Ultraviolet Light Exposure Decreases Thyroid Cancer Risk: A National Perspective
Abstract
:1. Introduction
2. Methods
2.1. Study Sample
2.2. Data Variables
3. Statistical Analysis
4. Results
4.1. Thyroid Cancer Incidence by Race/Ethnicity
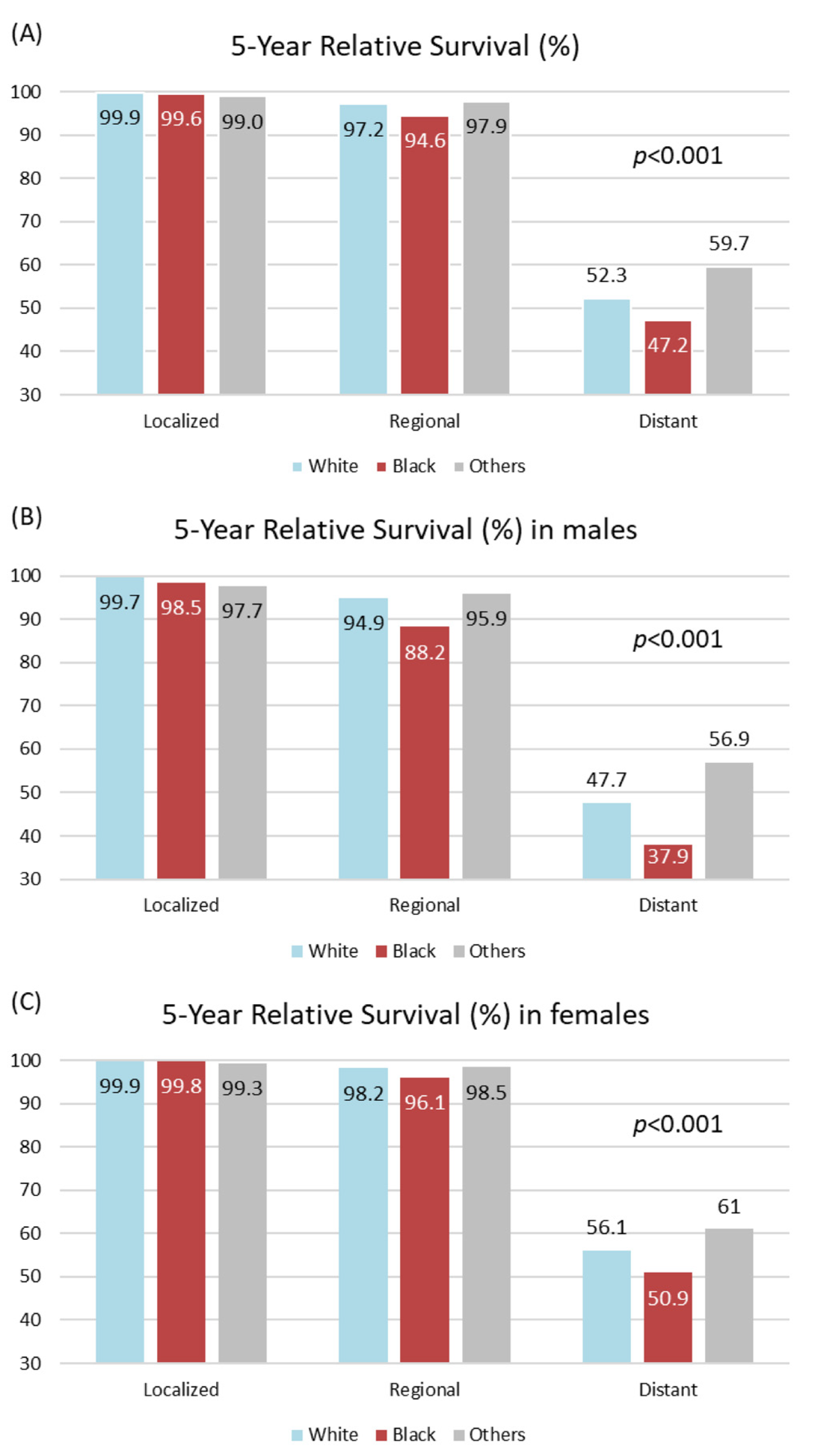
4.2. Thyroid Cancer 5-Year Survival by Race/Ethnicity
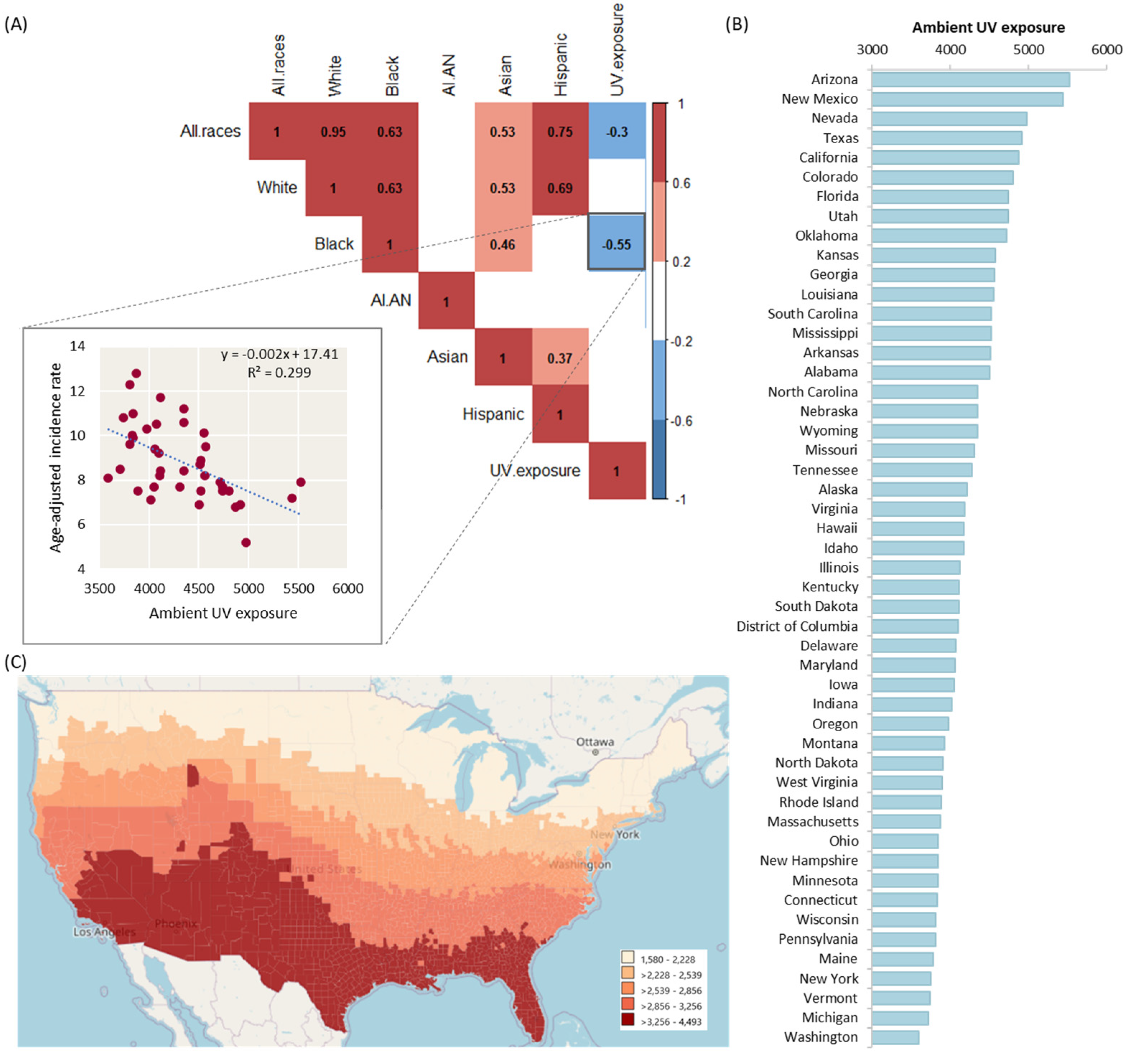
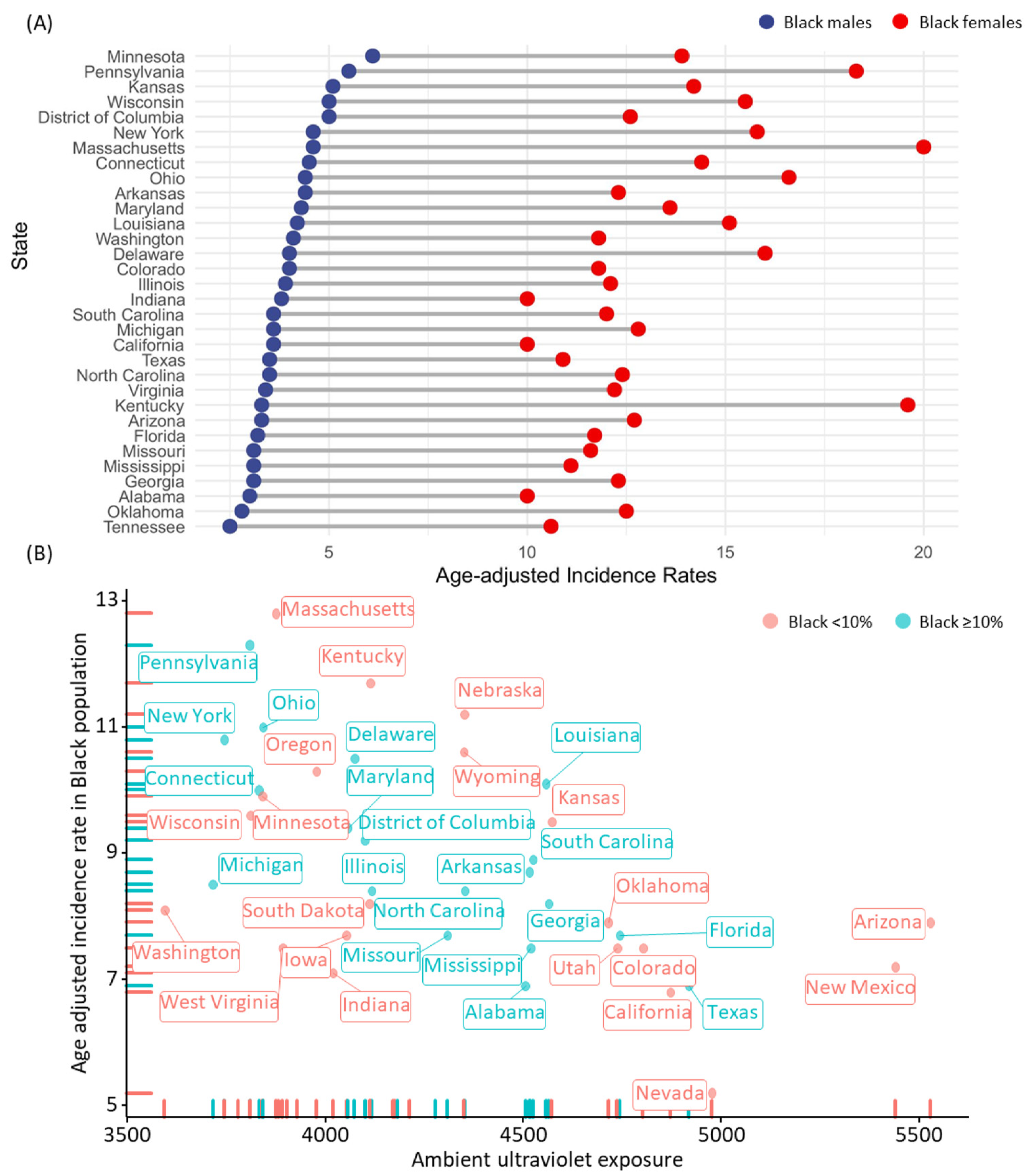
4.3. Ultraviolet Exposure and Thyroid Cancer Incidence Rate by Geographical Correlation
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davies, L.; Welch, H.G. Current Thyroid Cancer Trends in the United States. JAMA Otolaryngol. –Head Neck Surg. 2014, 140, 317–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, L.G.; Sikora, A.G.; Tosteson, T.D.; Davies, L. The Increasing Incidence of Thyroid Cancer: The Influence of Access to Care. Thyroid 2013, 23, 885–891. [Google Scholar] [CrossRef]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, R.L.; de Souza, J.A.; Cohen, E.E. Thyroid Cancer: Burden of Illness and Management of Disease. J. Cancer 2011, 2, 193. [Google Scholar] [CrossRef] [PubMed]
- Zamoiski, R.D.; Cahoon, E.K.; Freedman, D.M.; Linet, M.S.; Kitahara, C.M. Prospective Study of Ultraviolet Radiation Exposure and Thyroid Cancer Risk in the United States. Cancer Epidemiol. Biomark. Prev. 2017, 26, 684–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sample, A.; He, Y. Mechanisms and Prevention of UV-induced Melanoma. Photodermatol. Photoimmunol. Photomed. 2018, 34, 13–24. [Google Scholar] [CrossRef] [Green Version]
- DeLuca, H.F. Overview of General Physiologic Features and Functions of Vitamin D. Am. J. Clin. Nutr. 2004, 80, 1689S–1696S. [Google Scholar] [CrossRef] [Green Version]
- Nair-Shalliker, V.; Bang, A.; Egger, S.; Clements, M.; Gardiner, R.A.; Kricker, A.; Seibel, M.J.; Chambers, S.K.; Kimlin, M.G.; Armstrong, B.K. Post-Treatment Levels of Plasma 25-and 1, 25-Dihydroxy Vitamin D and Mortality in Men with Aggressive Prostate Cancer. Sci. Rep. 2020, 10, 7736. [Google Scholar] [CrossRef]
- Shui, I.M.; Mucci, L.A.; Kraft, P.; Tamimi, R.M.; Lindstrom, S.; Penney, K.L.; Nimptsch, K.; Hollis, B.W.; DuPre, N.; Platz, E.A. Vitamin d–Related Genetic Variation, Plasma Vitamin d, and Risk of Lethal Prostate Cancer: A Prospective Nested Case–Control Study. J. Natl. Cancer Inst. 2012, 104, 690–699. [Google Scholar] [CrossRef] [Green Version]
- Hossain, S.; Beydoun, M.A.; Beydoun, H.A.; Chen, X.; Zonderman, A.B.; Wood, R.J. Vitamin D and Breast Cancer: A Systematic Review and Meta-Analysis of Observational Studies. Clin. Nutr. ESPEN 2019, 30, 170–184. [Google Scholar] [CrossRef]
- Knight, J.A.; Lesosky, M.; Barnett, H.; Raboud, J.M.; Vieth, R. Vitamin D and Reduced Risk of Breast Cancer: A Population-Based Case-Control Study. Cancer Epidemiol. Biomark. Prev. 2007, 16, 422–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedrich, M.; Rafi, L.; Mitschele, T.; Tilgen, W.; Schmidt, W.; Reichrath, J. Analysis of the Vitamin D System in Cervical Carcinomas, Breast Cancer and Ovarian Cancer. In Vitamin D Analogs in Cancer Prevention and Therapy; Springer: Berlin/Heidelberg, Germany, 2003; pp. 239–246. [Google Scholar]
- Mesrine, S.; Kvaskoff, M.; Bah, T.; Wald, L.; Clavel-Chapelon, F.; Boutron-Ruault, M.-C. Nevi, Ambient Ultraviolet Radiation, and Thyroid Cancer Risk. Epidemiology 2017, 28, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Duggan, M.A.; Anderson, W.F.; Altekruse, S.; Penberthy, L.; Sherman, M.E. The Surveillance, Epidemiology and End Results (SEER) Program and Pathology: Towards Strengthening the Critical Relationship. Am. J. Surg. Pathol. 2016, 40, e94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milano, A.F. Thyroid Cancer: 20-Year Comparative Mortality and Survival Analysis of Six Thyroid Cancer Histologic Subtypes by Age, Sex, Race, Stage, Cohort Entry Time-Period and Disease Duration (SEER* Stat 8.3. 2) a Systematic Review of 145,457 Cases for Diagnosis Years 1993–2013. J. Insur. Med. 2018, 47, 143–158. [Google Scholar] [PubMed]
- Sung, H.; Hyun, N.; Leach, C.R.; Yabroff, K.R.; Jemal, A. Association of First Primary Cancer with Risk of Subsequent Primary Cancer among Survivors of Adult-Onset Cancers in the United States. JAMA 2020, 324, 2521–2535. [Google Scholar] [CrossRef]
- Hussein, M.; Mueller, L.; Issa, P.P.; Haidari, M.; Trinh, L.; Toraih, E.; Kandil, E. Latency Trend Analysis as a Guide to Screening Malignancy Survivors for Second Primary Thyroid Cancer. Biomedicines 2022, 10, 1984. [Google Scholar] [CrossRef]
- Megwalu, U.C.; Moon, P.K. Thyroid Cancer Incidence and Mortality Trends in the United States: 2000–2018. Thyroid 2022, 32, 560–570. [Google Scholar] [CrossRef]
- Powers, A.E.; Marcadis, A.R.; Lee, M.; Morris, L.G.; Marti, J.L. Changes in Trends in Thyroid Cancer Incidence in the United States, 1992 to 2016. Jama 2019, 322, 2440–2441. [Google Scholar] [CrossRef]
- Issa, P.P.; Omar, M.; Buti, Y.; Issa, C.P.; Chabot, B.; Carnabatu, C.J.; Munshi, R.; Hussein, M.; Aboueisha, M.; Shama, M. Hashimoto’s Thyroiditis Minimizes Lymph Node Metastasis in BRAF Mutant Papillary Thyroid Carcinomas. Biomedicines 2022, 10, 2051. [Google Scholar] [CrossRef]
- Liu, Y.; Su, L.; Xiao, H. Review of Factors Related to the Thyroid Cancer Epidemic. Int. J. Endocrinol. 2017, 2017, 530–539. [Google Scholar] [CrossRef] [Green Version]
- Tufano, R.P.; Noureldine, S.I.; Angelos, P. Incidental Thyroid Nodules and Thyroid Cancer: Considerations before Determining Management. JAMA Otolaryngol. –Head Neck Surg. 2015, 141, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Powers, A.E.; Morris, L.G.; Marti, J.L. Reversal in Thyroid Cancer Incidence Trends in the United States, 2000–2017. Thyroid 2020, 30, 1226. [Google Scholar] [CrossRef] [PubMed]
- Magreni, A.; Bann, D.V.; Schubart, J.R.; Goldenberg, D. The Effects of Race and Ethnicity on Thyroid Cancer Incidence. JAMA Otolaryngol. Head Neck Surg. 2015, 141, 319–323. [Google Scholar] [CrossRef] [Green Version]
- Noureldine, S.I.; Tufano, R.P. Association of Hashimoto’s Thyroiditis and Thyroid Cancer. Curr. Opin. Oncol. 2015, 27, 21–25. [Google Scholar] [CrossRef]
- Okayasu, I.; Fujiwara, M.; Hara, Y.; Tanaka, Y.; Rose, N.R. Association of Chronic Lymphocytic Thyroiditis and Thyroid Papillary Carcinoma. A Study of Surgical Cases among Japanese, and White and African Americans. Cancer 1995, 76, 2312–2318. [Google Scholar] [CrossRef]
- Singh, G.K.; Jemal, A. Socioeconomic and Racial/Ethnic Disparities in Cancer Mortality, Incidence, and Survival in the United States, 1950–2014: Over Six Decades of Changing Patterns and Widening Inequalities. J. Environ. Public Health 2017, 2017, 102–121. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.K.; Miller, B.A.; Hankey, B.F.; Edwards, B.K. Persistent Area Socioeconomic Disparities in US Incidence of Cervical Cancer, Mortality, Stage, and Survival, 1975–2000. Cancer 2004, 101, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Ghori, F.Y.; Gutterman-Litofsky, D.R.; Jamal, A.; Yeung, S.-C.J.; Arem, R.; Sherman, S.I. Socioeconomic Factors and the Presentation, Management, and Outcome of Patients with Differentiated Thyroid Carcinoma. Thyroid 2002, 12, 1009–1016. [Google Scholar] [CrossRef]
- Fowler, H.; Belot, A.; Ellis, L.; Maringe, C.; Luque-Fernandez, M.A.; Njagi, E.N.; Navani, N.; Sarfati, D.; Rachet, B. Comorbidity Prevalence among Cancer Patients: A Population-Based Cohort Study of Four Cancers. BMC Cancer 2020, 20, 2. [Google Scholar] [CrossRef] [Green Version]
- Moore, N.; Wright, M.; Gipson, J.; Jordan, G.; Harsh, M.; Reed, D.; Murray, M.; Keeter, M.K.; Murphy, A. A Survey of African American Men in Chicago Barbershops: Implications for the Effectiveness of the Barbershop Model in the Health Promotion of African American Men. J. Community Health 2016, 41, 772–779. [Google Scholar] [CrossRef] [Green Version]
- Murphy, A.; Moore, N.; Wright, M.; Gipson, J.; Keeter, M.; Cornelious, T.; Reed, D.; Russell, J.; Watson, K.; Murray, M. Alternative Locales for the Health Promotion of African American Men: A Survey of African American Men in Chicago Barbershops. J. Community Health 2017, 42, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Releford, B.J.; Frencher Jr, S.K.; Yancey, A.K.; Norris, K. Cardiovascular Disease Control through Barbershops: Design of a Nationwide Outreach Program. J. Natl. Med. Assoc. 2010, 102, 336–345. [Google Scholar] [CrossRef] [Green Version]
- Whiteman, D.C.; Brown, R.M.; Purdie, D.M.; Hughes, M.-C. Melanocytic Nevi in Very Young Children: The Role of Phenotype, Sun Exposure, and Sun Protection. J. Am. Acad. Dermatol. 2005, 52, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. An Ecologic Study of Cancer Mortality Rates in Spain with Respect to Indices of Solar UVB Irradiance and Smoking. Int. J. Cancer 2007, 120, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Greenlee, H.; White, E.; Patterson, R.E.; Kristal, A.R. Supplement Use among Cancer Survivors in the Vitamins and Lifestyle (VITAL) Study Cohort. J. Altern. Complement. Med. 2004, 10, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Cook, N.R.; Lee, I.-M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med. 2019, 380, 33–44. [Google Scholar] [CrossRef]
- VanWeelden, K.; Flanagan, L.; Binderup, L.; Tenniswood, M.; Welsh, J. Apoptotic Regression of MCF-7 Xenografts in Nude Mice Treated with the Vitamin D3 Analog, EB1089. Endocrinology 1998, 139, 2102–2110. [Google Scholar] [CrossRef]
- Clinckspoor, I.; Verlinden, L.; Mathieu, C.; Bouillon, R.; Verstuyf, A.; Decallonne, B. Vitamin D in Thyroid Tumorigenesis and Development. Prog. Histochem. Cytochem. 2013, 48, 65–98. [Google Scholar] [CrossRef]
- Fan, F.S.; Yu, W.C. 1, 25-Dihydroxyvitamin D3 Suppresses Cell Growth, DNA Synthesis, and Phosphorylation of Retinoblastoma Protein in a Breast Cancer Cell Line. Cancer Investig. 1995, 13, 280–286. [Google Scholar] [CrossRef]
- Boughanem, H.; Canudas, S.; Hernandez-Alonso, P.; Becerra-Tomás, N.; Babio, N.; Salas-Salvadó, J.; Macias-Gonzalez, M. Vitamin D Intake and the Risk of Colorectal Cancer: An Updated Meta-Analysis and Systematic Review of Case-Control and Prospective Cohort Studies. Cancers 2021, 13, 2814. [Google Scholar] [CrossRef]
- Mizoue, T.; Kimura, Y.; Toyomura, K.; Nagano, J.; Kono, S.; Mibu, R.; Tanaka, M.; Kakeji, Y.; Maehara, Y.; Okamura, T. Calcium, Dairy Foods, Vitamin D, and Colorectal Cancer Risk: The Fukuoka Colorectal Cancer Study. Cancer Epidemiol. Biomark. Prev. 2008, 17, 2800–2807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleming, J.B.; Shen, G.-L.; Holloway, S.E.; Davis, M.; Brekken, R.A. Molecular Consequences of Silencing Mutant K-Ras in Pancreatic Cancer Cells: Justification for K-Ras–Directed Therapy. Mol. Cancer Res. 2005, 3, 413–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, D.; Wang, L.; Zuo, X.; Bresalier, R.S. Vitamin D: Promises on the Horizon and Challenges Ahead for Fighting Pancreatic Cancer. Cancers 2021, 13, 2716. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Lin, J.; Fu, R.; Qi, S.; Fu, X.; Yuan, L.; Qian, L. The Role of Vitamin D Intake on the Prognosis and Incidence of Lung Cancer: A Systematic Review and Meta-Analysis. J. Nutr. Sci. Vitaminol. 2021, 67, 273–282. [Google Scholar] [CrossRef]
- Kwak, J.H.; Paik, J.K. Vitamin D Status and Gastric Cancer: A Cross-Sectional Study in Koreans. Nutrients 2020, 12, 2004. [Google Scholar] [CrossRef]
- Vyas, N.; Companioni, R.C.; Tiba, M.; Alkhawam, H.; Catalano, C.; Sogomonian, R.; Baum, J.; Walfish, A. Association between Serum Vitamin D Levels and Gastric Cancer: A Retrospective Chart Analysis. World J. Gastrointest. Oncol. 2016, 8, 688. [Google Scholar] [CrossRef]
- Eggermont, A.M.; Spatz, A.; Robert, C. Cutaneous Melanoma. Lancet 2014, 383, 816–827. [Google Scholar] [CrossRef]

| Characteristics | Age-Adjusted Incidence Rate Cases per 100,000 | Average Annual Count | Recent 5-Year Trend in Incidence Rates | |
|---|---|---|---|---|
| All races | Overall | 14.1 (14.0, 14.1) | 47,728 | −2.9 (−4.1, −1.7) * |
| Male | 7.3 (7.2, 7.3) | 12,303 | −1.9 (−3.3, −0.5) * | |
| Female | 20.7 (20.6, 20.8) | 35,425 | −3.1 (−4.4, −1.8) * | |
| White | Overall | 14.7 (14.6, 14.7) | 39,484 | −3.1 (−4.3, −1.8) * |
| Male | 7.7 (7.6, 7.7) | 10,580 | −1.9 (−3.4, −0.4) * | |
| Female | 21.7 (21.5, 21.8) | 28,904 | −3.4 (−4.7, −2.2) * | |
| Black | Overall | 8.7 (8.6, 8.8) | 3745 | −5.4 (−7.7, −3.2) * |
| Male | 3.8 (3.6, 3.9) | 727 | −4.3 (−6.7, −1.9) * | |
| Female | 13.0 (12.8, 13.2) | 3018 | −5.1 (−7.6, −2.6) * | |
| American Indian/Alaskan Native | Overall | 8.0 (7.6, 8.4) | 332 | −0.7 (−3.6, 2.4) |
| Male | 3.8 (3.4, 4.2) | 73 | 3.0 (1.0, 5.1) # | |
| Female | 12.2 (11.5, 12.9) | 259 | −0.3 (−3.9, 3.4) | |
| Asian or Pacific Islander | Overall | 14.9 (14.7, 15.2) | 3156 | −1.5 (−2.5, −0.4) * |
| Male | 7.3 (7.0, 7.5) | 700 | −1.8 (−3.7, 0.2) | |
| Female | 21.7 (21.3, 22.1) | 2456 | −1.8 (−3.1, −0.4) * | |
| Hispanic | Overall | 13.4 (13.2, 13.5) | 6536 | −0.9 (−2.7, 1.0) |
| Male | 5.8 (5.6, 5.9) | 1286 | 1.4 (0.3, 2.5) # | |
| Female | 20.9 (20.7, 21.2) | 5250 | −1.0 (−3.0, 1.0) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavorgna, T.R.; Hussein, M.; Issa, P.P.; Toraih, E.; Kandil, E. Ultraviolet Light Exposure Decreases Thyroid Cancer Risk: A National Perspective. Biomedicines 2022, 10, 2452. https://doi.org/10.3390/biomedicines10102452
Lavorgna TR, Hussein M, Issa PP, Toraih E, Kandil E. Ultraviolet Light Exposure Decreases Thyroid Cancer Risk: A National Perspective. Biomedicines. 2022; 10(10):2452. https://doi.org/10.3390/biomedicines10102452
Chicago/Turabian StyleLavorgna, Tessa R., Mohammad Hussein, Peter P. Issa, Eman Toraih, and Emad Kandil. 2022. "Ultraviolet Light Exposure Decreases Thyroid Cancer Risk: A National Perspective" Biomedicines 10, no. 10: 2452. https://doi.org/10.3390/biomedicines10102452
APA StyleLavorgna, T. R., Hussein, M., Issa, P. P., Toraih, E., & Kandil, E. (2022). Ultraviolet Light Exposure Decreases Thyroid Cancer Risk: A National Perspective. Biomedicines, 10(10), 2452. https://doi.org/10.3390/biomedicines10102452






