence of p53 accelerates atherosclerosis by increasing cell proliferation in vivo. Nat. Med. 1999, 5, 335–339. [Google Scholar] [CrossRef]
- Soliman, M.; Oredein, O.; Dass, C.R. Update on Safety and Efficacy of HPV Vaccines: Focus on Gardasil. Int. J. Mol. Cell. Med. 2021, 10, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, P.; Reeves, M. Pathogenesis of human cytomegalovirus in the immunocompromised host. Nat. Rev. Microbiol. 2021, 19, 759–773. [Google Scholar] [CrossRef]
- Zuhair, M.; Smit, G.S.A.; Wallis, G.; Jabbar, F.; Smith, C.; Devleesschauwer, B.; Griffiths, P. Estimation of the worldwide seroprevalence of cytomegalovirus: A systematic review and meta-analysis. Rev. Med. Virol. 2019, 29, e20341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffiths, P.; Baraniak, I.; Reeves, M. The pathogenesis of human cytomegalovirus. J. Pathol. 2015, 235, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Forte, E.; Zhang, Z.; Thorp, E.B.; Hummel, M. Cytomegalovirus Latency and Reactivation: An Intricate Interplay With the Host Immune Response. Front. Cell. Infect. Microbiol. 2020, 10, 130. [Google Scholar] [CrossRef]
- Poole, E.; Sinclair, J. Understanding HCMV Latency Using Unbiased Proteomic Analyses. Pathogens 2020, 9, 590. [Google Scholar] [CrossRef] [PubMed]
- Vasilieva, E.; Gianella, S.; Freeman, M.L. Novel Strategies to Combat CMV-Related Cardiovascular Disease. Pathog. Immun. 2020, 5, 240–274. [Google Scholar] [CrossRef] [PubMed]
- Horváth, R.; Cerný, J.; Benedík, J., Jr.; Hökl, J.; Jelínková, I.; Benedík, J. The possible role of human cytomegalovirus (HCMV) in the origin of atherosclerosis. J. Clin. Virol. 2000, 16, 17–24. [Google Scholar] [CrossRef]
- Hendrix, M.G.; Salimans, M.M.; van Boven, C.P.; Bruggeman, C.A. High prevalence of latently present cytomegalovirus in arterial walls of patients suffering from grade III atherosclerosis. Am. J. Pathol. 1990, 136, 23–28. [Google Scholar]
- Carlquist, J.F.; Muhlestein, J.B.; Horne, B.D.; Hart, N.I.; Lim, T.; Habashi, J.; Anderson, J.G.; Anderson, J.L. Cytomegalovirus stimulated mRNA accumulation and cell surface expression of the oxidized LDL scavenger receptor, CD36. Atherosclerosis 2004, 177, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Streblow, D.N.; Soderberg-Naucler, C.; Vieira, J.; Smith, P.; Wakabayashi, E.; Ruchti, F.; Mattison, K.; Altschuler, Y.; Nelson, J.A. The human cytomegalovirus chemokine receptor US28 mediates vascular smooth muscle cell migration. Cell 1999, 99, 511–520. [Google Scholar] [CrossRef]
- Tanaka, K.; Zou, J.P.; Takeda, K.; Ferrans, V.J.; Sandford, G.R.; Johnson, T.M.; Finkel, T.; Epstein, S.E. Effects of human cytomegalovirus immediate-early proteins on p53-mediated apoptosis in coronary artery smooth muscle cells. Circulation 1999, 99, 1656–1659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bason, C.; Corrocher, R.; Lunardi, C.; Puccetti, P.; Olivieri, O.; Girelli, D.; Navone, R.; Beri, R.; Millo, E.; Margonato, A.; et al. Interaction of antibodies against cytomegalovirus with heat-shock protein 60 in pathogenesis of atherosclerosis. Lancet 2003, 362, 1971–1977. [Google Scholar] [CrossRef]
- Pera, A.; Caserta, S.; Albanese, F.; Blowers, P.; Morrow, G.; Terrazzini, N.; Smith, H.E.; Rajkumar, C.; Reus, B.; Msonda, J.R.; et al. CD28null pro-atherogenic CD4 T-cells explain the link between CMV infection and an increased risk of cardiovascular death. Theranostics 2018, 8, 4509–4519. [Google Scholar] [CrossRef]
- Burnett, M.S.; Gaydos, C.A.; Madico, G.E.; Glad, S.M.; Paigen, B.; Quinn, T.C.; Epstein, S.E. Atherosclerosis in apoE knockout mice infected with multiple pathogens. J. Infect. Dis. 2001, 183, 226–231. [Google Scholar] [CrossRef]
- Tang-Feldman, Y.J.; Lochhead, S.R.; Lochhead, G.R.; Yu, C.; George, M.; Villablanca, A.C.; Pomeroy, C. Murine cytomegalovirus (MCMV) infection upregulates P38 MAP kinase in aortas of Apo E KO mice: A molecular mechanism for MCMV-induced acceleration of atherosclerosis. J. Cardiovasc. Transl. Res. 2013, 6, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Vliegen, I.; Duijvestijn, A.; Grauls, G.; Herngreen, S.; Bruggeman, C.; Stassen, F. Cytomegalovirus infection aggravates atherogenesis in apoE knockout mice by both local and systemic immune activation. Microbes Infect. 2004, 6, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Vliegen, I.; Herngreen, S.B.; Grauls, G.E.; Bruggeman, C.A.; Stassen, F.R. Mouse cytomegalovirus antigenic immune stimulation is sufficient to aggravate atherosclerosis in hypercholesterolemic mice. Atherosclerosis 2005, 181, 39–44. [Google Scholar] [CrossRef]
- Biron, K.K. Antiviral drugs for cytomegalovirus diseases. Antivir. Res. 2006, 71, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Perera, M.R.; Wills, M.R.; Sinclair, J.H. HCMV Antivirals and Strategies to Target the Latent Reservoir. Viruses 2021, 13, 817. [Google Scholar] [CrossRef]
- Effros, R.B.; Fletcher, C.V.; Gebo, K.; Halter, J.B.; Hazzard, W.R.; Horne, F.M.; Huebner, R.E.; Janoff, E.N.; Justice, A.C.; Kuritzkes, D.; et al. Aging and infectious diseases: Workshop on HIV infection and aging: What is known and future research directions. Clin. Infect. Dis. 2008, 47, 542–553. [Google Scholar] [CrossRef]
- Stone, S.F.; Price, P.; Keane, N.M.; Murray, R.J.; French, M.A. Levels of IL-6 and soluble IL-6 receptor are increased in HIV patients with a history of immune restoration disease after HAART. HIV Med. 2002, 3, 21–27. [Google Scholar] [CrossRef]
- Park, I.W.; Wang, J.F.; Groopman, J.E. HIV-1 Tat promotes monocyte chemoattractant protein-1 secretion followed by transmigration of monocytes. Blood 2001, 97, 352–358. [Google Scholar] [CrossRef]
- Shrestha, S.; Irvin, M.R.; Grunfeld, C.; Arnett, D.K. HIV, inflammation, and calcium in atherosclerosis. Arter. Thromb. Vasc. Biol. 2014, 34, 244–250. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.; Miyoshi, T.; Bao, Y.; Sheehan, J.P.; Matsumoto, A.H.; Shi, W. Microarray analysis of gene expression in mouse aorta reveals role of the calcium signaling pathway in control of atherosclerosis susceptibility. Am. J. Physiol. Circ. Physiol. 2009, 296, H1336–H1343. [Google Scholar] [CrossRef] [Green Version]
- Dickhout, J.G.; Sood, S.K.; Austin, R.C. Role of endoplasmic reticulum calcium disequilibria in the mechanism of homocysteine-induced ER stress. Antioxidants Redox Signal. 2007, 9, 1863–1873. [Google Scholar] [CrossRef]
- Tabas, I. The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ. Res. 2010, 107, 839–850. [Google Scholar] [CrossRef] [Green Version]
- Strategies for Management of Antiretroviral Therapy (SMART) Study Group; El-Sadr, W.M.; Lundgren, J.; Neaton, J.D.; Gordin, F.; Abrams, D.; Arduino, R.C.; Babiker, A.; Burman, W.; Clumeck, N.; et al. CD4+ count-guided interruption of antiretroviral treatment. N. Engl. J. Med. 2006, 355, 2283–2296. [Google Scholar] [CrossRef] [Green Version]
- SMART Study Group; El-Sadr, W.M.; Grund, B.; Neuhaus, J.; Babiker, A.; Cohen, C.J.; Darbyshire, J.; Emery, S.; Lundgren, J.D.; Phillips, A.; et al. Risk for opportunistic disease and death after reinitiating continuous antiretroviral therapy in patients with HIV previously receiving episodic therapy: A randomized trial. Ann. Intern. Med. 2008, 149, 289–299. [Google Scholar] [CrossRef]
- Cunha, R.F.; Simões, S.; Carvalheiro, M.; Pereira, J.M.A.; Costa, Q.; Ascenso, A. Novel Antiretroviral Therapeutic Strategies for HIV. Molecules 2021, 26, 5305. [Google Scholar] [CrossRef]
- Bares, S.H.; Scarsi, K.K. A new paradigm for antiretroviral delivery: Long-acting cabotegravir and rilpivirine for the treatment and prevention of HIV. Curr. Opin. HIV AIDS 2022, 17, 22–31. [Google Scholar] [CrossRef]
- The Centers for Disease Control and Prevention. Preexposure Prophylaxis for the Prevention of HIV Infection in The United States–2021 Update. Available online: https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf (accessed on 24 August 2022).
- The Centers for Disease Control and Prevention. HIV–Pre-Exposure Prophyljaxis (PrEP). Available online: https://www.cdc.gov/hiv/clinicians/prevention/prep.html (accessed on 24 August 2022).
- The United States Food and Drug Administration. FDA NEWS RELEASE–FDA Approves First Injectable Treatment for HIV Pre-Exposure Prevention. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-injectable-treatment-hiv-pre-exposure-prevention (accessed on 24 August 2022).
- Poznyak, A.V.; Bezsonov, E.E.; Borisov, E.E.; Grechko, A.V.; Kartuesov, A.G.; Orekhov, A.N. Atherosclerosis in HIV Patients: What Do We Know so Far? Int. J. Mol. Sci. 2022, 23, 2504. [Google Scholar] [CrossRef]
- Kovacs, L.; Kress, T.C.; Belin de Chantemèle, E.J. HIV, Combination Antiretroviral Therapy, and Vascular Diseases in Men and Women. JACC: Basic Transl. Sci. 2022, 7, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Hemmat, N.; Ebadi, A.; Badalzadeh, R.; Memar, M.Y.; Baghi, H.B. Viral infection and atherosclerosis. Eur. J. Clin. Microbiol. 2018, 37, 2225–2233. [Google Scholar] [CrossRef]
- Rosenfeld, M.E.; Campbell, L.A. Pathogens and atherosclerosis: Update on the potential contribution of multiple infectious organisms to the pathogenesis of atherosclerosis. Thromb. Haemost. 2011, 106, 858–867. [Google Scholar] [CrossRef]
- Magadum, A.; Kishore, R. Cardiovascular Manifestations of COVID-19 Infection. Cells 2020, 9, 2508. [Google Scholar] [CrossRef]
- Maitz, T.; Parfianowicz, D.; Vojtek, A.; Rajeswaran, Y.; Vyas, A.V.; Gupta, R. COVID-19 Cardiovascular Connection: A Review of Cardiac Manifestations in COVID-19 Infection and Treatment Modalities. Curr. Probl. Cardiol. 2022, 101186. [Google Scholar] [CrossRef]
- Parise, R.S.; Ramesh, S.; Govindarajulu, M.; Ajoolabady, A.; Moore, T.; Dhanasekaran, M. COVID-19-induced cardiovascular damage differs from other prevalent viruses. Cardiol. Plus 2021, 6, 231–245. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, S.-H.; Lee, K.-T. Atherosclerosis by Virus Infection—A Short Review. Biomedicines 2022, 10, 2634. https://doi.org/10.3390/biomedicines10102634
Jung S-H, Lee K-T. Atherosclerosis by Virus Infection—A Short Review. Biomedicines. 2022; 10(10):2634. https://doi.org/10.3390/biomedicines10102634
Chicago/Turabian StyleJung, Seang-Hwan, and Kyung-Tae Lee. 2022. "Atherosclerosis by Virus Infection—A Short Review" Biomedicines 10, no. 10: 2634. https://doi.org/10.3390/biomedicines10102634
APA StyleJung, S.-H., & Lee, K.-T. (2022). Atherosclerosis by Virus Infection—A Short Review. Biomedicines, 10(10), 2634. https://doi.org/10.3390/biomedicines10102634





