Empagliflozin Is Not Renoprotective in Non-Diabetic Rat Models of Chronic Kidney Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
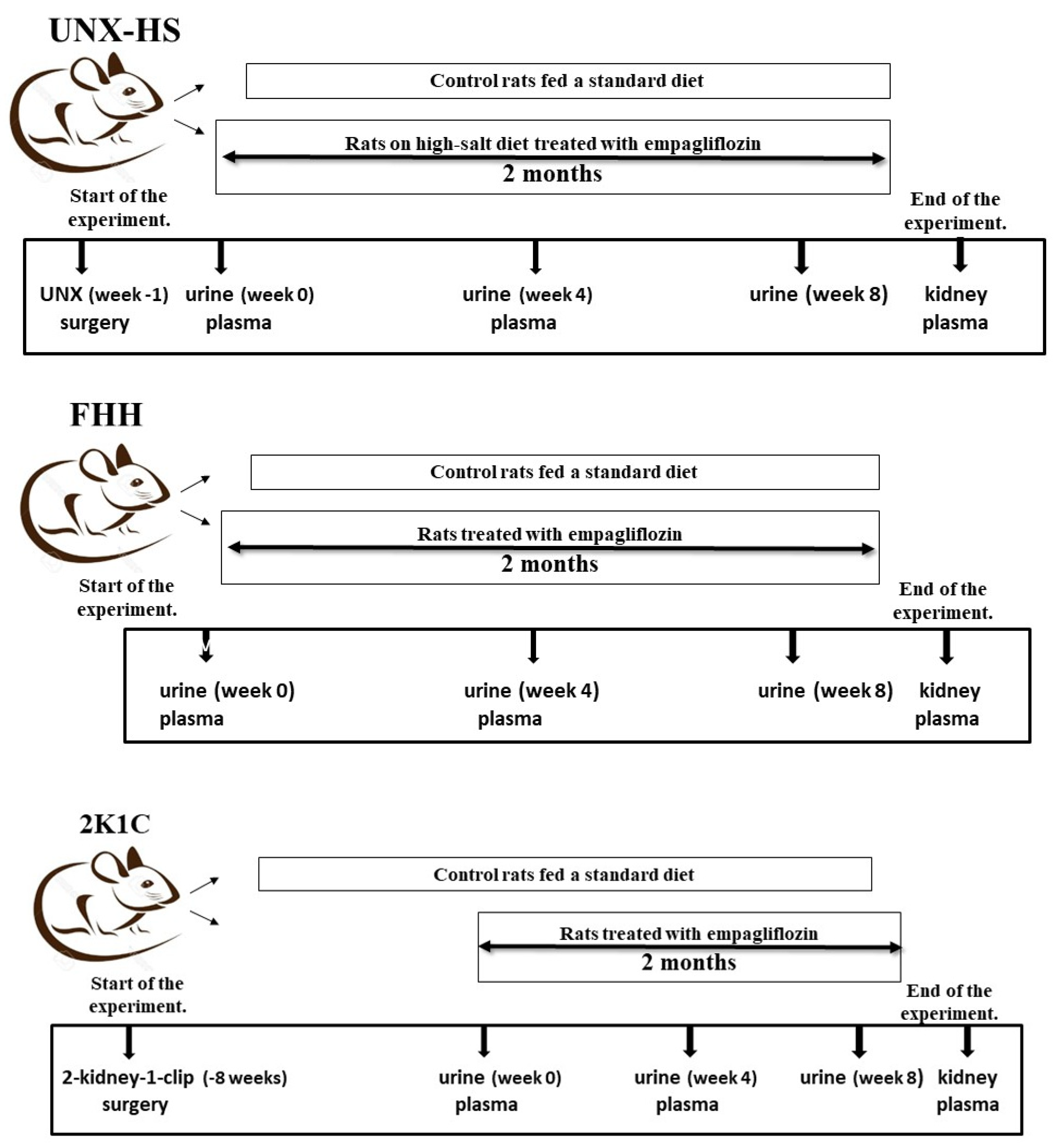
2.1.1. Fawn-Hooded Hypertensive Rats
2.1.2. Two-Kidney One-Clip (2K1C) Goldblatt Hypertension
2.1.3. Uninephrectomized Rats on High-Salt Intake
2.2. Biochemical Analysis
2.3. Statistical Analysis
3. Results
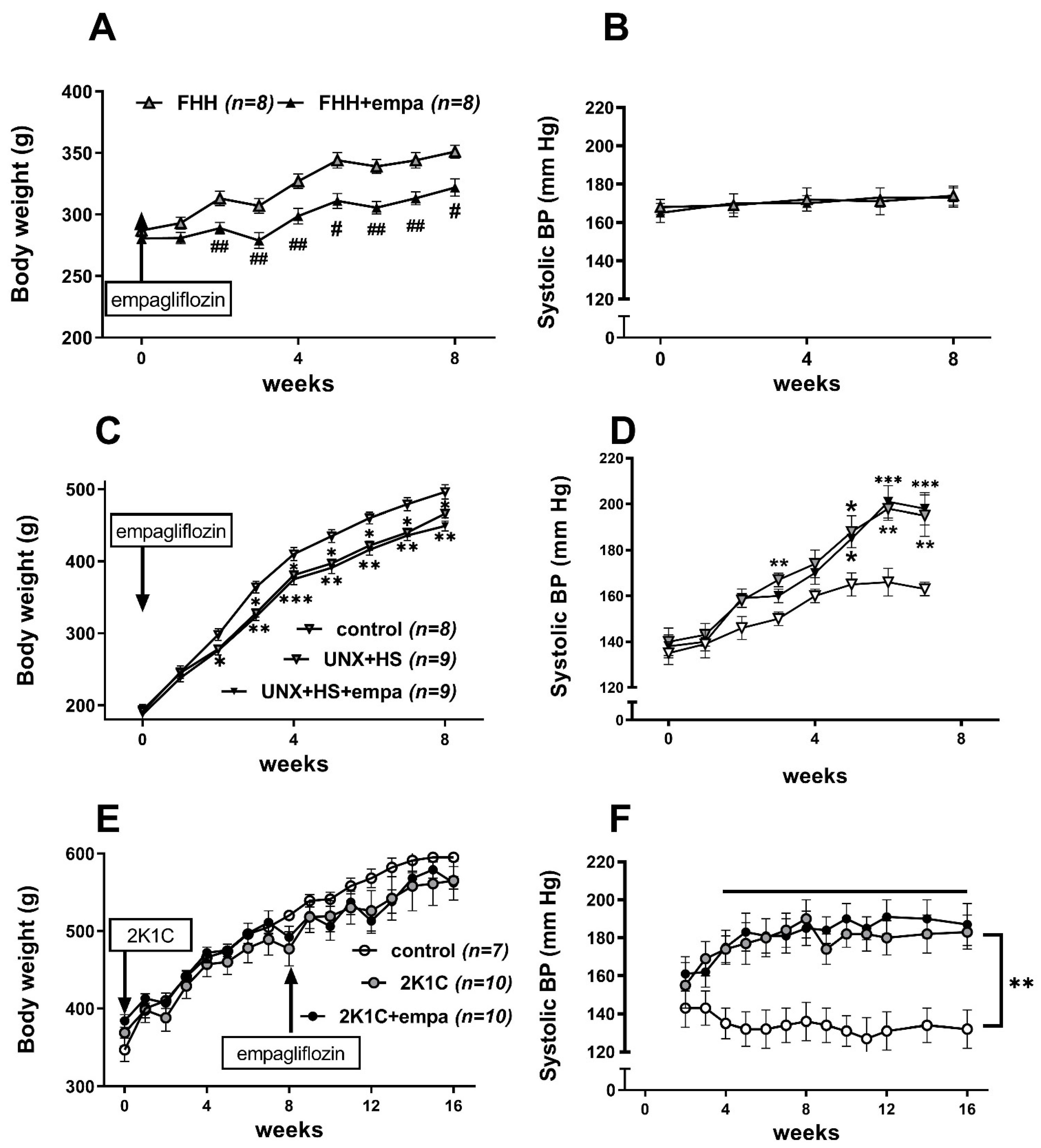
3.1. Effects of Empagliflozin on Body Weight, Weights of Fat Depots, and Blood Pressure
3.2. Effects of Empagliflozin on Renal Parameters
3.3. Effects of Empagliflozin on Oxidative Stress and ROS Production
4. Discussion
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board and Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. EMPA-REG OUTCOME Investigators, Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. CANVAS Program Collaborative Group, Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef] [PubMed]
- El Din, U.A.A.S.; Salem, M.M.; Abdulazim, D.O. Sodium-glucose cotransporter 2 inhibitors as the first universal treatment of chronic kidney disease. Nefrologia 2021, 42, 390–403. [Google Scholar] [CrossRef]
- Cannon, C.P.; Perkovic, V.; Agarwal, R.; Baldassarre, J.; Bakris, G.; Charytan, D.M.; De Zeeuw, D.; Edwards, R.; Greene, T.; Heerspink, H.J.; et al. Evaluating the Effects of Canagliflozin on Cardiovascular and Renal Events in Patients With Type 2 Diabetes Mellitus and Chronic Kidney Disease According to Baseline HbA1c, Including Those With HbA1c <7%: Results From the CREDENCE Trial. Circulation 2020, 141, 407–410. [Google Scholar] [CrossRef]
- Wheeler, D.C.; Stefansson, B.V.; Batiushin, M.; Bilchenko, O.; Cherney, D.Z.I.; Chertow, G.M.; Douthat, W.; Dwyer, J.P.; Escudero, E.; Pecoits-Filho, R.; et al. The dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) trial: Baseline characteristics. Nephrol. Dial. Transplant. 2020, 35, 1700–1711. [Google Scholar] [CrossRef] [PubMed]
- Zannad, F.; Ferreira, J.P.; Pocock, S.J.; Anker, S.D.; Butler, J.; Filippatos, G.; Brueckmann, M.; Ofstad, A.P.; Pfarr, E.; Jamal, W.; et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: A meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 2020, 396, 819–829. [Google Scholar] [CrossRef]
- Ojima, A.; Matsui, T.; Nishino, Y.; Nakamura, N.; Yamagishi, S. Empagliflozin, an Inhibitor of Sodium-Glucose Cotransporter 2 Exerts Anti-Inflammatory and Antifibrotic Effects on Experimental Diabetic Nephropathy Partly by Suppressing AGEs-Receptor Axis. Horm. Metab. Res. 2015, 47, 686–692. [Google Scholar] [CrossRef]
- Shin, S.J.; Chung, S.; Kim, S.J.; Lee, E.-M.; Yoo, Y.-H.; Kim, J.-W.; Ahn, Y.-B.; Kim, E.-S.; Moon, S.-D.; Kim, M.-J.; et al. Effect of Sodium-Glucose Co-Transporter 2 Inhibitor, Dapagliflozin, on Renal Renin-Angiotensin System in an Animal Model of Type 2 Diabetes. PLoS ONE 2016, 11, e0165703. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Oelze, M.; Hanf, A.; Kröller-Schön, S.; Kashani, F.; Roohani, S.; Welschof, P.; Kopp, M.; Gödtel-Armbrust, U.; Xia, N.; et al. The SGLT2 inhibitor empagliflozin improves the primary diabetic complications in ZDF rats. Redox Biol. 2017, 13, 370–385. [Google Scholar] [CrossRef] [PubMed]
- Gallo, L.A.; Ward, M.S.; Fotheringham, A.K.; Zhuang, A.; Borg, D.J.; Flemming, N.B.; Harvie, B.M.; Kinneally, T.L.; Yeh, S.-M.; McCarthy, D.A.; et al. Once daily administration of the SGLT2 inhibitor, empagliflozin, attenuates markers of renal fibrosis without improving albuminuria in diabetic db/db mice. Sci. Rep. 2016, 26, 26428. [Google Scholar] [CrossRef]
- Tang, L.; Wu, Y.; Tian, M.; Sjöström, C.D.; Johansson, U.; Peng, X.-R.; Smith, D.M.; Huang, Y. Dapagliflozin slows the progression of the renal and liver fibrosis associated with type 2 diabetes. Am. J. Physiol. Metab. 2017, 313, E563–E576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Thai, K.; Kepecs, D.M.; Gilbert, R.E. Sodium-Glucose Linked Cotransporter-2 Inhibition Does Not Attenuate Disease Progression in the Rat Remnant Kidney Model of Chronic Kidney Disease. PLoS ONE 2016, 11, e0144640. [Google Scholar] [CrossRef] [PubMed]
- Rajasekeran, H.; Reich, H.N.; Hladunewich, M.A.; Cattran, D.; Lovshin, J.A.; Lytvyn, Y.; Bjornstad, P.; Lai, V.; Tse, J.; Cham, L.; et al. Dapagliflozin in focal segmental glomerulosclerosis: A combined human-rodent pilot study. Am. J. Physiol. Renal Physiol. 2018, 314, F412–F422. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Konishi, Y.; Morikawa, T.; Zhang, Y.; Kitabayashi, C.; Kobara, H.; Masaki, T.; Nakano, D.; Hitomi, H.; Kobori, H.; et al. Effect of a SGLT2 inhibitor on the systemic and intrarenal renin–angiotensin system in subtotally nephrectomized rats. J. Pharmacol. Sci. 2018, 137, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jo, C.H.; Kim, G.-H. Effects of empagliflozin on nondiabetic salt-sensitive hypertension in uninephrectomized rats. Hypertens. Res. 2019, 42, 1905–1915. [Google Scholar] [CrossRef] [PubMed]
- Wan, N.; Fujisawa, Y.; Kobara, H.; Masaki, T.; Nakano, D.; Rahman, A.; Nishiyama, A. Effects of an SGLT2 inhibitor on the salt sensitivity of blood pressure and sympathetic nerve activity in a nondiabetic rat model of chronic kidney disease. Hypertens. Res. 2020, 43, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Delic, D.; Chu, C.; Xiong, Y.; Luo, T.; Chen, X.; Gaballa, M.M.; Xue, Y.; Chen, X.; Cao, Y.; et al. Antifibrotic effects of low dose SGLT2 Inhibition with empagliflozin in comparison to Ang II receptor blockade with telmisartan in 5/6 nephrectomised rats on high salt diet. Biomed. Pharmacother. 2022, 146, 112606. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.H.; Al-Salam, S.; Al Suleimani, Y.; Al Za’Abi, M.; Abdelrahman, A.M.; Ashique, M.; Manoj, P.; Adham, S.A.; Hartmann, C.; Schupp, N.; et al. Effects of the SGLT-2 Inhibitor Canagliflozin on Adenine-Induced Chronic Kidney Disease in Rats. Cell. Physiol. Biochem. 2019, 52, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Castoldi, G.; Carletti, R.; Ippolito, S.; Colzani, M.; Barzaghi, F.; Stella, A.; Zerbini, G.; Perseghin, G.; di Gioia, C.R. Renal Anti-Fibrotic Effect of Sodium Glucose Cotransporter 2 Inhibition in Angiotensin II-Dependent Hypertension. Am. J. Nephrol. 2020, 51, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Pardo, H.; Bautista, R.; Vargas-Robles, H.; Rios, A.; Sanchez, D.; Escalante, B. Role of sodium/glucose cotransporter inhibition on a rat model of angiotensin II–dependent kidney damage. BMC Nephrol. 2019, 20, 292. [Google Scholar] [CrossRef] [PubMed]
- Huttl, M.; Markova, I.; Miklankova, D.; Oliyarnyk, O.; Trnovska, J.; Kucera, J.; Sedlacek, R.; Haluzik, M.; Malinska, H. Metabolic cardio- and reno-protective effects of empagliflozin in a prediabetic rat model. J. Physiol. Pharmacol. 2020, 71, 635–645. [Google Scholar] [CrossRef]
- Hojná, S.; Rauchová, H.; Malínská, H.; Marková, I.; Hüttl, M.; Papoušek, F.; Behuliak, M.; Miklánková, D.; Vaňourková, Z.; Neckář, J.; et al. Antihypertensive and metabolic effects of empagliflozin in Ren-2 transgenic rats, an experimental non-diabetic model of hypertension. Biomed. Pharmacother. 2021, 144, 112246. [Google Scholar] [CrossRef] [PubMed]
- Malínská, H.; Hüttl, M.; Marková, I.; Miklánková, D.; Hojná, S.; Papoušek, F.; Šilhavý, J.; Mlejnek, P.; Zicha, J.; Hrdlička, J.; et al. Beneficial Effects of Empagliflozin Are Mediated by Reduced Renal Inflammation and Oxidative Stress in Spontaneously Hypertensive Rats Expressing Human C-Reactive Protein. Biomedicines 2022, 10, 2066. [Google Scholar] [CrossRef] [PubMed]
- Provoost, A.P. Spontaneous glomerulosclerosis: Insights from the fawn-hooded rat. Kidney Int. Suppl. 1994, 45, S2–S5. [Google Scholar]
- Drábková, N.; Hojná, S.; Zicha, J.; Vaněčková, I. Contribution of Selected Vasoactive Systems to Blood Pressure Regulation in Two Models of Chronic Kidney Disease. Physiol. Res. 2020, 69, 405–414. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Vaněčková, I.; Dobešová, Z.; Kuneš, J.; Zicha, J. The effects of repeated delivery of angiotensin II AT1 receptor antisense on distinct vasoactive systems in Ren-2 transgenic rats: Young vs. adult animals. Hypertens. Res. 2012, 35, 761–768. [Google Scholar] [CrossRef]
- Abbas, N.A.T.; El Salem, A.; Awad, M.M. Empagliflozin, SGLT-2 inhibitor, attenuates renal fibrosis in rats exposed to unilateral ureteric obstruction: Potential role of klotho expression. Naunyn Schmiedebergs Arch Pharmacol. 2018, 391, 1347–1360. [Google Scholar] [CrossRef]
- Cruz, C.; Correa-Rotter, R.; Sánchez-González, D.J.; Hernández-Pando, R.; Maldonado, P.D.; Martínez-Martínez, C.M.; Medina-Campos, O.N.; Tapia, E.; Aguilar, D.; Chirino, Y.I.; et al. Renoprotective and antihypertensive effects of S-allylcysteine in 5/6 nephrectomized rats. Am. J. Physio.l Renal Physiol. 2007, 293, F1691–F1698. [Google Scholar] [CrossRef]
- Kujal, P.; Chábová, V.Č.; Vernerová, Z.; Walkowska, A.; Kompanowska-Jezierska, E.; Sadowski, J.; Vaňourková, Z.; Husková, Z.; Opočenský, M.; Skaroupková, P.; et al. Similar renoprotection after renin-angiotensin-dependent and –independent antihypertensive therapy in 5/6-nephrectomized Ren-2 transgenic rats: Are there blood pressure-independent effects? Clin. Exp. Pharmacol. Physiol. 2010, 37, 1159–1169. [Google Scholar] [CrossRef]
- Hao, L.; Kanno, Y.; Fukushima, R.; Watanabe, Y.; Ishida, Y.; Suzuki, H. Effects of eplerenone on heart and kidney in two-kidney, one-clip rats. Am. J. Nephrol. 2004, 24, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Imamura, A.; Mackenzie, H.S.; Lacy, E.R.; Hutchison, F.N.; Fitzgibbon, W.R.; Ploth, D.W. Effects of chronic treatment with angiotensin converting enzyme inhibitor or an angiotensin receptor antagonist in two-kidney, one-clip hypertensive rats. Kidney Int. 1995, 47, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Chábová, V.C.; Červenka, L. The dilemma of dual renin-angiotensin system blockade in chronic kidney disease: Why beneficial in animal experiments but not in the clinic? Physiol. Res. 2017, 66, 181–192. [Google Scholar] [CrossRef] [PubMed]



| FHH Untreated | FHH + empa | |
|---|---|---|
| Body weight (g) | 351 ± 5 | 322 ± 7 # |
| Relative heart weight (g/100 g BW) | 0.287 ± 0.007 | 0.286 ± 0.007 |
| Relative kidneys weight (g/100 g BW) | 0.767 ± 0.028 | 0.900 ± 0.036 # |
| Relative weight of epididymal fat (g/100 g BW) | 0.724 ± 0.016 | 0.603 ± 0.025 # |
| Relative weight of perirenal fat (g/100 g BW) | 0.289 ± 0.018 | 0.176 ± 0.018 # |
| Systolic BP (mm Hg) | 180 ± 7 | 173 ± 5 |
| TBARS in kidneys | 36.8 ± 1.4 | 39.3 ± 1.3 |
| HanSD Control | UNX + HS | UNX + HS + empa | |
|---|---|---|---|
| Body weight (g) | 472 ± 10 | 461 ± 6 | 447 ± 7 |
| Relative heart weight (g/100 g BW) | 0.27 ± 0.02 | 0.33 ± 0.02 * | 0.32 ± 0.02 * |
| Relative left ventricle (g/100g) | 0.212 ± 0.005 | 0.264 ± 0.007 * | 0.261 ± 0.009 * |
| Relative left kidney weight (g/100 g BW) | 0.37 ± 0.01 | 0.74 ± 0.94 * | 0.94 ± 0.04 *,# |
| Relative epididymal fat (g/100 g BW) | 1.356 ± 0.081 | 0.891 ± 0.056 * | 0.812 ± 0.028 * |
| Relative retroperitoneal fat (g/100 g BW) | 1.349 ± 0.076 | 0.795 ± 0.077 * | 0.678 ± 0.051 * |
| Systolic BP (mm Hg) | 127 ± 57 | 166 ± 8 * | 179 ± 8 * |
| TBARS in kidney | 33 ± 3 | 44 ± 4 * | 45 ± 2 * |
| Wistar Control | 2K1C | 2K1C + empa | |
|---|---|---|---|
| Body weight (g) | 595 ± 12 | 565 ± 26 | 562 ± 13 |
| Relative heart weight (g/100 g BW) | 0.212 ± 0.004 | 0.269 ± 0.017 * | 0.269 ± 0.011 * |
| Relative left ventricle weight (g/100 g BW) | 0.173 ± 0.003 | 0.226 ± 0.017 * | 0.223 ± 0.010 * |
| Relative left kidney weight (g/100 g BW) | 0.303 ± 0.006 | 0.180 ± 0.032 * | 0.220 ± 0.030 * |
| Relative right kidney weight (g/100 g BW) | 0.307 ± 0.007 | 0.419 ± 0.022 * | 0.491 ± 0.028 * |
| Relative epididymal fat (g/100 g BW) | 1.47 ± 0.06 | 1.33 ± 0.17 | 1.33 ± 0.13 |
| Relative perirenal fat (g/100 g BW) | 1.29 ± 0.10 | 1.15 ± 0.24 | 1.20 ± 0.17 |
| Systolic BP (mm Hg) | 120 ± 4 | 163 ± 9 * | 157 ± 10 * |
| TBARS in kidney | 31 ± 3 | 20 ± 2 | 24 ± 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hojná, S.; Kotsaridou, Z.; Vaňourková, Z.; Rauchová, H.; Behuliak, M.; Kujal, P.; Kadlecová, M.; Zicha, J.; Vaněčková, I. Empagliflozin Is Not Renoprotective in Non-Diabetic Rat Models of Chronic Kidney Disease. Biomedicines 2022, 10, 2509. https://doi.org/10.3390/biomedicines10102509
Hojná S, Kotsaridou Z, Vaňourková Z, Rauchová H, Behuliak M, Kujal P, Kadlecová M, Zicha J, Vaněčková I. Empagliflozin Is Not Renoprotective in Non-Diabetic Rat Models of Chronic Kidney Disease. Biomedicines. 2022; 10(10):2509. https://doi.org/10.3390/biomedicines10102509
Chicago/Turabian StyleHojná, Silvie, Zoe Kotsaridou, Zdeňka Vaňourková, Hana Rauchová, Michal Behuliak, Petr Kujal, Michaela Kadlecová, Josef Zicha, and Ivana Vaněčková. 2022. "Empagliflozin Is Not Renoprotective in Non-Diabetic Rat Models of Chronic Kidney Disease" Biomedicines 10, no. 10: 2509. https://doi.org/10.3390/biomedicines10102509
APA StyleHojná, S., Kotsaridou, Z., Vaňourková, Z., Rauchová, H., Behuliak, M., Kujal, P., Kadlecová, M., Zicha, J., & Vaněčková, I. (2022). Empagliflozin Is Not Renoprotective in Non-Diabetic Rat Models of Chronic Kidney Disease. Biomedicines, 10(10), 2509. https://doi.org/10.3390/biomedicines10102509






