Persistent Depletion of Neuroprotective Factors Accompanies Neuroinflammatory, Neurodegenerative, and Vascular Remodeling Spectra in Serum Three Months after Non-Emergent Cardiac Surgery
Abstract
:1. Introduction
2. Results
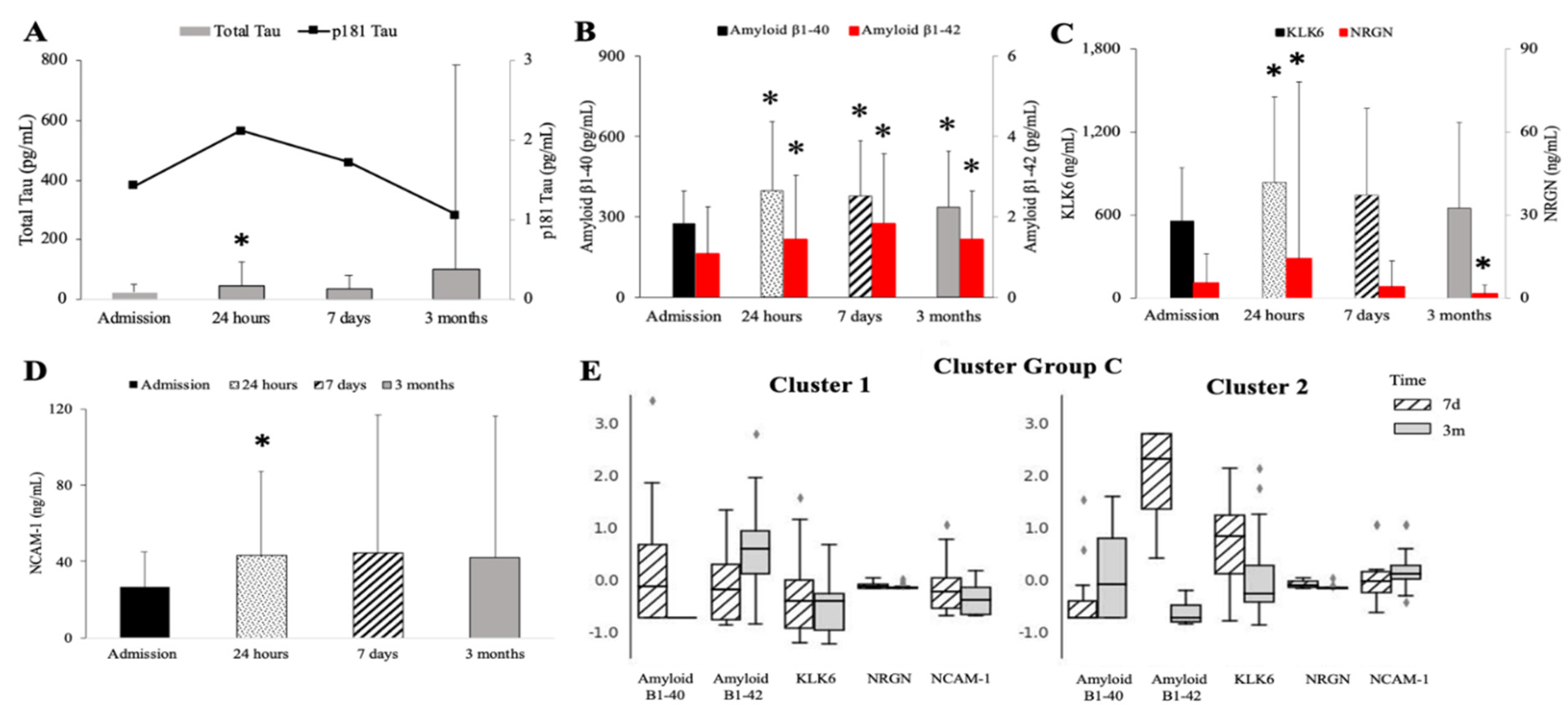
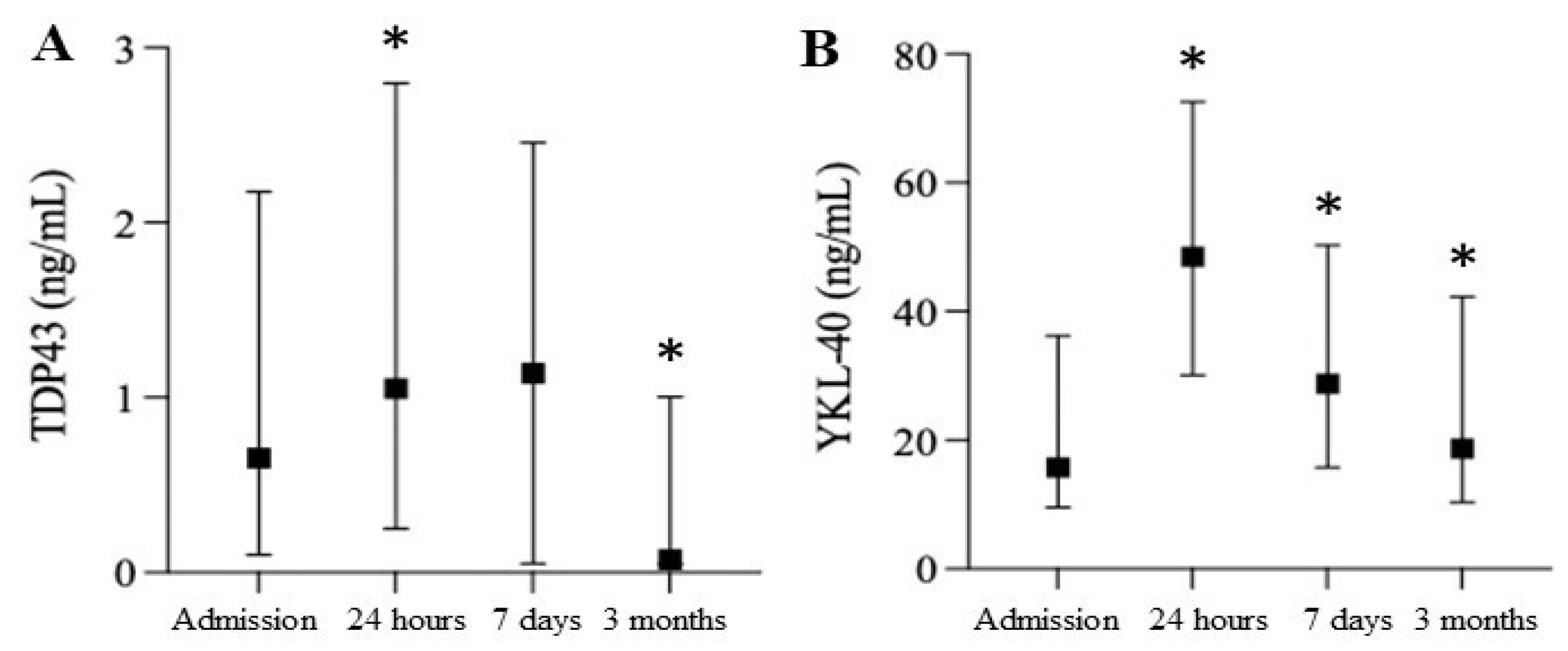
2.1. Assessment of Neurodegenerative Markers
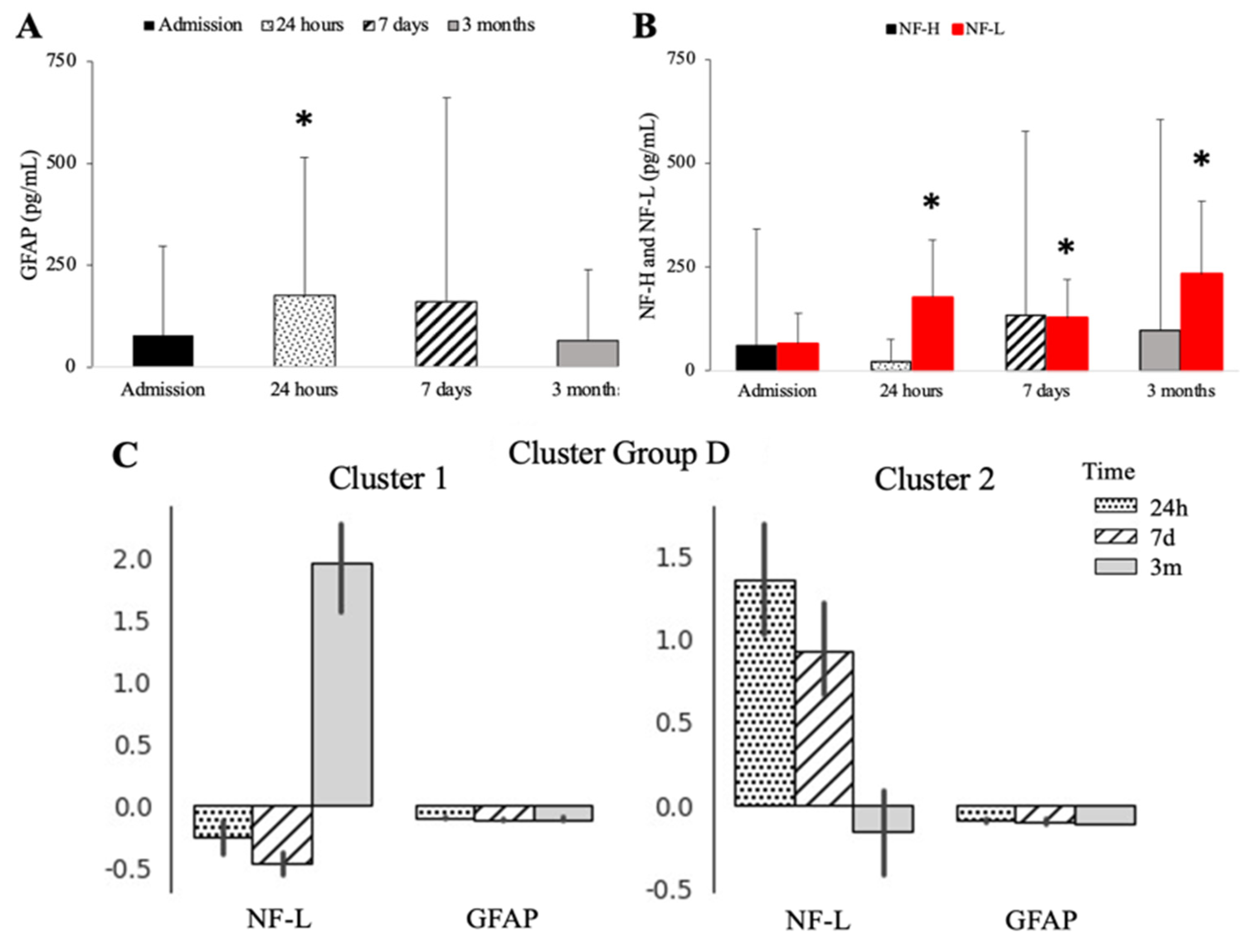
2.2. Severe Depletion of Neuro-Protective Markers Is Seen after Cardiac Surgery
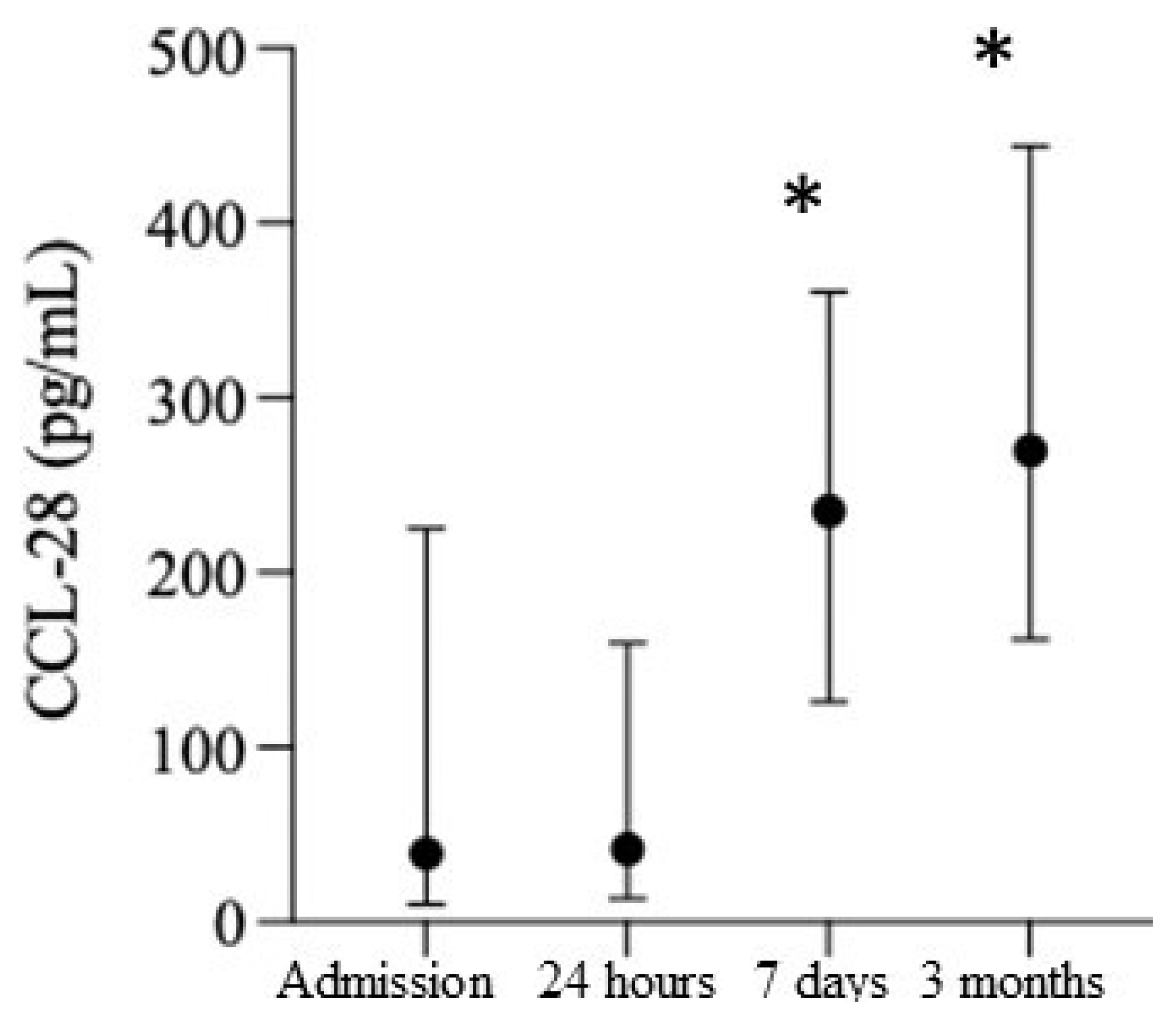
2.3. Smoldering Vascular Inflammation Persists after Cardiac Surgery and Is Accompanied by CNS Leakage of the DAMPs
2.4. Peri-Operative Management and Changes in Neuroinflammatory, Neuroprotective, and Neurodegenerative Markers
2.5. Incidence of CVA and Cognitive Dysfunction
3. Discussion
4. Materials and Methods
4.1. Consent
4.2. Patient Population
4.3. Clinical Data Collection
4.4. Study Procedure
4.5. Assessment of Biomarkers
4.6. Cognitive and Daily-Living Performance Testing
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melly, L.; Torregrossa, G.; Lee, T.; Jansens, J.-L.; Puskas, J.D. Fifty years of coronary artery bypass grafting. J. Thorac. Dis. 2018, 10, 1960–1967. [Google Scholar] [CrossRef] [PubMed]
- How Many Cardiac Surgeries Are Performed Each Year?—New Study by iData Research. Available online: https://idataresearch.com/over-900000-cardiac-surgeries-performed-every-year-in-the-united-states/#:~:text=%E2%80%93%20New%20Study%20by%20iData%20Research,-06%2F01%2F2021&text=According%20to%20a%20new%20cardiac,year%20in%20the%20United%20States (accessed on 24 August 2022).
- Elixhauser, A.; Andrews, R.M. Profile of Inpatient Operating Room Procedures in US Hospitals in 2007. Arch. Surg. 2010, 145, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Puskas, J.D.; Kilgo, P.D.; Thourani, V.H.; Lattouf, O.M.; Chen, E.; Vega, J.D.; Cooper, W.; Guyton, R.A.; Halkos, M. The society of thoracic surgeons 30-day predicted risk of mortality score also predicts long-term survival. Ann. Thorac. Surg. 2012, 93, 26–33, discussion 33–35. [Google Scholar] [CrossRef] [PubMed]
- Dieleman, J.M.; Peelen, L.M.; Coulson, T.G.; Tran, L.; Reid, C.M.; Smith, J.A.; Myles, P.S.; Pilcher, D. Age and other perioperative risk factors for postoperative systemic inflammatory response syndrome after cardiac surgery. Br. J. Anaesth. 2017, 119, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Ad, N.; Luc, J.G.Y.; Nguyen, T.C. Cardiac surgery in North America and coronavirus disease 2019 (COVID-19): Regional variability in burden and impact. J. Thorac. Cardiovasc. Surg. 2020, 162, 893–903.e4. [Google Scholar] [CrossRef]
- Weiss, A.J.; Elixhauser, A. Trends in Operating Room Procedures in US Hospitals, 2001–2011: Statistical Brief# 171; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2017.
- Ahmad, F.B.; Cisewski, J.A.; Miniño, A.; Anderson, R.N. Provisional Mortality Data—United States, 2020. MMWR Morb. Mortal Wkly. Rep. 2021, 70, 519–522. [Google Scholar] [CrossRef]
- Glumac, S.; Kardum, G.; Karanovic, N. Postoperative Cognitive Decline After Cardiac Surgery: A Narrative Review of Current Knowledge in 2019. Med. Sci. Monit. 2019, 25, 3262–3270. [Google Scholar] [CrossRef]
- Maheshwari, A.; McCormick, P.J.; Sessler, D.I.; Reich, D.L.; You, J.; Mascha, E.J.; Castillo, J.G.; Levin, M.A.; Duncan, A.E. Prolonged concurrent hypotension and low bispectral index (‘double low’) are associated with mortality, serious complications, and prolonged hospitalization after cardiac surgery. Br. J. Anaesth. 2017, 119, 40–49. [Google Scholar] [CrossRef]
- McCormick, P.J.; Levin, M.A.; Lin, H.M.; Sessler, D.I.; Reich, D.L. Effectiveness of an Electronic Alert for Hypotension and Low Bispectral Index on 90-day Postoperative Mortality: A Prospective, Randomized Trial. Anesthesiology 2016, 125, 1113–1120. [Google Scholar] [CrossRef]
- Murabito, P.; Astuto, M.; Sanfilippo, F.; La Via, L.; Vasile, F.; Basile, F.; Cappellani, A.; Longhitano, L.; Distefano, A.; Li Volti, G. Proactive Management of Intraoperative Hypotension Reduces Biomarkers of Organ Injury and Oxidative Stress during Elective Non-Cardiac Surgery: A Pilot Randomized Controlled Trial. J. Clin. Med. 2022, 11, 392. [Google Scholar] [CrossRef]
- Evered, L.; Atkins, K.; Silbert, B.; Scott, D.A. Acute peri-operative neurocognitive disorders: A narrative review. Anaesthesia 2022, 77, 34–42. [Google Scholar] [CrossRef]
- Laudanski, K.; Zawadka, M.; Polosak, J.; Modi, J.; DiMeglio, M.; Gutsche, J.; Szeto, W.Y.; Puzianowska-Kuznicka, M. Acquired immunological imbalance after surgery with cardiopulmonary bypass due to epigenetic over-activation of PU.1/M-CSF. J. Transl. Med. 2018, 16, 143. [Google Scholar] [CrossRef]
- Zawadka, M.; Wahome, J.; Oszkiel, H.; Szeto, W.Y.; Cobb, B.; Laudanski, K. Long-term alterations in monocyte function after elective cardiac surgery. Anesthesia 2017, 72, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Cappabianca, G.; Paparella, D.; Visicchio, G.; Capone, G.; Lionetti, G.; Numis, F.; Ferrara, P.; D’Agostino, C.; de Luca Tupputi Schinosa, L. Preoperative C-Reactive Protein Predicts Mid-Term Outcome After Cardiac Surgery. Ann. Thorac. Surg. 2006, 82, 2170–2178. [Google Scholar] [CrossRef] [PubMed]
- Squiccimarro, E.; Stasi, A.; Lorusso, R.; Paparella, D. Narrative review of the systemic inflammatory reaction to cardiac surgery and cardiopulmonary bypass. Artif. Organs 2022, 46, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Squiccimarro, E.; Labriola, C.; Malvindi, P.G.; Margari, V.; Guida, P.; Visicchio, G.; Kounakis, G.; Favale, A.; Dambruoso, P.; Mastrototaro, G.; et al. Prevalence and Clinical Impact of Systemic Inflammatory Reaction After Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2019, 33, 1682–1690. [Google Scholar] [CrossRef]
- DiMeglio, M.; Furey, W.; Hajj, J.; Lindekens, J.; Patel, S.; Acker, M.; Bavaria, J.; Szeto, W.Y.; Atluri, P.; Haber, M.; et al. Observational study of long-term persistent elevation of neurodegeneration markers after cardiac surgery. Sci. Rep. 2019, 9, 7177. [Google Scholar] [CrossRef]
- Bellaver, B.; Ferrari-Souza, J.P.; Uglione da Ros, L.; Carter, S.F.; Rodriguez-Vieitez, E.; Nordberg, A.; Pellerin, L.; Rosa-Neto, P.; Leffa, D.T.; Zimmer, E.R. Astrocyte Biomarkers in Alzheimer Disease: A Systematic Review and Meta-analysis. Neurology 2021, 96, e2944–e2955. [Google Scholar] [CrossRef]
- Georgiadis, D.; Berger, A.; Kowatschev, E.; Lautenschläger, C.; Börner, A.; Lindner, A.; Schulte-Mattler, W.; Zerkowski, H.-R.; Zierz, S.; Deufel, T. Predictive value of S-100β and neuron-specific enolase serum levels for adverse neurologic outcome after cardiac surgery. J. Thorac. Cardiovasc. Surg. 2000, 119, 138–147. [Google Scholar] [CrossRef]
- Požgain, Z.; Dulić, G.; Kondža, G.; Bogović, S.; Šerić, I.; Hil, D.; Trogrlić, B.; Bednjanić, A.; Perković-Kovačević, M.; Šahinović, I. Is postoperative cognitive decline after cardiac surgery associated with plasma beta amyloid 1–42 levels? J. Cardiothorac. Surg. 2022, 17, 6. [Google Scholar] [CrossRef]
- Alifier, M.; Olsson, B.; Andreasson, U.; Cullen, N.C.; Czyżewska, J.; Jakubów, P.; Sieśkiewicz, A.; Stasiak-Barmuta, A.; Hirnle, T.; Kornhuber, J.; et al. Cardiac Surgery is Associated with Biomarker Evidence of Neuronal Damage. J. Alzheimers Dis. 2020, 74, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Wiberg, S.; Holmgaard, F.; Zetterberg, H.; Nilsson, J.C.; Kjaergaard, J.; Wanscher, M.; Langkilde, A.R.; Hassager, C.; Rasmussen, L.S.; Blennow, K.; et al. Biomarkers of Cerebral Injury for Prediction of Postoperative Cognitive Dysfunction in Patients Undergoing Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2022, 36, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Barbu, M.; Jónsson, K.; Zetterberg, H.; Blennow, K.; Kolsrud, O.; Ricksten, S.E.; Dellgren, G.; Björk, K.; Jeppsson, A. Serum biomarkers of brain injury after uncomplicated cardiac surgery: Secondary analysis from a randomized trial. Acta Anaesthesiol. Scand. 2022, 66, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.B. Perioperative stroke: Pathophysiology and management. Korean J. Anesth. 2018, 71, 3–11. [Google Scholar] [CrossRef]
- Sanders, R.D.; Jørgensen, M.E.; Mashour, G.A. Perioperative stroke: A question of timing? Br. J. Anaesth. 2015, 115, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, C.; Smailagic, N.; Noel-Storr, A.H.; Ukoumunne, O.; Ladds, E.C.; Martin, S. CSF tau and the CSF tau/ABeta ratio for the diagnosis of Alzheimer’s disease dementia and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst. Rev. 2017, 3, Cd010803. [Google Scholar] [CrossRef]
- Mattsson, N.; Insel, P.S.; Palmqvist, S.; Portelius, E.; Zetterberg, H.; Weiner, M.; Blennow, K.; Hansson, O.; Alzheimer’s Disease Neuroimaging, I. Cerebrospinal fluid tau, neurogranin, and neurofilament light in Alzheimer’s disease. EMBO Mol. Med. 2016, 8, 1184–1196. [Google Scholar] [CrossRef]
- Hatsuta, H.; Takao, M.; Nogami, A.; Uchino, A.; Sumikura, H.; Takata, T.; Morimoto, S.; Kanemaru, K.; Adachi, T.; Arai, T.; et al. Tau and TDP-43 accumulation of the basal nucleus of Meynert in individuals with cerebral lobar infarcts or hemorrhage. Acta Neuropathol. Commun. 2019, 7, 49. [Google Scholar] [CrossRef]
- Rubenstein, R.; Chang, B.; Yue, J.K.; Chiu, A.; Winkler, E.A.; Puccio, A.M.; Diaz-Arrastia, R.; Yuh, E.L.; Mukherjee, P.; Valadka, A.B.; et al. Comparing Plasma Phospho Tau, Total Tau, and Phospho Tau-Total Tau Ratio as Acute and Chronic Traumatic Brain Injury Biomarkers. JAMA Neurol. 2017, 74, 1063–1072. [Google Scholar] [CrossRef]
- Saller, T.; Petzold, A.; Zetterberg, H.; Kuhle, J.; Chappell, D.; von Dossow, V.; Klawitter, F.; Schurholz, T.; Hagl, C.; Reuter, D.A.; et al. A case series on the value of tau and neurofilament protein levels to predict and detect delirium in cardiac surgery patients. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech. Repub. 2019, 163, 241–246. [Google Scholar] [CrossRef] [Green Version]
- Abu-Rumeileh, S.; Steinacker, P.; Polischi, B.; Mammana, A.; Bartoletti-Stella, A.; Oeckl, P.; Baiardi, S.; Zenesini, C.; Huss, A.; Cortelli, P.; et al. CSF biomarkers of neuroinflammation in distinct forms and subtypes of neurodegenerative dementia. Alzheimers Res. 2019, 12, 2. [Google Scholar] [CrossRef]
- Kaźmierski, J.; Miler, P.; Pawlak, A.; Jerczyńska, H.; Woźniak, J.; Frankowska, E.; Brzezińska, A.; Woźniak, K.; Krejca, M.; Wilczyński, M. Elevated Monocyte Chemoattractant Protein-1 as the Independent Risk Factor of Delirium after Cardiac Surgery. A Prospective Cohort Study. J. Clin. Med. 2021, 10, 1587. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Morillo, E.; Diamandis, A.; Romaschin, A.D.; Diamandis, E.P. Kallikrein 6 as a serum prognostic marker in patients with aneurysmal subarachnoid hemorrhage. PLoS ONE 2012, 7, e45676. [Google Scholar] [CrossRef] [PubMed]
- Ashby, E.L.; Kehoe, P.G.; Love, S. Kallikrein-related peptidase 6 in Alzheimer’s disease and vascular dementia. Brain Res. 2010, 1363, 1–10. [Google Scholar] [CrossRef]
- Goldhardt, O.; Warnhoff, I.; Yakushev, I.; Begcevic, I.; Förstl, H.; Magdolen, V.; Soosaipillai, A.; Diamandis, E.; Alexopoulos, P.; Grimmer, T. Kallikrein-related peptidases 6 and 10 are elevated in cerebrospinal fluid of patients with Alzheimer’s disease and associated with CSF-TAU and FDG-PET. Transl. Neurodegener. 2019, 8, 25. [Google Scholar] [CrossRef]
- De Vos, A.; Bjerke, M.; Brouns, R.; De Roeck, N.; Jacobs, D.; Van den Abbeele, L.; Guldolf, K.; Zetterberg, H.; Blennow, K.; Engelborghs, S.; et al. Neurogranin and tau in cerebrospinal fluid and plasma of patients with acute ischemic stroke. BMC Neurol. 2017, 17, 170. [Google Scholar] [CrossRef]
- Headley, A.; De Leon-Benedetti, A.; Dong, C.; Levin, B.; Loewenstein, D.; Camargo, C.; Rundek, T.; Zetterberg, H.; Blennow, K.; Wright, C.B.; et al. Neurogranin as a predictor of memory and executive function decline in MCI patients. Neurology 2018, 90, e887–e895. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.E.; Liu, D.; Li, J.; Schimmel, S.J.; Cambronero, F.E.; Terry, J.G.; Nair, S.; Pechman, K.R.; Moore, M.E.; Bell, S.P.; et al. Association of Aortic Stiffness With Biomarkers of Neuroinflammation, Synaptic Dysfunction, and Neurodegeneration. Neurology 2021, 97, e329–e340. [Google Scholar] [CrossRef]
- Kjaergaard, A.D.; Johansen, J.S.; Bojesen, S.E.; Nordestgaard, B.G. Elevated plasma YKL-40, lipids and lipoproteins, and ischemic vascular disease in the general population. Stroke J. Cereb. Circ. 2015, 46, 329–335. [Google Scholar] [CrossRef]
- Craig-Schapiro, R.; Perrin, R.J.; Roe, C.M.; Xiong, C.; Carter, D.; Cairns, N.J.; Mintun, M.A.; Peskind, E.R.; Li, G.; Galasko, D.R.; et al. YKL-40: A novel prognostic fluid biomarker for preclinical Alzheimer’s disease. Biol. Psychiatry 2010, 68, 903–912. [Google Scholar] [CrossRef] [Green Version]
- Jovanova-Nesic, K.; Shoenfeld, Y. MMP-2, VCAM-1 and NCAM-1 expression in the brain of rats with experimental autoimmune encephalomyelitis as a trigger mechanism for synaptic plasticity and pathology. J. Neuroimmunol. 2006, 181, 112–121. [Google Scholar] [CrossRef]
- Giegling, I.; Chiesa, A.; Mandelli, L.; Gibiino, S.; Hartmann, A.M.; Möller, H.J.; Schneider, B.; Schnabel, A.; Maurer, K.; De Ronchi, D.; et al. Influence of neuronal cell adhesion molecule (NCAM1) variants on suicidal behaviour and correlated traits. Psychiatry Res. 2010, 179, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Armentero, M.T.; Levandis, G.; Bazzini, E.; Cerri, S.; Ghezzi, C.; Blandini, F. Adhesion molecules as potential targets for neuroprotection in a rodent model of Parkinson’s disease. Neurobiol. Dis. 2011, 43, 663–668. [Google Scholar] [CrossRef]
- Yu, P.; Zhao, J.; Jiang, H.; Liu, M.; Yang, X.; Zhang, B.; Yu, Y.; Zhang, L.; Tong, R.; Liu, G.; et al. Neural cell adhesion molecule-1 may be a new biomarker of coronary artery disease. Int. J. Cardiol. 2018, 257, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Yuyama, K.; Takahashi, K.; Usuki, S.; Mikami, D.; Sun, H.; Hanamatsu, H.; Furukawa, J.; Mukai, K.; Igarashi, Y. Plant sphingolipids promote extracellular vesicle release and alleviate amyloid-β pathologies in a mouse model of Alzheimer’s disease. Sci. Rep. 2019, 9, 16827. [Google Scholar] [CrossRef] [PubMed]
- Kjaergaard, A.D.; Johansen, J.S.; Bojesen, S.E.; Nordestgaard, B.G. Role of inflammatory marker YKL-40 in the diagnosis, prognosis and cause of cardiovascular and liver diseases. Crit. Rev. Clin. Lab. Sci. 2016, 53, 396–408. [Google Scholar] [CrossRef]
- Davidson, Y.S.; Raby, S.; Foulds, P.G.; Robinson, A.; Thompson, J.C.; Sikkink, S.; Yusuf, I.; Amin, H.; DuPlessis, D.; Troakes, C.; et al. TDP-43 pathological changes in early onset familial and sporadic Alzheimer’s disease, late onset Alzheimer’s disease and Down’s syndrome: Association with age, hippocampal sclerosis and clinical phenotype. Acta Neuropathol. 2011, 122, 703–713. [Google Scholar] [CrossRef]
- Brahmachari, S.; Fung, Y.K.; Pahan, K. Induction of Glial Fibrillary Acidic Protein Expression in Astrocytes by Nitric Oxide. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 4930–4939. [Google Scholar] [CrossRef]
- Skaper, S.D.; Facci, L.; Zusso, M.; Giusti, P. An Inflammation-Centric View of Neurological Disease: Beyond the Neuron. Front. Cell. Neurosci. 2018, 12, 72. [Google Scholar] [CrossRef]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, regulation, and involvement in disease. Int Immunopharmacol. 2021, 101, 107598. [Google Scholar] [CrossRef] [PubMed]
- Marogianni, C.; Sokratous, M.; Dardiotis, E.; Hadjigeorgiou, G.M.; Bogdanos, D.; Xiromerisiou, G. Neurodegeneration and Inflammation-An Interesting Interplay in Parkinson’s Disease. Int J. Mol. Sci 2020, 21, 8421. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Yu, Y.; Wang, L.; Zhu, Z.; Lu, R.; Yao, Z. Hypoxia-induced CCL28 promotes recruitment of regulatory T cells and tumor growth in liver cancer. Oncotarget 2016, 7, 75763–75773. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.X.; Cao, X.; Liu, Y.; Tang, F.R. CCL28 in the mouse hippocampal CA1 area and the dentate gyrus during and after pilocarpine-induced status epilepticus. Neurochem. Int. 2012, 61, 1094–1101. [Google Scholar] [CrossRef]
- Monitoring of Protein Biomarkers of Inflammation in Human Traumatic Brain Injury Using Microdialysis and Proximity Extension Assay Technology in Neurointensive Care. J. Neurotraumm. 2019, 36, 2872–2885. [CrossRef] [PubMed]
- Bhalala, U.S.; Koehler, R.C.; Kannan, S. Neuroinflammation and neuroimmune dysregulation after acute hypoxic-ischemic injury of developing brain. Front. Pediatr. 2014, 2, 144. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.C.; Rizer, J.; Selenica, M.L.; Reid, P.; Kraft, C.; Johnson, A.; Blair, L.; Gordon, M.N.; Dickey, C.A.; Morgan, D. LPS- induced inflammation exacerbates phospho-tau pathology in rTg4510 mice. J. Neuroinflamm. 2010, 7, 56. [Google Scholar] [CrossRef]
- Monson, N.L.; Ortega, S.B.; Ireland, S.J.; Meeuwissen, A.J.; Chen, D.; Plautz, E.J.; Shubel, E.; Kong, X.; Li, M.K.; Freriks, L.H.; et al. Repetitive hypoxic preconditioning induces an immunosuppressed B cell phenotype during endogenous protection from stroke. J. Neuroinflamm. 2014, 11, 22. [Google Scholar] [CrossRef]
- Younger, D.S. The Blood-Brain Barrier: Implications for Vasculitis. Neurol. Clin. 2019, 37, 235–248. [Google Scholar] [CrossRef]
- Hao, A.J.; Dheen, S.T.; Ling, E.A. Expression of macrophage colony-stimulating factor and its receptor in microglia activation is linked to teratogen-induced neuronal damage. Neuroscience 2002, 112, 889–900. [Google Scholar] [CrossRef]
- Mitrasinovic, O.M.; Perez, G.V.; Zhao, F.; Lee, Y.L.; Poon, C.; Murphy, G.M., Jr. Overexpression of macrophage colony-stimulating factor receptor on microglial cells induces an inflammatory response. J. Biol. Chem. 2001, 276, 30142–30149. [Google Scholar] [CrossRef] [Green Version]
- Kitazawa, M.; Oddo, S.; Yamasaki, T.R.; Green, K.N.; LaFerla, F.M. Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer’s disease. J. Neurosci. 2005, 25, 8843–8853. [Google Scholar] [CrossRef]
- Okada, T.; Suzuki, H. The Role of Tenascin-C in Tissue Injury and Repair After Stroke. Front. Immunol 2020, 11, 587. [Google Scholar] [CrossRef] [PubMed]
- Thammisetty, S.S.; Pedragosa, J.; Weng, Y.C.; Calon, F.; Planas, A.; Kriz, J. Age-related deregulation of TDP-43 after stroke enhances NF-κB-mediated inflammation and neuronal damage. J. Neuroinflamm. 2018, 15, 312. [Google Scholar] [CrossRef]
- Rathcke, C.N.; Vestergaard, H. YKL-40—An emerging biomarker in cardiovascular disease and diabetes. Cardiovasc. Diabetol. 2009, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.B.; Lee, V.M.Y.; Trojanowski, J.Q.; Neumann, M. TDP-43 immunoreactivity in anoxic, ischemic and neoplastic lesions of the central nervous system. Acta Neuropathol. 2008, 115, 305–311. [Google Scholar] [CrossRef]
- Moller, J.T.; Cluitmans, P.; Rasmussen, L.S.; Houx, P.; Rasmussen, H.; Canet, J.; Rabbitt, P.; Jolles, J.; Larsen, K.; Hanning, C.D.; et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet 1998, 351, 857–861. [Google Scholar] [CrossRef]
- Bryant, S.R.; Bjercke, R.J.; Erichsen, D.A.; Rege, A.; Lindner, V. Vascular Remodeling in Response to Altered Blood Flow Is Mediated by Fibroblast Growth Factor-2. Circ. Res. 1999, 84, 323–328. [Google Scholar] [CrossRef]
- Giacinto, O.; Satriano, U.; Nenna, A.; Spadaccio, C.; Lusini, M.; Mastroianni, C.; Nappi, F.; Chello, M. Inflammatory Response and Endothelial Dysfunction Following Cardiopulmonary Bypass: Pathophysiology and Pharmacological Targets. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13, 158–173. [Google Scholar] [CrossRef]
- Soares, H.D.; Potter, W.Z.; Pickering, E.; Kuhn, M.; Immermann, F.W.; Shera, D.M.; Ferm, M.; Dean, R.A.; Simon, A.J.; Swenson, F.; et al. Plasma biomarkers associated with the apolipoprotein E genotype and Alzheimer disease. Arch. Neurol. 2012, 69, 1310–1317. [Google Scholar] [CrossRef]
- Raha, A.A.; Henderson, J.W.; Stott, S.R.; Vuono, R.; Foscarin, S.; Friedland, R.P.; Zaman, S.H.; Raha-Chowdhury, R. Neuroprotective Effect of TREM-2 in Aging and Alzheimer’s Disease Model. J. Alzheimers Dis. 2017, 55, 199–217. [Google Scholar] [CrossRef] [Green Version]
- Clarkson, A.N.; Parker, K.; Nilsson, M.; Walker, F.R.; Gowing, E.K. Combined ampakine and BDNF treatments enhance poststroke functional recovery in aged mice via AKT-CREB signaling. J. Cereb. Blood Flow Metab. 2015, 35, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Cagni, F.C.; Campelo, C.L.; Coimbra, D.G.; Barbosa, M.R.; Junior, L.G.; Neto, A.B.; Ribeiro, A.M.; Junior, C.O.; Gomes de Andrade, T.; Silva, R.H. Association of BDNF Val66MET Polymorphism With Parkinson’s Disease and Depression and Anxiety Symptoms. J. Neuropsychiatry Clin. Neurosci. 2016, 29, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.R.; Nilforooshan, R.; Weaving, G.; Tabet, N. Plasma fetuin-A is associated with the severity of cognitive impairment in mild-to-moderate Alzheimer’s disease. J. Alzheimers Dis. 2011, 24, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Akyüz, A. Association of Fetuin-A with Carotid Intima-Media Thickness and Vascular Diseases. In Biomarkers in Cardiovascular Disease; Springer: Berlin/Heidelberg, Germany, 2016; pp. 177–196. [Google Scholar]
- Humphreys, D.T.; Carver, J.A.; Easterbrook-Smith, S.B.; Wilson, M.R. Clusterin Has Chaperone-like Activity Similar to That of Small Heat Shock Proteins. J. Biol. Chem. 1999, 274, 6875–6881. [Google Scholar] [CrossRef]
- Desikan, R.S.; Thompson, W.K.; Holland, D.; Hess, C.P.; Brewer, J.B.; Zetterberg, H.; Blennow, K.; Andreassen, O.A.; McEvoy, L.K.; Hyman, B.T.; et al. The Role of Clusterin in Amyloid-β–Associated Neurodegeneration. JAMA Neurol. 2014, 71, 180–187. [Google Scholar] [CrossRef]
- Guo, J.; Guan, Q.; Liu, X.; Wang, H.; Gleave, M.E.; Nguan, C.Y.; Du, C. Relationship of clusterin with renal inflammation and fibrosis after the recovery phase of ischemia-reperfusion injury. BMC Nephrol. 2016, 17, 133. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Qi, X.; Xu, G.; Liu, J.; Guo, J.; Li, X.; Ma, X.; Sun, H. CCL28 promotes locomotor recovery after spinal cord injury via recruiting regulatory T cells. Aging 2019, 11, 7402–7415. [Google Scholar] [CrossRef]
- Romero, J.R.; Demissie, S.; Beiser, A.; Himali, J.J.; DeCarli, C.; Levy, D.; Seshadri, S. Relation of plasma β-amyloid, clusterin, and tau with cerebral microbleeds: Framingham Heart Study. Ann. Clin. Transl. Neurol. 2020, 7, 1083–1091. [Google Scholar] [CrossRef]
- Brorsson, B.; Asberg, K.H. Katz index of independence in ADL. Reliability and validity in short-term care. Scand. J. Rehabil. Med. 1984, 16, 125–132. [Google Scholar]
- Brovman, E.Y.; James, M.-E.; Alexander, B.; Rao, N.; Cobey, F.C. The Association Between Institutional Mortality After Coronary Artery Bypass Grafting at One Year and Mortality Rates at 30 Days. J. Cardiothorac. Vasc. Anesth. 2022, 36, 86–90. [Google Scholar] [CrossRef]
- Li, W.; Zhu, S.; Li, J.; Huang, Y.; Rongrong, Z.; Fan, X.; Yang, H.; Gong, X.; Eissa, N.T.; Jahnen-Dechent, W. A hepatic protein, fetuin-A, occupies a protective role in lethal systemic inflammation. PLoS ONE 2011, 6, e16945. [Google Scholar] [CrossRef] [Green Version]
- Jirak, P.; Stechemesser, L.; Moré, E.; Franzen, M.; Topf, A.; Mirna, M.; Paar, V.; Pistulli, R.; Kretzschmar, D.; Wernly, B.; et al. Chapter Three—Clinical implications of fetuin-A. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 89, pp. 79–130. [Google Scholar]
- Nuutinen, T.; Suuronen, T.; Kauppinen, A.; Salminen, A. Clusterin: A forgotten player in Alzheimer’s disease. Brain Res. Rev. 2009, 61, 89–104. [Google Scholar] [CrossRef]
- Falgarone, G.; Chiocchia, G. Chapter 8 Clusterin: A Multifacet Protein at the Crossroad of Inflammation and Autoimmunity. In Advances in Cancer Research; Academic Press: Cambridge, MA, USA, 2009; Volume 104, pp. 139–170. [Google Scholar]
- Gong, T.; Liu, L.; Jiang, W.; Zhou, R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 2020, 20, 95–112. [Google Scholar] [CrossRef]
- Laudanski, K.; Hajj, J.; Riedel, C.; Da, L.; Restrepo, M.; Siddiq, M. Long-term effects of Critical Care Insults on Lipoprotein Metabolism. Trans. Periop. Pain Med. 2021, 8, 385. [Google Scholar] [CrossRef]
- Namboori, P.K.; Vineeth, K.V.; Rohith, V.; Hassan, I.; Sekhar, L.; Sekhar, A.; Nidheesh, M. The ApoE gene of Alzheimer’s disease (AD). Funct. Integr. Genom. 2011, 11, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Deary, I.J.; Whiteman, M.C.; Pattie, A.; Starr, J.M.; Hayward, C.; Wright, A.F.; Carothers, A.; Whalley, L.J. Cognitive change and the APOE epsilon 4 allele. Nature 2002, 418, 932. [Google Scholar] [CrossRef] [PubMed]
- Thundyil, J.; Lim, K.-L. DAMPs and neurodegeneration. Ageing Res. Rev. 2015, 24, 17–28. [Google Scholar] [CrossRef]
- Scarisbrick, I.A.; Yoon, H.; Panos, M.; Larson, N.; Blaber, S.I.; Blaber, M.; Rodriguez, M. Kallikrein 6 regulates early CNS demyelination in a viral model of multiple sclerosis. Brain Pathol. 2012, 22, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Daiello, L.A.; Racine, A.M.; Yun Gou, R.; Marcantonio, E.R.; Xie, Z.; Kunze, L.J.; Vlassakov, K.V.; Inouye, S.K.; Jones, R.N.; Alsop, D. Postoperative Delirium and Postoperative Cognitive Dysfunction: Overlap and Divergence. Anesthesiology 2019, 131, 477–491. [Google Scholar] [CrossRef]
- Goyagi, T. Postoperative delirium and postoperative cognitive dysfunction. Jpn. J. Anesthesiol. 2015, 64, S41–S50. [Google Scholar]
- Patra, K.; Soosaipillai, A.; Sando, S.B.; Lauridsen, C.; Berge, G.; Møller, I.; Grøntvedt, G.R.; Bråthen, G.; Begcevic, I.; Moussaud, S.; et al. Assessment of kallikrein 6 as a cross-sectional and longitudinal biomarker for Alzheimer’s disease. Alzheimers Res. 2018, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.M. S100 and S100beta: Biomarkers of cerebral damage in cardiac surgery with or without the use of cardiopulmonary bypass. Rev. Bras. Cir. Cardiovasc 2014, 29, 630–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKenny, M.; Ryan, T.; Tate, H.; Graham, B.; Young, V.K.; Dowd, N. Age of transfused blood is not associated with increased postoperative adverse outcome after cardiac surgery. BJA Br. J. Anaesth. 2011, 106, 643–649. [Google Scholar] [CrossRef]
- Nguyen, L.; Lucke-Wold, B.P.; Logsdon, A.F.; Scandinaro, A.L.; Huber, J.D.; Matsumoto, R.R. Behavioral and biochemical effects of ketamine and dextromethorphan relative to its antidepressant-like effects in Swiss Webster mice. Neuroreport 2016, 27, 1004–1011. [Google Scholar] [CrossRef]
- Jiang, J.; Jiang, H. Effect of the inhaled anesthetics isoflurane, sevoflurane and desflurane on the neuropathogenesis of Alzheimer’s disease (Review). Mol. Med. Rep. 2015, 12, 3–12. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, X.; Xu, Z.; Zhang, Y.; Xie, Z. Anesthetic Isoflurane Increases Phosphorylated Tau Levels Mediated by Caspase Activation and Aβ Generation. PLoS ONE 2012, 7, e39386. [Google Scholar] [CrossRef] [PubMed]
- Belrose, J.C.; Noppens, R.R. Anesthesiology and cognitive impairment: A narrative review of current clinical literature. BMC Anesthesiol. 2019, 19, 241. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Yang, L.; Chen, X.; Li, S. Tau hyperphosphorylation: A downstream effector of isoflurane-induced neuroinflammation in aged rodents. Med. Hypotheses 2014, 82, 94–96. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Peres Bota, D.; Melot, C.; Lopes Ferreira, F.; Nguyen Ba, V.; Vincent, J.L. The Multiple Organ Dysfunction Score (MODS) versus the Sequential Organ Failure Assessment (SOFA) score in outcome prediction. Intensive Care Med. 2002, 28, 1619–1624. [Google Scholar] [CrossRef]

| Demographics | N = 158 | |
|---|---|---|
| Age (years) | ||
| Mean ± SD | 64.2 ± 12.1 | |
| Over 60 [%] | 70.2% | |
| Sex | ||
| Male [%] | 74.05% | |
| Female [%] | 25.31% | |
| Not reported [%] | 0.64% | |
| Race | ||
| Black [%] | 3.8% | |
| White [%] | 87.0% | |
| Other/Asian/Unknown [%] | 9.2% | |
| Pre-Existing Conditions | ||
| Weight (kg) [mean ± SD] | 86.1 ± 21.71 | |
| BMI (mean ± SD) | 28.4 ± 6.09 | |
| Charleston Comorbidity Index [mean ± SD] | 3.89 ± 2.13 | |
| ACS/MI [%] | 13.3% | |
| CHF [%] | 19.6% | |
| PVD [%] | 9.4% | |
| CVA/TIA [%] | 7.6% | |
| Dementia [%] | 0% | |
| COPD [%] | 6.96% | |
| DM [%] | 27.8% | |
| Anesthesia and Surgery Data | ||
| Duration of anesthesia (min) [mean ± SD] | 374.8 ± 107.77 | |
| Duration of surgery (min) [mean ± SD] | 265.5 ± 100.74 | |
| Duration of cardiopulmonary bypass (min) [mean ± SD] | 130.6 ± 65.69 | |
| Coronary artery bypass surgery [n] | 108 | |
| Mitral valvuloplasty and replacement [n] | 36 | |
| Aortic valvuloplasty and replacement [n] | 60 | |
| Aortic aneurysm repair [n] | 19 | |
| Other [n] | 9 | |
| Estimated Blood Loss (mL) [mean ± SD] | 205.5 ± 291 | |
| Peri-operative management | ||
| Transfusions during surgery | ||
| Packed red blood cells (mL) [mean ± SD] | 120 ± 270 | |
| Fresh frozen plasma (mL) [mean ± SD] | 87 ± 260 | |
| Total crystalloid during surgery (mL) [mean ± SD] | 1297 ± 291 | |
| Clinical Care during 24 h post-surgery | ||
| Packed Red Blood Cells (mL) [mean ± SD] | 17 ± 85 | |
| Fresh Frozen Plasma (mL) [mean ± SD] | 5.8 ± 75 | |
| Opioids Administration (mg) [mean ± SD] | 698 ± 233 | |
| Benzodiazepine administration (mg) [mean ± SD] | 3.67 ± 1.73 | |
| APACHE II scores measured in ICU | mean ± SD | |
| 1 h | 16.8 ± 6.02 | |
| 24 h | 9.4 ± 4.91 | |
| 48 h | 9.1 ± 4.66 | |
| Outcome at 28 days | ||
| LOS ICU (day) [mean ± SD] | 4.36 ± 16.26 | |
| LOS Hospital (day) [mean ± SD] | 10.3 ± 18.49 | |
| Discharged | 87.3% | |
| In the healthcare facility | 6.39% | |
| Mortality | 1.26% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laudanski, K.; Liu, D.; Okeke, T.; Restrepo, M.; Szeto, W.Y. Persistent Depletion of Neuroprotective Factors Accompanies Neuroinflammatory, Neurodegenerative, and Vascular Remodeling Spectra in Serum Three Months after Non-Emergent Cardiac Surgery. Biomedicines 2022, 10, 2364. https://doi.org/10.3390/biomedicines10102364
Laudanski K, Liu D, Okeke T, Restrepo M, Szeto WY. Persistent Depletion of Neuroprotective Factors Accompanies Neuroinflammatory, Neurodegenerative, and Vascular Remodeling Spectra in Serum Three Months after Non-Emergent Cardiac Surgery. Biomedicines. 2022; 10(10):2364. https://doi.org/10.3390/biomedicines10102364
Chicago/Turabian StyleLaudanski, Krzysztof, Da Liu, Tony Okeke, Mariana Restrepo, and Wilson Y. Szeto. 2022. "Persistent Depletion of Neuroprotective Factors Accompanies Neuroinflammatory, Neurodegenerative, and Vascular Remodeling Spectra in Serum Three Months after Non-Emergent Cardiac Surgery" Biomedicines 10, no. 10: 2364. https://doi.org/10.3390/biomedicines10102364
APA StyleLaudanski, K., Liu, D., Okeke, T., Restrepo, M., & Szeto, W. Y. (2022). Persistent Depletion of Neuroprotective Factors Accompanies Neuroinflammatory, Neurodegenerative, and Vascular Remodeling Spectra in Serum Three Months after Non-Emergent Cardiac Surgery. Biomedicines, 10(10), 2364. https://doi.org/10.3390/biomedicines10102364







