Obesity-Related Genes Expression in Testes and Sperm Parameters Respond to GLP-1 and Caloric Restriction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model and Experimental Design
2.2. Assessment of Sperm Quality Parameters
2.3. Assessment of Blood Glucose and Hormonal Levels
2.4. Polymerase Chain Reaction (PCR) Conditions
2.5. Immunohistochemistry (IHC)
2.6. Ferric-Reducing Antioxidant Power Assay (FRAP)
2.7. Statistical Analysis
3. Results
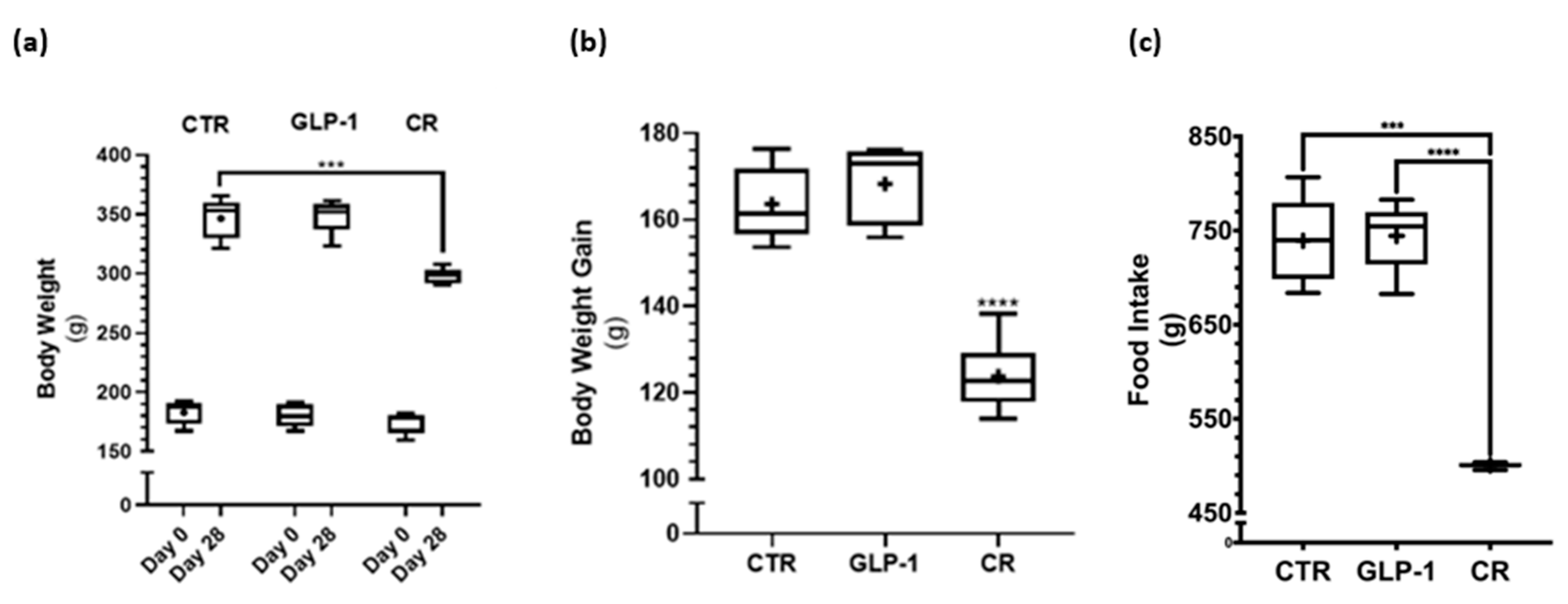
3.1. Caloric Restriction Decreased Body Weight Gain While No Difference in Body Weight Gain Was Detected after GLP-1 Administration
3.2. Caloric Restriction Resulted in Lower Insulin Resistance and Altered Adiposity Hormone Profile
3.3. GLP-1 Administration Resulted in a Lower Percentage of Sperm Morphology Defects
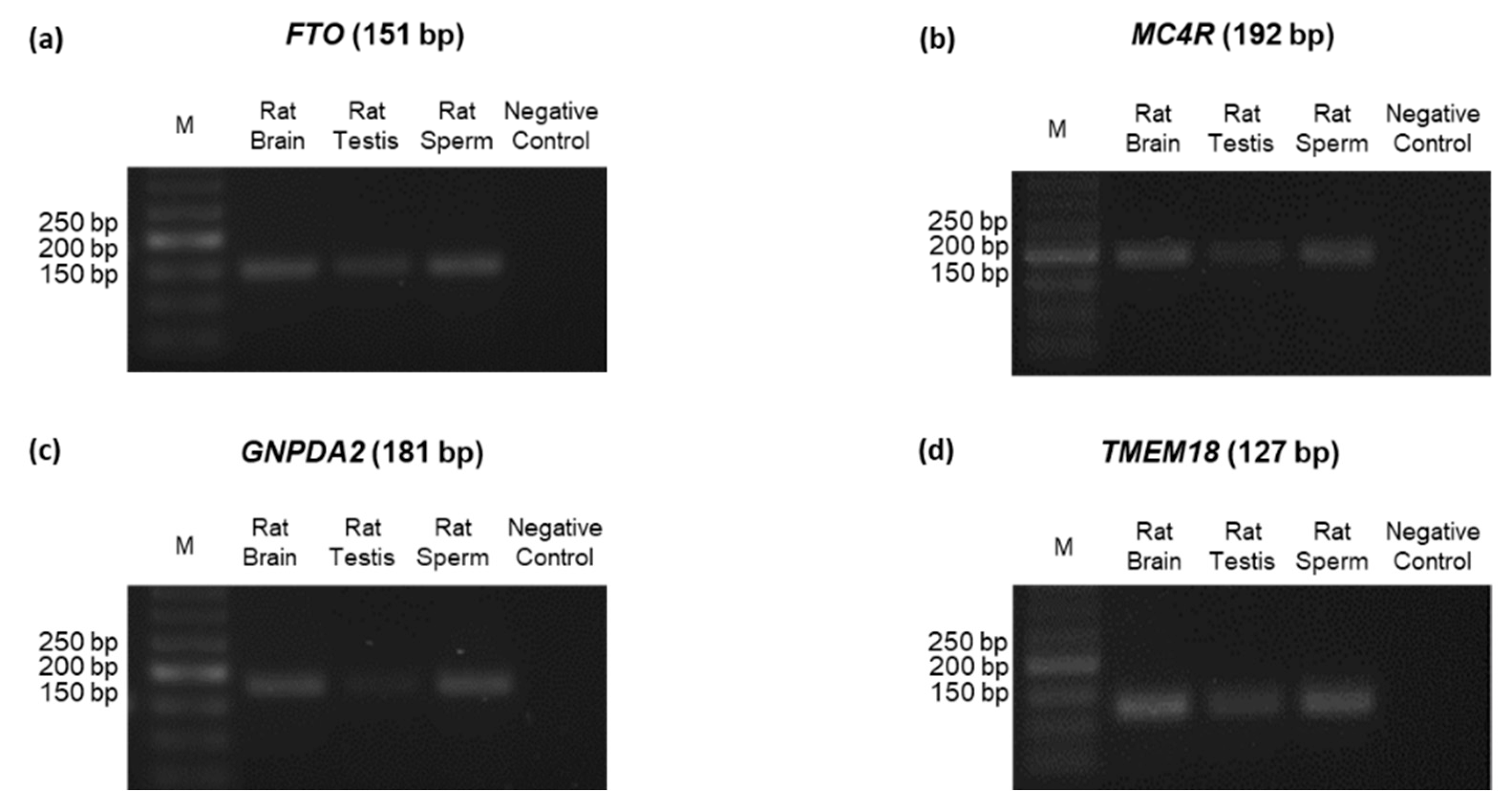
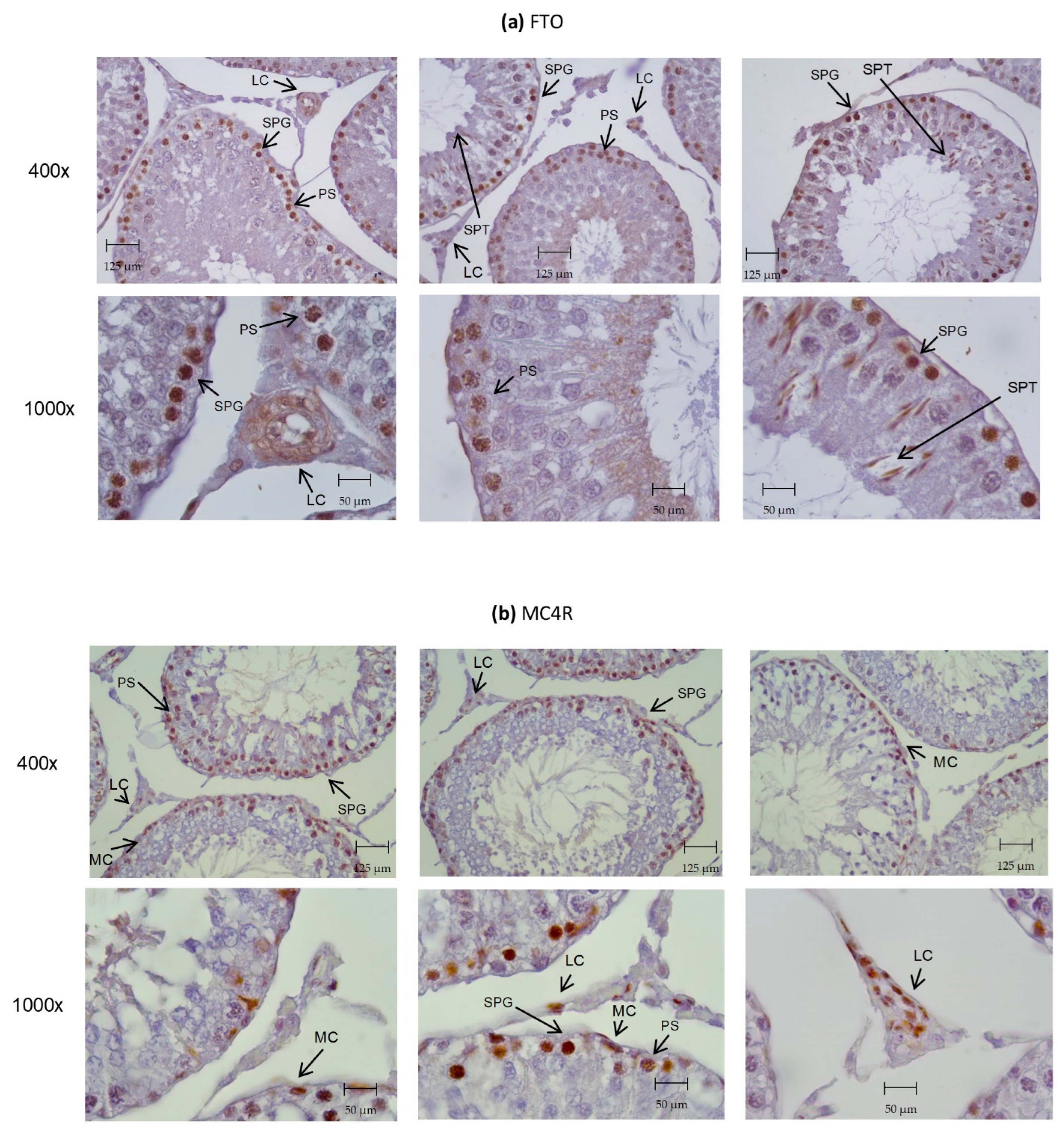
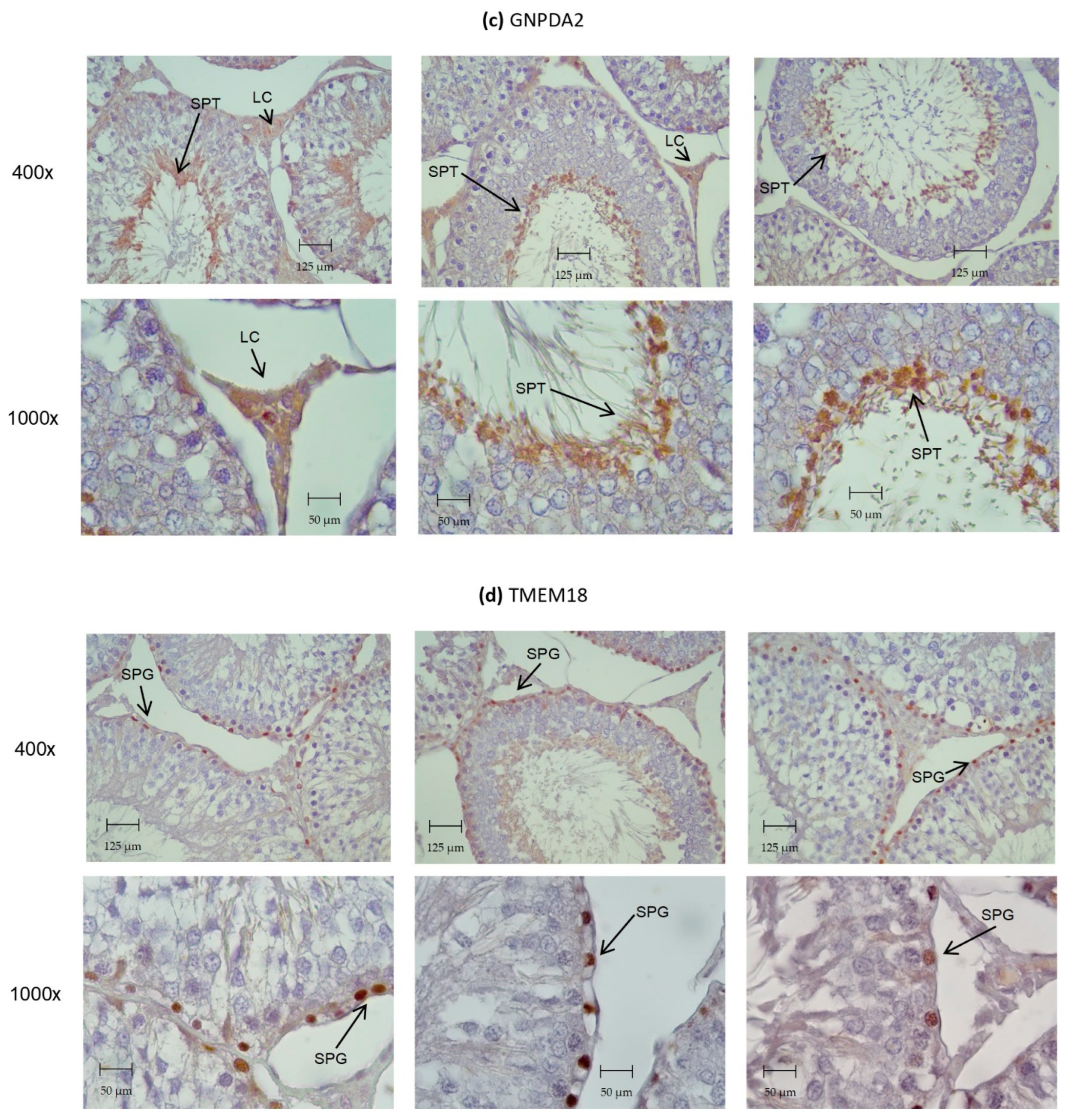
3.4. Identification of ORG Transcripts and Protein on the Testes of Wistar Rats
3.5. Caloric Restriction and GLP-1 Administration Increased ORG Expression in Testes
3.6. CR and GLP-1 Administration Increased Testicular NEF2L2 Expression but Did Not Alter Testes’ Total Antioxidant Capacity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Havel, P.J. Peripheral Signals Conveying Metabolic Information to the Brain: Short-Term and Long-Term Regulation of Food Intake and Energy Homeostasis. Exp. Biol. Med. 2001, 226, 963–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roh, E.; Kim, M.-S. Brain Regulation of Energy Metabolism. Endocrinol. Metab. 2016, 31, 519–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, R.F. Long- and short-term regulation of feeding patterns in the rat. J. Comp. Physiol. Psychol. 1977, 91, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Niswender, K.D.; Schwartz, M.W. Insulin and leptin revisited: Adiposity signals with overlapping physiological and intracellular signaling capabilities. Front. Neuroendocr. 2003, 24, 1–10. [Google Scholar] [CrossRef]
- Rogge, G.; Jones, D.; Hubert, G.W.; Lin, Y.; Kuhar, M.J. CART peptides: Regulators of body weight, reward and other functions. Nat. Rev. Neurosci. 2008, 9, 747–758. [Google Scholar] [CrossRef] [Green Version]
- Dimitriadis, G.; Mitrou, P.; Lambadiari, V.; Maratou, E.; Raptis, S.A. Insulin effects in muscle and adipose tissue. Diabetes Res. Clin. Pract. 2011, 93, S52–S59. [Google Scholar] [CrossRef]
- Cowley, M.A.; Smith, R.G.; Diano, S.; Tschöp, M.; Pronchuk, N.; Grove, K.L.; Strasburger, C.J.; Bidlingmaier, M.; Esterman, M.; Heiman, M.L.; et al. The Distribution and Mechanism of Action of Ghrelin in the CNS Demonstrates a Novel Hypothalamic Circuit Regulating Energy Homeostasis. Neuron 2003, 37, 649–661. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, P.E.; El-Kholy, W.; Riedel, M.J.; Salapatek, A.M.F.; Light, P.E.; Wheeler, M.B. The Multiple Actions of GLP-1 on the Process of Glucose-Stimulated Insulin Secretion. Diabetes 2002, 51, S434–S442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burcelin, R.; Gourdy, P.; Dalle, S. GLP-1-Based Strategies: A Physiological Analysis of Differential Mode of Action. Physiology 2014, 29, 108–121. [Google Scholar] [CrossRef] [Green Version]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- Garvey, W.T.; Birkenfeld, A.L.; Dicker, D.; Mingrone, G.; Pedersen, S.D.; Satylganova, A.; Skovgaard, D.; Sugimoto, D.; Jensen, C.; Mosenzon, O. Efficacy and Safety of Liraglutide 3.0 mg in Individuals With Overweight or Obesity and Type 2 Diabetes Treated With Basal Insulin: The SCALE Insulin Randomized Controlled Trial. Diabetes Care 2020, 43, 1085–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, M.; Færch, L.; Jeppesen, O.K.; Pakseresht, A.; Pedersen, S.D.; Perreault, L.; Rosenstock, J.; Shimomura, I.; Viljoen, A.; Wadden, T.A.; et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): A randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet 2021, 397, 971–984. [Google Scholar] [CrossRef]
- Andersen, E.; Juhl, C.R.; Kjøller, E.T.; Lundgren, J.R.; Janus, C.; Dehestani, Y.; Saupstad, M.; Ingerslev, L.R.; Duun, O.M.; Jensen, S.B.K.; et al. Sperm count is increased by diet-induced weight loss and maintained by exercise or GLP-1 analogue treatment: A randomized controlled trial. Hum. Reprod. 2022, 37, 1414–1422. [Google Scholar] [CrossRef]
- Martins, A.D.; Monteiro, M.P.; Silva, B.M.; Barros, A.; Sousa, M.; Carvalho, R.A.; Oliveira, P.F.; Alves, M.G. Metabolic dynamics of human Sertoli cells are differentially modulated by physiological and pharmacological concentrations of GLP-1. Toxicol. Appl. Pharmacol. 2018, 362, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gow, M.L.; Baur, L.; Johnson, N.; Cowell, C.T.; Garnett, S. Reversal of type 2 diabetes in youth who adhere to a very-low-energy diet: A pilot study. Diabetologia 2016, 60, 406–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koubova, J.; Guarente, L. How does calorie restriction work? Genes Dev. 2003, 17, 313–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Fernandez, R.; Martini, A.; Navarro, V.; Castellano, J.M.; Dieguez, C.; Aguilar, E.; Pinilla, L.; Tena-Sempere, M. Novel signals for the integration of energy balance and reproduction. Mol. Cell. Endocrinol. 2006, 254–255, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.K.; Andersson, A.-M.; Jørgensen, N.; Andersen, A.-G.; Carlsen, E.; Petersen, J.H.; Skakkebæk, N.E. Body mass index in relation to semen quality and reproductive hormonesamong 1,558 Danish men. Fertil. Steril. 2004, 82, 863–870. [Google Scholar] [CrossRef]
- Guo, D.; Xu, M.; Zhou, Q.; Wu, C.; Ju, R.; Dai, J. Is low body mass index a risk factor for semen quality? A PRISMA-compliant meta-analysis. Medicine 2019, 98, e16677. [Google Scholar] [CrossRef]
- Martins, A.D.; Jarak, I.; Morais, T.; Carvalho, R.; Oliveira, P.F.; Monteiro, M.; Alves, M.G. Caloric restriction alters the hormonal profile and testicular metabolome, resulting in alterations of sperm head morphology. Am. J. Physiol. Metab. 2020, 318, E33–E43. [Google Scholar] [CrossRef]
- Pereira, S.C.; Martins, A.C.; Moreira, B.P.; Bernardino, R.L.; Barros, A.; Monteiro, M.P.; Oliveira, P.F.; Alves, M.G. Obesity-related genes are expressed in human Sertoli cells and modulated by energy homeostasis regulating hormones. J. Cell. Physiol. 2020, 236, 5265–5277. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.C.; Martins, A.D.; Monteiro, M.P.; Pinto, S.; Barros, A.; Oliveira, P.F.; Alves, M.G. Expression of obesity-related genes in human spermatozoa affects the outcomes of reproductive treatments. F&S Sci. 2021, 2, 164–175. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Zhao, T.-X.; Wang, J.-K.; Shen, L.-J.; Long, C.-L.; Liu, B.; Wei, Y.; Han, L.-D.; Wei, Y.-X.; Wu, S.-D.; Wei, G.-H. Increased m6A RNA modification is related to the inhibition of the Nrf2-mediated antioxidant response in di-(2-ethylhexyl) phthalate-induced prepubertal testicular injury. Environ. Pollut. 2020, 259, 113911. [Google Scholar] [CrossRef] [PubMed]
- Mountjoy, K.G.; Wu, C.-S.J.; Dumont, L.M.; Wild, J.M. Melanocortin-4 Receptor Messenger Ribonucleic Acid Expression in Rat Cardiorespiratory, Musculoskeletal, and Integumentary Systems. Endocrinology 2003, 144, 5488–5496. [Google Scholar] [CrossRef] [Green Version]
- Almén, M.S.; Jacobsson, J.A.; Shaik, J.H.; Olszewski, P.K.; Cedernaes, J.; Alsiö, J.; Sreedharan, S.; Levine, A.S.; Fredriksson, R.; Marcus, C.; et al. The obesity gene, TMEM18, is of ancient origin, found in majority of neuronal cells in all major brain regions and associated with obesity in severely obese children. BMC Med. Genet. 2010, 11, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farhud, D.; Yeganeh, M.Z.; Yeganeh, M.Z. Nutrigenomics and Nutrigenetics. Iran. J. Public Health 2010, 39, 1–14. [Google Scholar]
- Flint, A.; Raben, A.; Astrup, A.; Holst, J.J. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J. Clin. Investig. 1998, 101, 515–520. [Google Scholar] [CrossRef]
- Vettor, R.; Di Vincenzo, A.; Maffei, P.; Rossato, M. Regulation of energy intake and mechanisms of metabolic adaptation or maladaptation after caloric restriction. Rev. Endocr. Metab. Disord. 2020, 21, 399–409. [Google Scholar] [CrossRef]
- Dias, T.R.; Alves, M.G.; Bernardino, R.; Martins, A.D.; Moreira, A.C.; Silva, J.; Barros, A.; Sousa, M.; Silva, B.M.; Oliveira, P.F. Dose-dependent effects of caffeine in human Sertoli cells metabolism and oxidative profile: Relevance for male fertility. Toxicology 2014, 328, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Wajda, A.; Łapczuk, J.; Grabowska, M.; Słojewski, M.; Laszczyńska, M.; Urasińska, E.; Droździk, M. Nuclear factor E2-related factor-2 (Nrf2) expression and regulation in male reproductive tract. Pharmacol. Rep. 2016, 68, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.F.; Sousa, M.; Silva, B.M.; Monteiro, M.P.; Alves, M.G. Obesity, energy balance and spermatogenesis. Reproduction 2017, 153, R173–R185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoed, M.D.; Luan, J.; Langenberg, C.; Cooper, C.; Sayer, A.A.; Jameson, K.; Kumari, M.; Kivimaki, M.; Hingorani, A.; Grøntved, A.; et al. Evaluation of common genetic variants identified by GWAS for early onset and morbid obesity in population-based samples. Int. J. Obes. 2012, 37, 191–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlauch, K.A.; Kulick, D.; Subramanian, K.; De Meirleir, K.L.; Palotás, A.; Lombardi, V.C. Single-nucleotide polymorphisms in a cohort of significantly obese women without cardiometabolic diseases. Int. J. Obes. 2018, 43, 253–262. [Google Scholar] [CrossRef]
- Yoganathan, P.; Karunakaran, S.; Ho, M.M.; Clee, S.M. Nutritional regulation of genome-wide association obesity genes in a tissue-dependent manner. Nutr. Metab. 2012, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Larsen, P.J.; Fledelius, C.; Knudsen, L.B.; Tang-Christensen, M. Systemic Administration of the Long-Acting GLP-1 Derivative NN2211 Induces Lasting and Reversible Weight Loss in Both Normal and Obese Rats. Diabetes 2001, 50, 2530–2539. [Google Scholar] [CrossRef] [Green Version]
- Hinnen, D. Glucagon-like peptide 1 receptor agonists for type 2 diabetes. Diabetes Spectr. 2017, 30, 202–210. [Google Scholar] [CrossRef] [Green Version]
- Berthoud, H.-R.; Morrison, C. The brain, appetite, and obesity. Annu. Rev. Psychol 2008, 59, 55–92. [Google Scholar] [CrossRef]
- Boender, A.J.; Van Rozen, A.J.; Adan, R.A. Nutritional state affects the expression of the obesity-associated genes Etv5, Faim2, Fto, and Negr1. Obesity 2012, 20, 2420–2425. [Google Scholar] [CrossRef]
- Olszewski, P.K.; Fredriksson, R.; Olszewska, A.M.; Stephansson, O.; Alsiö, J.; Radomska, K.J.; Levine, A.S.; Schiöth, H.B. Hypothalamic FTO is associated with the regulation of energy intake not feeding reward. BMC Neurosci. 2009, 10, 129. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, R.; Hägglund, M.; Olszewski, P.K.; Stephansson, O.; Jacobsson, J.A.; Olszewska, A.M.; Levine, A.S.; Lindblom, J.; Schiöth, H.B. The Obesity Gene, FTO, Is of Ancient Origin, Up-Regulated during Food Deprivation and Expressed in Neurons of Feeding-Related Nuclei of the Brain. Endocrinology 2008, 149, 2062–2071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poritsanos, N.J.; Lew, P.S.; Fischer, J.; Mobbs, C.; Nagy, J.I.; Wong, D.; Rüther, U.; Mizuno, T.M. Impaired hypothalamic Fto expression in response to fasting and glucose in obese mice. Nutr. Diabetes 2011, 1, e19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gredilla, R.; Barja, G. Minireview: The Role of Oxidative Stress in Relation to Caloric Restriction and Longevity. Endocrinology 2005, 146, 3713–3717. [Google Scholar] [CrossRef]
- Gantz, I.; Miwa, H.; Konda, Y.; Shimoto, Y.; Tashiro, T.; Watson, S.; DelValle, J.; Yamada, T. Molecular cloning, expression, and gene localization of a fourth melanocortin receptor. J. Biol. Chem. 1993, 268, 15174–15179. [Google Scholar] [CrossRef]
- Wu, L.; Ma, F.; Zhao, X.; Zhang, M.-X.; Wu, J.; Mi, J. GNPDA2 Gene Affects Adipogenesis and Alters the Transcriptome Profile of Human Adipose-Derived Mesenchymal Stem Cells. Int. J. Endocrinol. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Cheon, Y.-P.; Kim, C.-H. Impact of glycosylation on the unimpaired functions of the sperm. Clin. Exp. Reprod. Med. 2015, 42, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Majzoub, A.; Arafa, M.; Mahdi, M.; Agarwal, A.; Al Said, S.; Al-Emadi, I.; El Ansari, W.; Alattar, A.; Al Rumaihi, K.; Elbardisi, H. Oxidation–reduction potential and sperm DNA fragmentation, and their associations with sperm morphological anomalies amongst fertile and infertile men. Arab J. Urol. 2018, 16, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Kahraman, S.; Akarsu, C.; Cengiz, G.; Dirican, K.; Sözen, E.; Can, B.; Güven, C.; Vanderzwalmen, P. Fertility of ejaculated and testicular megalohead spermatozoa with intracytoplasmic sperm injection. Hum. Reprod. 1999, 14, 726–730. [Google Scholar] [CrossRef] [Green Version]
- Batra, V.; Dagar, K.; Nayak, S.; Kumaresan, A.; Kumar, R.; Datta, T.K. A Higher Abundance of O-Linked Glycans Confers a Selective Advantage to High Fertile Buffalo Spermatozoa for Immune-Evasion From Neutrophils. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Landgraf, K.; Klöting, N.; Gericke, M.; Maixner, N.; Guiu-Jurado, E.; Scholz, M.; Witte, A.V.; Beyer, F.; Schwartze, J.T.; Lacher, M.; et al. The Obesity-Susceptibility Gene TMEM18 Promotes Adipogenesis through Activation of PPARG. Cell Rep. 2020, 33, 108295. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Liu, L.-L.; Xian, H.; Cao, J.-C.; Zhang, C.; Zhang, Y.-H.; Chen, M.-M.; Qian, Y. Peroxisome proliferator-activated receptor gamma signaling in human sperm physiology. Asian J. Androl. 2015, 17, 942–947. [Google Scholar] [CrossRef]
- Kvandová, M.; Majzúnová, M.; Dovinová, I. The Role of PPARγ in Cardiovascular Diseases. Physiol. Res. 2016, 65, S343–S363. [Google Scholar] [CrossRef] [PubMed]
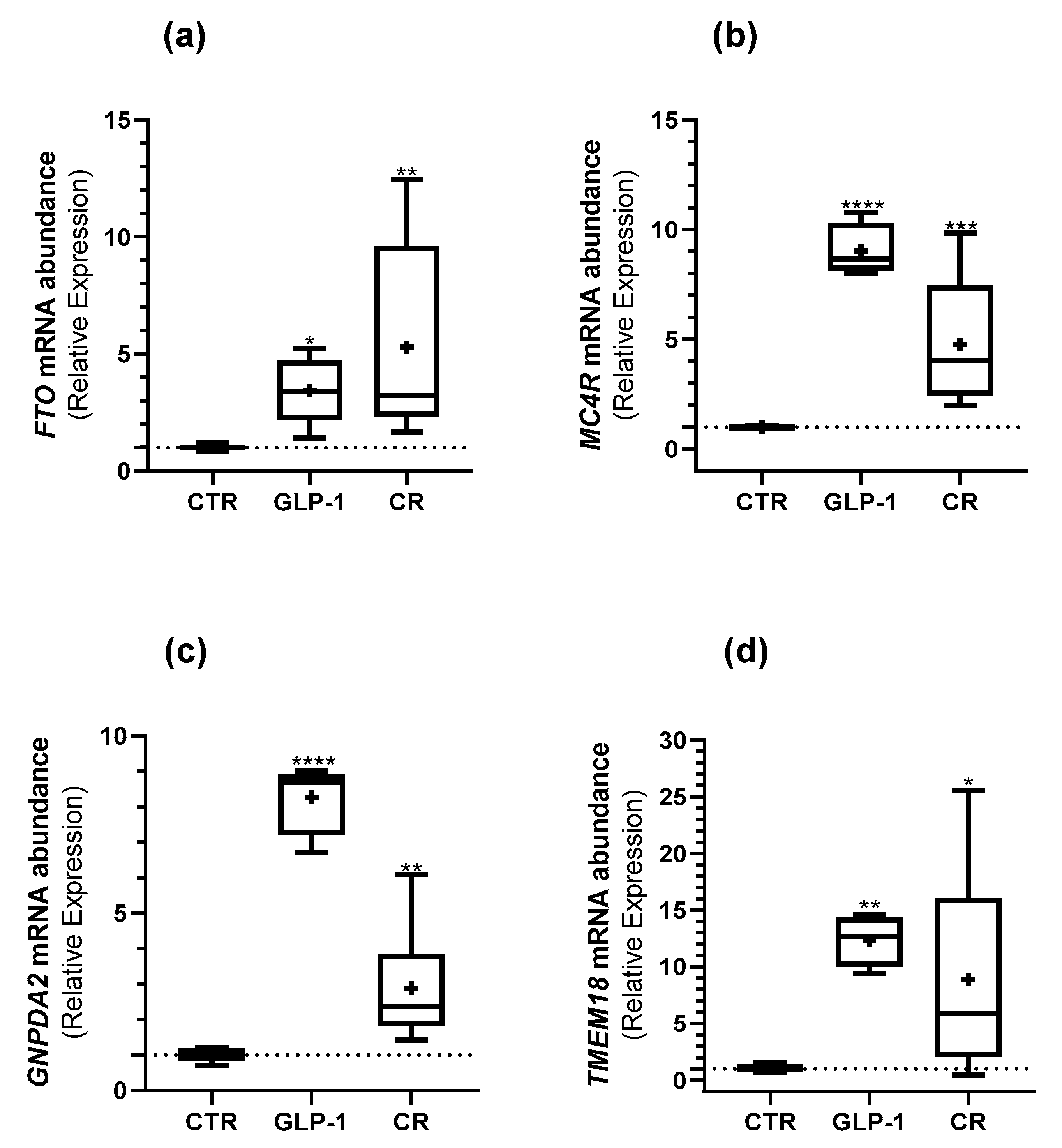
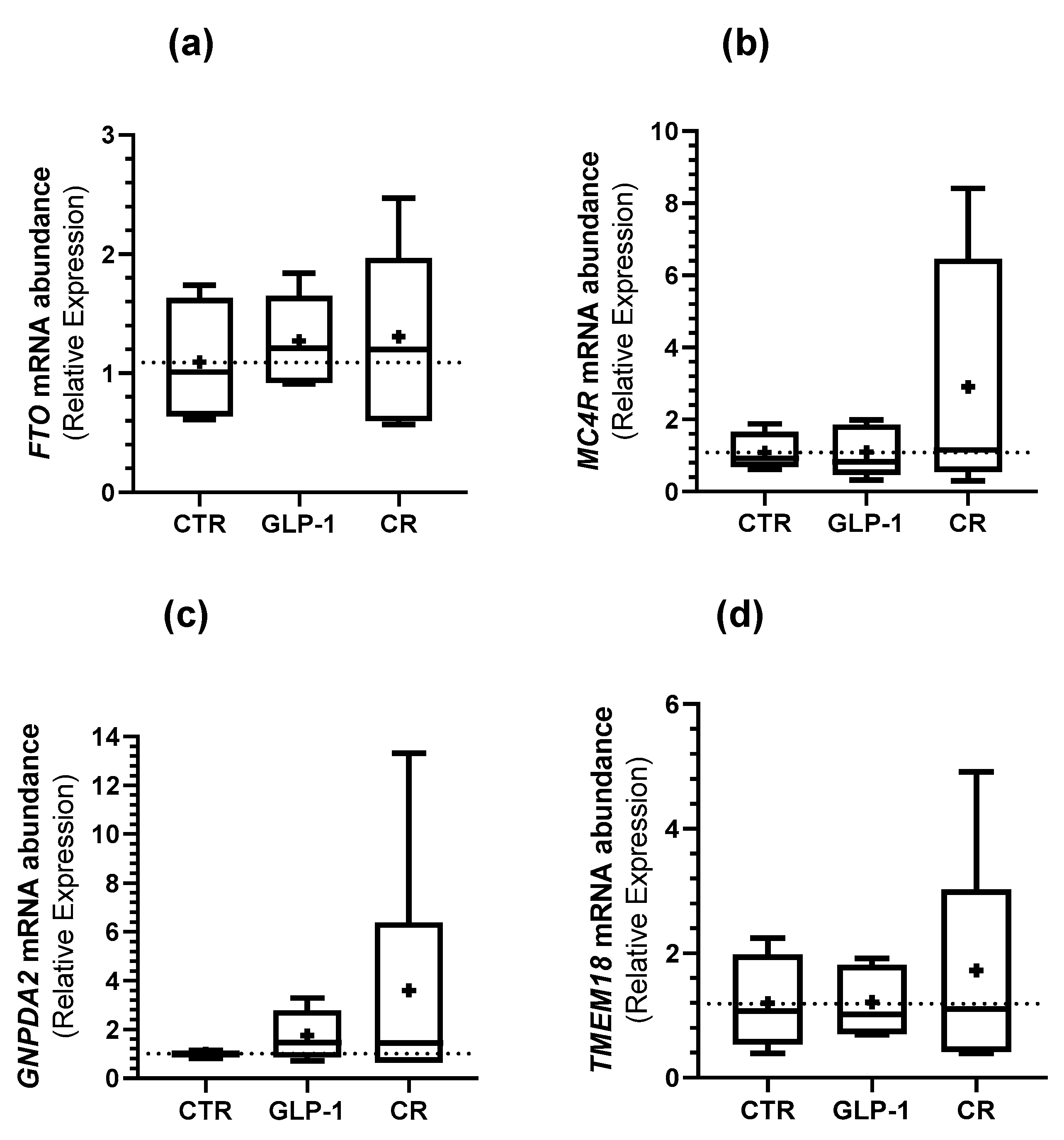

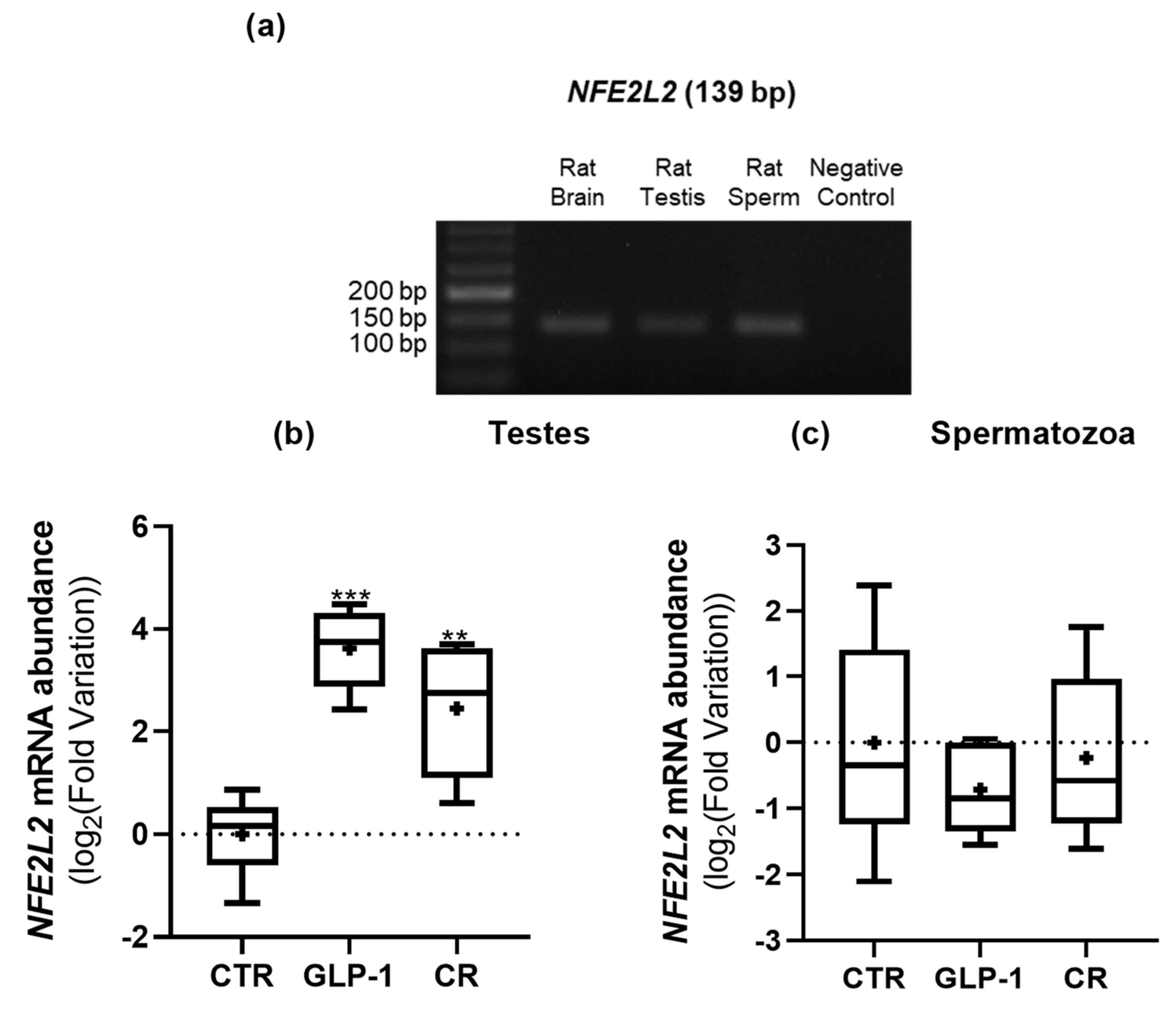
| Gene Name | GenBank Accession Number | Primer sequence (5′–3’) | Amplicon Length | Annealing Temperature | Cycles |
|---|---|---|---|---|---|
| β2M | NM_012512.1 | Forward: CCGTGATCTTTCTGGTGCTTGTC | 150 bp | 58.0 °C | 35 |
| Reverse: CTATCTGAGGTGGGTGGAACTGAG | |||||
| MC4R | NM_013099.3 | Forward: ACAAGAACCTGCACTCACCC | 192 bp | 62.0 °C | 35 |
| Reverse: ATGCGAGCAAGGAGCTACAG | |||||
| FTO | NM_001039713.1 | Forward: CAGAGATCCCGATACGTGGC | 151 bp | 57.6 °C | 35 |
| Reverse: CTGTGAGCCAGCCAAAACAC | |||||
| GNPDA2 | NM_001106005.1 | Forward: ACCATCCCGAAAGCTACCAT | 181 bp | 58.4 °C | 35 |
| Reverse: GGACCTATTCCTCCAACAAAAAGAT | |||||
| TMEM18 | NM_001007748.1 | Forward: AAGCATGGTGAATGGGGACC | 127 bp | 56.4 °C | 35 |
| Reverse: CACACTCAAACCTGCGTGAC | |||||
| NFE2L2 | NM_031789.2 | Forward: CAATGACTCTGACTCCGGCA | 139 bp | 56.6 °C | 35 |
| Reverse: AGGGGCACTGTCTAGCTCTT |
| Antibody | Company | Catalog Number | Concentration for IHC |
|---|---|---|---|
| Anti-FTO | Abcam | ab92821 | 1:300 |
| Anti-MC4R | Abcam | ab24233 | 1:75 |
| Anti-GNPDA2 | Abcam | ab106363 | 1:150 |
| Anti-TMEM18 | Abcam | ab100954 | 1:150 |
| Parameter | Control | GLP-1 Administration | Caloric Restriction |
|---|---|---|---|
| Active GLP-1 (pM) | 2.5 ± 0.2 | 15.7 ± 4.4 ↑ ** | 5.0 ± 1.5 ↔ |
| Glucose (mg/dL) | 89.6 ± 2.2 | 102.6 ± 7.1 ↔ | 88.0 ± 3.2 ↔ |
| Insulin (ng/mL) | 5.6 ± 0.5 | 5.2 ± 0.9 ↔ | 2.0 ± 0.2 ↓ *** |
| HOMA-IR | 35.3 ± 2.4 | 38.8 ± 8.1 ↔ | 12.3 ± 0.9 ↓ ** |
| Ghrelin (ng/mL) | 0.6 ± 0.1 | 0.6 ± 0.2 ↔ | 1.0 ± 0.2 ↑ * |
| Leptin (ng/mL) | 6.9 ± 0.7 | 6.3 ± 0.8 ↔ | 2.1 ± 0.3 ↓ *** |
| Parameter | Control | GLP-1 Administration | Caloric Restriction | |
|---|---|---|---|---|
| Testes Weight (g) | 3.6 ± 0.1 | 3.3 ± 0.2 | 3.2 ± 0.2 | |
| Epididymis Weight (g) | 1.3 ± 0.1 | 1.1 ± 0.1 | 1.0 ± 0.1 | |
| Gonadosomatic Index (%) | 1.0 ± 05 | 0.9 ± 0.6 | 1.1 ± 0.8 | |
| Sperm Concentration (Spermatozoa/mL × 106) | 39.5 ± 4.6 | 45.1 ± 4.2 | 30.8 ± 4.7 | |
| Sperm Vitality (% Spermatozoa) | Viable | 42.6 | 43.8 | 36.2 |
| Non-viable | 57.4 | 56.2 | 63.8 | |
| Sperm Morphology (% Spermatozoa) | Normal | 60.6 ± 2.3 | 66.2 ± 2.1 * | 59.5 ± 1.7 |
| Head Defects | 5.7 ± 1.3 | 5.3 ± 1.0 | 9.4 ± 0.8 * | |
| Neck Defects | 19.6 ± 3.2 | 15.9 ± 2.7 | 19.1 ± 3.3 | |
| Tail Defects | 13.7 ± 1.1 | 12.0 ± 2.2 | 12.6 ± 2.2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correia, A.S.; Pereira, S.C.; Morais, T.; Martins, A.D.; Monteiro, M.P.; Alves, M.G.; Oliveira, P.F. Obesity-Related Genes Expression in Testes and Sperm Parameters Respond to GLP-1 and Caloric Restriction. Biomedicines 2022, 10, 2609. https://doi.org/10.3390/biomedicines10102609
Correia AS, Pereira SC, Morais T, Martins AD, Monteiro MP, Alves MG, Oliveira PF. Obesity-Related Genes Expression in Testes and Sperm Parameters Respond to GLP-1 and Caloric Restriction. Biomedicines. 2022; 10(10):2609. https://doi.org/10.3390/biomedicines10102609
Chicago/Turabian StyleCorreia, Ana S., Sara C. Pereira, Tiago Morais, Ana D. Martins, Mariana P. Monteiro, Marco G. Alves, and Pedro F. Oliveira. 2022. "Obesity-Related Genes Expression in Testes and Sperm Parameters Respond to GLP-1 and Caloric Restriction" Biomedicines 10, no. 10: 2609. https://doi.org/10.3390/biomedicines10102609
APA StyleCorreia, A. S., Pereira, S. C., Morais, T., Martins, A. D., Monteiro, M. P., Alves, M. G., & Oliveira, P. F. (2022). Obesity-Related Genes Expression in Testes and Sperm Parameters Respond to GLP-1 and Caloric Restriction. Biomedicines, 10(10), 2609. https://doi.org/10.3390/biomedicines10102609








