A Review on Therapeutic Potential of Natural Phytocompounds for Stroke
Abstract
1. Introduction
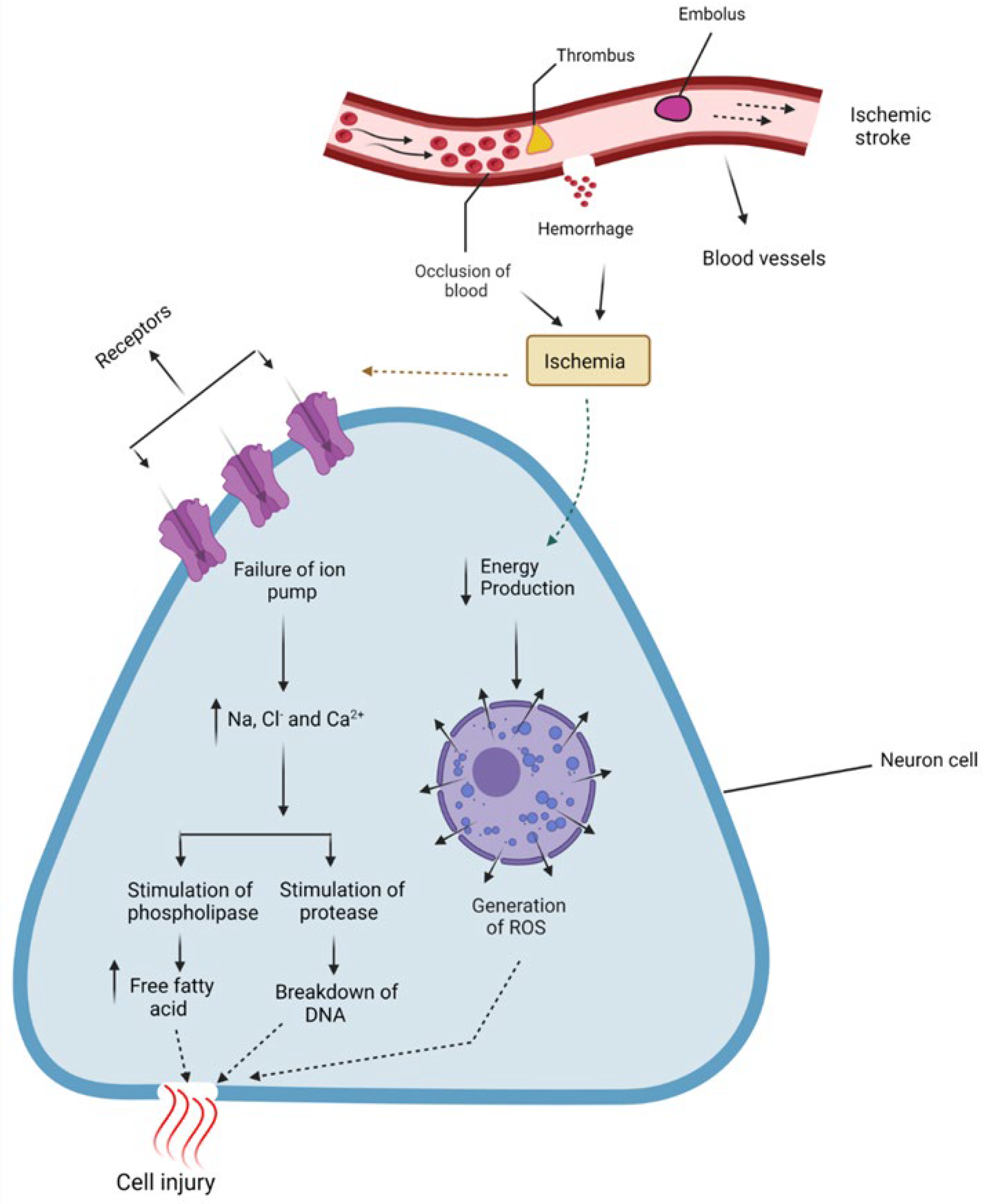
2. Pathophysiology
3. FDA-Approved Drugs for Stroke
4. Promising Phytocompounds for Stroke
4.1. Vitexin
4.2. Eriodictyol
4.3. Carveol
4.4. Ferulic Acid
4.5. Rosamirinic Acid
4.6. Paeoniflorin
4.7. Allicin
4.8. Curcumin
4.9. Ginkgolide K
4.10. 6″-O-succinylapigenin
4.11. Forsythiaside A
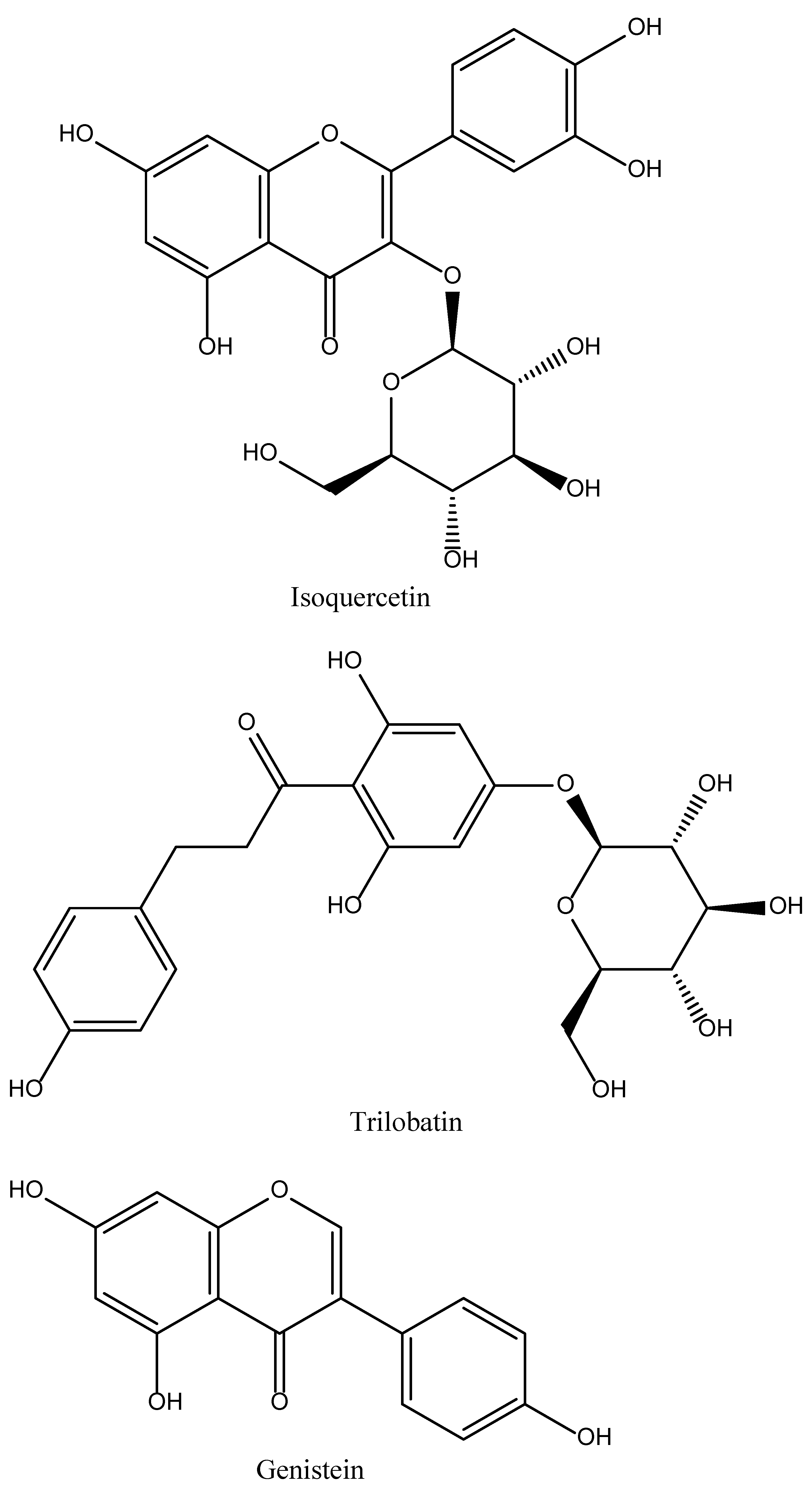
4.12. Isoquercetin
4.13. Trilobatin
4.14. Genistein
4.15. Tocotrienol
5. Conclusions and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gujjar, A.R.; El-Tigani, M.; Lal, D.; Kakaria, A.K.; Al-Asmi, A.R. Different Strokes: A management dilemma. Sultan Qaboos Univ. Med. J. 2018, 18, e202–e207. [Google Scholar] [CrossRef] [PubMed]
- Petrea, R.E.; Beiser, A.; Seshadri, S.; Kelly-Hayes, M.; Kase, C.S.; Wolf, P.A. Gender Differences in Stroke Incidence and Poststroke Disability in the Framingham Heart Study. Stroke 2009, 40, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Béjot, Y.; Bailly, H.; Durier, J.; Giroud, M. Epidemiology of stroke in Europe and trends for the 21st century. La Presse Méd. 2016, 45 Pt 2, e391–e398. [Google Scholar] [CrossRef] [PubMed]
- Béjot, Y.; Daubail, B.; Giroud, M. Epidemiology of stroke and transient ischemic attacks: Current knowledge and perspectives. Rev. Neurol. 2016, 172, 59–68. [Google Scholar] [CrossRef]
- Kuriakose, D.; Xiao, Z. Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 7609. [Google Scholar] [CrossRef] [PubMed]
- Bushnell, C.D.; Chaturvedi, S.; Gage, K.R.; Herson, P.S.; Hurn, P.D.; Jiménez, M.C.; Kittner, S.J.; Madsen, T.E.; McCullough, L.D.; McDermott, M.; et al. Sex differences in stroke: Challenges and opportunities. J. Cereb Blood Flow Metab Off. J. Int. Soc. Cereb. Blood Flow Metab. 2018, 38, 2179–2191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Saatman, K.E.; Chen, L. Therapeutic potential of natural compounds from Chinese medicine in acute and subacute phases of ischemic stroke. Neural Regen. Res. 2020, 15, 416. [Google Scholar] [PubMed]
- Kirkman, M.A.; Citerio, G.; Smith, M. The intensive care management of acute ischemic stroke: An overview. Intensive Care Med. 2014, 40, 640–653. [Google Scholar] [CrossRef]
- Ntaios, G. Embolic Stroke of Undetermined Source: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 75, 333–340. [Google Scholar] [CrossRef]
- Deb, P.; Sharma, S.; Hassan, K. Pathophysiologic mechanisms of acute ischemic stroke: An overview with emphasis on therapeutic significance beyond thrombolysis. Pathophysiology 2010, 17, 197–218. [Google Scholar] [CrossRef]
- Usman, H.; Tan, Z.; Gul, M.; Rashid, S.; Ali, T.; Shah, F.A.; Li, S.; Li, J. B Identification of novel and potential PPARγ stimulators as repurposed drugs for MCAO associated brain degeneration. Toxicol. Appl. Pharmacol. 2022, 446, 116055. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.; Arai, K.; Lo, E.H.; Hommel, M. Pathophysiologic cascades in ischemic stroke. Int. J. Stroke 2012, 7, 378–385. [Google Scholar] [CrossRef]
- Moustafa, R.R.; Baron, J.C. Pathophysiology of ischaemic stroke: Insights from imaging, and implications for therapy and drug discovery. Br. J. Pharmacol. 2008, 153 (Suppl. S1), S44–S54. [Google Scholar] [CrossRef]
- Dirnagl, U.; Iadecola, C.; Moskowitz, M.A. Pathobiology of ischaemic stroke: An integrated view. Trends Neurosci. 1999, 22, 391–397. [Google Scholar] [CrossRef]
- Sekerdag, E.; Solaroglu, I.; Gursoy-Ozdemir, Y. Cell Death Mechanisms in Stroke and Novel Molecular and Cellular Treatment Options. Curr. Neuropharmacol. 2018, 16, 1396–1415. [Google Scholar] [CrossRef]
- George, A.K.; Singh, M.; Homme, R.P.; Majumder, A.; Sandhu, H.S.; Tyagi, S.C. A hypothesis for treating inflammation and oxidative stress with hydrogen sulfide during age-related macular degeneration. Int. J. Ophthalmol. 2018, 11, 881–887. [Google Scholar] [PubMed]
- Xu, D.-S.; Bai, Y.-L.; Gao, B.-Y.; Sun, C.-C.; Xia, G.-H.; Zhou, S.-T.; Zhang, Y.; Mao, Y.-R.; Liu, P.-L.; Zheng, Y.; et al. Paired associated magnetic stimulation promotes neural repair in the rat middle cerebral artery occlusion model of stroke. Neural Regen. Res. 2020, 15, 2047–2056. [Google Scholar] [CrossRef]
- Dilek, N.; Papapetropoulos, A.; Toliver-Kinsky, T.; Szabo, C. Hydrogen sulfide: An endogenous regulator of the immune system. Pharmacol. Res. 2020, 161, 105119. [Google Scholar] [CrossRef]
- Zhao, F.-L.; Fang, F.; Qiao, P.-F.; Yan, N.; Gao, D.; Yan, Y. AP39, a Mitochondria-Targeted Hydrogen Sulfide Donor, Supports Cellular Bioenergetics and Protects against Alzheimer’s Disease by Preserving Mitochondrial Function in APP/PS1 Mice and Neurons. Oxidative Med. Cell. Longev. 2016, 2016, 8360738. [Google Scholar] [CrossRef]
- Carbajo, J.M.; Maraver, F. Salt water and skin interactions: New lines of evidence. Int. J. Biometeorol. 2018, 62, 1345–1360. [Google Scholar] [CrossRef] [PubMed]
- Jeong, N.Y.; Jung, J.; Tabassum, R. Protective effect of hydrogen sulfide on oxidative stress-induced neurodegenerative diseases. Neural Regen. Res. 2020, 15, 232–241. [Google Scholar] [CrossRef]
- Onose, G.; Anghelescu, A.; Blendea, D.; Ciobanu, V.; Daia, C.; Firan, F.C.; Oprea, M.; Spinu, A.; Popescu, C.; Ionescu, A.; et al. Cellular and Molecular Targets for Non-Invasive, Non-Pharmacological Therapeutic/Rehabilitative Interventions in Acute Ischemic Stroke. Int. J. Mol. Sci. 2022, 23, 907. [Google Scholar] [CrossRef]
- Hatcher, M.A.; Starr, J.A. Role of tissue plasminogen activator in acute ischemic stroke. Ann. Pharmacother. 2011, 45, 364–371. [Google Scholar] [CrossRef]
- Roth, J.M. Recombinant tissue plasminogen activator for the treatment of acute ischemic stroke. Proceedings 2011, 24, 257–259. [Google Scholar] [CrossRef]
- Borghi, S.M.; Carvalho, T.T.; Staurengo-Ferrari, L.; Hohmann, M.S.; Pinge-Filho, P.; Casagrande, R.; Verri, W.A., Jr. Vitexin inhibits inflammatory pain in mice by targeting TRPV1, oxidative stress, and cytokines. J. Nat. Prod. 2013, 76, 1141–1149. [Google Scholar] [CrossRef]
- Jiang, J.; Dai, J.; Cui, H. Vitexin reverses the autophagy dysfunction to attenuate MCAO-induced cerebral ischemic stroke via mTOR/Ulk1 pathway. Biomed. Pharmacother. 2018, 99, 583–590. [Google Scholar] [CrossRef]
- Xue, H.-F.; Ying, Z.-M.; Zhang, W.-J.; Meng, Y.-H.; Ying, X.-X.; Kang, T.-G. Hepatic, gastric, and intestinal first-pass effects of vitexin in rats. Pharm. Biol. 2014, 52, 967–971. [Google Scholar] [CrossRef]
- Ferreira, E.D.O.; Fernandes, M.Y.S.D.; de Lima, N.M.R.; Neves, K.R.T.; Carmo, M.R.S.D.; Lima, F.A.V.; Fonteles, A.A.; Menezes, A.P.F.; de Andrade, G.M. Neuroinflammatory response to experimental stroke is inhibited by eriodictyol. Behav. Brain Res. 2016, 312, 321–332. [Google Scholar] [CrossRef]
- Jing, X.; Ren, D.; Wei, X.; Shi, H.; Zhang, X.; Perez, R.G.; Lou, H.; Lou, H. Eriodictyol-7-O-glucoside activates Nrf2 and protects against cerebral ischemic injury. Toxicol. Appl. Pharm. 2013, 273, 672–679. [Google Scholar] [CrossRef]
- Malik, I.; Shah, F.A.; Ali, T.; Tan, Z.; Alattar, A.; Ullah, N.; Khan, A.-U.; Alshaman, R.; Li, S. Potent Natural Antioxidant Carveol Attenuates MCAO-Stress Induced Oxidative, Neurodegeneration by Regulating the Nrf-2 Pathway. Front. Neurosci. 2020, 14, 659. [Google Scholar] [CrossRef]
- Suzuki, A.; Yamamoto, M.; Jokura, H.; Fujii, A.; Tokimitsu, I.; Hase, T.; Saito, I. Ferulic acid restores endothelium-dependent vasodilation in aortas of spontaneously hypertensive rats. Am. J. Hypertens 2007, 20, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Anson, N.M.; Berg, R.V.D.; Havenaar, R.; Bast, A.; Haenen, G.R. Bioavailability of ferulic acid is determined by its bioaccessibility. J. Cereal Sci. 2009, 49, 296–300. [Google Scholar] [CrossRef]
- Wu, C.-F.; Hong, C.; Klauck, S.M.; Lin, Y.-L.; Efferth, T. Molecular mechanisms of rosmarinic acid from Salvia miltiorrhiza in acute lymphoblastic leukemia cells. J. Ethnopharmacol. 2015, 176, 55–68. [Google Scholar] [CrossRef]
- Blažević, T.; Reznicek, G.; Ding, L.; Yang, G.; Haiss, P.; Heiss, E.H.; Dirsch, V.M.; Liu, R. Short Chain (≤C4) Esterification Increases Bioavailability of Rosmarinic Acid and Its Potency to Inhibit Vascular Smooth Muscle Cell Proliferation. Front. Pharm. 2021, 11, 609756. [Google Scholar] [CrossRef]
- Luan, H.; Kan, Z.; Xu, Y.; Lv, C.; Jiang, W. Rosmarinic acid protects against experimental diabetes with cerebral ischemia: Relation to inflammation response. J. Neuroinflammat. 2013, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-B.; Zhao, Z.-X.; Peng, R.; Pan, L.-B.; Fu, J.; Ma, S.-R.; Han, P.; Cong, L.; Zhang, Z.-W.; Sun, L.-X.; et al. Gut microbiota-based pharmacokinetics and the antidepressant mechanism of paeoniflorin. Front. Pharm. 2019, 10, 268. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-F.; Li, W.-T.; Han, H.-C.; Gao, D.-K.; He, X.-S.; Li, L.; Song, J.-N.; Fei, Z. Allicin protects rat cortical neurons against mechanical trauma injury by regulating nitric oxide synthase pathways. Brain Res. Bull. 2014, 100, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Lawson, L. Allicin bioavailability from alliinase-inhibited garlic. Planta Medica 2009, 75, 95. [Google Scholar] [CrossRef]
- Huang, L.; Chen, C.; Zhang, X.; Li, X.; Chen, Z.; Yang, C.; Liang, X.; Zhu, G.; Xu, Z. Neuroprotective Effect of Curcumin Against Cerebral Ischemia-Reperfusion Via Mediating Autophagy and Inflammation. J. Mol. Neurosci. 2018, 64, 129–139. [Google Scholar] [CrossRef]
- Bavarsad, K.; Barreto, G.E.; Hadjzadeh, M.-A.; Sahebkar, A. Protective Effects of Curcumin Against Ischemia-Reperfusion Injury in the Nervous System. Mol. Neurobiol. 2019, 56, 1391–1404. [Google Scholar] [CrossRef]
- Lopresti, A.L. The problem of curcumin and its bioavailability: Could its gastrointestinal influence contribute to its overall health-enhancing effects? Adv. Nutr. 2018, 9, 41–50. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Fan, Q.; Guo, J.; Galli, G.; Du, G.; Wang, X.; Xiao, W. Ginkgolide K protects the heart against endoplasmic reticulum stress injury by activating the inositol-requiring enzyme 1α/X box-binding protein-1 pathway. Br. J. Pharmacol. 2016, 173, 2402–2418. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, S.; Duan, H.; Zhu, Z.; Yang, Z.; Cao, J.; Zhao, Y.; Huang, Z.; Wu, Q.; Duan, J. A novel, highly-water-soluble apigenin derivative provides neuroprotection following ischemia in male rats by regulating the ERK/Nrf2/HO-1 pathway. Eur. J. Pharmacol. 2019, 855, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Ben Salem, M.; Affes, H.; Ksouda, K.; Dhouibi, R.; Sahnoun, Z.; Hammami, S.; Zeghal, K.M. Pharmacological studies of artichoke leaf extract and their health benefits. Plant Foods Hum. Nutr. 2015, 70, 441–453. [Google Scholar] [CrossRef]
- Valentová, K.; Vrba, J.; Bancířová, M.; Ulrichová, J.; Křen, V. Isoquercitrin: Pharmacology, toxicology, and metabolism. Food Chem. Toxicol. 2014, 68, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Appleton, J. Evaluating the bioavailability of isoquercetin. Nat. Med. J. 2010, 2, 1–6. [Google Scholar]
- Dai, Y.; Zhang, H.; Zhang, J.; Yan, M. Isoquercetin attenuates oxidative stress and neuronal apoptosis after ischemia/reperfusion injury via Nrf2-mediated inhibition of the NOX4/ROS/NF-κB pathway. Chem. Biol. Interact. 2018, 284, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, J.; Wei, H.; Gu, T.; Wang, J.; Wu, Z.; Yang, Q. Genistein Attenuates Acute Cerebral Ischemic Damage by Inhibiting the NLRP3 Inflammasome in Reproductively Senescent Mice. Front. Aging Neurosci. 2020, 12, 153. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Kulkarni, K.; Zhu, W.; Hu, M. Bioavailability and pharmacokinetics of genistein: Mechanistic studies on its ADME. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2012, 12, 1264–1280. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, C.C.; Da Fonseca, M.M.R. Carvone: Why and how should one bother to produce this terpene. Food Chemistry 2006, 95, 413–422. [Google Scholar] [CrossRef]
- Crowell, P.L.; Kennan, W.S.; Haag, J.D.; Ahmad, S.; Vedejs, E.; Gould, M.N. Chemoprevention of mammary carcinogenesis by hydroxylated derivatives of d-limonene. Carcinogenesis 1992, 13, 1261–1264. [Google Scholar] [CrossRef]
- Cheng, C.-Y.; Ho, T.-Y.; Lee, E.-J.; Su, S.-Y.; Tang, N.-Y.; Hsieh, C.-L. Ferulic acid reduces cerebral infarct through its antioxidative and anti-inflammatory effects following transient focal cerebral ischemia in rats. Am. J. Chin. Med. 2008, 36, 1105–1119. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.Y.; Liu, C.H.; Hsieh, C.T.; Hsieh, C.L. The anti-inflammatory effect of paeoniflorin on cerebral infarction induced by ischemia-reperfusion injury in Sprague-Dawley rats. Am. J. Chin. Med. 2010, 38, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.Y.-Y.; Tsui, H.-T.; Chung, I.Y.-M.; Chan, R.Y.-K.; Kwan, Y.-W.; Chan, S.-W. Allicin protects rat cardiomyoblasts (H9c2 cells) from hydrogen peroxide-induced oxidative injury through inhibiting the generation of intracellular reactive oxygen species. Int. J. Food Sci. Nutr. 2014, 65, 868–873. [Google Scholar] [CrossRef]
- Zhang, B.; Li, F.; Zhao, W.-J.; Li, J.; Li, Q.; Wang, W. Protective effects of allicin against ischemic stroke in a rat model of middle cerebral artery occlusion. Mol. Med. Rep. 2015, 12, 3734–3738. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Bordoloi, D.; Harsha, C.; Banik, K.; Gupta, S.C.; Aggarwal, B.B. Curcumin mediates anticancer effects by modulating multiple cell signaling pathways. Clin. Sci. 2017, 131, 1781–1799. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Han, J.; Li, T.; Xin, Z.; Ma, Z.; Di, W.; Hu, W.; Gong, B.; Di, S.; Wang, D.; et al. Curcumin as a potential protective compound against cardiac diseases. Pharmacol. Res. 2017, 119, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.K.; Yang, W.Z.; Chen, J.P.; Chen, Y.; Ouyang, L.Q.; Xu, Y.C.; Shi, S.S. Curcumin inhibits TLR2/4-NF-κB signaling pathway and attenuates brain damage in permanent focal cerebral ischemia in rats. Inflammation 2014, 37, 1544–1551. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, H.-Y.; Wu, B.; Cheng, C.-Y.; Xiao, W.; Wang, Z.-Z.; Yang, Y.-Y.; Li, P.; Yang, H. Ginkgolide K attenuates neuronal injury after ischemic stroke by inhibiting mitochondrial fission and GSK-3β-dependent increases in mitochondrial membrane permeability. Oncotarget 2017, 8, 44682. [Google Scholar] [CrossRef]
- Ma, T.; Shi, Y.; Wang, Y. Forsythiaside A protects against focal cerebral ischemic injury by mediating the activation of the Nrf2 and endoplasmic reticulum stress pathways. Mol. Med. Rep. 2019, 20, 1313–1320. [Google Scholar] [CrossRef]
- Gao, J.; Chen, N.; Li, N.; Xu, F.; Wang, W.; Lei, Y.; Shi, J.; Gong, Q. Neuroprotective effects of trilobatin, a novel naturally occurring Sirt3 agonist from Lithocarpus polystachyus Rehd., mitigate cerebral ischemia/reperfusion injury: Involvement of TLR4/NF-κB and Nrf2/Keap-1 signaling. Antioxid. Red. Ssignal. 2020, 33, 117–143. [Google Scholar] [CrossRef] [PubMed]
- Schreihofer, D.A.; Oppong-Gyebi, A. Genistein: Mechanisms of action for a pleiotropic neuroprotective agent in stroke. Nutr. Neurosci. 2019, 22, 375–391. [Google Scholar] [CrossRef]
- Park, H.A.; Kubicki, N.; Gnyawali, S.; Chan, Y.C.; Roy, S.; Khanna, S.; Sen, C.K. Natural Vitamin E α-Tocotrienol protects against ischaemic stroke by induction of Multidrug Resistance-Associated Protein 1. Stroke 2011, 42, 2308–2314. [Google Scholar] [CrossRef] [PubMed]
- Leppala, J.M.; Virtamo, J.; Fogelholm, R.; Huttunen, J.K.; Albanes, D.; Taylor, P.R.; Heinonen, O.P. Controlled trial of alpha-tocopherol and beta-carotene supplements on stroke incidence and mortality in male smokers. Arterioscl. Thromb. Vas. Biol. 2000, 20, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.J.; Tsuchiya, M.; Wassall, S.R. Structural and dynamic membrane properties of alpha-tocopherol and alpha-tocotrienol: Implication to the molecular mechanism of their antioxidant potency. Biochemistry 1993, 32, 10692–10699. [Google Scholar] [CrossRef]
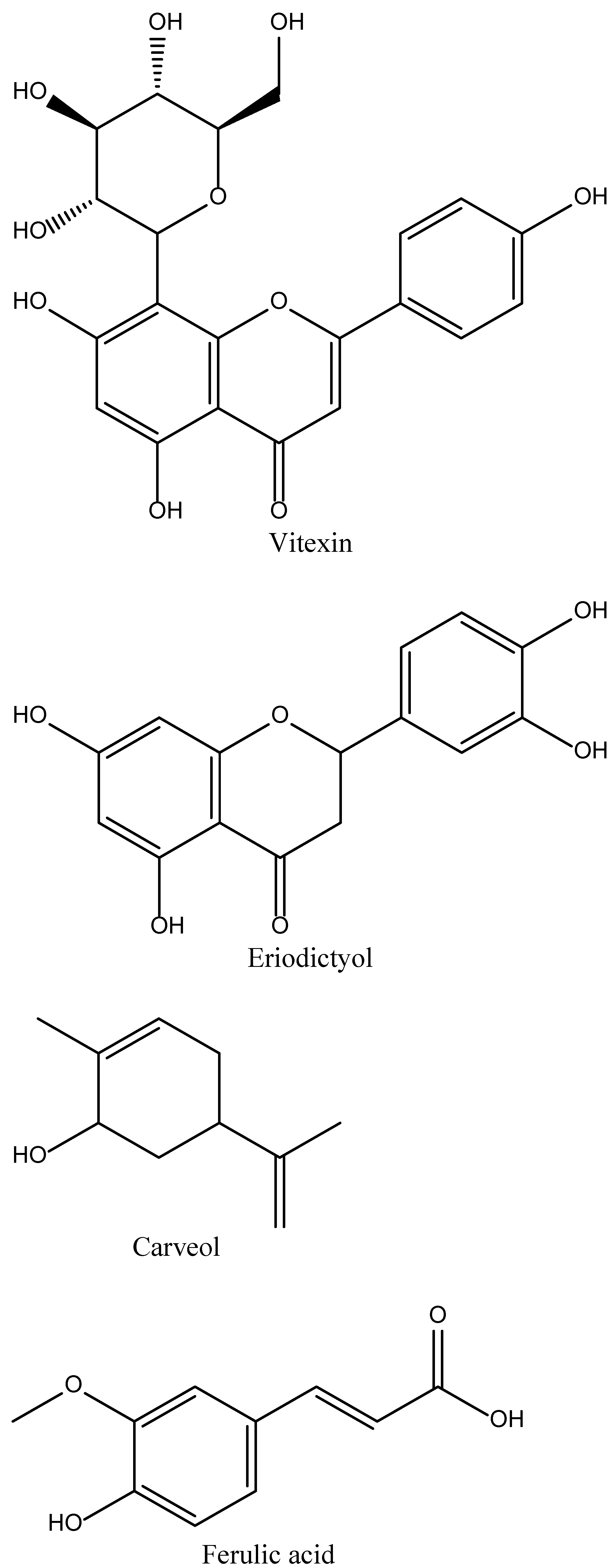
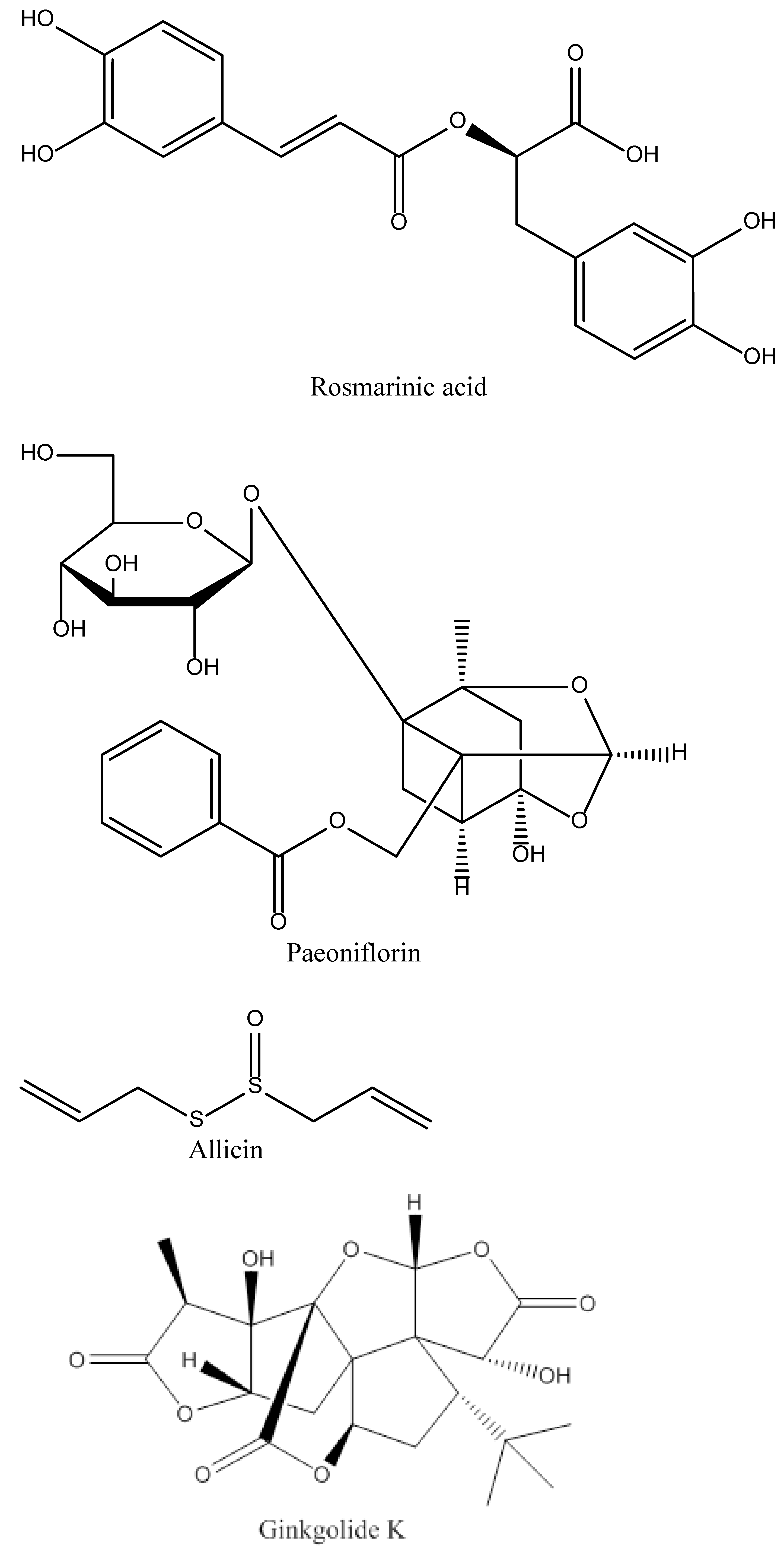
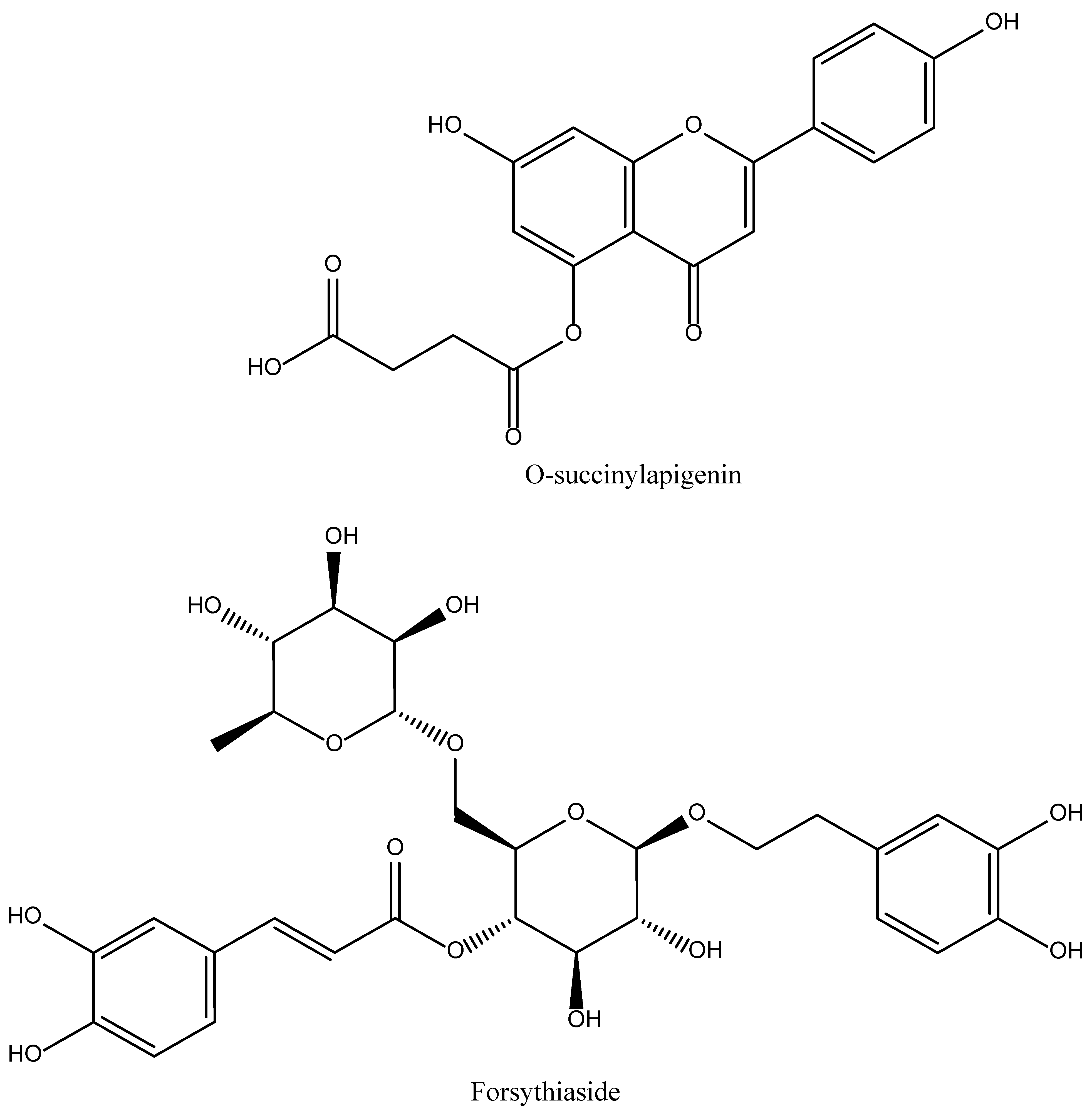

| Name of Natural Compound | Botanical Source | Nature of Compound | Dose | Route of Administration | Oral Bioavailability | Animal Model | References |
|---|---|---|---|---|---|---|---|
| Vitexin | Vitux agnus-castus, Vitex negundo | Flavone C-glycoside (apigenin-8-C-β-D-glucopyranoside) | 2 mg/kg | Intravenous | Poor due to very high 1st pass metabolism | MCAO induced Rats stroke model | [25,27] |
| Eriodictyol | Dracocephalum rupestre | Flavonoid | 1,2 and 4 mg/kg | Oral | Good | Male Swiss mice model and Rat model of focal cerebral ischemia | [26,28] |
| Carveol | Caraway seeds (Carum carvi), Dill seeds, Spearmint seed | Monocyclic monoterpenoid | 20 mg/kg | Intraperitoneal | Good | MCAO-induced ischemic stroke model | [29,30] |
| Ferulic acid | Ferula foetida (asafoetida) | Phenolic phytochemical | 80 and 100 mg/kg | Intravenous | Highly variable (0.4–98%) | MCAO-induced stroke model | [31,32] |
| Rosamirinic acid | Salvia miltiorrhia | Polyphenol | 50 mg/kg | Intravenous | Extremely poor (1.57%) | Rat models of cerebral ischemia-reperfusion injury | [33,34] |
| Paeoniflorin | Paeinia lactiflora Pall. | Monoterpene glycoside | 20 mg/kg | Intravenous | Very poor (2.32%) | MCAO ischemic stroke rat model | [35,36] |
| Allicin | Garlic species such as Allium sativum | Organosulfur compounds | 50 mg/kg | Intraperitoneal | 18% | MCAO ischemic stroke rat model | [37,38] |
| Curcumin | Curcuma longa (turmeric) | Diarylheptanoid | 300 mg/kg 50 mg/kg | Intraperitoneal | Very poor | MCAO ischemic stroke rat model | [39,40,41] |
| Ginkgolide K | Gingko biloba | Terpenoids | 2.4 and 8 mg/kg | Intraperitoneal | Approximately 80% | Rat Ischemia model | [42] |
| 6″-O-succinylapigenin | Cynara cardunculus var. scolymus L | Flavonoid | 20, 40 and 60 mg/kg | Intraperitoneal | Good | MCAO-induced rat models | [43] |
| Forsythiaside A | Fruit of Forsythia suspense. | Hydroxycinnamic acid | 50 mg/kg | Intraperitoneal | Moderate | MCAO-induced rat models | [44] |
| Isoquercetin | Citrus fruits, apples, onions, parsley, sage, tea, and red wine. Olive oil, grapes, dark cherries | O-Glucoside | 20 mg/kg | Intravenous | Moderate (51%) | MCAO-induced rat models | [45,46] |
| Trilobatin | Lithocarpus polystachyus | Polyphenol | 20 mg/kg | Intravenous | -- | MCAO-induced rat models | [47] |
| Genistein | Plants including lupin, fava beans, soybeans, kudzu, | 7-hydroxyisoflavone | 10 mg/kg | Intraperitoneal | 23.4% | [48,49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almutairi, F.M.; Ullah, A.; Althobaiti, Y.S.; Irfan, H.M.; Shareef, U.; Usman, H.; Ahmed, S. A Review on Therapeutic Potential of Natural Phytocompounds for Stroke. Biomedicines 2022, 10, 2566. https://doi.org/10.3390/biomedicines10102566
Almutairi FM, Ullah A, Althobaiti YS, Irfan HM, Shareef U, Usman H, Ahmed S. A Review on Therapeutic Potential of Natural Phytocompounds for Stroke. Biomedicines. 2022; 10(10):2566. https://doi.org/10.3390/biomedicines10102566
Chicago/Turabian StyleAlmutairi, Farooq M., Aman Ullah, Yusuf S. Althobaiti, Hafiz Muhammad Irfan, Usman Shareef, Halima Usman, and Sagheer Ahmed. 2022. "A Review on Therapeutic Potential of Natural Phytocompounds for Stroke" Biomedicines 10, no. 10: 2566. https://doi.org/10.3390/biomedicines10102566
APA StyleAlmutairi, F. M., Ullah, A., Althobaiti, Y. S., Irfan, H. M., Shareef, U., Usman, H., & Ahmed, S. (2022). A Review on Therapeutic Potential of Natural Phytocompounds for Stroke. Biomedicines, 10(10), 2566. https://doi.org/10.3390/biomedicines10102566





