Chronic Kidney Disease—An Underestimated Risk Factor for Antimicrobial Resistance in Patients with Urinary Tract Infections
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
2.3. Variables and Definitions
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Variable (n = 564) | (n, %) |
|---|---|
| Infectious complications | |
| Superinfection | 23 (4.1%) |
| Local complications (prostatitis, kidney abscess) | 5 (0.9%) |
| Clostridioides difficile enterocolitis | 16 (2.8%) |
Concurring bacterial/viral infections
| 34 (6.03%) 20 (58.9%) 6 (17.6%) 6 (17.6%) 2 (5.9%) |
| Sepsis (non-related to UTIs) | 7 (8.2%) |
| Non-Infectious complications | |
| Acute kidney injury | 197 (34.9%) |
| AKI on CKD (n = 197) | 129 (65.5%) |
| AKI stage (KDIGO) (n = 196) | |
| 53 (27%) 44 (22.4%) 99 (50.5%) |
| Acute hemodialysis | 52 (9.2%) |
Appendix B
| Uropathogen | Total (n = 564) (n, %) | General Antibiotic Resistance (n = 307, 54.4%) (n, %) | Multiple-Drug Resistance (n = 169, 30%) (n, %) |
|---|---|---|---|
| Gram negative | 516 (91.5%) | 275 (53.3%) | 150 (29.1%) |
| Gram positive | 48 (8.5%) | 32 (66.7%) | 19 (39.6%) |
| Escherichia coli | 385 (68.3%) | 176 (45.7%) | 87 (22.6%) |
| Klebsiella spp. | 63 (11.2%) | 46 (73%) | 30 (47.6%) |
| Enterococcus spp. | 36 (6.4%) | 26 (72.2%) | 18 (50%) |
| Pseudomonas spp. | 34 (6%) | 31 (91.2%) | 23 (67.6%) |
| Proteus spp. | 34 (6%) | 22 (64.7%) | 10 (29.4%) |
| Staphylococcus spp. | 8 (1.4%) | 4 (50%) | 1 (12.5%) |
| Streptococcus spp. | 4 (0.7%) | 2 (50%) | 0 (0%) |
References
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef] [PubMed]
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Su, G.; Xu, H.; Riggi, E.; He, Z.; Lu, L.; Lindholm, B.; Marrone, G.; Wen, Z.; Liu, X.; Johnson, D.W.; et al. Association of Kidney Function with Infections by Multidrug-Resistant Organisms: An Electronic Medical Record Analysis. Sci. Rep. 2018, 8, 13372. [Google Scholar] [CrossRef] [PubMed]
- McDonald, H.I.; Thomas, S.L.; Nitsch, D. Chronic kidney disease as a risk factor for acute community-acquired infections in high-income countries: A systematic review. BMJ Open 2014, 4, e004100. [Google Scholar] [CrossRef]
- Dalrymple, L.S.; Go, A.S. Epidemiology of Acute Infections among Patients with Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1487–1493. [Google Scholar] [CrossRef]
- Foley, R.N. Infections in Patients with Chronic Kidney Disease. Infect. Dis. Clin. N. Am. 2007, 21, 659–672. [Google Scholar] [CrossRef]
- Foley, R.N. Infections and Cardiovascular Disease in Patients with Chronic Kidney Disease. Adv. Chronic Kidney Dis. 2006, 13, 205–208. [Google Scholar] [CrossRef]
- EAU Guidelines Office. EAU Guidelines. In Proceedings of the EAU Annual Congress, Amsterdam, The Netherlands, 1–4 July 2022; ISBN 978-94-92671-16-5. [Google Scholar]
- Abraham, S.N.; Miao, Y. The nature of immune responses to urinary tract infections. Nat. Rev. Immunol. 2015, 15, 655–663. [Google Scholar] [CrossRef]
- Garcia-Bustos, V.; Escrig, A.I.R.; López, C.C.; Estellés, R.A.; Jerusalem, K.; Cabañero-Navalón, M.D.; Massó, V.M.; Sigona-Giangreco, I.-A.; Sahuquillo-Arce, J.M.; Hernández, I.C.; et al. Prospective cohort study on hospitalised patients with suspected urinary tract infection and risk factors por multidrug resistance. Sci. Rep. 2021, 11, 11927. [Google Scholar] [CrossRef]
- Gadalean, F.; Parv, F.; Morariu, V.; Popa, A.; Timar, R.; Petrica, L.; Schiller, O.; Velciov, S.; Gluhovschi, C.; Mihaescu, A.; et al. MP379 Chronic Kidney Disease as A Risk Factor for Antimicrobial Multidrug Resistance of Uropathogenic Bacteria. Nephrol. Dial. Transplant. 2017, 32, iii567. [Google Scholar] [CrossRef]
- WHO. Ten Threats to Global Health in 2019. Available online: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 (accessed on 20 August 2022).
- European Commisssion. A European One Health Action Plan against Antimicrobial Resistance (AMR). 2017. Available online: https://health.ec.europa.eu/system/files/2020-01/amr_2017_action-plan_0.pdf (accessed on 20 August 2022).
- United Nations. Political Declaration of the High-Level Meeting of the General Assembly on Antimicrobial Resistance; United Nations: New York, NY, USA, 2016; Available online: https://digitallibrary.un.org/record/842813?ln=en (accessed on 20 August 2022).
- WHO. Global Action Plan on Antimicrobial Resistance. 2015. Available online: http://www.who.int/antimicrobial-resistance/global-actionplan/en/ (accessed on 20 August 2022).
- Calfee, D.P. Multidrug-Resistant Organisms Within the Dialysis Population: A Potentially Preventable Perfect Storm. Am. J. Kidney Dis. 2015, 65, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Yelin, I.; Snitser, O.; Novich, G.; Katz, R.; Tal, O.; Parizade, M.; Chodick, G.; Koren, G.; Shalev, V.; Kishony, R. Personal clinical history predicts antibiotic resistance of urinary tract infections. Nat. Med. 2019, 25, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.Z.; Kodiyanplakkal, R.P.L.; Calfee, D.P. Antimicrobial resistance in nephrology. Nat. Rev. Nephrol. 2019, 15, 463–481. [Google Scholar] [CrossRef]
- D’Agata, E.M. Addressing the Problem of Multidrug-Resistant Organisms in Dialysis. Clin. J. Am. Soc. Nephrol. 2018, 13, 666–668. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- James, M.T.; Laupland, K.B. Examining Noncardiovascular Morbidity in CKD: Estimated GFR and the Risk of Infection. Am. J. Kidney Dis. 2012, 59, 327–329. [Google Scholar] [CrossRef]
- Dalrymple, L.S.; Katz, R.; Kestenbaum, B.; de Boer, I.H.; Fried, L.; Sarnak, M.J.; Shlipak, M.G. The Risk of Infection-Related Hospitalization With Decreased Kidney Function. Am. J. Kidney Dis. 2012, 59, 356–363. [Google Scholar] [CrossRef]
- Coussement, J.; Argudín, M.A.; Heinrichs, A.; Racapé, J.; De Mendonça, R.; Nienhaus, L.; Le Moine, A.; Roisin, S.; Dodémont, M.; Jacobs, F.; et al. Host and microbial factors in kidney transplant recipients with Escherichia coli acute pyelonephritis or asymptomatic bacteriuria: A prospective study using whole-genome sequencing. Nephrol. Dial. Transplant. 2019, 34, 878–885. [Google Scholar] [CrossRef] [Green Version]
- Dimitrijevic, Z.; Paunovic, G.; Tasic, D.; Mitic, B.; Basic, D. Risk factors for urosepsis in chronic kidney disease patients with urinary tract infections. Sci. Rep. 2021, 11, 14414. [Google Scholar] [CrossRef] [PubMed]
- Wagenlehner, F.M.E.; Bjerklund Johansen, T.E.; Cai, T.; Koves, B.; Kranz, J.; Pilatz, A.; Tandogdu, Z. Epidemiology, definition and treatment of complicated urinary tract infections. Nat. Rev. Urol. 2020, 17, 586–600. [Google Scholar] [CrossRef] [PubMed]
- Kuo, I.-C.; Lee, J.-J.; Hwang, D.-Y.; Lim, L.-M.; Lin, H.Y.-H.; Hwang, S.-J.; Chen, H.-C.; Hung, C.-C. Pyuria, urinary tract infection and renal outcome in patients with chronic kidney disease stage 3–5. Sci. Rep. 2020, 10, 19460. [Google Scholar] [CrossRef]
- Hamilton, C.; Tan, L.; Miethke, T.; Anand, P. Immunity to uropathogens: The emerging roles of inflammasomes. Nat. Rev. Urol. 2017, 14, 284–295. [Google Scholar] [CrossRef]
- Pak, J.; Pu, Y.; Zhang, Z.-T.; Hasty, D.L.; Wu, X.-R. Tamm-Horsfall Protein Binds to Type 1 Fimbriated Escherichia coli and Prevents E. coli from Binding to Uroplakin Ia and Ib Receptors. J. Biol. Chem. 2001, 276, 9924–9930. [Google Scholar] [CrossRef]
- Micanovic, R.; LaFavers, K.; Garimella, P.S.; Wu, X.-R.; El-Achkar, T.M. Uromodulin (Tamm–Horsfall protein): Guardian of urinary and systemic homeostasis. Nephrol. Dial. Transplant. 2020, 35, 33–43. [Google Scholar] [CrossRef]
- Roelofs, J.J.T.H.; Rouschop, K.M.A.; Teske, G.J.D.; Wagenaar, G.T.M.; Claessen, N.; Weening, J.J.; Van Der Poll, T.; Florquin, S. Endogenous tissue-type plasminogen activator is protective during ascending urinary tract infection. Nephrol. Dial. Transplant. 2009, 24, 801–808. [Google Scholar] [CrossRef]
- Ragnarsdóttir, B.; Lutay, N.; Grönberg-Hernandez, J.; Köves, B.; Svanborg, C. Genetics of innate immunity and UTI susceptibility. Nat. Rev. Urol. 2011, 8, 449–468. [Google Scholar] [CrossRef]
- Zacharioudakis, I.M.; Zervou, F.N.; Ziakas, P.D.; Mylonakis, E. Meta-Analysis of Methicillin-Resistant Staphylococcus aureus Colonization and Risk of Infection in Dialysis Patients. J. Am. Soc. Nephrol. 2014, 25, 2131–2141. [Google Scholar] [CrossRef]
- Crowley, L.; Wilson, J.; Guy, R.; Pitcher, D.; Fluck, R. Chapter 12 Epidemiology of Staphylococcus aureus Bacteraemia Amongst Patients Receiving Dialysis for Established Renal Failure in England in 2009 to 2011: A Joint Report from the Health Protection Agency and the UK Renal Registry. Nephron Clin. Pract. 2012, 120 (Suppl. S1), c233–c245. [Google Scholar] [CrossRef]
- Babich, T.; Eliakim-Raz, N.; Turjeman, A.; Pujol, M.; Carratalà, J.; Shaw, E.; Grange, A.G.; Vuong, C.; Addy, I.; Wiegand, I.; et al. Risk factors for hospital readmission following complicated urinary tract infection. Sci. Rep. 2021, 11, 6926. [Google Scholar] [CrossRef] [PubMed]
- Chibelean, C.B.; Petca, R.-C.; Mareș, C.; Popescu, R.-I.; Enikő, B.; Mehedințu, C.; Petca, A. A Clinical Perspective on the Antimicrobial Resistance Spectrum of Uropathogens in a Romanian Male Population. Microorganisms 2020, 8, 848. [Google Scholar] [CrossRef] [PubMed]
- Mareș, C.; Petca, R.-C.; Petca, A.; Popescu, R.-I.; Jinga, V. Does the COVID Pandemic Modify the Antibiotic Resistance of Uropathogens in Female Patients? A New Storm? Antibiotics 2022, 11, 376. [Google Scholar] [CrossRef] [PubMed]
- Królicki, T.; Bardowska, K.; Kudla, T.; Królicka, A.; Letachowicz, K.; Mazanowska, O.; Krajewski, W.; Poznański, P.; Krajewska, M.; Kamińska, D. Acute kidney injury secondary to urinary tract infection in kidney transplant recipients. Sci. Rep. 2022, 12, 10858. [Google Scholar] [CrossRef]
| Variable (n = 564) | |
|---|---|
| Age (Mean ± SD, Median (IQR)) | 68.63 ± 17.2, 72 (61–81) |
| Age ≥ 65, (n, %) | 391 (69.3%) |
| Male sex (n, %) | 179 (31.8%) |
| Infection type (n = 562) (n, %) | |
| Cystitis | 43 (7.6%) |
| Pyelonephritis | 436 (77.4%) |
| Urosepsis | 93 (16.5%) |
| Recurrent UTIs | 173 (30.7%) |
| CAUTI | 72 (12.8%) |
| Host-related risk factors for complicated UTIs | |
| CKD | 308 (54.6%) |
| Diabetes mellitus | 174 (30.9%) |
| Urolithiasis (n = 561, NA = 3) | 106 (18.9%) |
| Upper urinary tract obstruction | 113 (20.1%) |
| Lower urinary tract obstruction | 81 (14.4%) |
| Indwelling urinary catheter | 72 (12.8%) |
| Ureteral stent | 33 (5.9%) |
| Nephrostomy tube | 19 (3.4%) |
| Cystostomy tube | 2 (0.4%) |
| Pregnancy (n = 380) | 9 (2.4%) |
Urinary tract abnormalities (n = 563)
| 96 (17.1%) 58 (60.42%) 21 (21.88%) 17 (17.7%) |
| ADPKD | 14 (2.5%) |
| Immunosuppression | 19 (3.4%) |
| Nursing home residents | 15 (2.7%) |
| Prolonged immobilization | 61 (10.8%) |
| Decubitus ulcer | 20 (3.5%) |
| Malignancy | 50 (8.9%) |
| Malnutrition | 34 (6%) |
| Laboratory data (Mean ± SD, Median (IQR)) | |
| WBC count (103/mm3) (n = 561) | 10 ± 7.18, 9.3 (5.94–14.2) |
| CRP (mg/dL) (n = 373) | 17.91 ± 47.4, 5.19 (0.75–17.35) |
| Fibrinogen (mg/dL) (n = 483) | 574.06 ± 198.1, 546 (438–700) |
| Serum albumin (g/dL) (n = 154) | 3.58 ± 0.92, 3.68 (2.99–4.30) |
| Outcomes | |
| Length of hospital stay(days) (Mean ± SD, Median (IQR)) | 8.33 ± 5.82, 7 (4–10.75) |
| All-cause mortality | 42 (7.5%) |
| Etiologic Agent (n = 564) | CKD (n, %) | Non-CKD (n, %) | p * |
|---|---|---|---|
| Escherichia coli | 186 (72.7%) | 199 (64.6%) | 0.413 |
| Pseudomonas spp. | 12 (4.7%) | 22 (7.1%) | |
| Staphylococcus spp. | 3 (1.2%) | 5 (1.6%) | |
| Streptococcus spp. | 2 (0.8%) | 2 (0.6%) | |
| Proteus spp. | 16 (6.3%) | 18 (5.8%) | |
| Klebsiella spp. | 22 (8.6%) | 41 (13.3%) | |
| Enterococcus spp. | 15 (5.9%) | 21 (6.8%) |
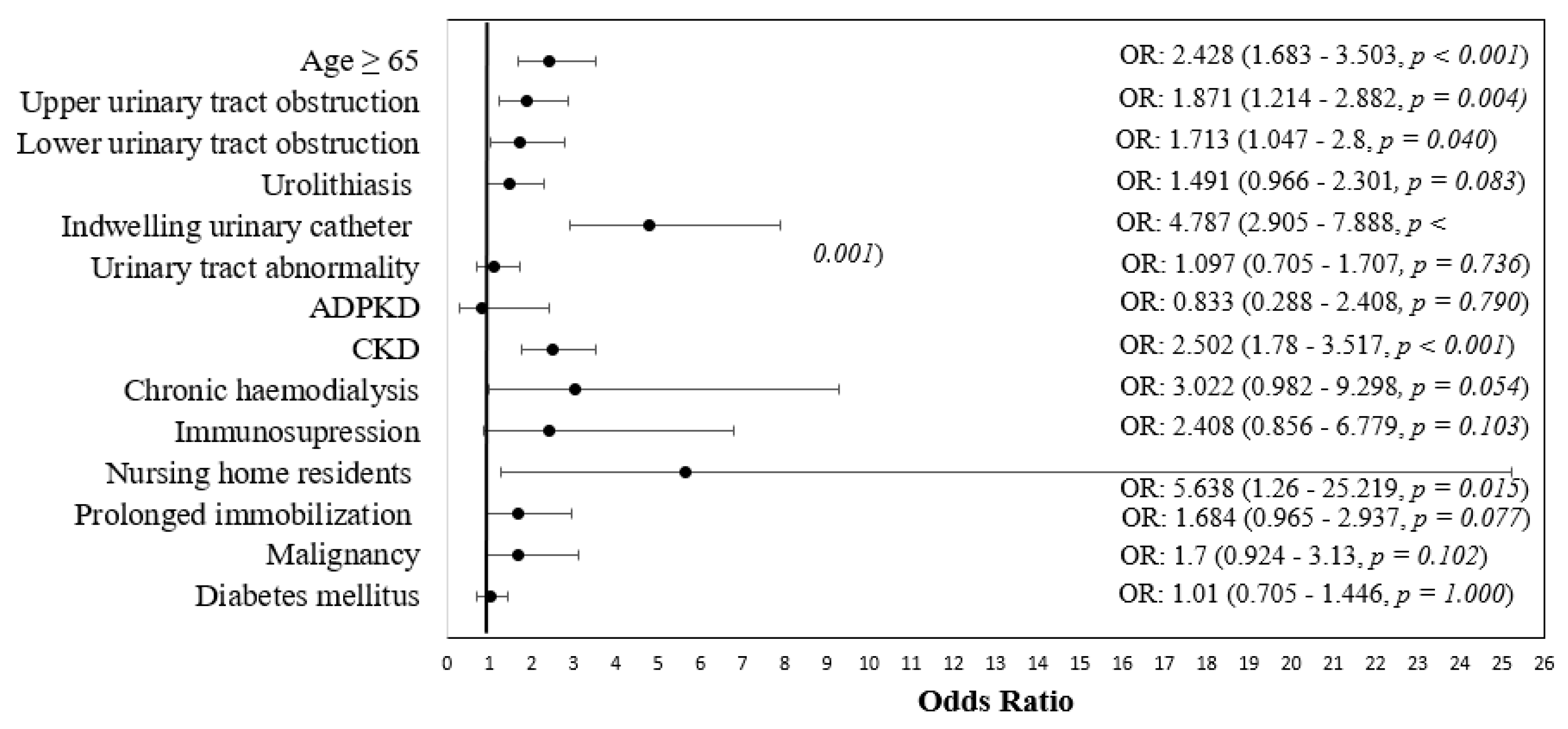
| Variable | Sensitive | Resistant | p * |
|---|---|---|---|
| Age ≥ 65 | 152 (59.1%) | 239 (77.9%) | <0.001 |
| Upper urinary tract obstruction | 38 (14.8%) | 75 (24.6%) | 0.004 |
| Lower urinary tract obstruction | 28 (10.9%) | 53 (17.4%) | 0.040 |
| Urolithiasis | 40 (15.6%) | 66 (21.6%) | 0.083 |
| Indwelling urinary catheter | 22 (8.6%) | 95 (30.9%) | <0.001 |
| Urinary tract abnormality | 42 (16.3%) | 54 (17.6%) | 0.736 |
| Autosomal dominant polycystic kidney disease | 7 (2.7%) | 7 (2.3%) | 0.790 |
| CKD | 109 (42.4%) | 199 (64.8%) | <0.001 |
| CKD stage | |||
| KDIGO G1 | 5 (4.8%) | 1 (0.5%) | 0.021 |
| KDIGO G2 | 10 (9.5%) | 29 (15.1%) | |
| KDIGO G3 | 47 (44.8%) | 66 (34.4%) | |
| KDIGO G4 | 20 (19%) | 54 (28.1%) | |
| KDIGO G5 | 23 (21.9%) | 42 (21.9%) | |
| Chronic hemodialysis | 4 (1.6%) | 14 (4.6%) | 0.054 |
| Immunosuppression | 5 (1.9%) | 14 (4.6%) | 0.103 |
| Nursing home residents | 2 (0.8%) | 13 (4.2%) | 0.015 |
| Prolonged immobilization | 21 (8.2%) | 40 (13%) | 0.077 |
| Malignancy | 17 (6.6%) | 33 (10.7%) | 0.102 |
| Diabetes mellitus | 79 (30.7%) | 95 (30.9%) | 1.000 |
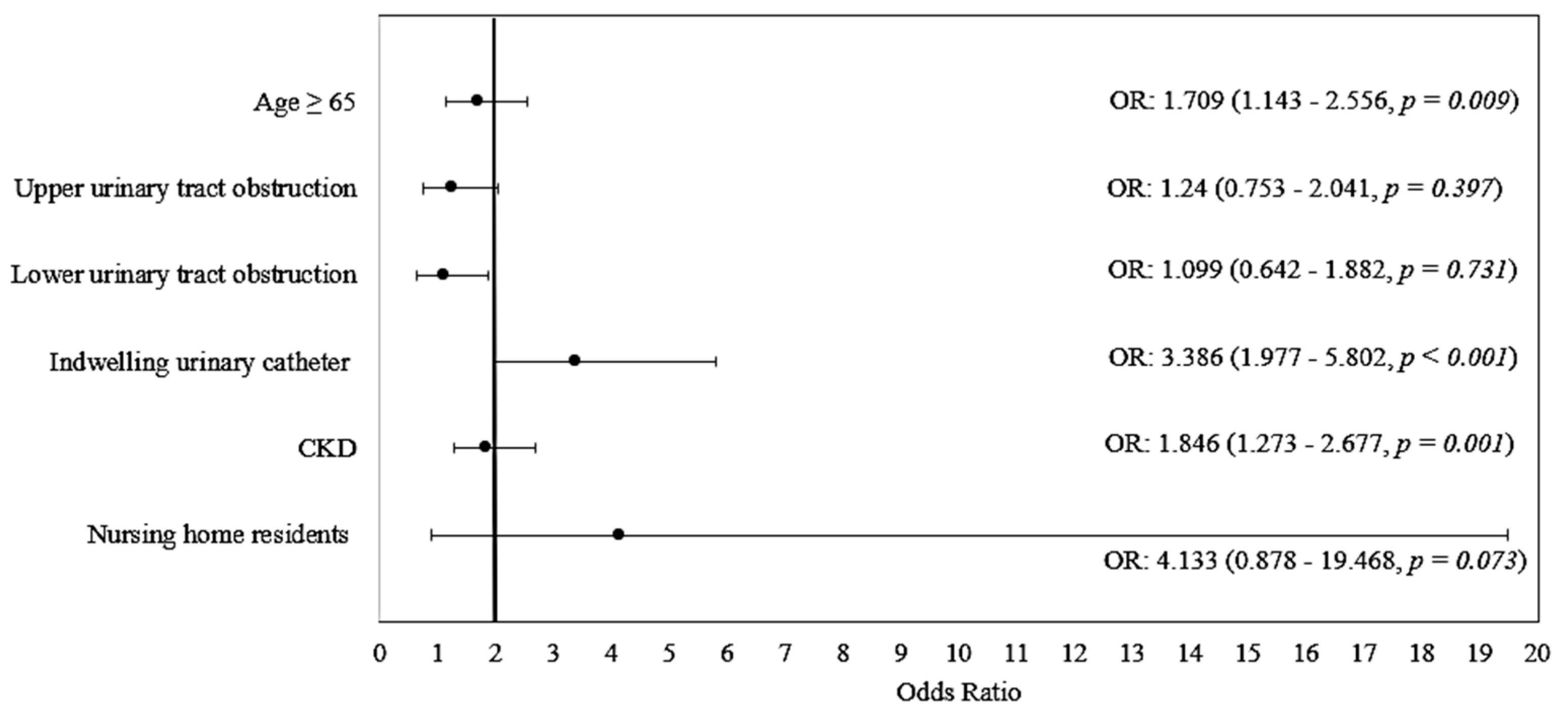
| Variable | OR (95% CI) | p | Model Parameters |
|---|---|---|---|
| Age ≥ 65 | 1.709 (1.143–2.556) | 0.009 | Χ2(6) = 75.085 p < 0.001 Nagelkerge R2 = 0.167 Hosmer–Lemeshow Test: p = 0.857 Se: 68.5%, Sp: 61.3% Accuracy: 65.2% |
| Upper urinary tract obstruction | 1.240 (0.753–2.041) | 0.397 | |
| Lower urinary tract obstruction | 1.099 (0.642–1.882) | 0.731 | |
| Indwelling urinary catheter | 3.386 (1.977–5.802) | <0.001 | |
| CKD | 1.846 (1.273–2.677) | 0.001 | |
| Nursing home residents | 4.133 (0.878–19.468) | 0.073 | |
| Constant | 0.439 | <0.001 |
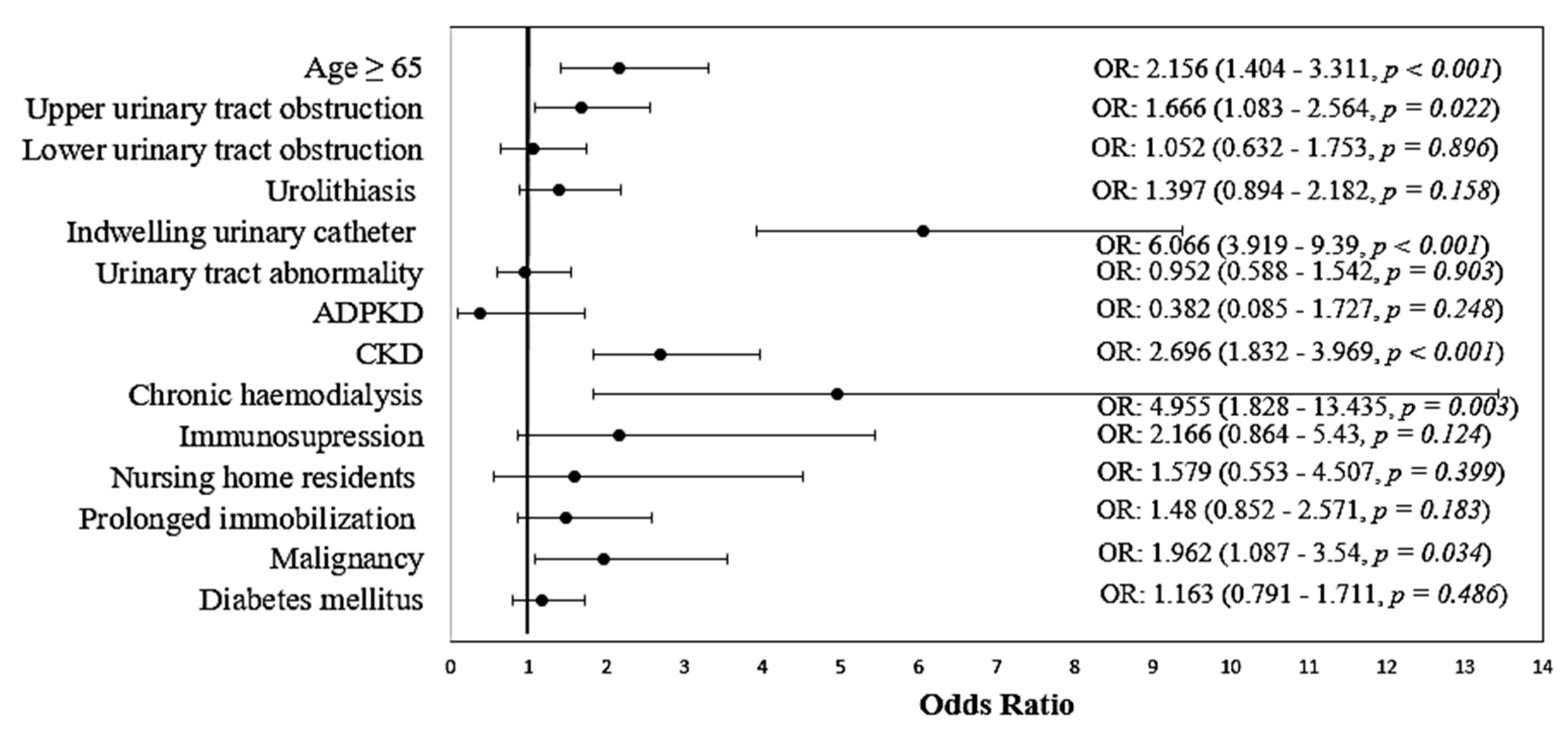
| Variable | Non-MDR | MDR | p * |
|---|---|---|---|
| Age ≥ 65 | 256 (64.8%) | 135 (79.9%) | <0.001 |
| Upper urinary tract obstruction | 69 (17.6%) | 44 (26.2%) | 0.022 |
| Lower urinary tract obstruction | 56 (14.2%) | 25 (14.9%) | 0.896 |
| Urolithiasis | 68 (17.3%) | 38 (22.6%) | 0.158 |
| Indwelling urinary catheter | 44 (11.1%) | 73 (43.2%) | <0.001 |
| Urinary tract abnormalities | 68 (17.3%) | 28 (16.6%) | 0.903 |
| Autosomal dominant polycystic kidney disease | 12 (3%) | 2 (1.2%) | 0.248 |
| CKD | 188 (47.6%) | 120 (71%) | <0.001 |
| CKD stage | |||
| KDIGO G1 | 6 (3.3%) | 0 (0%) | 0.037 |
| KDIGO G2 | 30 (16.5%) | 9 (7.8%) | |
| KDIGO G3 | 69 (37.9%) | 44 (38.3%) | |
| KDIGO G4 | 42 (23.1%) | 32 (27.8%) | |
| KDIGO G5 | 35 (19.2%) | 30 (26.1%) | |
| Chronic hemodialysis | 6 (1.5%) | 12 (7.1%) | 0.003 |
| Immunosuppression | 10 (2.5%) | 9 (5.3%) | 0.124 |
| Nursing home residents | 9 (2.3%) | 6 (3.6%) | 0.399 |
| Prolonged immobilization | 38 (9.6%) | 23 (13.6%) | 0.183 |
| Malignancy | 28 (7.1%) | 22 (13%) | 0.034 |
| Diabetes mellitus | 118 (29.9%) | 56 (33.1%) | 0.486 |
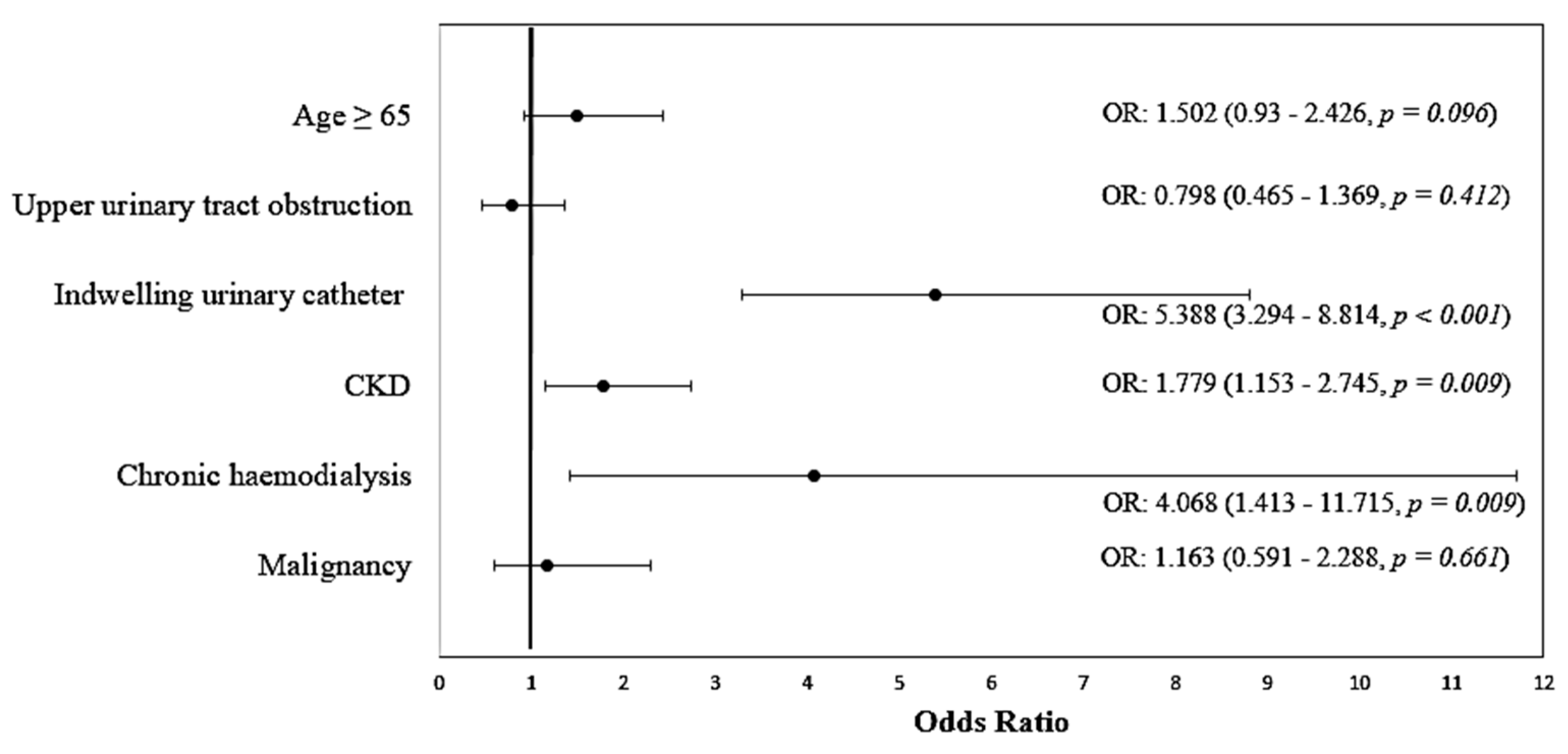
| Variable | OR (95% CI) | p | Model Parameters |
|---|---|---|---|
| Age ≥ 65 | 1.502 (0.930–2.426) | 0.096 | Χ2(6) = 90.689 p < 0.001 Nagelkerge R2 = 0.212 Hosmer–Lemeshow Test: p = 0.543 Se: 45.8%, Sp: 88.8% Accuracy: 75.9% |
| Upper urinary tract obstruction | 0.798 (0.465–1.369) | 0.412 | |
| Indwelling urinary catheter | 5.388 (3.294–8.814) | <0.001 | |
| CKD | 1.779 (1.153–2.745) | 0.009 | |
| Chronic hemodialysis | 4.068 (1.413–11.715) | 0.009 | |
| Malignancies | 1.163 (0.591–2.288) | 0.661 | |
| Constant | 0.146 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vacaroiu, I.A.; Cuiban, E.; Geavlete, B.F.; Gheorghita, V.; David, C.; Ene, C.V.; Bulai, C.; Lupusoru, G.E.; Lupusoru, M.; Balcangiu-Stroescu, A.E.; et al. Chronic Kidney Disease—An Underestimated Risk Factor for Antimicrobial Resistance in Patients with Urinary Tract Infections. Biomedicines 2022, 10, 2368. https://doi.org/10.3390/biomedicines10102368
Vacaroiu IA, Cuiban E, Geavlete BF, Gheorghita V, David C, Ene CV, Bulai C, Lupusoru GE, Lupusoru M, Balcangiu-Stroescu AE, et al. Chronic Kidney Disease—An Underestimated Risk Factor for Antimicrobial Resistance in Patients with Urinary Tract Infections. Biomedicines. 2022; 10(10):2368. https://doi.org/10.3390/biomedicines10102368
Chicago/Turabian StyleVacaroiu, Ileana Adela, Elena Cuiban, Bogdan Florin Geavlete, Valeriu Gheorghita, Cristiana David, Cosmin Victor Ene, Catalin Bulai, Gabriela Elena Lupusoru, Mircea Lupusoru, Andra Elena Balcangiu-Stroescu, and et al. 2022. "Chronic Kidney Disease—An Underestimated Risk Factor for Antimicrobial Resistance in Patients with Urinary Tract Infections" Biomedicines 10, no. 10: 2368. https://doi.org/10.3390/biomedicines10102368
APA StyleVacaroiu, I. A., Cuiban, E., Geavlete, B. F., Gheorghita, V., David, C., Ene, C. V., Bulai, C., Lupusoru, G. E., Lupusoru, M., Balcangiu-Stroescu, A. E., Feier, L. F., Simion, I. S., & Radulescu, D. (2022). Chronic Kidney Disease—An Underestimated Risk Factor for Antimicrobial Resistance in Patients with Urinary Tract Infections. Biomedicines, 10(10), 2368. https://doi.org/10.3390/biomedicines10102368









