Dual Role of Extracellular Vesicles in Sepsis-Associated Kidney and Lung Injury
Abstract
:1. Introduction
1.1. General Features and Biological Activities of Extracellular Vesicles
1.2. Sepsis and Multi-Organ Failure—New Potential Mechanisms
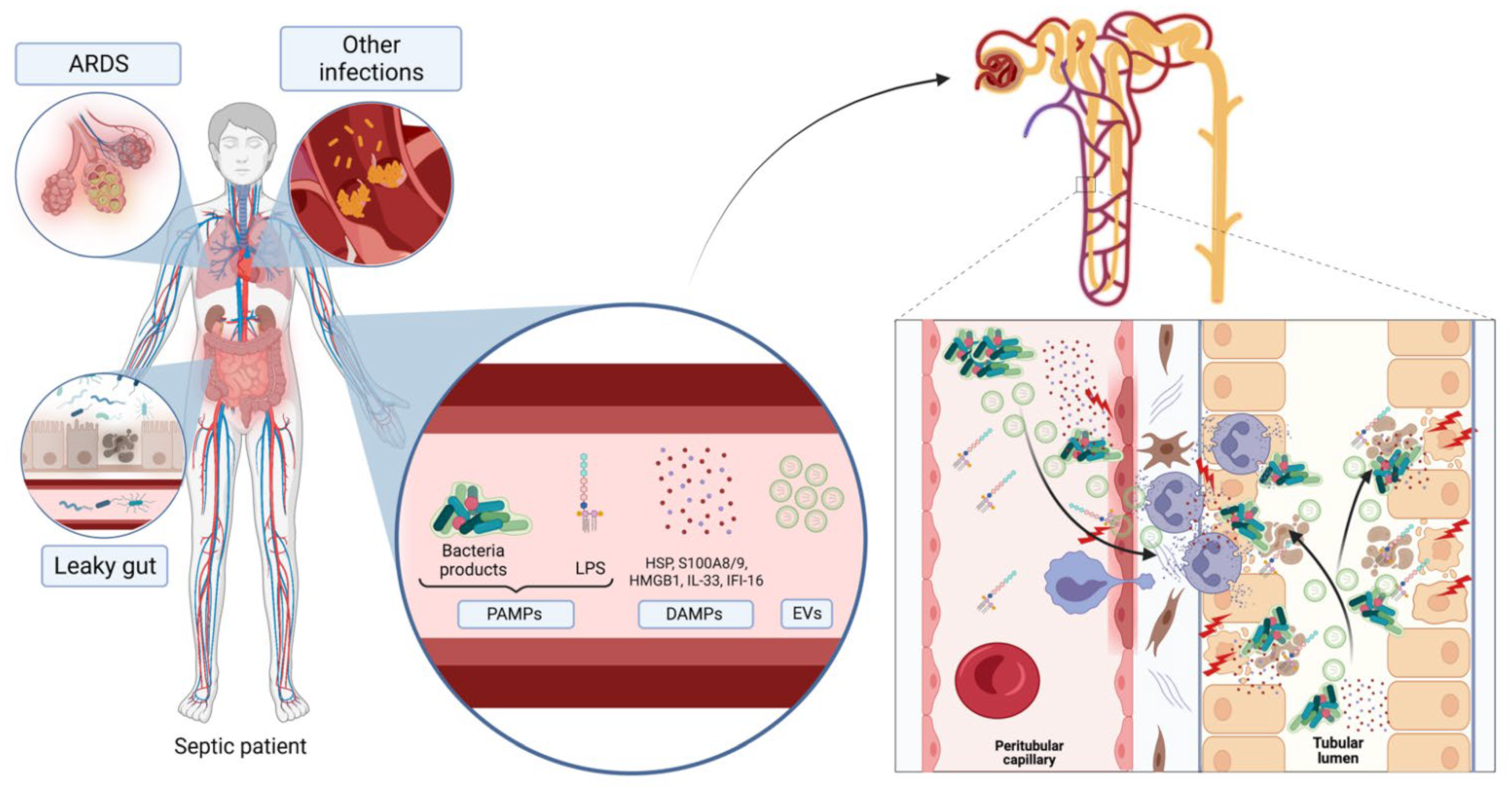
2. Sepsis-Associated Acute Kidney Injury
2.1. Role of EVs as Mediator of Renal Damage in s-AKI
2.1.1. EVs and Microvascular Dysfunction
2.1.2. EVs and Oxidative Stress
2.1.3. EVs and Immune Dysfunction
2.2. Role of Stem Cell-Derived Extracellular Vesicles as a Potential Therapeutic Tool in s-AKI
3. Role of Extracellular Vesicles in Sepsis-Associated ARDS
3.1. Role of EVs as Mediators of Lung Damage in Sepsis-Associated ARDS
3.2. Role of Stem Cell-Derived Extracellular Vescicles as a Potential Therapeutic Tool in Sepsis-Associated ARDS
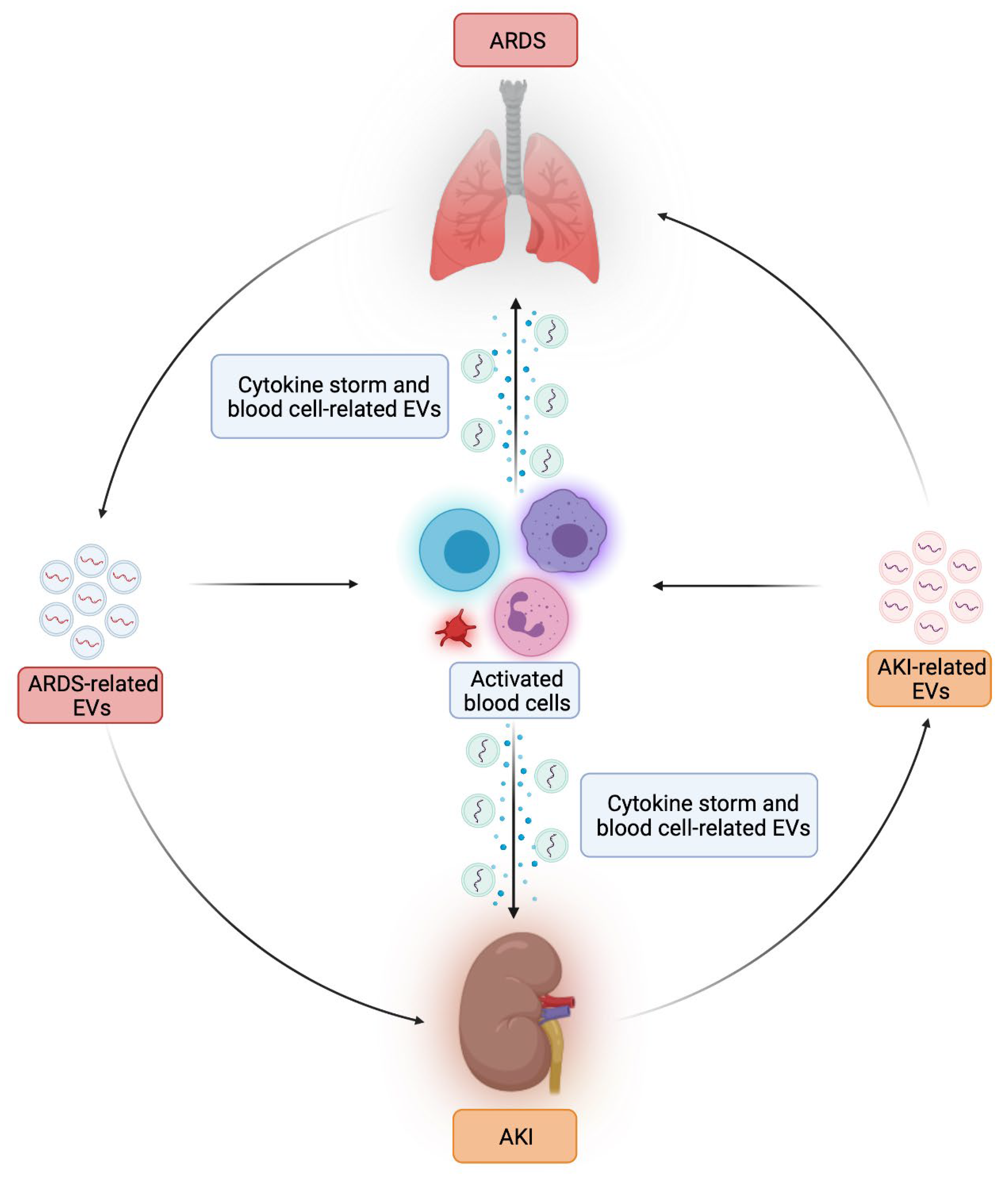
4. Potential Role of Extracellular Vesicles in Kidney-Lung Crosstalk and Future Therapeutic Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKI | acute kidney injury |
| ALI | acute lung injury |
| BALF | bronco-alveolar lavage fluid |
| CKD | chronic kidney disease |
| CLP | cecal ligation and puncture |
| DAMP | damage-associated molecular pattern |
| DC | dendritic cell |
| EC | endothelial cell |
| EMT | epithelial-to-mesenchymal transition |
| EndMT | endothelial-to-mesenchymal transition |
| EPC | endothelial progenitor cell |
| EV | extracellular vesicles |
| ICU | intensive care unit |
| IL | interleukin |
| IRI | ischemia-reperfusion injury |
| LPS | lipopolysaccharide |
| mDNA | mitochondrial DNA |
| MOD | multi-organ dysfunction |
| miRNA | microRNA |
| NEAT-1 | nuclear-enriched abundant transcript 1 |
| NRF-2 | nuclear factor erythroid 2-related factor 2 |
| PAMP | pathogen-associated molecular pattern |
| PMN | polymorphonuclear cell |
| PMT | pericyte-to-mesenchymal transition |
| ROS | reactive oxygen species |
| RRT | renal replacement therapy |
| RTEC | renal tubular epithelial cells |
| RUNX1 | runt-related transcription factor 1 axis |
| s-AKI | sepsis-associated AKI |
| SASP | senescence-associated secretory phenotype |
| SC | stem cell |
| SOCS-1 | suppressor of cytokine signaling-1 |
| TGFβ-1 | transforming growth factor β-1 |
| TLR | Toll-like receptors |
| TXNIP | thioredoxin-interacting protein |
References
- Raposo, G.; Stoorvogel, W. Extracellular Vesicles: Exosomes, Microvesicles, and Friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Cocucci, E.; Meldolesi, J. Ectosomes and Exosomes: Shedding the Confusion between Extracellular Vesicles. Trends Cell Biol. 2015, 25, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Bobrie, A.; Colombo, M.; Krumeich, S.; Raposo, G.; Théry, C. Diverse Subpopulations of Vesicles Secreted by Different Intracellular Mechanisms Are Present in Exosome Preparations Obtained by Differential Ultracentrifugation. J. Extracell. Vesicles 2012, 1, 18397. [Google Scholar] [CrossRef] [PubMed]
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell. Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Hristov, M.; Erl, W.; Linder, S.; Weber, P.C. Apoptotic Bodies from Endothelial Cells Enhance the Number and Initiate the Differentiation of Human Endothelial Progenitor Cells in Vitro. Blood 2004, 104, 2761–2766. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Urabe, F.; Kosaka, N.; Ito, K.; Kimura, T.; Egawa, S.; Ochiya, T. Extracellular Vesicles as Biomarkers and Therapeutic Targets for Cancer. Am. J. Physiol. Cell Physiol. 2020, 318, C29–C39. [Google Scholar] [CrossRef]
- Ratajczak, J.; Miekus, K.; Kucia, M.; Zhang, J.; Reca, R.; Dvorak, P.; Ratajczak, M.Z. Embryonic Stem Cell-Derived Microvesicles Reprogram Hematopoietic Progenitors: Evidence for Horizontal Transfer of MRNA and Protein Delivery. Leukemia 2006, 20, 847–856. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-Mediated Transfer of MRNAs and MicroRNAs Is a Novel Mechanism of Genetic Exchange between Cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [Green Version]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of Secretion and Uptake of Exosomes and Other Extracellular Vesicles for Cell-to-Cell Communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Kodam, S.P.; Ullah, M. Diagnostic and Therapeutic Potential of Extracellular Vesicles. Technol. Cancer Res. Treat. 2021, 20, 15330338211041204. [Google Scholar] [CrossRef] [PubMed]
- Claridge, B.; Lozano, J.; Poh, Q.H.; Greening, D.W. Development of Extracellular Vesicle Therapeutics: Challenges, Considerations, and Opportunities. Front. Cell Dev. Biol. 2021, 9, 734720. [Google Scholar] [CrossRef] [PubMed]
- Rady, D.; Abbass, M.M.S.; El-Rashidy, A.A.; El Moshy, S.; Radwan, I.A.; Dörfer, C.E.; Fawzy El-Sayed, K.M. Mesenchymal Stem/Progenitor Cells: The Prospect of Human Clinical Translation. Stem Cells Int. 2020, 2020, 8837654. [Google Scholar] [CrossRef]
- Salybekov, A.A.; Kunikeyev, A.D.; Kobayashi, S.; Asahara, T. Latest Advances in Endothelial Progenitor Cell-Derived Extracellular Vesicles Translation to the Clinic. Front. Cardiovasc. Med. 2021, 8, 734562. [Google Scholar] [CrossRef]
- Terriaca, S.; Fiorelli, E.; Scioli, M.G.; Fabbri, G.; Storti, G.; Cervelli, V.; Orlandi, A. Endothelial Progenitor Cell-Derived Extracellular Vesicles: Potential Therapeutic Application in Tissue Repair and Regeneration. Int. J. Mol. Sci. 2021, 22, 6375. [Google Scholar] [CrossRef]
- Cantaluppi, V.; Medica, D.; Mannari, C.; Stiaccini, G.; Figliolini, F.; Dellepiane, S.; Quercia, A.D.; Migliori, M.; Panichi, V.; Giovannini, L.; et al. Endothelial Progenitor Cell-Derived Extracellular Vesicles Protect from Complement-Mediated Mesangial Injury in Experimental Anti-Thy1.1 Glomerulonephritis. Nephrol. Dial. Transplant. 2015, 30, 410–422. [Google Scholar] [CrossRef]
- Qiu, P.; Zhou, J.; Zhang, J.; Dong, Y.; Liu, Y. Exosome: The Regulator of the Immune System in Sepsis. Front. Pharmacol. 2021, 12, 671164. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, Regional, and National Sepsis Incidence and Mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [Green Version]
- Kaukonen, K.-M.; Bailey, M.; Suzuki, S.; Pilcher, D.; Bellomo, R. Mortality Related to Severe Sepsis and Septic Shock among Critically Ill Patients in Australia and New Zealand, 2000–2012. JAMA 2014, 311, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.-L.; Marshall, J.C.; Namendys-Silva, S.A.; François, B.; Martin-Loeches, I.; Lipman, J.; Reinhart, K.; Antonelli, M.; Pickkers, P.; Njimi, H.; et al. Assessment of the Worldwide Burden of Critical Illness: The Intensive Care over Nations (ICON) Audit. Lancet Respir. Med. 2014, 2, 380–386. [Google Scholar] [CrossRef]
- Rossaint, J.; Zarbock, A. Pathogenesis of Multiple Organ Failure in Sepsis. Crit. Rev. Immunol. 2015, 35, 277–291. [Google Scholar] [CrossRef]
- May, C.N.; Bellomo, R.; Lankadeva, Y.R. Therapeutic Potential of Megadose Vitamin C to Reverse Organ Dysfunction in Sepsis and COVID-19. Br. J. Pharmacol. 2021, 178, 3864–3868. [Google Scholar] [CrossRef] [PubMed]
- Annane, D.; Bellissant, E.; Sebille, V.; Lesieur, O.; Mathieu, B.; Raphael, J.C.; Gajdos, P. Impaired Pressor Sensitivity to Noradrenaline in Septic Shock Patients with and without Impaired Adrenal Function Reserve. Br. J. Clin. Pharmacol. 1998, 46, 589–597. [Google Scholar] [CrossRef]
- Kellum, J.A.; Lameire, N.; KDIGO AKI Guideline Work Group. Diagnosis, Evaluation, and Management of Acute Kidney Injury: A KDIGO Summary (Part 1). Crit. Care 2013, 17, 204. [Google Scholar] [CrossRef]
- Poston, J.T.; Koyner, J.L. Sepsis Associated Acute Kidney Injury. BMJ 2019, 364, k4891. [Google Scholar] [CrossRef]
- Kellum, J.A.; Chawla, L.S.; Keener, C.; Singbartl, K.; Palevsky, P.M.; Pike, F.L.; Yealy, D.M.; Huang, D.T.; Angus, D.C.; ProCESS and ProGReSS-AKI Investigators. The Effects of Alternative Resuscitation Strategies on Acute Kidney Injury in Patients with Septic Shock. Am. J. Respir. Crit. Care Med. 2016, 193, 281–287. [Google Scholar] [CrossRef]
- Mehta, R.L.; Bouchard, J.; Soroko, S.B.; Ikizler, T.A.; Paganini, E.P.; Chertow, G.M.; Himmelfarb, J.; Program to Improve Care in Acute Renal Disease (PICARD) Study Group. Sepsis as a Cause and Consequence of Acute Kidney Injury: Program to Improve Care in Acute Renal Disease. Intensive Care Med. 2011, 37, 241–248. [Google Scholar] [CrossRef]
- Lee, S.A.; Cozzi, M.; Bush, E.L.; Rabb, H. Distant Organ Dysfunction in Acute Kidney Injury: A Review. Am. J. Kidney Dis. 2018, 72, 846–856. [Google Scholar] [CrossRef]
- Kurzhagen, J.T.; Dellepiane, S.; Cantaluppi, V.; Rabb, H. AKI: An Increasingly Recognized Risk Factor for CKD Development and Progression. J. Nephrol. 2020, 33, 1171–1187. [Google Scholar] [CrossRef]
- Quaglia, M.; Merlotti, G.; Colombatto, A.; Bruno, S.; Stasi, A.; Franzin, R.; Castellano, G.; Grossini, E.; Fanelli, V.; Cantaluppi, V. Stem Cell-Derived Extracellular Vesicles as Potential Therapeutic Approach for Acute Kidney Injury. Front. Immunol. 2022, 13, 849891. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Y.; Wei, W.; Liu, M.-L. Extracellular Vesicles in Autoimmune Vasculitis—Little Dirts Light the Fire in Blood Vessels. Autoimmun. Rev. 2019, 18, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Mariano, F.; Cantaluppi, V.; Stella, M.; Romanazzi, G.M.; Assenzio, B.; Cairo, M.; Biancone, L.; Triolo, G.; Ranieri, V.M.; Camussi, G. Circulating Plasma Factors Induce Tubular and Glomerular Alterations in Septic Burns Patients. Crit. Care 2008, 12, R42. [Google Scholar] [CrossRef] [PubMed]
- Gómez, H.; Kellum, J.A. Sepsis-Induced Acute Kidney Injury. Curr. Opin. Crit. Care 2016, 22, 546–553. [Google Scholar] [CrossRef]
- Cantaluppi, V.; Biancone, L.; Quercia, A.; Deregibus, M.C.; Segoloni, G.; Camussi, G. Rationale of Mesenchymal Stem Cell Therapy in Kidney Injury. Am. J. Kidney Dis. 2013, 61, 300–309. [Google Scholar] [CrossRef]
- Bussolati, B.; Camussi, G. Therapeutic Use of Human Renal Progenitor Cells for Kidney Regeneration. Nat. Rev. Nephrol. 2015, 11, 695–706. [Google Scholar] [CrossRef]
- Franzin, R.; Stasi, A.; Ranieri, E.; Netti, G.S.; Cantaluppi, V.; Gesualdo, L.; Stallone, G.; Castellano, G. Targeting Premature Renal Aging: From Molecular Mechanisms of Cellular Senescence to Senolytic Trials. Front. Pharmacol. 2021, 12, 630419. [Google Scholar] [CrossRef]
- Franzin, R.; Stasi, A.; Fiorentino, M.; Stallone, G.; Cantaluppi, V.; Gesualdo, L.; Castellano, G. Inflammaging and Complement System: A Link Between Acute Kidney Injury and Chronic Graft Damage. Front. Immunol. 2020, 11, 734. [Google Scholar] [CrossRef]
- Peerapornratana, S.; Manrique-Caballero, C.L.; Gómez, H.; Kellum, J.A. Acute Kidney Injury from Sepsis: Current Concepts, Epidemiology, Pathophysiology, Prevention and Treatment. Kidney Int. 2019, 96, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.C.P.; Yuen, P.S.T.; Star, R.A. Microparticles: Markers and Mediators of Sepsis-Induced Microvascular Dysfunction, Immunosuppression, and AKI. Kidney Int. 2015, 87, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Karpman, D.; Tontanahal, A. Extracellular Vesicles in Renal Inflammatory and Infectious Diseases. Free Radic. Biol. Med. 2021, 171, 42–54. [Google Scholar] [CrossRef]
- Zheng, D.; Zhang, J.; Zhang, Z.; Kuang, L.; Zhu, Y.; Wu, Y.; Xue, M.; Zhao, H.; Duan, C.; Liu, L.; et al. Endothelial Microvesicles Induce Pulmonary Vascular Leakage and Lung Injury During Sepsis. Front. Cell Dev. Biol. 2020, 8, 643. [Google Scholar] [CrossRef]
- Mastronardi, M.L.; Mostefai, H.A.; Meziani, F.; Martínez, M.C.; Asfar, P.; Andriantsitohaina, R. Circulating Microparticles from Septic Shock Patients Exert Differential Tissue Expression of Enzymes Related to Inflammation and Oxidative Stress. Crit. Care Med. 2011, 39, 1739–1748. [Google Scholar] [CrossRef]
- Mortaza, S.; Martinez, M.C.; Baron-Menguy, C.; Burban, M.; de la Bourdonnaye, M.; Fizanne, L.; Pierrot, M.; Calès, P.; Henrion, D.; Andriantsitohaina, R.; et al. Detrimental Hemodynamic and Inflammatory Effects of Microparticles Originating from Septic Rats. Crit. Care Med. 2009, 37, 2045–2050. [Google Scholar] [CrossRef]
- Raeven, P.; Zipperle, J.; Drechsler, S. Extracellular Vesicles as Markers and Mediators in Sepsis. Theranostics 2018, 8, 3348–3365. [Google Scholar] [CrossRef]
- Iba, T.; Ogura, H. Role of Extracellular Vesicles in the Development of Sepsis-Induced Coagulopathy. J. Intensive Care 2018, 6, 68. [Google Scholar] [CrossRef] [PubMed]
- Tőkés-Füzesi, M.; Woth, G.; Ernyey, B.; Vermes, I.; Mühl, D.; Bogár, L.; Kovács, G.L. Microparticles and Acute Renal Dysfunction in Septic Patients. J. Crit. Care 2013, 28, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Evans, R.G.; Iguchi, N.; Tare, M.; Parkington, H.C.; Bellomo, R.; May, C.N.; Lankadeva, Y.R. Sepsis-Induced Acute Kidney Injury: A Disease of the Microcirculation. Microcirculation 2019, 26, e12483. [Google Scholar] [CrossRef]
- Nadim, M.K.; Forni, L.G.; Mehta, R.L.; Connor, M.J.; Liu, K.D.; Ostermann, M.; Rimmelé, T.; Zarbock, A.; Bell, S.; Bihorac, A.; et al. COVID-19-Associated Acute Kidney Injury: Consensus Report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nat. Rev. Nephrol. 2020, 16, 747–764. [Google Scholar] [CrossRef]
- Legrand, M.; Bell, S.; Forni, L.; Joannidis, M.; Koyner, J.L.; Liu, K.; Cantaluppi, V. Pathophysiology of COVID-19-Associated Acute Kidney Injury. Nat. Rev. Nephrol. 2021, 17, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Petruk, G.; Puthia, M.; Petrlova, J.; Samsudin, F.; Strömdahl, A.-C.; Cerps, S.; Uller, L.; Kjellström, S.; Bond, P.J.; Schmidtchen, A.A. SARS-CoV-2 Spike Protein Binds to Bacterial Lipopolysaccharide and Boosts Proinflammatory Activity. J. Mol. Cell Biol. 2020, 12, 916–932. [Google Scholar] [CrossRef] [PubMed]
- Cappellano, G.; Raineri, D.; Rolla, R.; Giordano, M.; Puricelli, C.; Vilardo, B.; Manfredi, M.; Cantaluppi, V.; Sainaghi, P.P.; Castello, L.; et al. Circulating Platelet-Derived Extracellular Vesicles Are a Hallmark of SARS-CoV-2 Infection. Cells 2021, 10, e85. [Google Scholar] [CrossRef]
- Barberis, E.; Vanella, V.V.; Falasca, M.; Caneapero, V.; Cappellano, G.; Raineri, D.; Ghirimoldi, M.; de Giorgis, V.; Puricelli, C.; Vaschetto, R.; et al. Circulating Exosomes Are Strongly Involved in SARS-CoV-2 Infection. Front. Mol. Biosci. 2021, 8, 632290. [Google Scholar] [CrossRef]
- Janiszewski, M.; Do Carmo, A.O.; Pedro, M.A.; Silva, E.; Knobel, E.; Laurindo, F.R.M. Platelet-Derived Exosomes of Septic Individuals Possess Proapoptotic NAD(P)H Oxidase Activity: A Novel Vascular Redox Pathway. Crit. Care Med. 2004, 32, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Burger, D.; Turner, M.; Munkonda, M.N.; Touyz, R.M. Endothelial Microparticle-Derived Reactive Oxygen Species: Role in Endothelial Signaling and Vascular Function. Oxidative Med. Cell. Longev. 2016, 2016, 5047954. [Google Scholar] [CrossRef]
- Dolmatova, E.V.; Wang, K.; Mandavilli, R.; Griendling, K.K. The Effects of Sepsis on Endothelium and Clinical Implications. Cardiovasc. Res. 2021, 117, 60–73. [Google Scholar] [CrossRef]
- Burger, D.; Montezano, A.C.; Nishigaki, N.; He, Y.; Carter, A.; Touyz, R.M. Endothelial Microparticle Formation by Angiotensin II Is Mediated via Ang II Receptor Type I/NADPH Oxidase/Rho Kinase Pathways Targeted to Lipid Rafts. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1898–1907. [Google Scholar] [CrossRef]
- Sonoda, H.; Lee, B.R.; Park, K.-H.; Nihalani, D.; Yoon, J.-H.; Ikeda, M.; Kwon, S.-H. MiRNA Profiling of Urinary Exosomes to Assess the Progression of Acute Kidney Injury. Sci. Rep. 2019, 9, 4692. [Google Scholar] [CrossRef]
- Toro, J.; Manrique-Caballero, C.L.; Gómez, H. Metabolic Reprogramming and Host Tolerance: A Novel Concept to Understand Sepsis-Associated AKI. J. Clin. Med. 2021, 10, 4184. [Google Scholar] [CrossRef]
- Cantaluppi, V.; Quercia, A.D.; Dellepiane, S.; Ferrario, S.; Camussi, G.; Biancone, L. Interaction between Systemic Inflammation and Renal Tubular Epithelial Cells. Nephrol. Dial. Transplant. 2014, 29, 2004–2011. [Google Scholar] [CrossRef] [PubMed]
- Emma, F.; Montini, G.; Parikh, S.M.; Salviati, L. Mitochondrial Dysfunction in Inherited Renal Disease and Acute Kidney Injury. Nat. Rev. Nephrol. 2016, 12, 267–280. [Google Scholar] [CrossRef]
- Ralto, K.M.; Rhee, E.P.; Parikh, S.M. NAD+ Homeostasis in Renal Health and Disease. Nat. Rev. Nephrol. 2020, 16, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Youn, Y.-J.; Shrestha, S.; Lee, Y.-B.; Kim, J.-K.; Lee, J.H.; Hur, K.; Mali, N.M.; Nam, S.-W.; Kim, S.-H.; Lee, S.; et al. Neutrophil-Derived Trail Is a Proinflammatory Subtype of Neutrophil-Derived Extracellular Vesicles. Theranostics 2021, 11, 2770–2787. [Google Scholar] [CrossRef] [PubMed]
- Essandoh, K.; Li, Y.; Huo, J.; Fan, G.-C. MiRNA-Mediated Macrophage Polarization and Its Potential Role in the Regulation of Inflammatory Response. Shock 2016, 46, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Lyon, C.J.; Fletcher, J.K.; Tang, W.; Wan, M.; Hu, T.Y. Extracellular Vesicle Activities Regulating Macrophage- and Tissue-Mediated Injury and Repair Responses. Acta Pharm. Sin. B 2021, 11, 1493–1512. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.-L.; Feng, Y.; Wu, M.; Wang, B.; Li, Z.-L.; Zhong, X.; Wu, W.-J.; Chen, J.; Ni, H.-F.; Tang, T.-T.; et al. Exosomal MiRNA-19b-3p of Tubular Epithelial Cells Promotes M1 Macrophage Activation in Kidney Injury. Cell Death Differ. 2020, 27, 210–226. [Google Scholar] [CrossRef]
- Wang, Z.-W.; Zhu, X. Exosomal MiR-19b-3p Communicates Tubular Epithelial Cells and M1 Macrophage. Cell Death Dis. 2019, 10, 762. [Google Scholar] [CrossRef]
- Li, Z.-L.; Lv, L.-L.; Tang, T.-T.; Wang, B.; Feng, Y.; Zhou, L.-T.; Cao, J.-Y.; Tang, R.-N.; Wu, M.; Liu, H.; et al. HIF-1α Inducing Exosomal MicroRNA-23a Expression Mediates the Cross-Talk between Tubular Epithelial Cells and Macrophages in Tubulointerstitial Inflammation. Kidney Int. 2019, 95, 388–404. [Google Scholar] [CrossRef]
- Lv, L.-L.; Feng, Y.; Wen, Y.; Wu, W.-J.; Ni, H.-F.; Li, Z.-L.; Zhou, L.-T.; Wang, B.; Zhang, J.-D.; Crowley, S.D.; et al. Exosomal CCL2 from Tubular Epithelial Cells Is Critical for Albumin-Induced Tubulointerstitial Inflammation. J. Am. Soc. Nephrol. 2018, 29, 919–935. [Google Scholar] [CrossRef] [Green Version]
- Juan, C.-X.; Mao, Y.; Cao, Q.; Chen, Y.; Zhou, L.-B.; Li, S.; Chen, H.; Chen, J.-H.; Zhou, G.-P.; Jin, R. Exosome-Mediated Pyroptosis of MiR-93-TXNIP-NLRP3 Leads to Functional Difference between M1 and M2 Macrophages in Sepsis-Induced Acute Kidney Injury. J. Cell Mol. Med. 2021, 25, 4786–4799. [Google Scholar] [CrossRef]
- Ye, Z.; Zhang, L.; Li, R.; Dong, W.; Liu, S.; Li, Z.; Liang, H.; Wang, L.; Shi, W.; Malik, A.B.; et al. Caspase-11 Mediates Pyroptosis of Tubular Epithelial Cells and Septic Acute Kidney Injury. Kidney Blood Press. Res. 2019, 44, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Gildea, J.J.; Seaton, J.E.; Victor, K.G.; Reyes, C.M.; Bigler Wang, D.; Pettigrew, A.C.; Courtner, C.E.; Shah, N.; Tran, H.T.; van Sciver, R.E.; et al. Exosomal Transfer from Human Renal Proximal Tubule Cells to Distal Tubule and Collecting Duct Cells. Clin. Biochem. 2014, 47, 89–94. [Google Scholar] [CrossRef]
- Cricrì, G.; Bellucci, L.; Montini, G.; Collino, F. Urinary Extracellular Vesicles: Uncovering the Basis of the Pathological Processes in Kidney-Related Diseases. Int. J. Mol. Sci. 2021, 22, 6507. [Google Scholar] [CrossRef] [PubMed]
- Fatima, F.; Ekstrom, K.; Nazarenko, I.; Maugeri, M.; Valadi, H.; Hill, A.F.; Camussi, G.; Nawaz, M. Non-Coding RNAs in Mesenchymal Stem Cell-Derived Extracellular Vesicles: Deciphering Regulatory Roles in Stem Cell Potency, Inflammatory Resolve, and Tissue Regeneration. Front. Genet. 2017, 8, 161. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, K.; Kitamura, S.; Wada, J. Secretomes from Mesenchymal Stem Cells against Acute Kidney Injury: Possible Heterogeneity. Stem Cells Int. 2018, 2018, 8693137. [Google Scholar] [CrossRef]
- Li, J.-K.; Yang, C.; Su, Y.; Luo, J.-C.; Luo, M.-H.; Huang, D.-L.; Tu, G.-W.; Luo, Z. Mesenchymal Stem Cell-Derived Extracellular Vesicles: A Potential Therapeutic Strategy for Acute Kidney Injury. Front. Immunol. 2021, 12, 684496. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, G.P.; Shah, S.V. Autophagy in Acute Kidney Injury. Kidney Int. 2016, 89, 779–791. [Google Scholar] [CrossRef]
- Grange, C.; Skovronova, R.; Marabese, F.; Bussolati, B. Stem Cell-Derived Extracellular Vesicles and Kidney Regeneration. Cells 2019, 8, e1240. [Google Scholar] [CrossRef]
- Maccario, R.; Podestà, M.; Moretta, A.; Cometa, A.; Comoli, P.; Montagna, D.; Daudt, L.; Ibatici, A.; Piaggio, G.; Pozzi, S.; et al. Interaction of Human Mesenchymal Stem Cells with Cells Involved in Alloantigen-Specific Immune Response Favors the Differentiation of CD4+ T-Cell Subsets Expressing a Regulatory/Suppressive Phenotype. Haematologica 2005, 90, 516–525. [Google Scholar]
- Zou, X.; Gu, D.; Zhang, G.; Zhong, L.; Cheng, Z.; Liu, G.; Zhu, Y. NK Cell Regulatory Property Is Involved in the Protective Role of MSC-Derived Extracellular Vesicles in Renal Ischemic Reperfusion Injury. Hum. Gene Ther. 2016, 27, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Gatti, S.; Bruno, S.; Deregibus, M.C.; Sordi, A.; Cantaluppi, V.; Tetta, C.; Camussi, G. Microvesicles Derived from Human Adult Mesenchymal Stem Cells Protect against Ischaemia-Reperfusion-Induced Acute and Chronic Kidney Injury. Nephrol. Dial. Transplant. 2011, 26, 1474–1483. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.W.; Wang, J.; Lee, C.J.; Liu, M.; Neelamegham, S.; Canty, J.M.; Nguyen, J. The MicroRNA Regulatory Landscape of MSC-Derived Exosomes: A Systems View. Sci. Rep. 2018, 8, 1419. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Y.; Hong, Q.; Zhang, C.-Y.; Yang, Y.-J.; Cai, G.-Y.; Chen, X.-M. MiRNAs in Stem Cell-Derived Extracellular Vesicles for Acute Kidney Injury Treatment: Comprehensive Review of Preclinical Studies. Stem Cell Res. Ther. 2019, 10, 281. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, H.; Liu, W.; Liu, S.; Wang, X.Y.; Diao, Z.L.; Zhang, A.H.; Guo, W.; Han, X.; Dong, X.; et al. Endothelial Progenitor Cells-Derived Exosomal MicroRNA-21-5p Alleviates Sepsis-Induced Acute Kidney Injury by Inhibiting RUNX1 Expression. Cell Death Dis. 2021, 12, 335. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Wang, H.; Yue, L. Endothelial Progenitor Cells-Secreted Extracellular Vesicles Containing MicroRNA-93-5p Confer Protection against Sepsis-Induced Acute Kidney Injury via the KDM6B/H3K27me3/TNF-α Axis. Exp. Cell Res. 2020, 395, 112173. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Wang, P.; Zhou, X.; Liang, H.; Li, C. Knockdown of Circ-FANCA Alleviates LPS-Induced HK2 Cell Injury via Targeting MiR-93-5p/OXSR1 Axis in Septic Acute Kidney Injury. Diabetol. Metab. Syndr. 2021, 13, 7. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Kong, M.; Yang, J. MiR-22-3p Suppresses Sepsis-Induced Acute Kidney Injury by Targeting PTEN. Biosci. Rep. 2020, 40, BSR20200527. [Google Scholar] [CrossRef]
- Zhang, R.; Zhu, Y.; Li, Y.; Liu, W.; Yin, L.; Yin, S.; Ji, C.; Hu, Y.; Wang, Q.; Zhou, X.; et al. Human Umbilical Cord Mesenchymal Stem Cell Exosomes Alleviate Sepsis-Associated Acute Kidney Injury via Regulating MicroRNA-146b Expression. Biotechnol. Lett. 2020, 42, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Zuo, B.; Wang, Y.; Li, S.; Yang, J.; Sun, D. Protective Function of Exosomes from Adipose Tissue-Derived Mesenchymal Stem Cells in Acute Kidney Injury through SIRT1 Pathway. Life Sci. 2020, 255, 117719. [Google Scholar] [CrossRef]
- Pan, T.; Jia, P.; Chen, N.; Fang, Y.; Liang, Y.; Guo, M.; Ding, X. Delayed Remote Ischemic Preconditioning ConfersRenoprotection against Septic Acute Kidney Injury via Exosomal MiR-21. Theranostics 2019, 9, 405–423. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, S.; Wang, C.; Wang, Y.; Wan, M.; Liu, F.; Gong, M.; Yuan, Y.; Chen, Y.; Cheng, J.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles Attenuate Mitochondrial Damage and Inflammation by Stabilizing Mitochondrial DNA. ACS Nano 2021, 15, 1519–1538. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.-R.; Chan, W.P.A.; Nguyen, T.H.; Liu, S.; Procter, N.E.K.; Ngo, D.T.; Sverdlov, A.L.; Chirkov, Y.Y.; Horowitz, J.D. Thioredoxin-Interacting Protein: Pathophysiology and Emerging Pharmacotherapeutics in Cardiovascular Disease and Diabetes. Cardiovasc. Drugs Ther. 2014, 28, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wu, L.; Liu, S.; Hu, X.; Wang, Q.; Fang, L. Long Non-Coding RNA NEAT1 Promotes Lipopolysaccharide-Induced Injury in Human Tubule Epithelial Cells by Regulating MiR-93-5p/TXNIP Axis. Med. Microbiol. Immunol. 2021, 210, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S.; ARDS Definition Task Force. Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, Patterns of Care, and Mortality for Patients with Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef]
- Matthay, M.A.; Arabi, Y.M.; Siegel, E.R.; Ware, L.B.; Bos, L.D.J.; Sinha, P.; Beitler, J.R.; Wick, K.D.; Curley, M.A.Q.; Constantin, J.-M.; et al. Phenotypes and Personalized Medicine in the Acute Respiratory Distress Syndrome. Intensive Care Med. 2020, 46, 2136–2152. [Google Scholar] [CrossRef]
- Sheu, C.-C.; Gong, M.N.; Zhai, R.; Chen, F.; Bajwa, E.K.; Clardy, P.F.; Gallagher, D.C.; Thompson, B.T.; Christiani, D.C. Clinical Characteristics and Outcomes of Sepsis-Related vs Non-Sepsis-Related ARDS. Chest 2010, 138, 559–567. [Google Scholar] [CrossRef]
- Alhazzani, W.; Møller, M.H.; Arabi, Y.M.; Loeb, M.; Gong, M.N.; Fan, E.; Oczkowski, S.; Levy, M.M.; Derde, L.; Dzierba, A.; et al. Surviving Sepsis Campaign: Guidelines on the Management of Critically Ill Adults with Coronavirus Disease 2019 (COVID-19). Crit. Care Med. 2020, 48, e440–e469. [Google Scholar] [CrossRef]
- Aziz, S.; Arabi, Y.M.; Alhazzani, W.; Evans, L.; Citerio, G.; Fischkoff, K.; Salluh, J.; Meyfroidt, G.; Alshamsi, F.; Oczkowski, S.; et al. Managing ICU Surge during the COVID-19 Crisis: Rapid Guidelines. Intensive Care Med. 2020, 46, 1303–1325. [Google Scholar] [CrossRef]
- Fanelli, V.; Ranieri, V.M. Mechanisms and Clinical Consequences of Acute Lung Injury. Ann. Am. Thorac. Soc. 2015, 12 (Suppl. S1), S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.T.; Chambers, R.C.; Liu, K.D. Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2017, 377, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Ware, L.B.; Matthay, M.A. The Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2000, 342, 1334–1349. [Google Scholar] [CrossRef]
- Moon, H.-G.; Cao, Y.; Yang, J.; Lee, J.H.; Choi, H.S.; Jin, Y. Lung Epithelial Cell-Derived Extracellular Vesicles Activate Macrophage-Mediated Inflammatory Responses via ROCK1 Pathway. Cell Death Dis. 2015, 6, e2016. [Google Scholar] [CrossRef]
- Soni, S.; Wilson, M.R.; O’Dea, K.P.; Yoshida, M.; Katbeh, U.; Woods, S.J.; Takata, M. Alveolar Macrophage-Derived Microvesicles Mediate Acute Lung Injury. Thorax 2016, 71, 1020–1029. [Google Scholar] [CrossRef]
- Lee, H.; Zhang, D.; Laskin, D.L.; Jin, Y. Functional Evidence of Pulmonary Extracellular Vesicles in Infectious and Noninfectious Lung Inflammation. J. Immunol. 2018, 201, 1500–1509. [Google Scholar] [CrossRef] [PubMed]
- Bastarache, J.A.; Fremont, R.D.; Kropski, J.A.; Bossert, F.R.; Ware, L.B. Procoagulant Alveolar Microparticles in the Lungs of Patients with Acute Respiratory Distress Syndrome. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 297, L1035–L1041. [Google Scholar] [CrossRef]
- Mahida, R.Y.; Price, J.; Lugg, S.T.; Li, H.; Parekh, D.; Scott, A.; Harrison, P.; Matthay, M.A.; Thickett, D.R. CD14-Positive Extracellular Vesicles in Bronchoalveolar Lavage Fluid as a New Biomarker of Acute Respiratory Distress Syndrome. Am. J. Physiol. Lung Cell. Mol. Physiol. 2022, 322, L617–L624. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, S.; Kazepidou, E.; Antonelou, M.H.; Leondaritis, G.; Tsapinou, A.; Koulouras, V.P.; Avgeropoulos, A.; Nakos, G.; Lekka, M.E. Secretory Phospholipase A2-IIA Protein and MRNA Pools in Extracellular Vesicles of Bronchoalveolar Lavage Fluid from Patients with Early Acute Respiratory Distress Syndrome: A New Perception in the Dissemination of Inflammation? Pharmaceuticals 2020, 13, e415. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, J.; Xie, W.; Li, G.; Yao, L.; Zhang, R.; Xu, B. MiR-425 Reduction Causes Aberrant Proliferation and Collagen Synthesis through Modulating TGF-β/Smad Signaling in Acute Respiratory Distress Syndrome. Int. J. Clin. Exp. Pathol. 2019, 12, 2604–2612. [Google Scholar]
- Xu, X.; Liu, X.; Dong, X.; Qiu, H.; Yang, Y.; Liu, L. Secretory Autophagosomes from Alveolar Macrophages Exacerbate Acute Respiratory Distress Syndrome by Releasing IL-1β. J. Inflamm. Res. 2022, 15, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Shikano, S.; Gon, Y.; Maruoka, S.; Shimizu, T.; Kozu, Y.; Iida, Y.; Hikichi, M.; Takahashi, M.; Okamoto, S.; Tsuya, K.; et al. Increased Extracellular Vesicle MiRNA-466 Family in the Bronchoalveolar Lavage Fluid as a Precipitating Factor of ARDS. BMC Pulm. Med. 2019, 19, 110. [Google Scholar] [CrossRef] [PubMed]
- Scheller, N.; Herold, S.; Kellner, R.; Bertrams, W.; Jung, A.L.; Janga, H.; Greulich, T.; Schulte, L.N.; Vogelmeier, C.F.; Lohmeyer, J.; et al. Proviral MicroRNAs Detected in Extracellular Vesicles from Bronchoalveolar Lavage Fluid of Patients with Influenza Virus-Induced Acute Respiratory Distress Syndrome. J. Infect. Dis. 2019, 219, 540–543. [Google Scholar] [CrossRef] [PubMed]
- Meidert, A.S.; Hermann, S.; Brandes, F.; Kirchner, B.; Buschmann, D.; Billaud, J.-N.; Klein, M.; Lindemann, A.; Aue, E.; Schelling, G.; et al. Extracellular Vesicle Associated MiRNAs Regulate Signaling Pathways Involved in COVID-19 Pneumonia and the Progression to Severe Acute Respiratory Corona Virus-2 Syndrome. Front. Immunol. 2021, 12, 784028. [Google Scholar] [CrossRef]
- Li, H.; Meng, X.; Liang, X.; Gao, Y.; Cai, S. Administration of Microparticles from Blood of the Lipopolysaccharide-Treated Rats Serves to Induce Pathologic Changes of Acute Respiratory Distress Syndrome. Exp. Biol. Med. 2015, 240, 1735–1741. [Google Scholar] [CrossRef] [PubMed]
- Buesing, K.L.; Densmore, J.C.; Kaul, S.; Pritchard, K.A.; Jarzembowski, J.A.; Gourlay, D.M.; Oldham, K.T. Endothelial Microparticles Induce Inflammation in Acute Lung Injury. J. Surg. Res. 2011, 166, 32–39. [Google Scholar] [CrossRef]
- Densmore, J.C.; Signorino, P.R.; Ou, J.; Hatoum, O.A.; Rowe, J.J.; Shi, Y.; Kaul, S.; Jones, D.W.; Sabina, R.E.; Pritchard, K.A.; et al. Endothelium-Derived Microparticles Induce Endothelial Dysfunction and Acute Lung Injury. Shock 2006, 26, 464–471. [Google Scholar] [CrossRef]
- Frank, J.A.; Briot, R.; Lee, J.W.; Ishizaka, A.; Uchida, T.; Matthay, M.A. Physiological and Biochemical Markers of Alveolar Epithelial Barrier Dysfunction in Perfused Human Lungs. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 293, L52–L59. [Google Scholar] [CrossRef]
- Liu, A.; Park, J.-H.; Zhang, X.; Sugita, S.; Naito, Y.; Lee, J.-H.; Kato, H.; Hao, Q.; Matthay, M.A.; Lee, J.-W. Therapeutic Effects of Hyaluronic Acid in Bacterial Pneumonia in Ex Vivo Perfused Human Lungs. Am. J. Respir. Crit. Care Med. 2019, 200, 1234–1245. [Google Scholar] [CrossRef]
- Morrison, T.J.; Jackson, M.V.; Cunningham, E.K.; Kissenpfennig, A.; McAuley, D.F.; O’Kane, C.M.; Krasnodembskaya, A.D. Mesenchymal Stromal Cells Modulate Macrophages in Clinically Relevant Lung Injury Models by Extracellular Vesicle Mitochondrial Transfer. Am. J. Respir. Crit. Care Med. 2017, 196, 1275–1286. [Google Scholar] [CrossRef]
- Harrington, E.O.; Braza, J.; Shil, A.; Chichger, H. Extracellular Vesicles Released from P18 Overexpressing Pulmonary Endothelial Cells Are Barrier Protective—Potential Implications for Acute Respiratory Distress Syndrome. Pulm. Circ. 2020, 10, 2045894020951759. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, S.; Xiang, H.; Liu, J.; Zhang, Y.; Zhou, S.; Du, T.; Shan, L. Microvesicles Derived from Human Wharton’s Jelly Mesenchymal Stem Cells Ameliorate Acute Lung Injury Partly Mediated by Hepatocyte Growth Factor. Int. J. Biochem. Cell Biol. 2019, 112, 114–122. [Google Scholar] [CrossRef]
- Deng, H.; Wu, L.; Liu, M.; Zhu, L.; Chen, Y.; Zhou, H.; Shi, X.; Wei, J.; Zheng, L.; Hu, X.; et al. Bone Marrow Mesenchymal Stem Cell-Derived Exosomes Attenuate LPS-Induced ARDS by Modulating Macrophage Polarization Through Inhibiting Glycolysis in Macrophages. Shock 2020, 54, 828–843. [Google Scholar] [CrossRef]
- Devaney, J.; Horie, S.; Masterson, C.; Elliman, S.; Barry, F.; O’Brien, T.; Curley, G.F.; O’Toole, D.; Laffey, J.G. Human Mesenchymal Stromal Cells Decrease the Severity of Acute Lung Injury Induced by E. coli in the Rat. Thorax 2015, 70, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Dutra Silva, J.; Su, Y.; Calfee, C.S.; Delucchi, K.L.; Weiss, D.; McAuley, D.F.; O’Kane, C.; Krasnodembskaya, A.D. Mesenchymal Stromal Cell Extracellular Vesicles Rescue Mitochondrial Dysfunction and Improve Barrier Integrity in Clinically Relevant Models of ARDS. Eur. Respir. J. 2021, 58, 2002978. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.; Curley, G.F.; Masterson, C.; Devaney, J.; O’Toole, D.; Laffey, J.G. Mesenchymal Stromal Cells Are More Effective than the MSC Secretome in Diminishing Injury and Enhancing Recovery Following Ventilator-Induced Lung Injury. Intensive Care Med. Exp. 2015, 3, 29. [Google Scholar] [CrossRef]
- Huang, R.; Qin, C.; Wang, J.; Hu, Y.; Zheng, G.; Qiu, G.; Ge, M.; Tao, H.; Shu, Q.; Xu, J. Differential Effects of Extracellular Vesicles from Aging and Young Mesenchymal Stem Cells in Acute Lung Injury. Aging 2019, 11, 7996–8014. [Google Scholar] [CrossRef]
- Ionescu, L.; Byrne, R.N.; van Haaften, T.; Vadivel, A.; Alphonse, R.S.; Rey-Parra, G.J.; Weissmann, G.; Hall, A.; Eaton, F.; Thébaud, B. Stem Cell Conditioned Medium Improves Acute Lung Injury in Mice: In Vivo Evidence for Stem Cell Paracrine Action. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 303, L967–L977. [Google Scholar] [CrossRef]
- Kaspi, H.; Semo, J.; Abramov, N.; Dekel, C.; Lindborg, S.; Kern, R.; Lebovits, C.; Aricha, R. MSC-NTF (NurOwn®) Exosomes: A Novel Therapeutic Modality in the Mouse LPS-Induced ARDS Model. Stem Cell Res. Ther. 2021, 12, 72. [Google Scholar] [CrossRef]
- Mao, G.-C.; Gong, C.-C.; Wang, Z.; Sun, M.-X.; Pei, Z.-P.; Meng, W.-Q.; Cen, J.-F.; He, X.-W.; Lu, Y.; Xu, Q.-Q.; et al. BMSC-Derived Exosomes Ameliorate Sulfur Mustard-Induced Acute Lung Injury by Regulating the GPRC5A-YAP Axis. Acta Pharmacol. Sin. 2021, 42, 2082–2093. [Google Scholar] [CrossRef]
- Monsel, A.; Zhu, Y.; Gennai, S.; Hao, Q.; Hu, S.; Rouby, J.-J.; Rosenzwajg, M.; Matthay, M.A.; Lee, J.W. Therapeutic Effects of Human Mesenchymal Stem Cell-Derived Microvesicles in Severe Pneumonia in Mice. Am. J. Respir. Crit. Care Med. 2015, 192, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.D.; de Castro, L.L.; Braga, C.L.; Oliveira, G.P.; Trivelin, S.A.; Barbosa-Junior, C.M.; Morales, M.M.; Dos Santos, C.C.; Weiss, D.J.; Lopes-Pacheco, M.; et al. Mesenchymal Stromal Cells Are More Effective Than Their Extracellular Vesicles at Reducing Lung Injury Regardless of Acute Respiratory Distress Syndrome Etiology. Stem Cells Int. 2019, 2019, 8262849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, V.Y.-F.; Lin, C.-S.; Hung, S.-C.; Yang, K.-Y. Mesenchymal Stem Cell-Conditioned Medium Induces Neutrophil Apoptosis Associated with Inhibition of the NF-ΚB Pathway in Endotoxin-Induced Acute Lung Injury. Int. J. Mol. Sci. 2019, 20, e2208. [Google Scholar] [CrossRef]
- Tang, X.-D.; Shi, L.; Monsel, A.; Li, X.-Y.; Zhu, H.-L.; Zhu, Y.-G.; Qu, J.-M. Mesenchymal Stem Cell Microvesicles Attenuate Acute Lung Injury in Mice Partly Mediated by Ang-1 MRNA. Stem Cells 2017, 35, 1849–1859. [Google Scholar] [CrossRef]
- Varkouhi, A.K.; Jerkic, M.; Ormesher, L.; Gagnon, S.; Goyal, S.; Rabani, R.; Masterson, C.; Spring, C.; Chen, P.Z.; Gu, F.X.; et al. Extracellular Vesicles from Interferon-γ-Primed Human Umbilical Cord Mesenchymal Stromal Cells Reduce Escherichia coli-Induced Acute Lung Injury in Rats. Anesthesiology 2019, 130, 778–790. [Google Scholar] [CrossRef]
- Wei, X.; Yi, X.; Lv, H.; Sui, X.; Lu, P.; Li, L.; An, Y.; Yang, Y.; Yi, H.; Chen, G. MicroRNA-377-3p Released by Mesenchymal Stem Cell Exosomes Ameliorates Lipopolysaccharide-Induced Acute Lung Injury by Targeting RPTOR to Induce Autophagy. Cell Death Dis. 2020, 11, 657. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, Z.; Hu, L.; Gu, W.; Zhu, L. Exosomes Derived from Endothelial Progenitor Cells Ameliorate Acute Lung Injury by Transferring MiR-126. Exp. Cell Res. 2018, 370, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-G.; Feng, X.-M.; Abbott, J.; Fang, X.-H.; Hao, Q.; Monsel, A.; Qu, J.-M.; Matthay, M.A.; Lee, J.W. Human Mesenchymal Stem Cell Microvesicles for Treatment of Escherichia coli Endotoxin-Induced Acute Lung Injury in Mice. Stem Cells 2014, 32, 116–125. [Google Scholar] [CrossRef]
- Shah, T.; Qin, S.; Vashi, M.; Predescu, D.N.; Jeganathan, N.; Bardita, C.; Ganesh, B.; diBartolo, S.; Fogg, L.F.; Balk, R.A.; et al. Alk5/Runx1 Signaling Mediated by Extracellular Vesicles Promotes Vascular Repair in Acute Respiratory Distress Syndrome. Clin. Transl. Med. 2018, 7, 19. [Google Scholar] [CrossRef]
- Guervilly, C.; Lacroix, R.; Forel, J.-M.; Roch, A.; Camoin-Jau, L.; Papazian, L.; Dignat-George, F. High Levels of Circulating Leukocyte Microparticles Are Associated with Better Outcome in Acute Respiratory Distress Syndrome. Crit. Care 2011, 15, R31. [Google Scholar] [CrossRef]
- Shaver, C.M.; Woods, J.; Clune, J.K.; Grove, B.S.; Wickersham, N.E.; McNeil, J.B.; Shemancik, G.; Ware, L.B.; Bastarache, J.A. Circulating Microparticle Levels Are Reduced in Patients with ARDS. Crit. Care 2017, 21, 120. [Google Scholar] [CrossRef]
- Park, J.; Kim, S.; Lim, H.; Liu, A.; Hu, S.; Lee, J.; Zhuo, H.; Hao, Q.; Matthay, M.A.; Lee, J.W. Therapeutic Effects of Human Mesenchymal Stem Cell Microvesicles in an Ex Vivo Perfused Human Lung Injured with Severe E. coli Pneumonia. Thorax 2019, 74, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Sengupta, V.; Sengupta, S.; Lazo, A.; Woods, P.; Nolan, A.; Bremer, N. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020, 29, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Dilogo, I.H.; Aditianingsih, D.; Sugiarto, A.; Burhan, E.; Damayanti, T.; Sitompul, P.A.; Mariana, N.; Antarianto, R.D.; Liem, I.K.; Kispa, T.; et al. Umbilical Cord Mesenchymal Stromal Cells as Critical COVID-19 Adjuvant Therapy: A Randomized Controlled Trial. Stem Cells Transl. Med. 2021, 10, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Lanzoni, G.; Linetsky, E.; Correa, D.; Messinger Cayetano, S.; Alvarez, R.A.; Kouroupis, D.; Alvarez Gil, A.; Poggioli, R.; Ruiz, P.; Marttos, A.C.; et al. Umbilical Cord Mesenchymal Stem Cells for COVID-19 Acute Respiratory Distress Syndrome: A Double-blind, Phase 1/2a, Randomized Controlled Trial. Stem Cells Transl. Med. 2021, 10, 660–673. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Huang, L.; Tong, H.; Shu, Q.; Hu, Y.; Ge, M.; Deng, K.; Zhang, L.; Zou, B.; Cheng, B.; et al. Treatment of Acute Respiratory Distress Syndrome with Allogeneic Adipose-Derived Mesenchymal Stem Cells: A Randomized, Placebo-Controlled Pilot Study. Respir. Res. 2014, 15, 39. [Google Scholar] [CrossRef]
- Yip, H.-K.; Fang, W.-F.; Li, Y.-C.; Lee, F.-Y.; Lee, C.-H.; Pei, S.-N.; Ma, M.-C.; Chen, K.-H.; Sung, P.-H.; Lee, M.S. Human Umbilical Cord-Derived Mesenchymal Stem Cells for Acute Respiratory Distress Syndrome. Crit. Care Med. 2020, 48, 391–399. [Google Scholar] [CrossRef]
- Chen, J.; Hu, C.; Chen, L.; Tang, L.; Zhu, Y.; Xu, X.; Chen, L.; Gao, H.; Lu, X.; Yu, L. Clinical Study of Mesenchymal Stem Cell Treatment for Acute Respiratory Distress Syndrome Induced by Epidemic Influenza A (H7N9) Infection: A Hint for COVID-19 Treatment. Engineering 2020, 6, 1153–1161. [Google Scholar] [CrossRef]
- Bellingan, G.; Jacono, F.; Bannard-Smith, J.; Brealey, D.; Meyer, N.; Thickett, D.; Young, D.; Bentley, A.; McVerry, B.J.; Wunderink, R.G.; et al. Safety and Efficacy of Multipotent Adult Progenitor Cells in Acute Respiratory Distress Syndrome (MUST-ARDS): A Multicentre, Randomised, Double-Blind, Placebo-Controlled Phase 1/2 Trial. Intensive Care Med. 2021, 48, 1–9. [Google Scholar] [CrossRef]
- Husain-Syed, F.; Slutsky, A.S.; Ronco, C. Lung-Kidney Cross-Talk in the Critically Ill Patient. Am. J. Respir. Crit. Care Med. 2016, 194, 402–414. [Google Scholar] [CrossRef]
- Mahida, R.Y.; Matsumoto, S.; Matthay, M.A. Extracellular Vesicles: A New Frontier for Research in Acute Respiratory Distress Syndrome. Am. J. Respir. Cell Mol. Biol. 2020, 63, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Dou, H.; Li, X.; Zhao, X.; Li, Y.; Liu, D.; Ji, J.; Liu, F.; Ding, L.; Ni, Y.; et al. Exosomal MiR-146a Contributes to the Enhanced Therapeutic Efficacy of Interleukin-1β-Primed Mesenchymal Stem Cells Against Sepsis. Stem Cells 2017, 35, 1208–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, Z.; Ma, J.; Wang, C.; Yu, J.; Qiao, Y.; Hei, F. Exosomes from IPSCs Delivering SiRNA Attenuate Intracellular Adhesion Molecule-1 Expression and Neutrophils Adhesion in Pulmonary Microvascular Endothelial Cells. Inflammation 2017, 40, 486–496. [Google Scholar] [CrossRef]
- Aghajani Nargesi, A.; Lerman, L.O.; Eirin, A. Mesenchymal Stem Cell-Derived Extracellular Vesicles for Kidney Repair: Current Status and Looming Challenges. Stem Cell Res. Ther. 2017, 8, 273. [Google Scholar] [CrossRef] [PubMed]

| EV Strain and Model | Mechanisms | Treatment Effects | Reference |
|---|---|---|---|
| EPC-EVs injected in a CLP rat model | EV-carried miR-21-5p modulates RUNX1 axis |
| [85] |
| EPC-EVs injected in LPS-induced mouse model of s-AKI with MOD | EV-carried mi-RNA-93-5p conferred endothelial protection via the KDM6B/H3K27me3/TNF-α axis |
| [86] |
| EPC-EVs injected in LPS-induced HK-2 cell injury | EV-carried mi-RNA-93-5p alleviates LPS-induced HK-2 cell injury targeting miR-93-5p/OXSR1 axis |
| [87] |
| Rat model of s-AKI in vivo; LPS-induced sepsis model in HK-2 cells in vitro | miR-22-3p downregulates HMGB1, p-p65, TLR4, and pro-inflammatory cytokines (IL-1β, IL-6, TNF-α), both in vivo and in vitro. It can also repress PTEN, a protein involved in mitophagy regulation |
| [88] |
| s-AKI mouse model through CLP | Human MSC-EVs increased expression of miR-146b in kidney tissue and consequently reduced IRAK1 level and NF-κB activity |
| [89] |
| s-AKI mouse model through CLP | AT-EVs activate SIRT1 signaling pathway blunting inflammation |
| [90] |
| s-AKI mouse model with remote ischemic pre-conditioning pre-treatment | Exosomal miR-21 integrates into RTECs and targets PDCD4/NF-κB and PTEN/AKT pathways |
| [91] |
| s-AKI mouse model | MSC-EVs from healthy controls transferred TFAM in recipient cells and restored TFAM-mtDNA complex stability, reversing mitochondrial oxidative phosphorylation defects after s-AKI |
| [92] |
| Population | EV Strain | Lung Injury Model | Intervention Arms | Intervention Details | Mechanism Analyzed | Treatment Effects | Reference |
|---|---|---|---|---|---|---|---|
| Rats | WJMSC-EV | BLM IT | MSC-EV vs. Neg shRNA MSC-EV vs. HGF shRNA MSC-EV | MSC-EV IT | Apoptosis modulation via PI3K/AKT/mTOR signaling pathway |
| [122] |
| Mice | bm-MSCs naïve | LPS IP | bm-MSCs EV naïve | bm-MSCs exosomes IT 50 μg or bm-MSCs exosomes IT 100 μg | Glycolysis through HIF-1α inhibition Macrophage polarization |
| [123] |
| Rats | h-MSCs | E. coli IT | 24 h CdM h-MSCs 48 h CdM h-MSCs vs. h-MSCs | CdM IV 300 μL | Macrophage phagocytosis |
| [124] |
| Mice | h-bm-MSCs | LPS IT | EV naïve vs. EV with dysfunctional mitochondria | EV from 5 × 105 and 1 × 106 MSCs | Restored mitochondrial function |
| [125] |
| Rats | mu-MSCs | VILI | mu-MSCs vs. CdM | MSCs IV 106, CdM IV 500 μL | IL-6 modulation |
| [126] |
| Mice | hu-ADSCs | LPS IT | ADSCs from young donor (25 YO) vs. ADSCs from older donor (72 YO) | MSCs EV 100 μg IV 30 min after LPS | Macrophage polarization |
| [127] |
| Mice | mu-MSCs | LPS IT | mu-MSCs CdM vs. mu-MSCs | CdM IT 30 µL | Macrophage polarization |
| [128] |
| Mice | hu-bm-MSCs | LPS IT | Exo MSCs naïve vs. Exo MSCs NTF | Exo MSCs NTF IT 50 µL, 3 h after LPS 3 days until 72 h post-injury | Immune modulation balancing factors |
| [129] |
| Mice | bm-MSCs | SM SC | bm-MSCs-EV naïve | bm-MSCs-EV IV 20 mg/kg 24 h after injection of SM | Tight junction dysfunction and apoptosis inhibition |
| [130] |
| Mice | hu-bm-MSCs | E. coli IT | EV vs. hu-bm-MSCs | EV IV 90 μL 4 h after injury | Enhanced macrophage-mediated bacterial phagocytosis |
| [131] |
| Mice | mu-bm-MSCs | LPS IT LPS IP | mu-bm-MSCs EV vs. mu-bm-MSCs | EV IV released by 105 cells | Effect of EV preconditioning with serum from ARDS mice EV effect in pulmonary or extrapulmonary ARDS |
| [132] |
| Mice | mu-MSCs | LPS IT | mu-MSCs CdM | CdM IV 200 µL | Neutrophil apoptosis |
| [133] |
| Mice | mu-bm-MSCs | LPS IT | HLMVECs vs. Neg SiRNA h-MSCs vs. Ang-1 SiRNA h-MSCs | HLMVECs IT 2 × 105 | Macrophage polarization |
| [134] |
| Rats | hu-MSCs naive | E. coli IT | IFNγ-primed EV vs. naïve EV | EV IT 100 × 106/Kg | Macrophage phagocytosis |
| [135] |
| Mice | hu-MSCs | LPS IT | hu-MSCs EV naïve vs. hu-MSCs EV + autophagy inhibitor | hu-MSCs exosomes IT 50 μg 4 h after LPS | Autophagy |
| [136] |
| Rats | bm-EPCs | LPS IT | EPC-EV vs. EPC-EV + GW4869 | EPC-EV IV 10 μg | miR-126-mediated modulation of RAF/ERK signaling pathway |
| [137] |
| Mice | hu-MSCs | E. coli IT | MSC-EV IT vs. MSC-EV IV vs. KGF siRNA- Pre-treated | MSC-EV 30 µL IT | KGF protein expression through mRNA modulation |
| [138] |
| Study Design | n | Clinical Context and Inclusion Criteria | Intervention | Treatment Effect | Reference |
|---|---|---|---|---|---|
| Prospective interventional (ex vivo) | n = 37 |
|
|
| [142] |
| Prospective interventional (single arm) | n = 24 |
|
|
| [143] |
| RCT | n = 40 (n = 20 C n = 20 T) |
|
|
| [144] |
| RCT | n = 24 (n = 12 C n = 12 T) |
|
|
| [145] |
| RCT | n = 12 (n = 6 C n = 6 T) |
|
|
| [146] |
| Prospective Phase 1 CT | n = 9 |
|
|
| [147] |
| Open label clinical trial | n = 61 (n = 44 C n = 17 T) |
|
|
| [148] |
| Phase 1/2 multicentre RCT Cohort 1 and 2 | n = 6 (n = 3 C1 n = 3 C2) |
|
|
| [149] |
| Phase 1/2 multicentre RCT Cohort 3 | n = 30 (n = 10 C n = 20 T) |
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quaglia, M.; Fanelli, V.; Merlotti, G.; Costamagna, A.; Deregibus, M.C.; Marengo, M.; Balzani, E.; Brazzi, L.; Camussi, G.; Cantaluppi, V. Dual Role of Extracellular Vesicles in Sepsis-Associated Kidney and Lung Injury. Biomedicines 2022, 10, 2448. https://doi.org/10.3390/biomedicines10102448
Quaglia M, Fanelli V, Merlotti G, Costamagna A, Deregibus MC, Marengo M, Balzani E, Brazzi L, Camussi G, Cantaluppi V. Dual Role of Extracellular Vesicles in Sepsis-Associated Kidney and Lung Injury. Biomedicines. 2022; 10(10):2448. https://doi.org/10.3390/biomedicines10102448
Chicago/Turabian StyleQuaglia, Marco, Vito Fanelli, Guido Merlotti, Andrea Costamagna, Maria Chiara Deregibus, Marita Marengo, Eleonora Balzani, Luca Brazzi, Giovanni Camussi, and Vincenzo Cantaluppi. 2022. "Dual Role of Extracellular Vesicles in Sepsis-Associated Kidney and Lung Injury" Biomedicines 10, no. 10: 2448. https://doi.org/10.3390/biomedicines10102448
APA StyleQuaglia, M., Fanelli, V., Merlotti, G., Costamagna, A., Deregibus, M. C., Marengo, M., Balzani, E., Brazzi, L., Camussi, G., & Cantaluppi, V. (2022). Dual Role of Extracellular Vesicles in Sepsis-Associated Kidney and Lung Injury. Biomedicines, 10(10), 2448. https://doi.org/10.3390/biomedicines10102448









