Perhexiline Therapy in Patients with Type 2 Diabetes: Incremental Insulin Resistance despite Potentiation of Nitric Oxide Signaling
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Study Design
2.3. Investigations
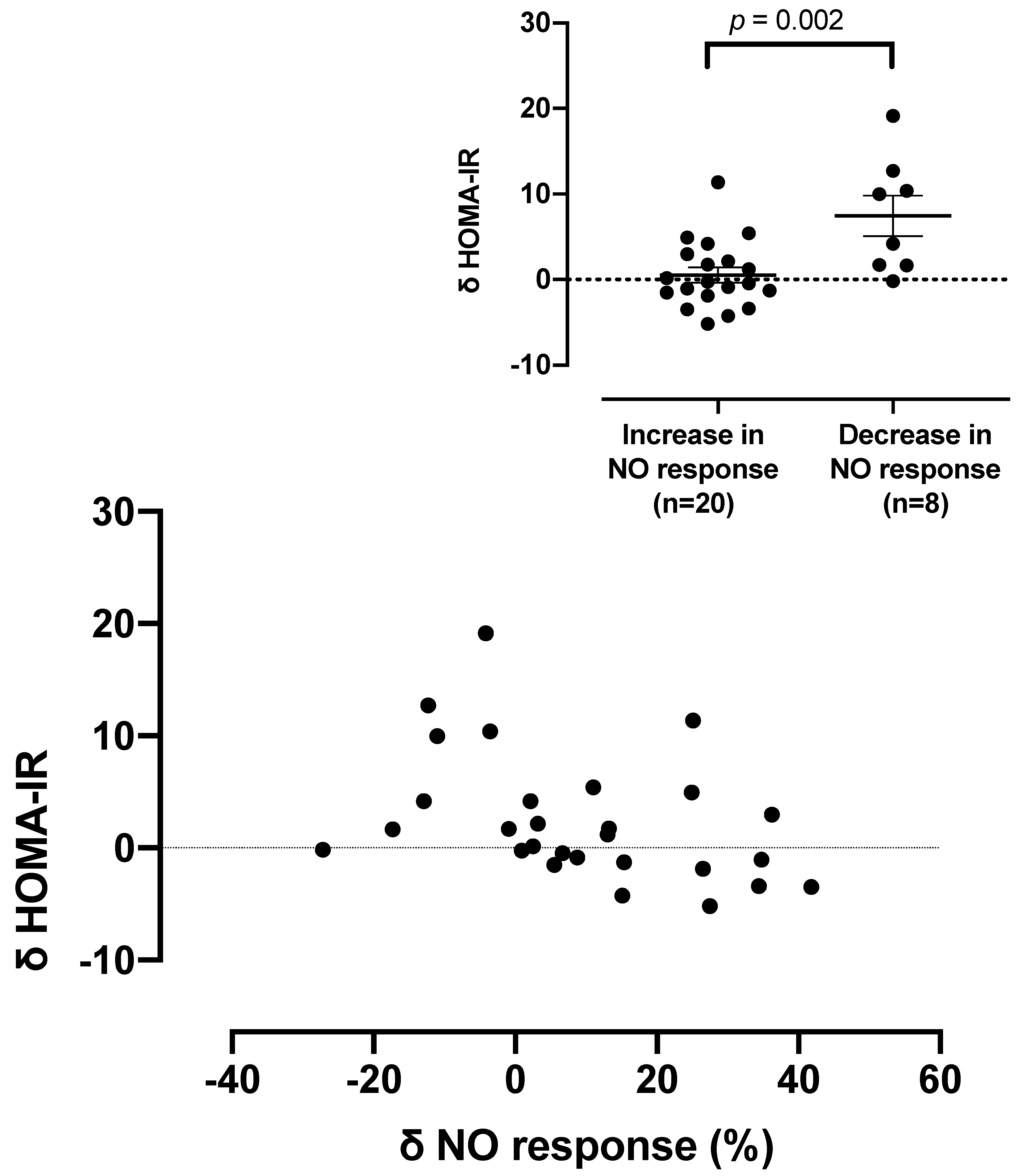
- Determination of fasting blood glucose levels and plasma insulin concentrations to measure insulin resistance as HOMA-IR, representing the primary endpoint, and insulin sensitivity by QUICKI score.
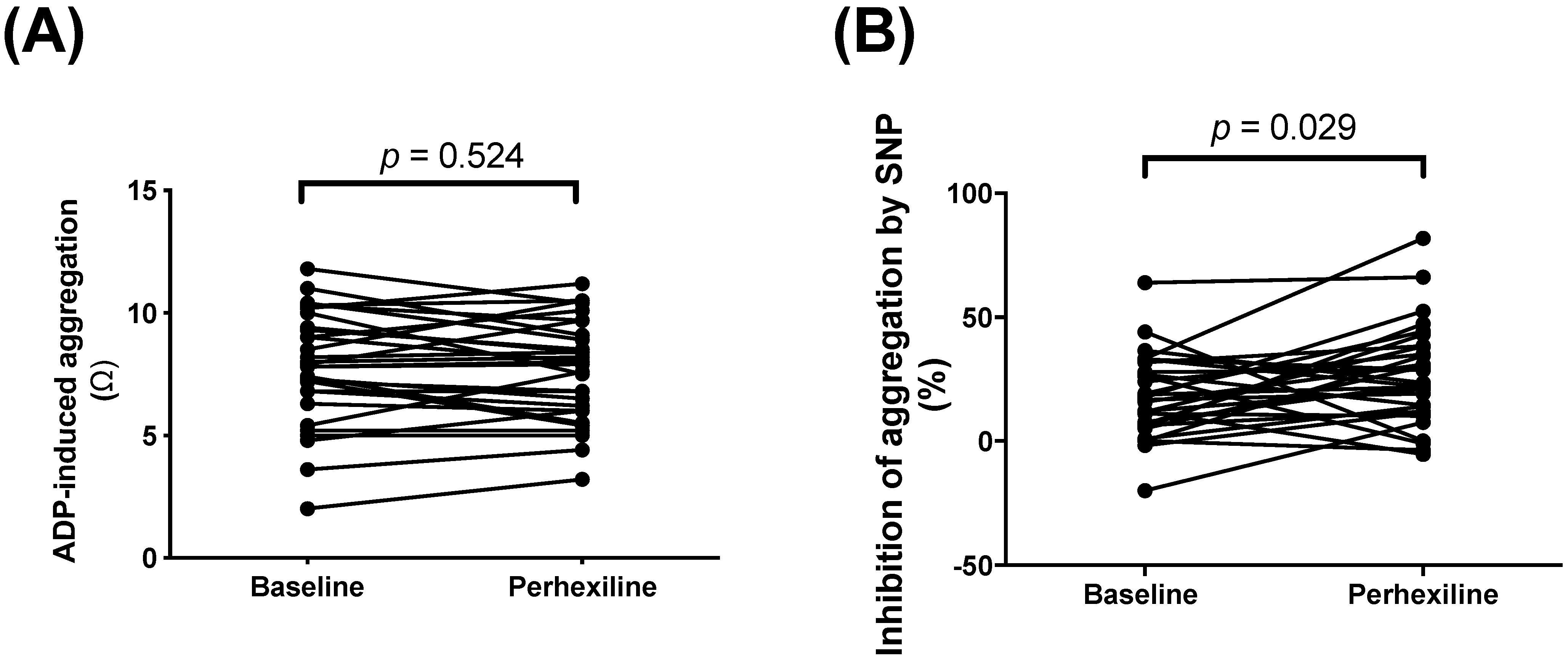
- Measurement of platelet pro-aggregatory responses to ADP and anti-aggregatory responses to the NO donor sodium nitroprusside (SNP). Whole blood impedance aggregometry (Model 560, Chrono-log®, Haverstown, PA, USA) was used to record platelet aggregation, in Ohms [36]. Blood samples were stirred at 900 rpm at 37 °C, and platelet aggregation was induced by 2.5 μM ADP; inhibition of aggregation was induced by 10 μM of SNP.
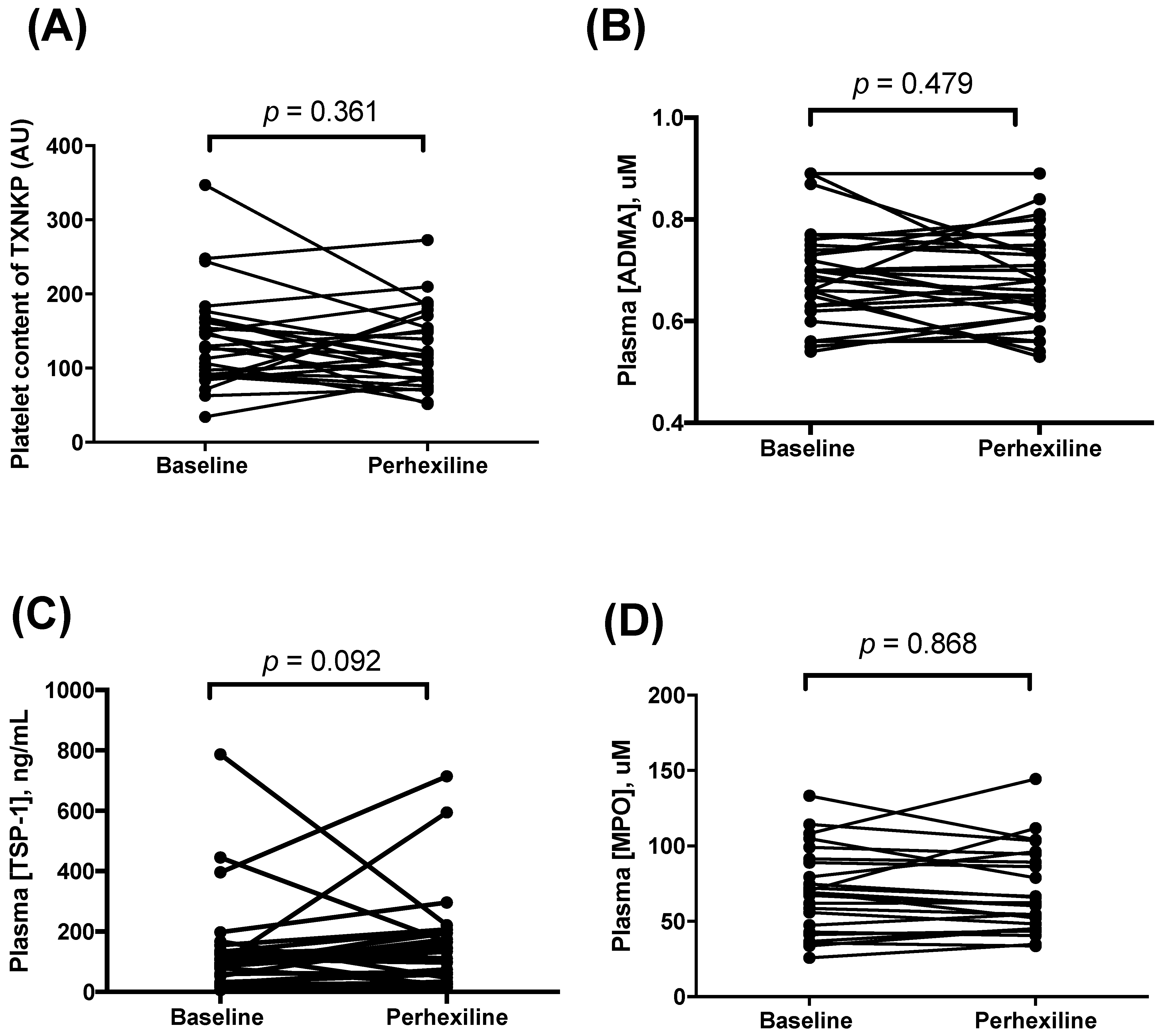
- Plasma concentrations of TSP-1 [37] were assayed with ELISA kit (R&D systems®, Minneapolis, MN, USA); ADMA was assayed using a previously published HPLC assay [38,39]; and MPO using an ELISA kit (Mercodia®, Uppsala, Sweden) [40]. Platelet content of TXNIP was also determined by immunohistochemistry [34,41].
2.4. Statistical Methodology
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giovannucci, E.; Harlan, D.M.; Archer, M.C.; Bergenstal, R.M.; Gapstur, S.M.; Habel, L.A.; Pollak, M.; Regensteiner, J.G.; Yee, D. Diabetes and cancer: A consensus report. Diabetes Care 2010, 33, 1674–1685. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.M.; Martínez-Rodríguez, J.; González-Lucán, M.; Fernández-Fernández, C.; Castro-Quintela, E. Insulin resistance is a cardiovascular risk factor in humans. Diabetes Metab. Syndr. 2019, 13, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Malmberg, K.A.; Efendic, S.; Rydén, L.E. Feasibility of insulin-glucose infusion in diabetic patients with acute myocardial infarction. A report from the multicenter trial: DIGAMI. Diabetes Care 1994, 17, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.; Oates, P.J.; Hammes, H.P.; et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000, 404, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, S.R.; Stewart, S.; Holmes, A.S.; Chirkov, Y.Y.; Horowitz, J.D. Platelet nitric oxide responsiveness: A novel prognostic marker in acute coronary syndromes. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2661–2666. [Google Scholar] [CrossRef]
- Schächinger, V.; Britten, M.B.; Zeiher, A.M. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation 2000, 101, 1899–1906. [Google Scholar] [CrossRef]
- Worthley, M.I.; Holmes, A.S.; Willoughby, S.R.; Kucia, A.M.; Heresztyn, T.; Stewart, S.; Chirkov, Y.Y.; Zeitz, C.J.; Horowitz, J.D. The deleterious effects of hyperglycemia on platelet function in diabetic patients with acute coronary syndromes mediation by superoxide production, resolution with intensive insulin administration. J. Am. Coll. Cardiol. 2007, 49, 304–310. [Google Scholar] [CrossRef]
- Chirkov, Y.Y.; Horowitz, J.D. Impaired tissue responsiveness to organic nitrates and nitric oxide: A new therapeutic frontier? Pharmacol. Ther. 2007, 116, 287–305. [Google Scholar] [CrossRef]
- Arrigoni, F.; Ahmetaj, B.; Leiper, J. The biology and therapeutic potential of the DDAH/ADMA pathway. Curr. Pharm. Des. 2010, 16, 4089–4102. [Google Scholar] [CrossRef]
- von Leitner, E.C.; Klinke, A.; Atzler, D.; Slocum, J.L.; Lund, N.; Kielstein, J.T.; Maas, R.; Schmidt-Haupt, R.; Pekarova, M.; Hellwinkel, O.; et al. Pathogenic cycle between the endogenous nitric oxide synthase inhibitor asymmetrical dimethylarginine and the leukocyte-derived hemoprotein myeloperoxidase. Circulation 2011, 124, 2735–2745. [Google Scholar] [CrossRef]
- Isenberg, J.S.; Romeo, M.J.; Yu, C.; Yu, C.K.; Nghiem, K.; Monsale, J.; Rick, M.E.; Wink, D.A.; Frazier, W.A.; Roberts, D.D. Thrombospondin-1 stimulates platelet aggregation by blocking the antithrombotic activity of nitric oxide/cGMP signaling. Blood 2008, 111, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, S.R.; Rajendran, S.; Chan, W.P.; Procter, N.; Leslie, S.; Liberts, E.A.; Heresztyn, T.; Chirkov, Y.Y.; Horowitz, J.D. Ramipril sensitizes platelets to nitric oxide: Implications for therapy in high-risk patients. J. Am. Coll. Cardiol. 2012, 60, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Imam, H.; Nguyen, T.H.; Stafford, I.; Liu, S.; Heresztyn, T.; Chirkov, Y.Y.; Horowitz, J.D. Impairment of platelet NO signalling in coronary artery spasm: Role of hydrogen sulphide. Br. J. Pharm. 2021, 178, 1639–1650. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, S.R.; Stewart, S.; Chirkov, Y.Y.; Kennedy, J.A.; Holmes, A.S.; Horowitz, J.D. Beneficial clinical effects of perhexiline in patients with stable angina pectoris and acute coronary syndromes are associated with potentiation of platelet responsiveness to nitric oxide. Eur. Heart J. 2002, 23, 1946–1954. [Google Scholar] [CrossRef][Green Version]
- Horowitz, J.D.; Sia, S.T.; Macdonald, P.S.; Goble, A.J.; Louis, W.J. Perhexiline maleate treatment for severe angina pectoris--correlations with pharmacokinetics. Int. J. Cardiol. 1986, 13, 219–229. [Google Scholar] [CrossRef]
- Phuong, H.; Choi, B.Y.; Chong, C.R.; Raman, B.; Horowitz, J.D. Can Perhexiline Be Utilized Without Long-Term Toxicity? A Clinical Practice Audit. Ther. Drug Monit. 2016, 38, 73–78. [Google Scholar] [CrossRef]
- Phan, T.T.; Shivu, G.N.; Choudhury, A.; Abozguia, K.; Davies, C.; Naidoo, U.; Ahmed, I.; Yousef, Z.; Horowitz, J.; Frenneaux, M. Multi-centre experience on the use of perhexiline in chronic heart failure and refractory angina: Old drug, new hope. Eur. J. Heart Fail. 2009, 11, 881–886. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Unger, S.A.; Horowitz, J.D. Inhibition of carnitine palmitoyltransferase-1 in rat heart and liver by perhexiline and amiodarone. Biochem. Pharmacol. 1996, 52, 273–280. [Google Scholar] [CrossRef]
- Randle, P.J.; Garland, P.B.; Hales, C.N.; Newsholme, E.A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963, 1, 785–789. [Google Scholar] [CrossRef]
- Beadle, R.M.; Williams, L.K.; Kuehl, M.; Bowater, S.; Abozguia, K.; Leyva, F.; Yousef, Z.; Wagenmakers, A.J.M.; Thies, F.; Horowitz, J.; et al. Improvement in cardiac energetics by perhexiline in heart failure due to dilated cardiomyopathy. JACC Heart Fail. 2015, 3, 202–211. [Google Scholar] [CrossRef]
- Lee, L.; Campbell, R.; Scheuermann-Freestone, M.; Taylor, R.; Gunaruwan, P.; Williams, L.; Ashrafian, H.; Horowitz, J.; Fraser, A.G.; Clarke, K.; et al. Metabolic modulation with perhexiline in chronic heart failure: A randomized, controlled trial of short-term use of a novel treatment. Circulation 2005, 112, 3280–3288. [Google Scholar] [CrossRef] [PubMed]
- Abozguia, K.; Elliott, P.; McKenna, W.; Phan, T.T.; Nallur-Shivu, G.; Ahmed, I.; Maher, A.R.; Kaur, K.; Taylor, J.; Henning, A.; et al. Metabolic modulator perhexiline corrects energy deficiency and improves exercise capacity in symptomatic hypertrophic cardiomyopathy. Circulation 2010, 122, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Flaig, T.W.; Salzmann-Sullivan, M.; Su, L.J.; Zhang, Z.; Joshi, M.; Gijón, M.A.; Kim, J.; Arcaroli, J.J.; Van Bokhoven, A.; Lucia, M.S.; et al. Lipid catabolism inhibition sensitizes prostate cancer cells to antiandrogen blockade. Oncotarget 2017, 8, 56051–56065. [Google Scholar] [CrossRef] [PubMed]
- Kant, S.; Kesarwani, P.; Guastella, A.R.; Kumar, P.; Graham, S.F.; Buelow, K.L.; Nakano, I.; Chinnaiyan, P. Perhexiline Demonstrates FYN-mediated Antitumor Activity in Glioblastoma. Mol. Cancer Ther. 2020, 19, 1415–1422. [Google Scholar] [CrossRef]
- Nassar, Z.D.; Mah, C.Y.; Centenera, M.M.; Irani, S.; Sadowski, M.C.; Scott, J.S.; Nguyen, E.V.; Nagarajan, S.R.; Moldovan, M.; Lynn, D.J.; et al. Fatty Acid Oxidation Is an Adaptive Survival Pathway Induced in Prostate Tumors by HSP90 Inhibition. Mol. Cancer Res. MCR 2020, 18, 1500–1511. [Google Scholar] [CrossRef]
- Ren, X.R.; Wang, J.; Osada, T.; Mook, R.A., Jr.; Morse, M.A.; Barak, L.S.; Lyerly, H.K.; Chen, W. Perhexiline promotes HER3 ablation through receptor internalization and inhibits tumor growth. Breast Cancer Res. BCR 2015, 17, 20. [Google Scholar] [CrossRef]
- Vella, S.; Penna, I.; Longo, L.; Pioggia, G.; Garbati, P.; Florio, T.; Rossi, F.; Pagano, A. Perhexiline maleate enhances antitumor efficacy of cisplatin in neuroblastoma by inducing over-expression of NDM29 ncRNA. Sci. Rep. 2015, 5, 18144. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Dally, S.; Lagier, G.; Assan, R.; Gaultier, M. Hypoglycemia in 2 patients treated with perhexiline maleate. La Nouv. Presse Med. 1977, 6, 1643–1644, 1649. [Google Scholar]
- Fournier, C.B.C.; Barrillon, A.; Gerbaux, A. Complications following perhexiline maleate treatment. Coeur Med. Interne 1978, 17, 553–559. [Google Scholar]
- Houdent, C.E.; Wolf, L.M.; Corriat, A. Liver during perhexiline hypoglycaemia. Lancet 1977, 2, 1028. [Google Scholar] [CrossRef]
- Roger, P.; Nogue, F.; Ragnaud, J.M.; Manciet, G.; Doumax, Y. Letter: Hypoglycemia after perhexiline maleate. La Nouv. Presse Med. 1975, 4, 2663. [Google Scholar]
- Unger, S.A.; Robinson, M.A.; Horowitz, J.D. Perhexiline improves symptomatic status in elderly patients with severe aortic stenosis. Aust. N. Z. J. Med. 1997, 27, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Sverdlov, A.L.; Chan, W.P.; Procter, N.E.; Chirkov, Y.Y.; Ngo, D.T.; Horowitz, J.D. Reciprocal regulation of NO signaling and TXNIP expression in humans: Impact of aging and ramipril therapy. Int. J. Cardiol. 2013, 168, 4624–4630. [Google Scholar] [CrossRef]
- Philpott, A.; Chandy, S.; Morris, R.; Horowitz, J.D. Development of a regimen for rapid initiation of perhexiline therapy in acute coronary syndromes. Intern. Med. J. 2004, 34, 361–363. [Google Scholar] [CrossRef]
- Chirkov, Y.Y.; Chirkova, L.P.; Sage, R.E.; Horowitz, J.D. Impaired responsiveness of platelets from patients with stable angina pectoris to antiaggregating and cyclicAMP-elevating effects of prostaglandin E1. J. Cardiovasc. Pharmacol. 1995, 25, 961–966. [Google Scholar] [CrossRef]
- Ramanathan, S.; Mazzalupo, S.; Boitano, S.; Montfort, W.R. Thrombospondin-1 and angiotensin II inhibit soluble guanylyl cyclase through an increase in intracellular calcium concentration. Biochemistry 2011, 50, 7787–7799. [Google Scholar] [CrossRef]
- Horowitz, J.D.; De Caterina, R.; Heresztyn, T.; Alexander, J.H.; Andersson, U.; Lopes, R.D.; Steg, P.G.; Hylek, E.M.; Mohan, P.; Hanna, M.; et al. Asymmetric and Symmetric Dimethylarginine Predict Outcomes in Patients With Atrial Fibrillation: An ARISTOTLE Substudy. J. Am. Coll. Cardiol. 2018, 72, 721–733. [Google Scholar] [CrossRef]
- Horowitz, J.D.; Heresztyn, T. An overview of plasma concentrations of asymmetric dimethylarginine (ADMA) in health and disease and in clinical studies: Methodological considerations. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 851, 42–50. [Google Scholar] [CrossRef]
- Lau, D.; Baldus, S. Myeloperoxidase and its contributory role in inflammatory vascular disease. Pharmacol. Ther. 2006, 111, 16–26. [Google Scholar] [CrossRef]
- Chong, C.R.; Chan, W.P.; Nguyen, T.H.; Liu, S.; Procter, N.E.; Ngo, D.T.; Sverdlov, A.L.; Chirkov, Y.Y.; Horowitz, J.D. Thioredoxin-interacting protein: Pathophysiology and emerging pharmacotherapeutics in cardiovascular disease and diabetes. Cardiovasc. Drugs Ther. 2014, 28, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.A.; Beck-Oldach, K.; McFadden-Lewis, K.; Murphy, G.A.; Wong, Y.W.; Zhang, Y.; Horowitz, J.D. Effect of the anti-anginal agent, perhexiline, on neutrophil, valvular and vascular superoxide formation. Eur. J. Pharmacol. 2006, 531, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.R.; Oates, N.S.; Idle, J.R.; Smith, R.L.; Lockhart, J.D. Impaired oxidation of debrisoquine in patients with perhexiline neuropathy. Br. Med. J. (Clin. Res. Ed.) 1982, 284, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Cole, P.L.; Beamer, A.D.; McGowan, N.; Cantillon, C.O.; Benfell, K.; Kelly, R.A.; Hartley, L.H.; Smith, T.W.; Antman, E.M. Efficacy and safety of perhexiline maleate in refractory angina. A double-blind placebo-controlled clinical trial of a novel antianginal agent. Circulation 1990, 81, 1260–1270. [Google Scholar] [CrossRef]
- Ashrafian, H.; Horowitz, J.D.; Frenneaux, M.P. Perhexiline. Cardiovasc. Drug Rev. 2007, 25, 76–97. [Google Scholar] [CrossRef]
- White, H.D.; Lowe, J.B. Antianginal efficacy of perhexiline maleate in patients refractory to beta-adrenoreceptor blockade. Int. J. Cardiol. 1983, 3, 145–155. [Google Scholar] [CrossRef]
- Sallustio, B.C.; Westley, I.S.; Morris, R.G. Pharmacokinetics of the antianginal agent perhexiline: Relationship between metabolic ratio and steady-state dose. Br. J. Clin. Pharmacol. 2002, 54, 107–114. [Google Scholar] [CrossRef]
- Westley, I.S.; Licari, G.; Sallustio, B.C. Validation of a High-Performance Liquid Chromatography-Tandem Mass Spectrometry Method for the Determination of Perhexiline and Cis-Hydroxy-Perhexiline Plasma Concentrations. Ther. Drug Monit. 2015, 37, 821–826. [Google Scholar] [CrossRef]
- Lai, B. Hypoglycaemia after treatment with perhexiline maleate: A case report. N. Z. Med. J. 2011, 124, 74–77. [Google Scholar]
- Chirkov, Y.Y.; Nguyen, T.H.; Horowitz, J.D. Impairment of Anti-Aggregatory Responses to Nitric Oxide and Prostacyclin: Mechanisms and Clinical Implications in Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 1042. [Google Scholar] [CrossRef]
- Ngo, D.T.; Chan, W.P.; Rajendran, S.; Heresztyn, T.; Amarasekera, A.; Sverdlov, A.L.; O’Loughlin, P.D.; Morris, H.A.; Chirkov, Y.Y.; Norman, R.J.; et al. Determinants of insulin responsiveness in young women: Impact of polycystic ovarian syndrome, nitric oxide, and vitamin D. Nitric Oxide Biol. Chem. 2011, 25, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Camici, P.; Ferrannini, E.; Opie, L.H. Myocardial metabolism in ischemic heart disease: Basic principles and application to imaging by positron emission tomography. Prog. Cardiovasc. Dis. 1989, 32, 217–238. [Google Scholar] [CrossRef]
- Song, J.D.; Alves, T.C.; Befroy, D.E.; Perry, R.J.; Mason, G.F.; Zhang, X.M.; Munk, A.; Zhang, Y.; Zhang, D.; Cline, G.W.; et al. Dissociation of Muscle Insulin Resistance from Alterations in Mitochondrial Substrate Preference. Cell Metab. 2020, 32, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Lehtihet, M.; Welsh, N.; Berggren, P.O.; Cook, G.A.; Sjoholm, A. Glibenclamide inhibits islet carnitine palmitoyltransferase 1 activity, leading to PKC-dependent insulin exocytosis. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E438–E446. [Google Scholar] [CrossRef] [PubMed]
- Nolan, C.J.; Prentki, M. Insulin resistance and insulin hypersecretion in the metabolic syndrome and type 2 diabetes: Time for a conceptual framework shift. Diabetes Vasc. Dis. Res. 2019, 16, 118–127. [Google Scholar] [CrossRef]
- Vigneri, P.; Frasca, F.; Sciacca, L.; Pandini, G.; Vigneri, R. Diabetes and cancer. Endocr.-Relat. Cancer 2009, 16, 1103–1123. [Google Scholar] [CrossRef]
| Patient Characteristics | |
|---|---|
| Age (years) | 70 ± 2.2 |
| Female (%) | 37 |
| HbA1c (%) | 7.1 ± 0.21 |
| Baseline serum creatinine (μmol/L) | 112.7 ± 13.74 |
| Major indication(s) for Px therapy | |
| Refractory angina (%) | 70 |
| Systolic heart failure (%) | 23 |
| Symptomatic aortic stenosis (%) | 7 |
| Concurrent pharmacotherapy | |
| ACE-inhibitor/ARB (%) | 73 |
| Calcium channel antagonist (%) | 43 |
| β-blocker (%) | 37 |
| Organic nitrate (%) | 40 |
| Metformin (%) | 60 |
| Insulin (%) | 33 |
| Sulphonylurea (%) | 30 |
| DPP-IV inhibitor or thiazolidinedione (%) | 20 |
| Parameter | Before Px | After Px | p Value |
|---|---|---|---|
| Fasting blood glucose level (mmol/L) | 6.8 (5.7, 8.6) | 7.0 (6.0, 8.7) | 0.366 |
| Fasting plasma insulin level (mU/L) | 16.5 (11.8, 25.3) | 19.0 (11.8, 37.3) | 0.014 |
| HOMA-IR score | 4.47 (3.42, 8.55) | 6.15 (3.05, 15.06) | 0.028 |
| QUICKI score | 0.158 ± 0.001 | 0.156 ± 0.002 | 0.078 |
| Effects | Toxicity |
|---|---|
|
|
| Known utility | |
|
|
| Potential for incremental use | |
| |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chong, C.-R.; Liu, S.; Imam, H.; Heresztyn, T.; Sallustio, B.C.; Chirkov, Y.Y.; Horowitz, J.D. Perhexiline Therapy in Patients with Type 2 Diabetes: Incremental Insulin Resistance despite Potentiation of Nitric Oxide Signaling. Biomedicines 2022, 10, 2381. https://doi.org/10.3390/biomedicines10102381
Chong C-R, Liu S, Imam H, Heresztyn T, Sallustio BC, Chirkov YY, Horowitz JD. Perhexiline Therapy in Patients with Type 2 Diabetes: Incremental Insulin Resistance despite Potentiation of Nitric Oxide Signaling. Biomedicines. 2022; 10(10):2381. https://doi.org/10.3390/biomedicines10102381
Chicago/Turabian StyleChong, Cher-Rin, Saifei Liu, Hasan Imam, Tamila Heresztyn, Benedetta C. Sallustio, Yuliy Y. Chirkov, and John D. Horowitz. 2022. "Perhexiline Therapy in Patients with Type 2 Diabetes: Incremental Insulin Resistance despite Potentiation of Nitric Oxide Signaling" Biomedicines 10, no. 10: 2381. https://doi.org/10.3390/biomedicines10102381
APA StyleChong, C.-R., Liu, S., Imam, H., Heresztyn, T., Sallustio, B. C., Chirkov, Y. Y., & Horowitz, J. D. (2022). Perhexiline Therapy in Patients with Type 2 Diabetes: Incremental Insulin Resistance despite Potentiation of Nitric Oxide Signaling. Biomedicines, 10(10), 2381. https://doi.org/10.3390/biomedicines10102381







