Phenotyping Post-COVID Pain as a Nociceptive, Neuropathic, or Nociplastic Pain Condition
Abstract
:1. Introduction
2. Phenotyping Post-COVID Pain
2.1. Nociceptive Pain
2.2. Neuropathic Pain
2.3. Nociplastic Pain
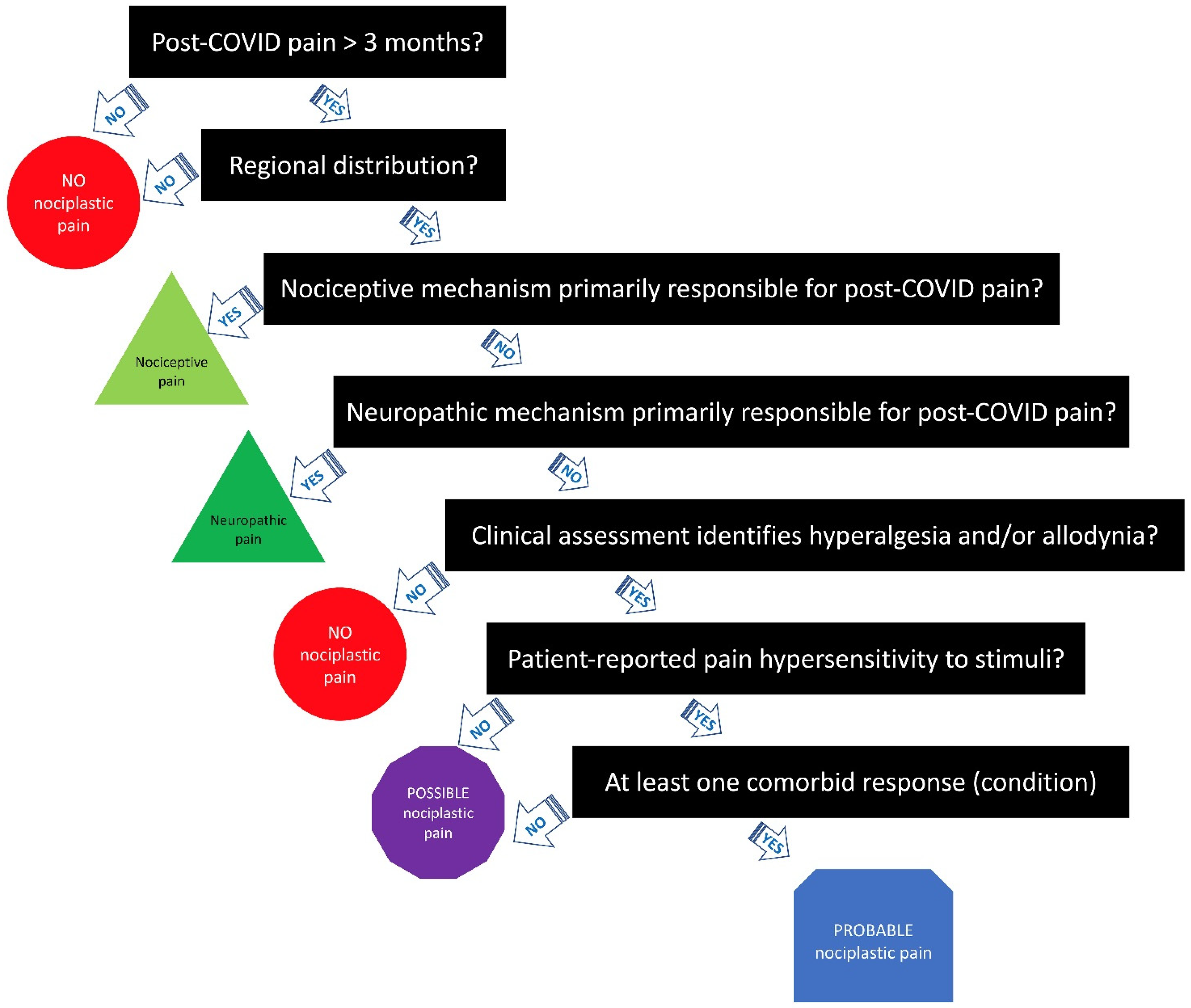
3. Clinical Criteria/Grading System for Nociplastic Pain in Post-COVID Pain
3.1. Step 1—Duration of Pain
3.2. Step 2—Distribution of Pain
3.3. Step 3—Determine Whether Nociceptive Pain Is Present
3.4. Step 4—Determine Whether Neuropathic Pain Is Present
3.5. Step 5—Elucidate the Presence of Pain Hypersensitivity
3.6. Step 6—Check for History of Pain Hypersensitivity
3.7. Step 7—Determine Whether Comorbidities Are Present
4. Toward Precision Pain Medicine for Post-COVID Pain?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernández-de-las-Peñas, C. Long COVID: Current definition. Infection 2022, 50, 285–286. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V.; WHO Clinical Case Definition Working Group on Post-COVID-19 Condition. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef]
- Hayes, L.D.; Ingram, J.; Sculthorpe, N.F. More Than 100 Persistent Symptoms of SARS-CoV-2 (Long COVID): A scoping review. Front Med. 2021, 8, 750378. [Google Scholar] [CrossRef]
- Michelen, M.; Manoharan, L.; Elkheir, N.; Cheng, V.; Dagens, A.; Hastie, C.; O’Hara, M.; Suett, J.; Dahmash, D.; Bugaeva, P.; et al. Characterising long COVID: A living systematic review. BMJ Glob. Health 2021, 6, e005427. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-las-Peñas, C.; Palacios-Ceña, D.; Gómez-Mayordomo, V.; Florencio, L.L.; Cuadrado, M.L.; Plaza-Manzano, G.; Navarro-Santana, M. Prevalence of post-COVID-19 symptoms in hospitalized and non-hospitalized COVID-19 survivors: A systematic review and meta-analysis. Eur. J. Inter. Med. 2021, 92, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Haupert, S.R.; Zimmermann, L.; Shi, X.; Fritsche, L.G.; Mukherjee, B. Global prevalence of post COVID-19 condition or long COVID: A meta-analysis and systematic review. J. Infect. Dis. 2022, jiac136. [Google Scholar] [CrossRef] [PubMed]
- Alkodaymi, M.S.; Omrani, O.A.; Fawzy, N.A.; Shaar, B.A.; Almamlouk, R.; Riaz, M.; Obeidat, M.; Obeidat, Y.; Gerberi, D.; Taha, R.M.; et al. Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2022, 28, 657–666. [Google Scholar] [CrossRef]
- Han, Q.; Zheng, B.; Daines, L.; Sheikh, A. Long-Term Sequelae of COVID-19: A Systematic Review and Meta-Analysis of One-Year Follow-Up Studies on Post-COVID Symptoms. Pathogens 2022, 11, 269. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Yan, M.Z.; Li, X.; Lau, E.H.Y. Sequelae of COVID-19 among previously hospitalized patients up to 1 year after discharge: A systematic review and meta-analysis. Infection 2022, 50, 1067–1109. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C.; Navarro-Santana, M.; Plaza-Manzano, G.; Palacios-Ceña, D.; Arendt-Nielsen, L. Time course prevalence of Post-COVID pain symptoms of musculoskeletal origin in patients who had survived to SARS-CoV-2 infection: A systematic review and meta-analysis. Pain 2022, 163, 1220–1231. [Google Scholar] [CrossRef]
- Bakılan, F.; Gökmen, İ.G.; Ortanca, B.; Uçan, A.; Eker Güvenç, Ş.; Şahin Mutlu, F.; Gökmen, H.M.; Ekim, A. Musculoskeletal symptoms and related factors in postacute COVID-19 patients. Int. J. Clin. Pract. 2021, 75, e14734. [Google Scholar] [CrossRef] [PubMed]
- Karaarslan, F.; Demircioğlu, G.F.; Kardeş, S. Post-discharge rheumatic and musculoskeletal symptoms following hospitalization for COVID-19: Prospective follow-up by phone interviews. Rheumatol. Int. 2021, 41, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Soares, F.H.C.; Kubota, G.T.; Fernandes, A.M.; Hojo, B.; Couras, C.; Costa, B.V.; Lapa, J.D.D.S.; Braga, L.M.; Almeida, M.M.; Cunha, P.H.M.D.; et al. “Pain in the Pandemic Initiative Collaborators”. Prevalence and characteristics of new-onset pain in COVID-19 survivours, a controlled study. Eur. J. Pain 2021, 25, 1342–1354. [Google Scholar] [CrossRef] [PubMed]
- Bileviciute-Ljungar, I.; Norrefalk, J.R.; Borg, K. Pain burden in post-COVID-19 syndrome following mild COVID-19 infection. J. Clin. Med. 2022, 11, 771. [Google Scholar] [CrossRef]
- National Research Council Committee on AFfDaNToD. The National Academies Collection: Reports Funded by National Institutes of Health. In Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Shraim, M.A.; Massé-Alarie, H.; Hodges, P.W. Methods to discriminate between mechanism-based categories of pain experienced in the musculoskeletal system: A systematic review. Pain 2021, 162, 1007–1037. [Google Scholar] [CrossRef]
- Kosek, E.; Cohen, M.; Baron, R.; Gebhart, G.F.; Mico, J.A.; Rice, A.S.; Rief, W.; Sluka, A.K. Do we need a third mechanistic descriptor for chronic pain states? Pain 2016, 157, 1382–1386. [Google Scholar] [CrossRef]
- Aydede, M.; Shriver, A. Recently introduced definition of nociplastic pain by the International Association for the Study of Pain needs better formulation. Pain 2018, 159, 1176–1177. [Google Scholar] [CrossRef]
- Kosek, E.; Clauw, D.; Nijs, J.; Baron, R.; Gilron, I.; Harris, R.E.; Mico, J.A.; Rice, A.S.C.; Sterling, M. Chronic nociplastic pain affecting the musculoskeletal system: Clinical criteria and grading system. Pain 2021, 162, 2629–2634. [Google Scholar] [CrossRef]
- Nijs, J.; Lahousse, A.; Kapreli, E.; Bilika, P.; Saraçoğlu, İ.; Malfliet, A.; Coppieters, I.; De Baets, L.; Leysen, L.; Roose, E.; et al. Nociplastic pain criteria or recognition of central sensitization? Pain phenotyping in the past, present and future. J. Clin. Med. 2021, 10, 3203. [Google Scholar]
- Nijs, J.; George, S.Z.; Clauw, D.J.; Fernández-de-las-Peñas, C.; Kosek, E.; Ickmans, K.; Fernández-Carnero, J.; Polli, A.; Kapreli, E.; Huysmans, E.; et al. Central sensitisation in chronic pain conditions: Latest discoveries and their potential for precision medicine. Lancet Rheumatol. 2021, 3, e383–e392. [Google Scholar] [CrossRef]
- Smart, K.M.; Blake, C.; Staines, A.; Doody, C. Clinical indicators of “nociceptive”, “peripheral neuropathic” and “central” mechanisms of musculoskeletal pain. A Delphi survey of expert clinicians. Man. Ther. 2010, 15, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Nijs, J.; Torres-Cueco, R.; van Wilgen, C.P.; Girbes, E.L.; Struyf, F.; Roussel, N.; van Oosterwijck, J.; Daenen, L.; Kuppens, K.; Vanwerweeen, L.; et al. Applying modern pain neuroscience in clinical practice: Criteria for the classification of central sensitization pain. Pain Phys. 2014, 17, 447–457. [Google Scholar] [CrossRef]
- Mulchandani, R.; Lyngdoh, T.; Kakkar, A.K. Deciphering the COVID-19 cytokine storm: Systematic review and meta-analysis. Eur. J. Clin. Investig. 2021, 51, e13429. [Google Scholar] [CrossRef] [PubMed]
- Coomes, E.A.; Haghbayan, H. Interleukin-6 in Covid-19: A systematic review and meta-analysis. Rev. Med. Virol. 2020, 30, 1–9. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, R.S.; Kilgore, A.E.; D’Souza, S. Manifestations of pain during the COVID-19 pandemic portrayed on social media: A cross-sectional study. Pain Med. 2021, 23, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-las-Peñas, C.; de-la-Llave-Rincón, A.I.; Ortega-Santiago, R.; Ambite-Quesada, S.; Gómez-Mayordomo, V.; Cuadrado, M.L.; Arias-Navalón, J.A.; Hernández-Barrera, V.; Martín-Guerrero, J.D.; Pellicer-Valero, O.J.; et al. Prevalence and risk factors of musculoskeletal pain symptoms as long-term post-COVID sequelae in hospitalized COVID-19 survivors: A multicenter study. Pain 2022, 163, e989–e996. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-las-Peñas, C.; Cancela-Cilleruelo, I.; Rodríguez-Jiménez, J.; Moro-López-Menchero, P.; Pellicer-Valero, O.J.; Martín-Guerrero, J.D.; Arendt-Nielsen, L. Exploring the trajectory curve of long-term musculoskeletal post-COVID pain symptoms in hospitalized COVID-19 survivors: A multicenter study. Pain 2022. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.N.; Hoang, V.T.; Dao, T.L.; Dudouet, P.; Eldin, C.; Gautret, P. Clinical patterns of somatic symptoms in patients suffering from post-acute long COVID: A systematic review. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 515–545. [Google Scholar] [CrossRef]
- Cui, D.; Wang, Y.; Huang, L.; Gu, X.; Huang, Z.; Mu, S.; Wang, C.; Cao, B. Rheumatic symptoms following Coronavirus Disease 2019 (COVID-19): A chronic post-COVID-19 condition. Open Forum Infect. Dis. 2022, 9, ofac170. [Google Scholar] [CrossRef]
- Pal, A.; Roongta, R.; Mondal, S.; Sinha, D.; Sinhamahapatra, P.; Ghosh, A.; Chattopadhyay, A. Does post-COVID reactive arthritis exist? Experience of a tertiary care centre with a review of the literature. Reumatol. Clin. 2022. [Google Scholar] [CrossRef]
- Khoja, O.; Silva Passadouro, B.; Mulvey, M.; Delis, I.; Astill, S.; Tan, A.L.; Sivan, M. Clinical characteristics and mechanisms of musculoskeletal pain in long COVID. J. Pain Res. 2022, 15, 1729–1748. [Google Scholar] [CrossRef] [PubMed]
- Gavrilova, N.; Soprun, L.; Lukashenko, M.; Ryabkova, V.; Fedotkina, T.V.; Churilov, L.P.; Shoenfeld, Y. New clinical phenotype of the post-covid syndrome: Fibromyalgia and joint hypermobility condition. Pathophysiology 2022, 29, 24–29. [Google Scholar] [PubMed]
- Scholz, J.; Finnerup, N.B.; Attal, N.; Aziz, Q.; Baron, R.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Cruccu, G.; Davis, K.D.; et al. Classification Committee of the Neuropathic Pain Special Interest Group (NeuPSIG). The IASP classification of chronic pain for ICD-11: Chronic neuropathic pain. Pain 2019, 160, 53–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torices, S.; Cabrera, R.; Stangis, M.; Naranjo, O.; Fattakhov, N.; Teglas, T.; Adesse, D.; Toborek, M. Expression of SARS-CoV-2-related receptors in cells of the neurovascular unit: Implications for HIV-1 infection. J. Neuroinflamm. 2021, 18, 167. [Google Scholar] [CrossRef]
- Oaklander, A.L. Clinical significance of angiotensin-converting enzyme 2 receptors for severe acute respiratory syndrome coronavirus 2 (COVID-19) on peripheral small-fiber sensory neurons is unknown today. Pain 2020, 161, 2431–2433. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.; Gyanpuri, V.; Pathak, A.; Chaurasia, R.N.; Mishra, V.N.; Kumar, A.; Singh, V.K.; Dhiman, N.R. Neuropathic pain associated with COVID-19: A systematic review of case reports. Curr. Pain Headache Rep. 2022, 22, 1–9. [Google Scholar] [CrossRef]
- Rodríguez, Y.; Vatti, N.; Ramirez-Santana, C.; Chang, C.; Mancera-Páez, O.; Gershwin, M.E.; Anaya, J.M. Chronic inflammatory demyelinating polyneuropathy as an autoimmune disease. J. Autoimmun. 2019, 102, 8–37. [Google Scholar] [CrossRef] [PubMed]
- Oguz-Akarsu, E.; Gullu, G.; Kilic, E.; Dinç, Y.; Ursavas, A.; Yilmaz, E.; Zarifoglu, M.; Karli, N. Pandemic Study Team. Insight into pain syndromes in acute phase of mild-to-moderate COVID-19: Frequency, clinical characteristics, and associated factors. Eur. J. Pain 2021, 26, 492–504. [Google Scholar] [CrossRef] [PubMed]
- Attal, N.; Bouhassira, D.; Baron, R. Diagnosis and assessment of neuropathic pain through questionnaires. Lancet Neurol. 2018, 17, 456–466. [Google Scholar] [CrossRef]
- Herrero-Montes, M.; Fernández-de-las-Peñas, C.; Ferrer-Pargada, D.; Tello-Mena, S.; Cancela-Cilleruelo, I.; Rodríguez-Jiménez, J.; Palacios-Ceña, D.; Parás-Bravo, P. Prevalence of neuropathic component in post-COVID pain symptoms in previously hospitalized COVID-19 survivors. Int. J. Clin. Pract. 2022, 2022, 3532917. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-las-Peñas, C.; Valera-Calero, J.A.; Herrero-Montes, M.; Del-Valle-Loarte, P.; Rodríguez-Rosado, R.; Ferrer-Pargada, D.; Arendt-Nielsen, L.; Parás-Bravo, P. The Self-Reported Leeds Assessment of Neuropathic Symptoms and Signs (S-LANSS) and PainDETECT questionnaires in COVID-19 survivors with post-COVID pain. Viruses 2022, 14, 1486. [Google Scholar] [CrossRef] [PubMed]
- Magdy, R.; Eid, R.A.; Fathy, W.; Abdel-Aziz, M.M.; Ibrahim, R.E.; Yehia, A.; Sheemy, M.S.; Hussein, M. Characteristics and risk factors of persistent neuropathic pain in recovered COVID-19 patients. Pain Med. 2022, 23, 774–781. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C.; Herrero-Montes, M.; Ferrer-Pargada, D.; Izquierdo-Cuervo, S.; Palacios-Ceña, D.; Arendt-Nielsen, L.; Torres-Macho, J.; Parás-Bravo, P. Development of neuropathic post-COVID pain symptoms is not associated with serological biomarkers at hospital admission in COVID-19 survivors: A secondary analysis. Pain Med. 2022, pnac086. [Google Scholar] [CrossRef] [PubMed]
- Oaklander, A.L.; Mills, A.J.; Kelley, M.; Toran, L.S.; Smith, B.; Dalakas, M.C.; Nath, A. Peripheral neuropathy evaluations of patients with prolonged long COVID. Neurol. Neuroimmunol. Neuroinflamm. 2022, 9, e1146. [Google Scholar] [CrossRef]
- Loeser, J.D.; Treede, R.D. The Kyoto protocol of IASP basic pain terminology. Pain 2008, 137, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Fitzcharles, M.A.; Cohen, S.P.; Clauw, D.J.; Littlejohn, G.; Usui, C.; Häuser, W. Nociplastic pain: Towards an understanding of prevalent pain conditions. Lancet 2021, 397, 2098–2110. [Google Scholar] [CrossRef]
- Ursini, F.; Ciaffi, J.; Mancarella, L.; Lisi, L.; Brusi, V.; Cavallari, C.; D’Onghia, M.; Mari, A.; Borlandelli, E.; Faranda Cordella, J.; et al. Fibromyalgia: A new facet of the post-COVID-19 syndrome spectrum? Results from a web-based survey. RMD Open 2021, 7, e001735. [Google Scholar] [CrossRef]
- Neblett, R.; Cohen, H.; Choi, Y.; Hartzell, M.M.; Williams, M.; Mayer, T.G.; Gatchel, R.J. The Central Sensitization Inventory (CSI): Establishing clinically significant values for identifying central sensitivity syndromes in an outpatient chronic pain sample. J. Pain 2013, 14, 438–445. [Google Scholar] [CrossRef] [Green Version]
- Mayer, T.G.; Neblett, R.; Cohen, H.; Howard, K.J.; Choi, Y.H.; Williams, M.J.; Perez, Y.; Gatchel, R.J. The development and psychometric validation of the central sensitization inventory. Pain Pract. 2012, 12, 276–285. [Google Scholar] [CrossRef] [Green Version]
- Goudman, L.; De Smedt, A.; Noppen, M.; Moens, M. Is central sensitisation the missing link of persisting symptoms after COVID-19 infection? J. Clin. Med. 2021, 10, 5594. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C.; Parás-Bravo, P.; Ferrer-Pargada, D.; Cancela-Cilleruelo, I.; Rodríguez-Jiménez, J.; Nijs, J.; Arendt-Nielsen, L.; Herrero-Montes, M. Sensitization symptoms are associated with psychological and cognitive variables in COVID-19 survivors exhibiting post-COVID pain. Pain Pract. 2022. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-las-Peñas, C.; Herrero-Montes, M.; Cancela-Cilleruelo, I.; Rodríguez-Jiménez, J.; Parás-Bravo, P.; Varol, U.; Del-Valle-Loarte, P.; Flox-Benítez, G.; Arendt-Nielsen, L.; Valera-Calero, J.A. Understanding sensitization, cognitive and neuropathic associated mechanisms behind post-COVID pain: A Network Analysis. Diagnostics 2022, 12, 1538. [Google Scholar] [CrossRef]
- Maglietta, G.; Diodati, F.; Puntoni, M.; Lazzarelli, S.; Marcomini, B.; Patrizi, L.; Caminiti, C. Prognostic Factors for Post-COVID-19 Syndrome: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 1541. [Google Scholar] [CrossRef]
- Barbero, M.; Navarro-Santana, M.J.; Palacios-Ceña, M.; Ortega-Santiago, R.; Cescon, C.; Falla, D.; Fernández-de-las-Peñas, C. Clinical significance and diagnostic value of pain extent extracted from pain drawings: A scoping review. Diagnostics 2020, 10, 604. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-las-Peñas, C.; Fuensalida-Novo, S.; Ortega-Santiago, S.; Valera-Calero, J.A.; Cescon, C.; Derboni, M.; Giuffrida, V.; Barbero, M. Pain extent is not associated with sensory-associated symptoms, cognitive and psychological variables in COVID-19 survivors suffering from post-COVID pain. J. Clin. Med. 2022, 11, 4633. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Haroutounian, S.; Kamerman, P.; Baron, R.; Bennett, D.L.H.; Bouhassira, D.; Cruccu, G.; Freeman, R.; Hansson, P.; Nurmikko, T.; et al. Neuropathic pain: An updated grading system for research and clinical practice. Pain 2016, 157, 1599–1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boadas-Vaello, P.; Castany, S.; Homs, J.; Álvarez-Pérez, B.; Deulofeu, M.; Verdú, E. Neuroplasticity of ascending and descending pathways after somatosensory system injury: Reviewing knowledge to identify neuropathic pain therapeutic targets. Spinal Cord 2016, 54, 330–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-de-las-Peñas, C.; Plaza-Manzano, G. Carpal tunnel syndrome: Just a peripheral neuropathy? Pain Manag. 2018, 8, 209–216. [Google Scholar] [CrossRef]
- Uddin, Z.; MacDermid, J.C. Quantitative sensory testing in chronic musculoskeletal pain. Pain Med. 2016, 17, 1694–1703. [Google Scholar] [CrossRef] [Green Version]
- Walk, D.; Sehgal, N.; Moeller-Bertram, T.; Edwards, R.R.; Wasan, A.; Wallace, M.; Irving, G.; Argoff, C.; Backonja, M.M. Quantitative sensory testing and mapping: A review of nonautomated quantitative methods for examination of the patient with neuropathic pain. Clin. J. Pain 2009, 25, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.T.; Riveros, C.; Clepier, B.; Desvarieux, M.; Collet, C.; Yordanov, Y.; Ravaud, P. Development and validation of the Long Coronavirus Disease (COVID) Symptom and Impact Tools: A Set of Patient-Reported Instruments Constructed from patients’ lived experience. Clin. Infect. Dis. 2022, 74, 278–287. [Google Scholar] [CrossRef]
- Bonanni, R.; Cariati, I.; Tancredi, V.; Iundusi, R.; Gasbarra, E.; Tarantino, U. Chronic pain in musculoskeletal diseases: Do you know your enemy? J. Clin. Med. 2022, 11, 2609. [Google Scholar] [CrossRef] [PubMed]
- Jimeno-Almazán, A.; Pallarés, J.G.; Buendía-Romero, Á.; Martínez-Cava, A.; Franco-López, F.; Sánchez-Alcaraz Martínez, B.J.; Bernal-Morel, E.; Courel-Ibáñez, J. Post-COVID-19 syndrome and the potential benefits of exercise. Int. J. Environ. Res. Public Health 2021, 18, 5329. [Google Scholar] [CrossRef]
- Ahmadi Hekmatikar, A.H.; Ferreira Júnior, J.B.; Shahrbanian, S.; Suzuki, K. Functional and psychological changes after exercise training in post-COVID-19 patients discharged from the hospital: A PRISMA-compliant systematic review. Int. J. Environ. Res. Public Health 2022, 19, 2290. [Google Scholar] [CrossRef] [PubMed]
- Ferro Moura Franco, K.; Lenoir, D.; Dos Santos Franco, Y.R.; Jandre Reis, F.J.; Nunes Cabral, C.M.; Meeus, M. Prescription of exercises for the treatment of chronic pain along the continuum of nociplastic pain: A systematic review with meta-analysis. Eur. J. Pain 2021, 25, 51–70. [Google Scholar] [CrossRef]
- Twomey, R.; DeMars, J.; Franklin, K.; Culos-Reed, S.N.; Weatherald, J.; Wrightson, J.G. Chronic fatigue and postexertional malaise in people living with long COVID: An observational study. Phys. Ther. 2022, 102, pzac005. [Google Scholar] [CrossRef]
- Cattadori, G.; Di Marco, S.; Baravelli, M.; Picozzi, A.; Ambrosio, G. Exercise training in post-COVID-19 patients: The need for a multifactorial protocol for a multifactorial pathophysiology. J. Clin. Med. 2022, 11, 2228. [Google Scholar] [CrossRef] [PubMed]
- Bailly, M.; Pélissier, L.; Coudeyre, E.; Evrard, B.; Bingula, R.; Rochette, C.; Mériade, L.; Blavignac, C.; Fournier, A.C.; Bignon, Y.J.; et al. Systematic review of COVID-19-related physical activity-based rehabilitations: Benefits to be confirmed by more robust methodological approaches. Int. J. Environ. Res. Public Health 2022, 19, 9025. [Google Scholar] [CrossRef] [PubMed]
- Bodes-Pardo, G.; Lluch-Girbés, E.; Roussel, N.A.; Gallego-Izquierdo, T.; Jiménez-Penick, V.; Pecos-Martín, D. Pain neurophysiology education and therapeutic exercise for patients with chronic low back pain: A single-blind randomized controlled trial. Arch. Phys. Med. Rehabil. 2018, 99, 338–347. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-de-las-Peñas, C.; Nijs, J.; Neblett, R.; Polli, A.; Moens, M.; Goudman, L.; Shekhar Patil, M.; Knaggs, R.D.; Pickering, G.; Arendt-Nielsen, L. Phenotyping Post-COVID Pain as a Nociceptive, Neuropathic, or Nociplastic Pain Condition. Biomedicines 2022, 10, 2562. https://doi.org/10.3390/biomedicines10102562
Fernández-de-las-Peñas C, Nijs J, Neblett R, Polli A, Moens M, Goudman L, Shekhar Patil M, Knaggs RD, Pickering G, Arendt-Nielsen L. Phenotyping Post-COVID Pain as a Nociceptive, Neuropathic, or Nociplastic Pain Condition. Biomedicines. 2022; 10(10):2562. https://doi.org/10.3390/biomedicines10102562
Chicago/Turabian StyleFernández-de-las-Peñas, César, Jo Nijs, Randy Neblett, Andrea Polli, Maarten Moens, Lisa Goudman, Madhura Shekhar Patil, Roger D. Knaggs, Gisele Pickering, and Lars Arendt-Nielsen. 2022. "Phenotyping Post-COVID Pain as a Nociceptive, Neuropathic, or Nociplastic Pain Condition" Biomedicines 10, no. 10: 2562. https://doi.org/10.3390/biomedicines10102562
APA StyleFernández-de-las-Peñas, C., Nijs, J., Neblett, R., Polli, A., Moens, M., Goudman, L., Shekhar Patil, M., Knaggs, R. D., Pickering, G., & Arendt-Nielsen, L. (2022). Phenotyping Post-COVID Pain as a Nociceptive, Neuropathic, or Nociplastic Pain Condition. Biomedicines, 10(10), 2562. https://doi.org/10.3390/biomedicines10102562








