COVID-19, Endothelium and the Cardiometabolic Patient: A Possible Role for Capillary Leak Syndrome
Abstract
:1. Introduction
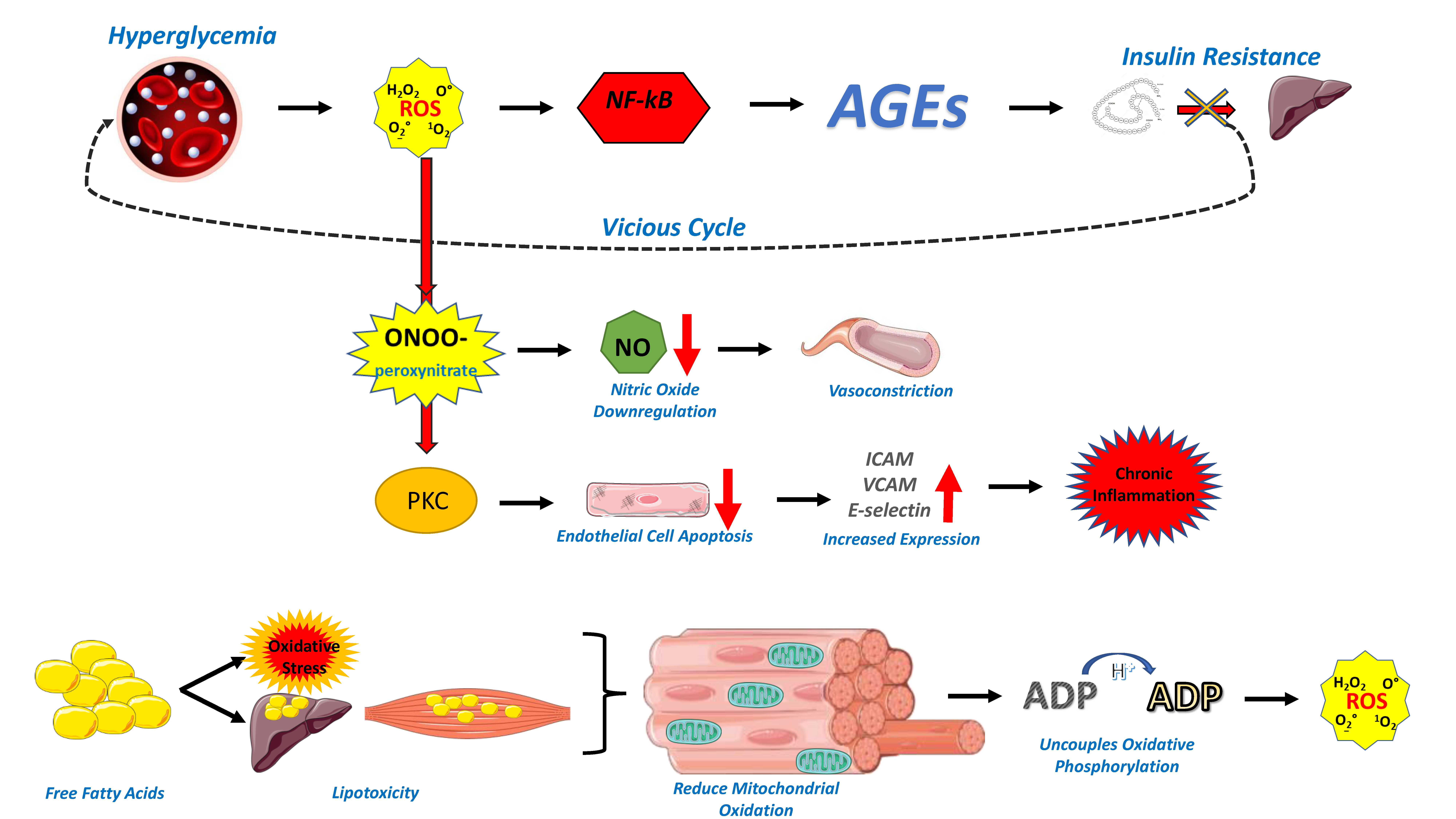
2. Endothelial Dysfunction in the Cardiometabolic Patient
3. Endothelial Dysfunction in COVID-19
4. Capillary Leak Syndrome
5. Capillary Leak Syndrome in the Cardiometabolic COVID-19 Patient
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Maruhashi, T.; Higashi, Y. Pathophysiological Association of Endothelial Dysfunction with Fatal Outcome in COVID-19. Int. J. Mol. Sci. 2021, 22, 5131. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Gao, Y.-D.; Ding, M.; Dong, X.; Zhang, J.; Azkur, A.K.; Azkur, D.; Gan, H.; Sun, Y.-L.; Fu, W.; Li, W.; et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy 2021, 76, 428–455. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, R.B.; Wandermurem, D.C.; Libório, F.C.; Machado, M.K.; Ushijima, N.M.; Narde, R.S.; Pecly, I.M.D.; Muxfeldt, E.S. Impact of Metabolic Risk Factors on Covid-19 Clinical Outcomes: An Extensive Review. Curr. Cardiol. Rev. 2022, 18. [Google Scholar] [CrossRef]
- Kaminska, H.; Szarpak, L.; Kosior, D.; Wieczorek, W.; Szarpak, A.; Al-Jeabory, M.; Gawel, W.; Gasecka, A.; Jaguszewski, M.J.; Jarosz-Chobot, P. Impact of diabetes mellitus on in-hospital mortality in adult patients with COVID-19: A systematic review and meta-analysis. Geol. Rundsch. 2021, 58, 1101–1110. [Google Scholar] [CrossRef]
- Yang, J.; Tian, C.; Chen, Y.; Zhu, C.; Chi, H.; Li, J. Obesity aggravates COVID-19: An updated systematic review and meta-analysis. J. Med Virol. 2021, 93, 2662–2674. [Google Scholar] [CrossRef]
- Siddall, E.; Khatri, M.; Radhakrishnan, J. Capillary leak syndrome: Etiologies, pathophysiology, and management. Kidney Int. 2017, 92, 37–46. [Google Scholar] [CrossRef]
- Soetedjo, N.N.M.; Iryaningrum, M.R.; Damara, F.A.; Permadhi, I.; Sutanto, L.B.; Hartono, H.; Rasyid, H. Prognostic properties of hypoalbuminemia in COVID-19 patients: A systematic review and diagnostic meta-analysis. Clin. Nutr. ESPEN 2021, 45, 120–126. [Google Scholar] [CrossRef]
- Kim, J.-A.; Montagnani, M.; Koh, K.K.; Quon, M. Reciprocal Relationships between Insulin Resistance and Endothelial Dysfunction. Circulation 2006, 113, 1888–1904. [Google Scholar] [CrossRef]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.-I.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.J.; Oates, P.J.; Hammes, H.-P.; et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000, 404, 787–790. [Google Scholar] [CrossRef]
- Sasso, F.C.; Carbonara, O.; Cozzolino, D.; Rambaldi, P.; Mansi, L.; Torella, D.; Gentile, S.; Turco, S.; Torella, R.; Salvatore, T. Effects of insulin-glucose infusion on left ventricular function at rest and during dynamic exercise in healthy subjects and noninsulin dependent diabetic patients: A radionuclide ventriculographic study. J. Am. Coll. Cardiol. 2000, 36, 219–226. [Google Scholar] [CrossRef]
- Sasso, F.C.; Carbonara, O.; Nasti, R.; Marfella, R.; Esposito, K.; Rambaldi, P.; Mansi, L.; Salvatore, T.; Torella, R.; Cozzolino, D. Effects of insulin on left ventricular function during dynamic exercise in overweight and obese subjects. Eur. Heart J. 2005, 26, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.A.; Yang, Y.; Zhang, L.; Sun, Z.; Jia, G.; Parrish, A.R.; Sowers, J.R. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism 2021, 119, 154766. [Google Scholar] [CrossRef]
- Prieto, D.; Contreras, C.; Sanchez, A. Endothelial Dysfunction, Obesity and Insulin Resistance. Curr. Vasc. Pharmacol. 2014, 12, 412–426. [Google Scholar] [CrossRef] [PubMed]
- Salt, I.P.; Morrow, V.A.; Brandie, F.M.; Connell, J.M.C.; Petrie, J.R. High Glucose Inhibits Insulin-stimulated Nitric Oxide Production without Reducing Endothelial Nitric-oxide Synthase Ser1177 Phosphorylation in Human Aortic Endothelial Cells. J. Biol. Chem. 2003, 278, 18791–18797. [Google Scholar] [CrossRef]
- Esposito, K.; Nappo, F.; Marfella, R.; Giugliano, G.; Giugliano, F.; Ciotola, M.; Quagliaro, L.; Ceriello, A.; Giugliano, D. Inflammatory Cytokine Concentrations Are Acutely Increased by Hyperglycemia in Humans. Circulation 2002, 106, 2067–2072. [Google Scholar] [CrossRef]
- Chavakis, T.; Bierhaus, A.; Nawroth, P.P. RAGE (receptor for advanced glycation end products): A central player in the inflammatory response. Microbes Infect. 2004, 6, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Inoguchi, T.; Li, P.; Umeda, F.; Yu, H.Y.; Kakimoto, M.; Imamura, M.; Aoki, T.; Etoh, T.; Hashimoto, T.; Naruse, M.; et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C--dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 2000, 49, 1939–1945. [Google Scholar] [CrossRef]
- Li, H.; Junk, P.; Huwiler, A.; Burkhardt, C.; Wallerath, T.; Pfeilschifter, J.; Förstermann, U. Dual Effect of Ceramide on Human Endothelial Cells. Circulation 2002, 106, 2250–2256. [Google Scholar] [CrossRef] [PubMed]
- Min, J.-K.; Kim, Y.-M.; Kim, S.W.; Kwon, M.-C.; Kong, Y.-Y.; Hwang, I.K.; Won, M.H.; Rho, J.; Kwon, Y.-G. TNF-Related Activation-Induced Cytokine Enhances Leukocyte Adhesiveness: Induction of ICAM-1 and VCAM-1 via TNF Receptor-Associated Factor and Protein Kinase C-Dependent NF-κB Activation in Endothelial Cells. J. Immunol. 2005, 175, 531–540. [Google Scholar] [CrossRef] [Green Version]
- Konukoglu, D.; Uzun, H. Endothelial Dysfunction and Hypertension. Adv. Exp. Med. Biol. 2016, 956, 511–540. [Google Scholar] [CrossRef]
- De Miguel, C.; Rudemiller, N.P.; Abais, J.M.; Mattson, D.L. Inflammation and Hypertension: New Understandings and Potential Therapeutic Targets. Curr. Hypertens. Rep. 2015, 17, 507. [Google Scholar] [CrossRef] [PubMed]
- Korakas, E.; Ikonomidis, I.; Kousathana, F.; Balampanis, K.; Kountouri, A.; Raptis, A.; Palaiodimou, L.; Kokkinos, A.; Lambadiari, V. Obesity and COVID-19: Immune and metabolic derangement as a possible link to adverse clinical outcomes. Am. J. Physiol. Metab. 2020, 319, E105–E109. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Miglio, C.; Eden, T.; Del Rio, D. Assessment of vascular and endothelial dysfunction in nutritional studies. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Santilli, F.; Vazzana, N.; Liani, R.; Guagnano, M.T.; Davì, G. Platelet activation in obesity and metabolic syndrome. Obes. Rev. 2011, 13, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, T.; Pafundi, P.C.; Galiero, R.; Rinaldi, L.; Caturano, A.; Vetrano, E.; Aprea, C.; Albanese, G.; Di Martino, A.; Ricozzi, C.; et al. Can Metformin Exert as an Active Drug on Endothelial Dysfunction in Diabetic Subjects? Biomedicines 2020, 9, 3. [Google Scholar] [CrossRef]
- Salvatore, T.; Galiero, R.; Caturano, A.; Rinaldi, L.; Di Martino, A.; Albanese, G.; Di Salvo, J.; Epifani, R.; Marfella, R.; Docimo, G.; et al. An Overview of the Cardiorenal Protective Mechanisms of SGLT2 Inhibitors. Int. J. Mol. Sci. 2022, 23, 3651. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Pavlidis, G.; Thymis, J.; Birba, D.; Kalogeris, A.; Kousathana, F.; Kountouri, A.; Balampanis, K.; Parissis, J.; Andreadou, I.; et al. Effects of Glucagon-Like Peptide-1 Receptor Agonists, Sodium-Glucose Cotransporter-2 Inhibitors, and Their Combination on Endothelial Glycocalyx, Arterial Function, and Myocardial Work Index in Patients with Type 2 Diabetes Mellitus after 12-Month Treatment. J. Am. Heart Assoc. 2020, 9, e015716. [Google Scholar] [CrossRef]
- Salt, I.; Hardie, D.G. AMP-Activated Protein Kinase. Circ. Res. 2017, 120, 1825–1841. [Google Scholar] [CrossRef]
- Bosselaar, M.; Boon, H.; Van Loon, L.J.C.; Broek, P.H.H.V.D.; Smits, P.; Tack, C.J. Intra-arterial AICA-riboside administration induces NO-dependent vasodilation in vivo in human skeletal muscle. Am. J. Physiol. Metab. 2009, 297, E759–E766. [Google Scholar] [CrossRef]
- Shiu, S.W.; Wong, Y.; Tan, K.C. Effect of Advanced Glycation End Products on Lectin-Like Oxidized Low Density Lipoprotein Receptor-1 Expression in Endothelial Cells. J. Atheroscler. Thromb. 2012, 19, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Batzias, K.; Antonopoulos, A.; Oikonomou, E.; Siasos, G.; Bletsa, E.; Stampouloglou, P.K.; Mistakidi, C.-V.; Noutsou, M.; Katsiki, N.; Karopoulos, P.; et al. Effects of Newer Antidiabetic Drugs on Endothelial Function and Arterial Stiffness: A Systematic Review and Meta-Analysis. J. Diabetes Res. 2018, 2018, 1232583. [Google Scholar] [CrossRef] [PubMed]
- Lambadiari, V.; Pavlidis, G.; Kousathana, F.; Varoudi, M.; Vlastos, D.; Maratou, E.; Georgiou, D.; Andreadou, I.; Parissis, J.; Triantafyllidi, H.; et al. Effects of 6-month treatment with the glucagon like peptide-1 analogue liraglutide on arterial stiffness, left ventricular myocardial deformation and oxidative stress in subjects with newly diagnosed type 2 diabetes. Cardiovasc. Diabetol. 2018, 17, 8. [Google Scholar] [CrossRef]
- Roberts, J.; Pritchard, A.L.; Treweeke, A.T.; Rossi, A.G.; Brace, N.; Cahill, P.; MacRury, S.M.; Wei, J.; Megson, I.L. Why Is COVID-19 More Severe in Patients With Diabetes? The Role of Angiotensin-Converting Enzyme 2, Endothelial Dysfunction and the Immunoinflammatory System. Front. Cardiovasc. Med. 2021, 7, 629933. [Google Scholar] [CrossRef] [PubMed]
- Bradley, B.T.; Maioli, H.; Johnston, R.; Chaudhry, I.; Fink, S.L.; Xu, H.; Najafian, B.; Deutsch, G.; Lacy, J.M.; Williams, T.; et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: A case series. Lancet 2020, 396, 320–332. [Google Scholar] [CrossRef]
- Menter, T.; Haslbauer, J.D.; Nienhold, R.; Savic, S.; Hopfer, H.; Deigendesch, N.; Frank, S.; Turek, D.; Willi, N.; Pargger, H.; et al. Post-mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology 2020, 77, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, J.; Gary, J.; Reagan-Steiner, S.; Estetter, L.B.; Tong, S.; Tao, Y.; Denison, A.M.; Lee, E.; DeLeon-Carnes, M.; Li, Y.; et al. Evidence of Severe Acute Respiratory Syndrome Coronavirus 2 Replication and Tropism in the Lungs, Airways, and Vascular Endothelium of Patients with Fatal Coronavirus Disease 2019: An Autopsy Case Series. J. Infect. Dis. 2021, 223, 752–764. [Google Scholar] [CrossRef]
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B.; Huan, Y.; Yang, P.; Zhang, Y.; Deng, W.; et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat. Med. 2005, 11, 875–879. [Google Scholar] [CrossRef]
- Moustafa, J.S.E.-S.; Jackson, A.U.; Brotman, S.M.; Guan, L.; Villicaña, S.; Roberts, A.L.; Zito, A.; Bonnycastle, L.; Erdos, M.R.; Narisu, N.; et al. ACE2 expression in adipose tissue is associated with cardio-metabolic risk factors and cell type composition—implications for COVID-19. Int. J. Obes. 2022, 46, 1478–1486. [Google Scholar] [CrossRef]
- Santos, R.A.S.; Sampaio, W.O.; Alzamora, A.C.; Motta-Santos, D.; Alenina, N.; Bader, M.; Campagnole-Santos, M.J. The ACE2/Angiotensin-(1–7)/MAS Axis of the Renin-Angiotensin System: Focus on Angiotensin-(1–7). Physiol. Rev. 2018, 98, 505–553. [Google Scholar] [CrossRef] [Green Version]
- Zaman, A.K.M.T.; Fujii, S.; Sawa, H.; Goto, D.; Ishimori, N.; Watano, K.; Kaneko, T.; Furumoto, T.; Sugawara, T.; Sakuma, I.; et al. Angiotensin-Converting Enzyme Inhibition Attenuates Hypofibrinolysis and Reduces Cardiac Perivascular Fibrosis in Genetically Obese Diabetic Mice. Circulation 2001, 103, 3123–3128. [Google Scholar] [CrossRef] [PubMed]
- De Mello, W.C. Chemical Communication between Heart Cells is Disrupted by Intracellular Renin and Angiotensin II: Implications for Heart Development and Disease. Front. Endocrinol. 2015, 6, 72. [Google Scholar] [CrossRef] [PubMed]
- Cenko, E.; Badimon, L.; Bugiardini, R.; Claeys, M.J.; De Luca, G.; de Wit, C.; Derumeaux, G.; Dorobantu, M.; Duncker, D.J.; Eringa, E.C.; et al. Cardiovascular disease and COVID-19: A consensus paper from the ESC Working Group on Coronary Pathophysiology & Microcirculation, ESC Working Group on Thrombosis and the Association for Acute CardioVascular Care (ACVC), in collaboration with the European Heart Rhythm Association (EHRA). Cardiovasc. Res. 2021, 117, 2705–2729. [Google Scholar] [CrossRef] [PubMed]
- Jenne, C.N.; Wong, C.H.; Zemp, F.J.; McDonald, B.; Rahman, M.M.; Forsyth, P.A.; McFadden, G.; Kubes, P. Neutrophils Recruited to Sites of Infection Protect from Virus Challenge by Releasing Neutrophil Extracellular Traps. Cell Host Microbe 2013, 13, 169–180. [Google Scholar] [CrossRef]
- Teuwen, L.-A.; Geldhof, V.; Pasut, A.; Carmeliet, P. COVID-19: The vasculature unleashed. Nat. Rev. Immunol. 2020, 20, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Pober, J.S.; Sessa, W.C. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 2007, 7, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka-Tojo, M. Vascular Endothelial Glycocalyx Damage in COVID-19. Int. J. Mol. Sci. 2020, 21, 9712. [Google Scholar] [CrossRef] [PubMed]
- Lambadiari, V.; Mitrakou, A.; Kountouri, A.; Thymis, J.; Katogiannis, K.; Korakas, E.; Varlamos, C.; Andreadou, I.; Tsoumani, M.; Triantafyllidi, H.; et al. Association of COVID-19 with impaired endothelial glycocalyx, vascular function and myocardial deformation 4 months after infection. Eur. J. Heart Fail. 2021, 23, 1916–1926. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Lambadiari, V.; Mitrakou, A.; Kountouri, A.; Katogiannis, K.; Thymis, J.; Korakas, E.; Pavlidis, G.; Kazakou, P.; Panagopoulos, G.; et al. Myocardial work and vascular dysfunction are partially improved at 12 months after COVID-19 infection. Eur. J. Heart Fail. 2022, 24, 727–729. [Google Scholar] [CrossRef]
- Druey, K.M.; Parikh, S.M. Idiopathic systemic capillary leak syndrome (Clarkson disease). J. Allergy Clin. Immunol. 2016, 140, 663–670. [Google Scholar] [CrossRef] [Green Version]
- Druey, K.M. Narrative Review: The Systemic Capillary Leak Syndrome. Ann. Intern. Med. 2010, 153, 90–98. [Google Scholar] [CrossRef]
- Lambert, M.; Launay, D.; Hachulla, E.; Morell-Dubois, S.; Soland, V.; Queyrel, V.; Fourrier, F.; Hatron, P.-Y. High-dose intravenous immunoglobulins dramatically reverse systemic capillary leak syndrome. Crit. Care Med. 2008, 36, 2184–2187. [Google Scholar] [CrossRef]
- Dejana, E.; Orsenigo, F.; Lampugnani, M.G. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J. Cell Sci. 2008, 121, 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.P.; Waldmann, T.A.; Stein, S.F.; Gelfand, J.A.; MacDONALD, W.J.; Heck, L.W.; Cohen, E.L.; Kaplan, A.P.; Frank, M.M. Systemic Capillary Leak Syndrome and Monoclonal Igg Gammopathy. Medicine 1977, 56, 225–240. [Google Scholar] [CrossRef]
- Janka, G.E.; Lehmberg, K. Hemophagocytic lymphohistiocytosis: Pathogenesis and treatment. Hematology 2013, 2013, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Kayaaslan, B.U.; Asilturk, D.; Eser, F.; Korkmaz, M.; Kucuksahin, O.; Pamukcuoglu, M.; Guner, R. A case of Hemophagocytic lymphohistiocytosis induced by COVID-19, and review of all cases reported in the literature. J. Infect. Dev. Ctries. 2021, 15, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Zanza, C.; Romenskaya, T.; Manetti, A.C.; Franceschi, F.; La Russa, R.; Bertozzi, G.; Maiese, A.; Savioli, G.; Volonnino, G.; Longhitano, Y. Cytokine Storm in COVID-19: Immunopathogenesis and Therapy. Medicina 2022, 58, 144. [Google Scholar] [CrossRef]
- Zhang, J.J.; Dong, X.; Cao, Y.Y.; Yuan, Y.D.; Yang, Y.B.; Yan, Y.Q.; Akdis, C.A.; Gao, Y.D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020, 75, 1730–1741. [Google Scholar] [CrossRef]
- Lambadiari, V.; Kousathana, F.; Raptis, A.; Katogiannis, K.; Kokkinos, A.; Ikonomidis, I. Pre-Existing Cytokine and NLRP3 Inflammasome Activation and Increased Vascular Permeability in Diabetes: A Possible Fatal Link with Worst COVID-19 Infection Outcomes? Front. Immunol. 2020, 11, 557235. [Google Scholar] [CrossRef]
- McLaughlin, T.; Ackerman, S.E.; Shen, L.; Engleman, E. Role of innate and adaptive immunity in obesity-associated metabolic disease. J. Clin. Investig. 2017, 127, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Clarkson, B.; Thompson, D.; Horwith, M.; Luckey, E. Cyclical edema and shock due to increased capillary permeability. Am. J. Med. 1960, 29, 193–216. [Google Scholar] [CrossRef]
- Kapoor, P.; Greipp, P.T.; Schaefer, E.W.; Mandrekar, S.J.; Kamal, A.H.; Gonzalez-Paz, N.C.; Kumar, S.; Greipp, P.R. Idiopathic Systemic Capillary Leak Syndrome (Clarkson’s Disease): The Mayo Clinic Experience. Mayo Clin. Proc. 2010, 85, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Gousseff, M. The Systemic Capillary Leak Syndrome: A Case Series of 28 Patients from a European Registry. Ann. Intern. Med. 2011, 154, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Kawabe, S.; Saeki, T.; Yamazaki, H.; Nagai, M.; Aoyagi, R.; Miyamura, S. Systemic Capillary Leak Syndrome. Intern. Med. 2002, 41, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Ghosh, C.C.; Patel, R.; Iwaki, S.; Gaskins, D.; Nelson, C.; Jones, N.; Greipp, P.R.; Parikh, S.M.; Druey, K.M. Vascular endothelial hyperpermeability induces the clinical symptoms of Clarkson disease (the systemic capillary leak syndrome). Blood 2012, 119, 4321–4332. [Google Scholar] [CrossRef]
- Corada, M.; Mariotti, M.; Thurston, G.; Smith, K.; Kunkel, R.; Brockhaus, M.; Lampugnani, M.G.; Martin-Padura, I.; Stoppacciaro, A.; Ruco, L.; et al. Vascular endothelial–cadherin is an important determinant of microvascular integrity in vivo. Proc. Natl. Acad. Sci. USA 1999, 96, 9815–9820. [Google Scholar] [CrossRef] [PubMed]
- Lesterhuis, W.J.; Rennings, A.J.; Leenders, W.P.; Nooteboom, A.; Punt, C.J.; Sweep, F.C.; Pickkers, P.; Geurts-Moespot, A.; Van Laarhoven, H.W.; Van der Vlag, J.; et al. Vascular Endothelial Growth Factor in Systemic Capillary Leak Syndrome. Am. J. Med. 2009, 122, e5–e7. [Google Scholar] [CrossRef]
- Leligdowicz, A.; Richard-Greenblatt, M.; Wright, J.; Crowley, V.; Kain, K.C. Endothelial Activation: The Ang/Tie Axis in Sepsis. Front. Immunol. 2018, 9, 838. [Google Scholar] [CrossRef]
- Albert, C.; Garrido, N.; Mercader, A.; Rao, C.; Remohí, J.; Simón, C.; Pellicer, A. The role of endothelial cells in the pathogenesis of ovarian hyperstimulation syndrome. Mol. Hum. Reprod. 2002, 8, 409–418. [Google Scholar] [CrossRef]
- Dispenzieri, A.; Moreno-Aspitia, A.; Suarez, G.A.; Lacy, M.Q.; Colon-Otero, G.; Tefferi, A.; Litzow, M.R.; Roy, V.; Hogan, W.J.; Kyle, R.A.; et al. Peripheral blood stem cell transplantation in 16 patients with POEMS syndrome, and a review of the literature. Blood 2004, 104, 3400–3407. [Google Scholar] [CrossRef] [Green Version]
- Zafar, M.I.; Mills, K.; Ye, X.; Blakely, B.; Min, J.; Kong, W.; Zhang, N.; Gou, L.; Regmi, A.; Hu, S.Q.; et al. Association between the expression of vascular endothelial growth factors and metabolic syndrome or its components: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2018, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Korakas, E.; Ikonomidis, I.; Markakis, K.; Raptis, A.; Dimitriadis, G.; Lambadiari, V. The Endothelial Glycocalyx as a Key Mediator of Albumin Handling and the Development of Diabetic Nephropathy. Curr. Vasc. Pharmacol. 2020, 18, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Paliogiannis, P.; Mangoni, A.A.; Cangemi, M.; Fois, A.G.; Carru, C.; Zinellu, A. Serum albumin concentrations are associated with disease severity and outcomes in coronavirus 19 disease (COVID-19): A systematic review and meta-analysis. Clin. Exp. Med. 2021, 21, 343–354. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Xiao, J.; Shi, Z.; He, J.; Li, T. Supplementation of enteral nutritional powder decreases surgical site infection, prosthetic joint infection, and readmission after hip arthroplasty in geriatric femoral neck fracture with hypoalbuminemia. J. Orthop. Surg. Res. 2019, 14, 292. [Google Scholar] [CrossRef]
- Bohl, D.D.; Shen, M.R.; Kayupov, E.; Cvetanovich, G.L.; Della Valle, C.J. Is Hypoalbuminemia Associated With Septic Failure and Acute Infection After Revision Total Joint Arthroplasty? A Study of 4517 Patients from the National Surgical Quality Improvement Program. J. Arthroplast. 2015, 31, 963–967. [Google Scholar] [CrossRef]
- Soeters, P.B.; Wolfe, R.R.; Shenkin, A. Hypoalbuminemia: Pathogenesis and Clinical Significance. J. Parenter. Enter. Nutr. 2018, 43, 181–193. [Google Scholar] [CrossRef]
- Uhlig, C.; Silva, P.L.; Deckert, S.; Schmitt, J.; De Abreu, M.G. Albumin versus crystalloid solutions in patients with the acute respiratory distress syndrome: A systematic review and meta-analysis. Crit. Care 2014, 18, 1–8. [Google Scholar] [CrossRef]
- Huang, J.; Cheng, A.; Kumar, R.; Fang, Y.; Chen, G.; Zhu, Y.; Lin, S. Hypoalbuminemia predicts the outcome of COVID-19 independent of age and co-morbidity. J. Med. Virol. 2020, 92, 2152–2158. [Google Scholar] [CrossRef]
- Wu, M.A.; Fossali, T.; Pandolfi, L.; Carsana, L.; Ottolina, D.; Frangipane, V.; Rech, R.; Tosoni, A.; Lopez, G.; Agarossi, A.; et al. Hypoalbuminemia in COVID-19: Assessing the hypothesis for underlying pulmonary capillary leakage. J. Intern. Med. 2021, 289, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Bassoli, C.; Oreni, L.; Ballone, E.; Foschi, A.; Perotti, A.; Mainini, A.; Casalini, G.; Galimberti, L.; Meroni, L.; Antinori, S.; et al. Role of serum albumin and proteinuria in patients with SARS-CoV-2 pneumonia. Int. J. Clin. Pract. 2021, 75, e13946. [Google Scholar] [CrossRef]
- Hundt, M.A.; Deng, Y.; Ciarleglio, M.M.; Nathanson, M.H.; Lim, J.K. Abnormal Liver Tests in COVID-19: A Retrospective Observational Cohort Study of 1827 Patients in a Major U.S. Hospital Network. Hepatology 2020, 72, 1169–1176. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Y.; Zhao, X.; Tao, M.; Yan, W.; Fu, Y. Hypoalbuminemia–An Indicator of the Severity and Prognosis of COVID-19 Patients: A Multicentre Retrospective Analysis. Infect. Drug Resist. 2021, 14, 3699–3710. [Google Scholar] [CrossRef] [PubMed]
- Viana-Llamas, M.C.; Arroyo-Espliguero, R.; Silva-Obregón, J.A.; Uribe-Heredia, G.; Núñez-Gil, I.; García-Magallón, B.; Torán-Martínez, C.G.; Castillo-Sandoval, A.; Díaz-Caraballo, E.; Rodríguez-Guinea, I.; et al. Hypoalbuminemia on admission in COVID-19 infection: An early predictor of mortality and adverse events. A Retrosp. Obs. Study 2021, 156, 428–436. [Google Scholar] [CrossRef]
- Hirashima, T.; Arai, T.; Kitajima, H.; Tamura, Y.; Yamada, T.; Hashimoto, S.; Morishita, H.; Minamoto, S.; Kawashima, K.; Kashiwa, Y.; et al. Factors significantly associated with COVID-19 severity in symptomatic patients: A retrospective single-center study. J. Infect. Chemother. 2021, 27, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Abdeen, Y.; Kaako, A.; Amin, Z.A.; Muhanna, A.; Froessl, L.J.; Alnabulsi, M.; Okeh, A.; Miller, R.A. The Prognostic Effect of Serum Albumin Level on Outcomes of Hospitalized COVID-19 Patients. Crit. Care Res. Pract. 2021, 2021, 9963274. [Google Scholar] [CrossRef]
- Arnau-Barrés, I.; Pascual-Dapena, A.; López-Montesinos, I.; Gómez-Zorrilla, S.; Sorlí, L.; Herrero, M.; Nogués, X.; Navarro-Valls, C.; Ibarra, B.; Canchucaja, L.; et al. Severe Hypoalbuminemia at Admission Is Strongly Associated with Worse Prognosis in Older Adults with SARS-CoV-2 Infection. J. Clin. Med. 2021, 10, 5134. [Google Scholar] [CrossRef]
- de Chambrun, M.P.; Cohen-Aubart, F.; Donker, D.W.; Cariou, P.-L.; Luyt, C.-E.; Combes, A.; Amoura, Z. SARS-CoV-2 Induces Acute and Refractory Relapse of Systemic Capillary Leak Syndrome (Clarkson’s Disease). Am. J. Med. 2020, 133, e663–e664. [Google Scholar] [CrossRef]
- Lacout, C.; Rogez, J.; Orvain, C.; Nicot, C.; Rony, L.; Julien, H.; Urbanski, G. A new diagnosis of systemic capillary leak syndrome in a patient with COVID-19. Rheumatology 2021, 60, e19–e20. [Google Scholar] [CrossRef]
- Concistrè, A.; Alessandri, F.; Rosato, E.; Pugliese, F.; Muscaritoli, M.; Letizia, C. A case of chronic systemic capillary leak syndrome (SCLS) exacerbated during SARS-CoV2 infection. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5922–5927. [Google Scholar] [CrossRef]
- Cheung, P.C.; Eisch, A.R.; Maleque, N.; Polly, D.M.; Auld, S.C.; Druey, K.M. Fatal Exacerbations of Systemic Capillary Leak Syndrome Complicating Coronavirus Disease. Emerg. Infect. Dis. 2021, 27, 2529–2534. [Google Scholar] [CrossRef]
- Knox, D.B.; Lee, V.; Leither, L.; Brown, S.M. New-Onset Systemic Capillary Leak Syndrome in an Adult Patient with COVID-19. Case Rep. Crit. Care 2021, 2021, 8098942. [Google Scholar] [CrossRef] [PubMed]
- Case, R.; Ramaniuk, A.; Martin, P.; Simpson, P.J.; Harden, C.; Ataya, A. Systemic Capillary Leak Syndrome Secondary to Coronavirus Disease 2019. Chest 2020, 158, e267–e268. [Google Scholar] [CrossRef] [PubMed]
- Beber, A.; Dellai, F.; Jaber, M.A.; Peterlana, D.; Brunori, G.; Maino, A. Systemic Capillary Leak Syndrome triggered by SARS-CoV2 infection: Case Report and Systematic Review. Scand. J. Rheumatol. 2022, 51, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Robichaud, J.; Côté, C.; Côté, F. Systemic capillary leak syndrome after ChAdOx1 nCOV-19 (Oxford–AstraZeneca) vaccination. Can. Med. Assoc. J. 2021, 193, E1341–E1344. [Google Scholar] [CrossRef]
- Yatsuzuka, K.; Murakami, M.; Kuroo, Y.; Fukui, M.; Yoshida, S.; Muto, J.; Shiraishi, K.; Sayama, K. Flare-up of generalized pustular psoriasis combined with systemic capillary leak syndrome after coronavirus disease 2019 mRNA vaccination. J. Dermatol. 2022, 49, 454–458. [Google Scholar] [CrossRef] [PubMed]
| Author | Type of Study | Patients Characteristics | Main Findings |
|---|---|---|---|
| Paliogiannis et al. [73] | Systematic review and meta-analysis |
|
|
| Soetedjo et al. [8] | Systematic review and meta-analysis |
|
|
| Huang et al. [78] | Retrospective cohort study |
|
|
| Wu et al. [79] | Retrospective cohort study |
|
|
| Bassoli et al. [80] | Retrospective cohort study |
|
|
| Chen et al. [82] | Retrospective cohort study |
|
|
| Viana et al. [83] | Retrospective cohort study |
|
|
| Hirashima et al. [84] | Retrospective cohort study |
|
|
| Abdeen et al. [85] | Retrospective cohort study |
|
|
| Arnau-Barrés et al. [86] | Retrospective cohort study |
|
|
| Author | Type of Study | Patient Characteristics | Cardiometabolic Risk Factors | Main Findings |
|---|---|---|---|---|
| Wu et al. [79] | Retrospective cohort study |
|
|
|
| Huang et al. [78] | Retrospective cohort study |
|
|
|
| Bassoli et al. [80] | Retrospective cohort study |
|
|
|
| Hundt et al. [81] | Retrospective cohort study |
|
|
|
| Chen et al. [82] | Retrospective cohort study |
|
|
|
| Viana et al. [83] | Retrospective cohort study |
|
|
|
| Hirashima et al. [84] | Retrospective cohort study |
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lambadiari, V.; Korakas, E.; Oikonomou, E.; Bletsa, E.; Kountouri, A.; Goliopoulou, A.; Ikonomidis, I.; Siasos, G. COVID-19, Endothelium and the Cardiometabolic Patient: A Possible Role for Capillary Leak Syndrome. Biomedicines 2022, 10, 2379. https://doi.org/10.3390/biomedicines10102379
Lambadiari V, Korakas E, Oikonomou E, Bletsa E, Kountouri A, Goliopoulou A, Ikonomidis I, Siasos G. COVID-19, Endothelium and the Cardiometabolic Patient: A Possible Role for Capillary Leak Syndrome. Biomedicines. 2022; 10(10):2379. https://doi.org/10.3390/biomedicines10102379
Chicago/Turabian StyleLambadiari, Vaia, Emmanouil Korakas, Evangelos Oikonomou, Evanthia Bletsa, Aikaterini Kountouri, Athina Goliopoulou, Ignatios Ikonomidis, and Gerasimos Siasos. 2022. "COVID-19, Endothelium and the Cardiometabolic Patient: A Possible Role for Capillary Leak Syndrome" Biomedicines 10, no. 10: 2379. https://doi.org/10.3390/biomedicines10102379
APA StyleLambadiari, V., Korakas, E., Oikonomou, E., Bletsa, E., Kountouri, A., Goliopoulou, A., Ikonomidis, I., & Siasos, G. (2022). COVID-19, Endothelium and the Cardiometabolic Patient: A Possible Role for Capillary Leak Syndrome. Biomedicines, 10(10), 2379. https://doi.org/10.3390/biomedicines10102379













