In Vitro Model for the Evaluation of Innovative Transcatheter Debridement Device (TDD): Pericardium-Based Scaffold and Stem Cells to Reproduce Calcificated Valves
Abstract
1. Introduction
2. Materials and Methods
2.1. Transcatheter Debridement Device (TDD)
2.2. Cell Culture
2.3. Real-Time Polymerase Chain Reaction
2.4. Cell Viability Assays
2.5. Decalcification Detection
2.6. Quantification of Secreted Factors
2.7. Statistical Analyses
3. Results
3.1. Shock Waves and Their Biophysical Effects
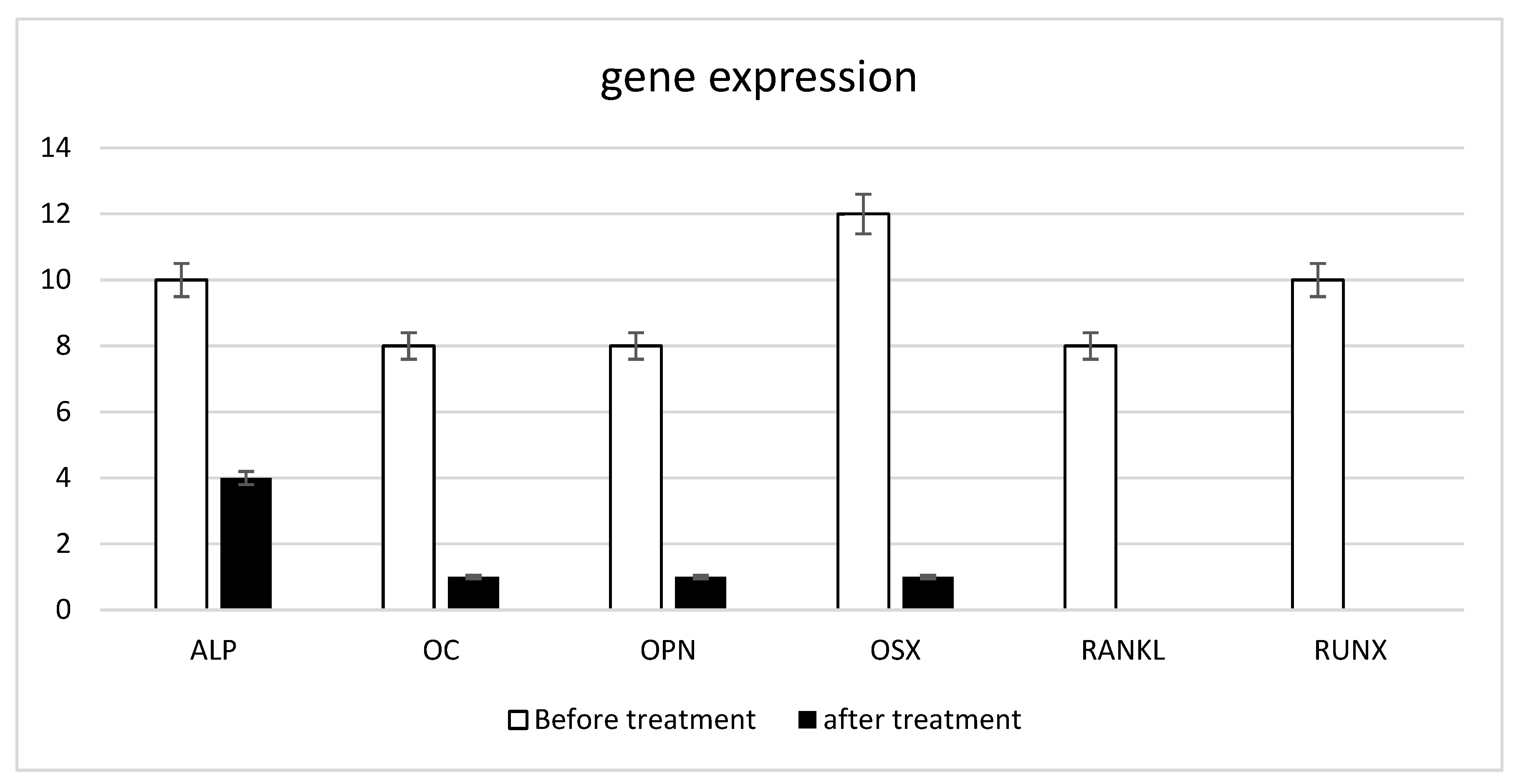
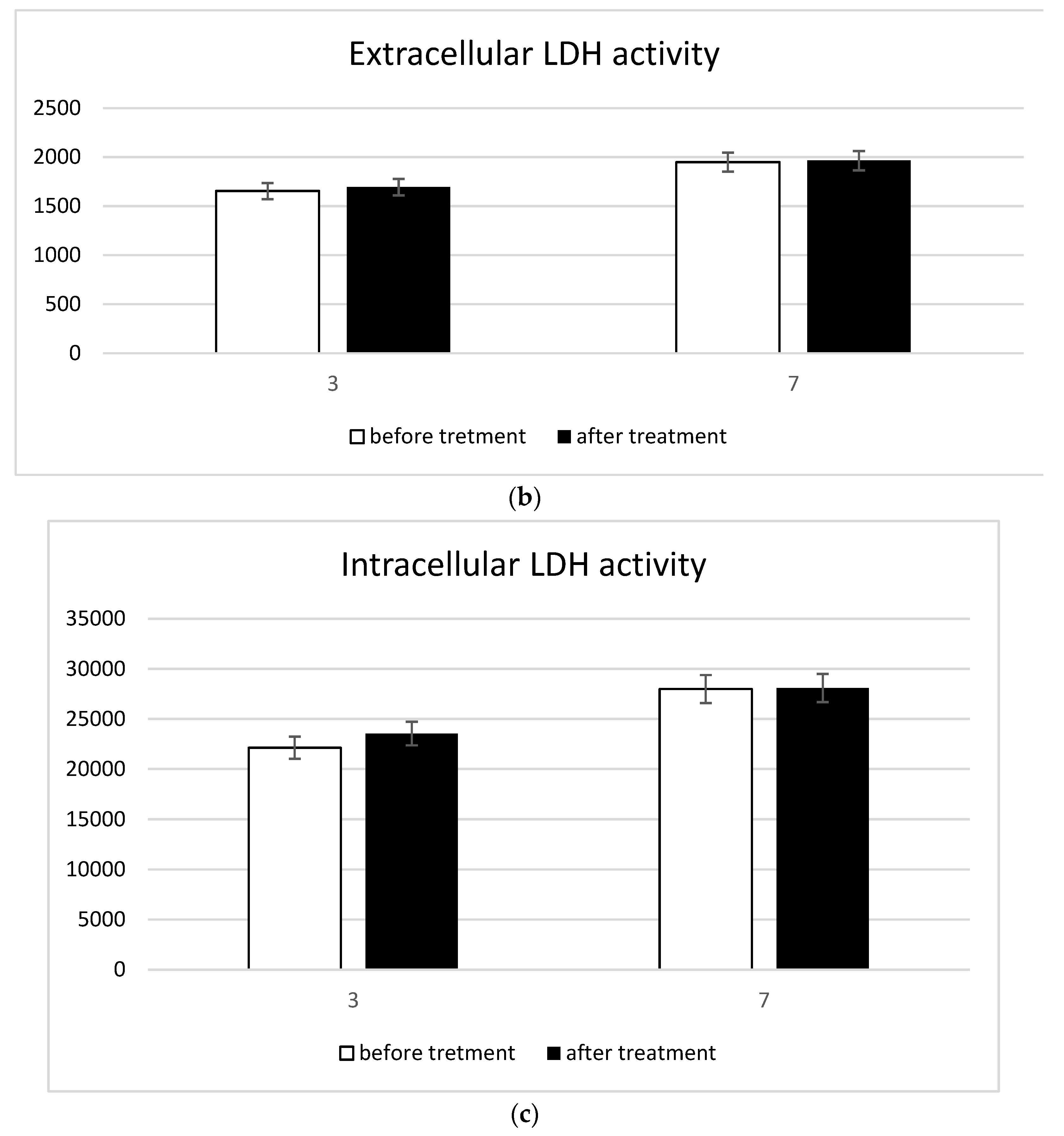
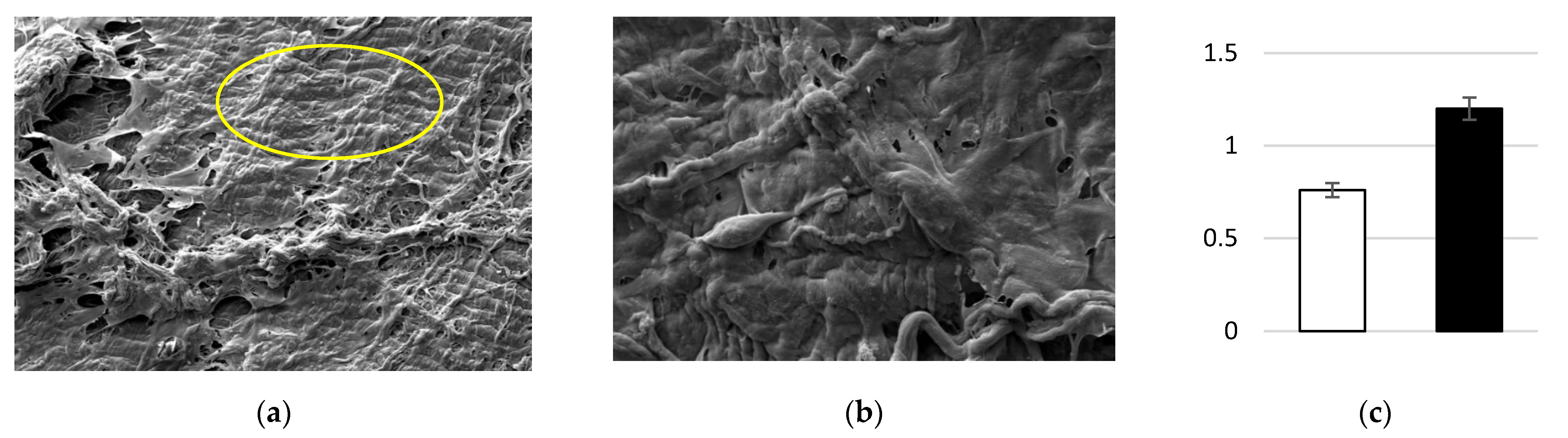
3.2. In Vitro Model Decalcification and Integrity
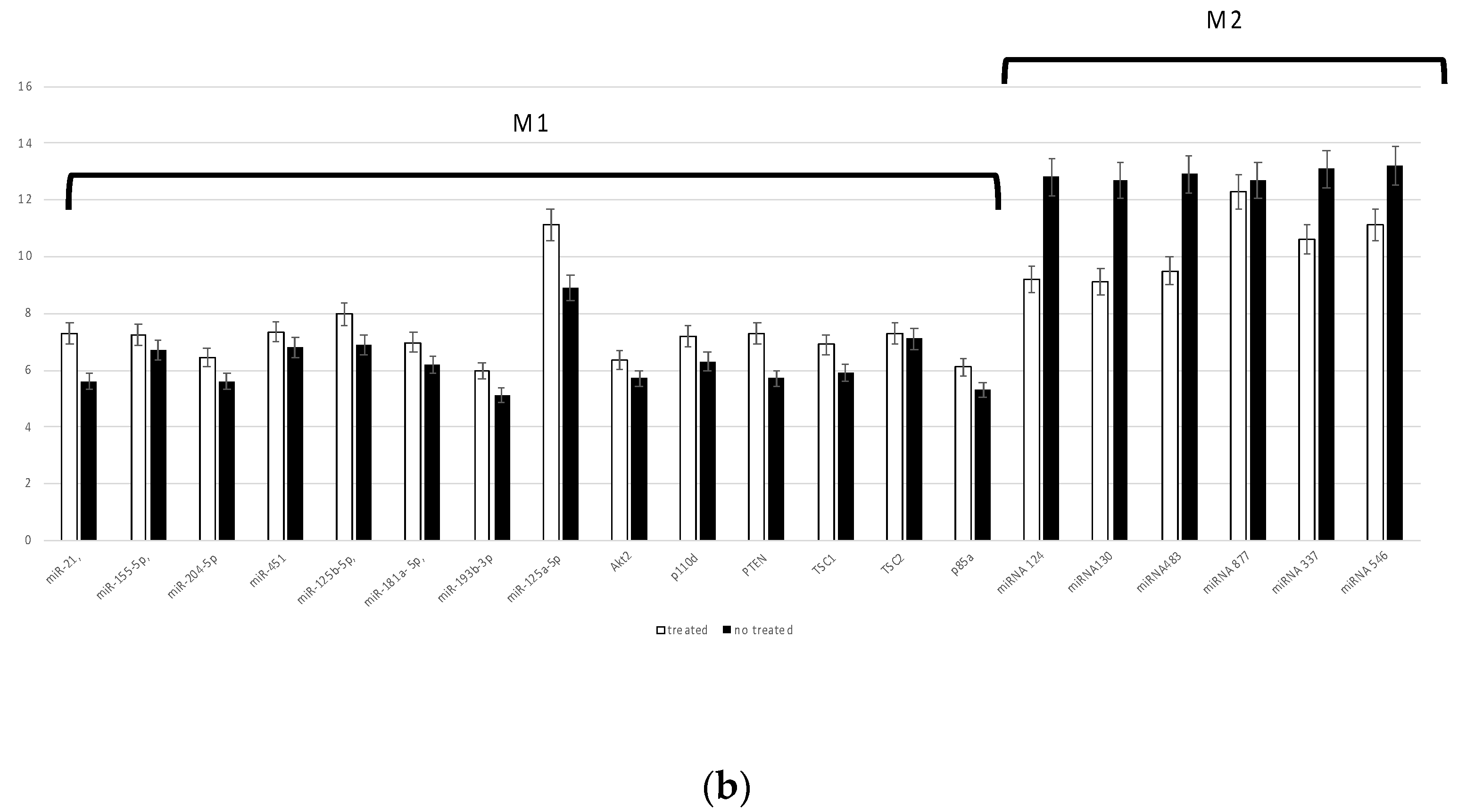
3.3. Inflammatory Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grave, C.; Juillière, Y.; Tuppin, P.; Weill, A.; Gabet, A.; Tribouilloy, C.; Olié, V. Epidemiological Features of Aortic Stenosis in a French Nationwide Study: 10-Year Trends and New Challenges. J. Am. Heart Assoc. 2020, 9, e017588. [Google Scholar] [CrossRef]
- Kruithof, B.P.T.; van Wijngaarden, A.L.; Mousavi Gourabi, B.; Hjortnaes, J.; Palmen, M.; Ajmone Marsan, N. Superimposed Tissue Formation in Human Aortic Valve Disease: Differences between Regurgitant and Stenotic Valves. J. Cardiovasc. Dev. Dis. 2021, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Lindman, B.R.; Clavel, M.A.; Mathieu, P.; Iung, B.; Lancellotti, P.; Otto, C.M.; Pibarot, P. Calcific aortic stenosis. Nat. Rev. Dis. Primers 2016, 2, 16006. [Google Scholar] [CrossRef] [PubMed]
- Messika-Zeitoun, D.; Lloyd, G. Aortic valve stenosis: Evaluation and management of patients with discordant grading. Eur. Soc. Cardiol. 2018, 15, 32. [Google Scholar]
- Kirmani, B.H.; Jones, S.G.; Malaisrie, S.C.; Chung, D.A.; Williams, R.J. Limited versus full sternotomy for aortic valve replacement. Cochrane Database Syst. Rev. 2017, 4, CD011793. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, A.; Wendler, O. TAVI 2018: From guidelines to practice. Eur. Soc. Cardiol. 2018, 15. [Google Scholar]
- Sarmento-Leite, R.; Junior, G.E.d.O. Transcatheter Aortic Valve Implantation: Where are we in 2020? Int. J. Cardiovasc. Sci. 2020, 33, 537–549. [Google Scholar] [CrossRef]
- Bernava, G.; Fermi, E.; Gelpi, G.; Rizzi, S.; Benettin, D.; Barbuto, M.; Romagnoni, C.; Ventrella, D.; Palmieri, M.C.; Agrifoglio, M.; et al. Lithotripsy of Calcified Aortic Valve Leaflets by a Novel Ultrasound Transcatheter-Based Device. Front. Cardiovasc. Med. 2022, 9, 850393. [Google Scholar] [CrossRef]
- Fermi, E.; Benettin, D.; Bernava, G.; Pesce, M.; Pasquino, E. Trans-Catheter Double-Frequency Ultrasound Ablator for The Treatment of Aortic Valve Leaflets Calcification. Biomed. J. Sci. Tech. Res. 2021, 33, 25952–25957. [Google Scholar] [CrossRef]
- Gardin, C.; Bosco, G.; Ferroni, L.; Quartesan, S.; Rizzato, A.; Tatullo, M.; Zavan, B. Hyperbaric Oxygen Therapy Improves the Osteogenic and Vasculogenic Properties of Mesenchymal Stem Cells in the Presence of Inflammation In Vitro. Int. J. Mol. Sci. 2020, 21, 1452. [Google Scholar] [CrossRef] [PubMed]
- Azzena, B.; Mazzoleni, F.; Abatangelo, G.; Zavan, B.; Vindigni, V. Autologous platelet-rich plasma as an adipocyte in vivo delivery system: Case report. Aesthet. Plast. Surg. 2008, 32, 155–158; discussion 159–161. [Google Scholar] [CrossRef] [PubMed]
- Fiocco, L.; Elsayed, H.; Ferroni, L.; Gardin, C.; Zavan, B.; Bernardo, E. Bioactive Wollastonite-Diopside Foams from Preceramic Polymers and Reactive Oxide Fillers. Materials 2015, 8, 2480–2494. [Google Scholar] [CrossRef]
- Sivolella, S.; Stellini, E.; Brunello, G.; Gardin, C.; Ferroni, L.; Bressan, E.; Zavan, B. Silver Nanoparticles in Alveolar Bone Surgery Devices. J. Nanomater. 2012, 2012, 975842. [Google Scholar] [CrossRef]
- Romano, M.; De Francesco, F.; Pirozzi, G.; Gringeri, E.; Boetto, R.; Di Domenico, M.; Zavan, B.; Ferraro, G.A.; Cillo, U. Expression of cancer stem cell biomarkers as a tool for a correct therapeutic approach to hepatocellular carcinoma. Oncoscience 2015, 2, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Leong, T.S.H.; Ashokkumar, M.; Kentish, S.E. The fundamentals of power ultrasound—A review. Acoust. Aust. 2011, 39, 54. [Google Scholar]
- Dong, H.; Zou, X.; Qian, S. Simulation Study on the Influence of Multifrequency Ultrasound on Transient Cavitation Threshold in Different Media. Appl. Sci. 2020, 10, 4778. [Google Scholar] [CrossRef]
- Iernetti, G.; Ciuti, P.; Dezhkunov, N.V.; Reali, M.; Francescutto, A.; Johri, G.K. Enhancement of high-frequency acoustic cavitation effects by a low-frequency stimulation. Ultrason. Sonochem. 1997, 4, 263–268. [Google Scholar] [CrossRef]
- Liu, H.-L.; Hsieh, C.-M. Single-transducer dual-frequency ultrasound generation to enhance acoustic cavitation. Ultrason. Sonochem. 2009, 16, 431–438. [Google Scholar] [CrossRef]
- Kereiakes, D.J.; Virmani, R.; Hokama, J.Y.; Illindala, U.; Mena-Hurtado, C.; Holden, A.; Hill, J.M.; Lyden, S.P.; Ali, Z.A. Principles of Intravascular Lithotripsy for Calcific Plaque Modification. JACC Cardiovasc. Interv. 2021, 14, 1275–1292. [Google Scholar] [CrossRef]
- Jaffe, H. Piezoelectric Ceramics. J. Am. Ceram. Soc. 1958, 41, 494–498. [Google Scholar] [CrossRef]
- Ogden, J.A.; Tóth-Kischkat, A.; Schultheiss, R. Principles of shock wave therapy. Clin. Orthop. Relat. Res. 2001, 387, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Zavan, B.; Michelotto, L.; Lancerotto, L.; Della Puppa, A.; D’Avella, D.; Abatangelo, G.; Vindigni, V.; Cortivo, R. Neural potential of a stem cell population in the adipose and cutaneous tissues. Neurol. Res. 2010, 32, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Zavan, B.; Vindigni, V.; Gardin, C.; D’Avella, D.; Della Puppa, A.; Abatangelo, G.; Cortivo, R. Neural potential of adipose stem cells. Discov. Med. 2010, 10, 37–43. [Google Scholar]
- Zavan, B.; Giorgi, C.; Bagnara, G.P.; Vindigni, V.; Abatangelo, G.; Cortivo, R. Osteogenic and chondrogenic differentiation: Comparison of human and rat bone marrow mesenchymal stem cells cultured into polymeric scaffolds. Eur. J. Histochem. 2007, 51 (Suppl. 1), 1–8. [Google Scholar]
- Ferroni, L.; Gardin, C.; Dolkart, O.; Salai, M.; Barak, S.; Piattelli, A.; Amir-Barak, H.; Zavan, B. Pulsed electromagnetic fields increase osteogenetic commitment of MSCs via the mTOR pathway in TNF-alpha mediated inflammatory conditions: An in-vitro study. Sci. Rep. 2018, 8, 5108. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, L.; Tocco, I.; De Pieri, A.; Menarin, M.; Fermi, E.; Piattelli, A.; Gardin, C.; Zavan, B. Pulsed magnetic therapy increases osteogenic differentiation of mesenchymal stem cells only if they are pre-committed. Life Sci. 2016, 152, 44–51. [Google Scholar] [CrossRef]
- Gardin, C.; Bressan, E.; Ferroni, L.; Nalesso, E.; Vindigni, V.; Stellini, E.; Pinton, P.; Sivolella, S.; Zavan, B. In vitro concurrent endothelial and osteogenic commitment of adipose-derived stem cells and their genomical analyses through comparative genomic hybridization array: Novel strategies to increase the successful engraftment of tissue-engineered bone grafts. Stem Cells Dev. 2012, 21, 767–777. [Google Scholar] [CrossRef]
- Ferroni, L.; Gardin, C.; Sivolella, S.; Brunello, G.; Berengo, M.; Piattelli, A.; Bressan, E.; Zavan, B. A hyaluronan-based scaffold for the in vitro construction of dental pulp-like tissue. Int. J. Mol. Sci. 2015, 16, 4666–4681. [Google Scholar] [CrossRef]
- Ettorre, V.; De Marco, P.; Zara, S.; Perrotti, V.; Scarano, A.; Di Crescenzo, A.; Petrini, M.; Hadad, C.; Bosco, D.; Zavan, B.; et al. In vitro and in vivo characterization of graphene oxide coated porcine bone granules. Carbon 2016, 103, 291–298. [Google Scholar] [CrossRef]
- Bressan, E.; Favero, V.; Gardin, C.; Ferroni, L.; Iacobellis, L.; Favero, L.; Vindigni, V.; Berengo, M.; Sivolella, S.; Zavan, B. Biopolymers for Hard and Soft Engineered Tissues: Application in Odontoiatric and Plastic Surgery Field. Polymers 2011, 3, 509–526. [Google Scholar] [CrossRef]
- Bressan, E.; Carraro, A.; Ferroni, L.; Gardin, C.; Sbricoli, L.; Guazzo, R.; Stellini, E.; Roman, M.; Pinton, P.; Sivolella, S.; et al. Nanotechnology to drive stem cell commitment. Nanomedicine 2013, 8, 469–486. [Google Scholar] [CrossRef]
- Elsayed, H.; Rebesan, P.; Giacomello, G.; Pasetto, M.; Gardin, C.; Ferroni, L.; Zavan, B.; Biasetto, L. Direct ink writing of porous titanium (Ti6Al4V) lattice structures. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109794. [Google Scholar] [CrossRef] [PubMed]
- Gardin, C.; Vindigni, V.; Bressan, E.; Ferroni, L.; Nalesso, E.; Della Puppa, A.; D’Avella, D.; Lops, D.; Pinton, P.; Zavan, B. Hyaluronan and Fibrin Biomaterial as Scaffolds for Neuronal Differentiation of Adult Stem Cells Derived from Adipose Tissue and Skin. Int. J. Mol. Sci. 2011, 12, 6749–6764. [Google Scholar] [CrossRef] [PubMed]
- Andjus, P.; Kosanović, M.; Milićević, K.; Gautam, M.; Vainio, S.J.; Jagečić, D.; Kozlova, E.N.; Pivoriūnas, A.; Chachques, J.-C.; Sakaj, M.; et al. Extracellular Vesicles as Innovative Tool for Diagnosis, Regeneration and Protection against Neurological Damage. Int. J. Mol. Sci. 2020, 21, 6859. [Google Scholar] [CrossRef]
- Ferroni, L.; Gardin, C.; Bellin, G.; Vindigni, V.; Mortellaro, C.; Zavan, B. Bovine pericardium membrane as new tool for mesenchymal stem cells commitment. J. Tissue Eng. Regen. Med. 2019, 13, 1805–1814. [Google Scholar] [CrossRef] [PubMed]
- Gardin, C.; Ricci, S.; Ferroni, L.; Guazzo, R.; Sbricoli, L.; De Benedictis, G.; Finotti, L.; Isola, M.; Bressan, E.; Zavan, B. Decellularization and Delipidation Protocols of Bovine Bone and Pericardium for Bone Grafting and Guided Bone Regeneration Procedures. PLoS ONE 2015, 10, e0132344. [Google Scholar] [CrossRef]
- Gardin, C.; Morciano, G.; Ferroni, L.; Mikus, E.; Tripodi, A.; Pin, M.; Tremoli, E.; Albertini, A.; Zavan, B. Biological Characterization of Human Autologous Pericardium Treated with the Ozaki Procedure for Aortic Valve Reconstruction. J. Clin. Med. 2021, 10, 3954. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, L.; Gardin, C.; Sivolella, S.; Bressan, E.; Zavan, B. A hyaluronan-based scaffold for the in vitro construction of dental pulp-like tissue. Int. J. Mol. Sci. 2015, 16, 4666–4681. [Google Scholar]
- Leopaldi, A.M.; Vismara, R.; van Tuijl, S.; Redaelli, A.; van de Vosse, F.N.; Fiore, G.B.; Rutten, M.C.M. A novel passive left heart platform for device testing and research. Med. Eng. Phys. 2015, 37, 361–366. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiengo, E.; Fermi, E.; Zanolla, I.; Zanotti, F.; Trentini, M.; Pasquino, E.; Palmieri, M.C.; Soliani, G.; Leo, S.; Tremoli, E.; et al. In Vitro Model for the Evaluation of Innovative Transcatheter Debridement Device (TDD): Pericardium-Based Scaffold and Stem Cells to Reproduce Calcificated Valves. Biomedicines 2022, 10, 2352. https://doi.org/10.3390/biomedicines10102352
Tiengo E, Fermi E, Zanolla I, Zanotti F, Trentini M, Pasquino E, Palmieri MC, Soliani G, Leo S, Tremoli E, et al. In Vitro Model for the Evaluation of Innovative Transcatheter Debridement Device (TDD): Pericardium-Based Scaffold and Stem Cells to Reproduce Calcificated Valves. Biomedicines. 2022; 10(10):2352. https://doi.org/10.3390/biomedicines10102352
Chicago/Turabian StyleTiengo, Elena, Enrico Fermi, Ilaria Zanolla, Federica Zanotti, Martina Trentini, Enrico Pasquino, Maria Chiara Palmieri, Giorgio Soliani, Sara Leo, Elena Tremoli, and et al. 2022. "In Vitro Model for the Evaluation of Innovative Transcatheter Debridement Device (TDD): Pericardium-Based Scaffold and Stem Cells to Reproduce Calcificated Valves" Biomedicines 10, no. 10: 2352. https://doi.org/10.3390/biomedicines10102352
APA StyleTiengo, E., Fermi, E., Zanolla, I., Zanotti, F., Trentini, M., Pasquino, E., Palmieri, M. C., Soliani, G., Leo, S., Tremoli, E., Ferroni, L., & Zavan, B. (2022). In Vitro Model for the Evaluation of Innovative Transcatheter Debridement Device (TDD): Pericardium-Based Scaffold and Stem Cells to Reproduce Calcificated Valves. Biomedicines, 10(10), 2352. https://doi.org/10.3390/biomedicines10102352






