Cellular Mechanisms of Coronary Artery Spasm
Abstract
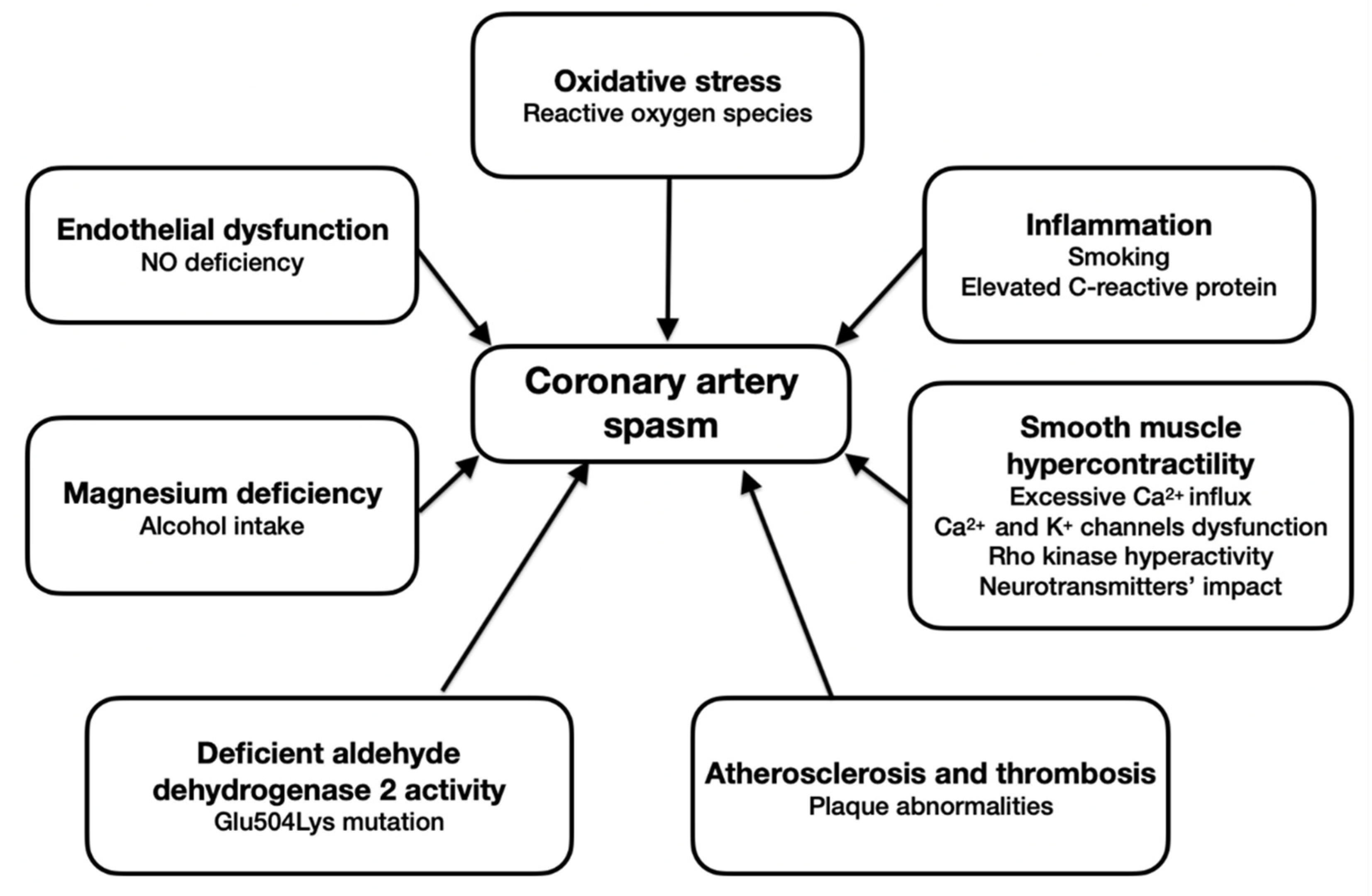
1. Introduction
2. Endothelial Dysfunction
3. Oxidative Stress
4. Inflammation
5. Smooth Muscle Hypercontractility
6. Atherosclerosis and Thrombosis
7. Deficient Aldehyde Dehydrogenase 2 Activity
8. The Role of Magnesium
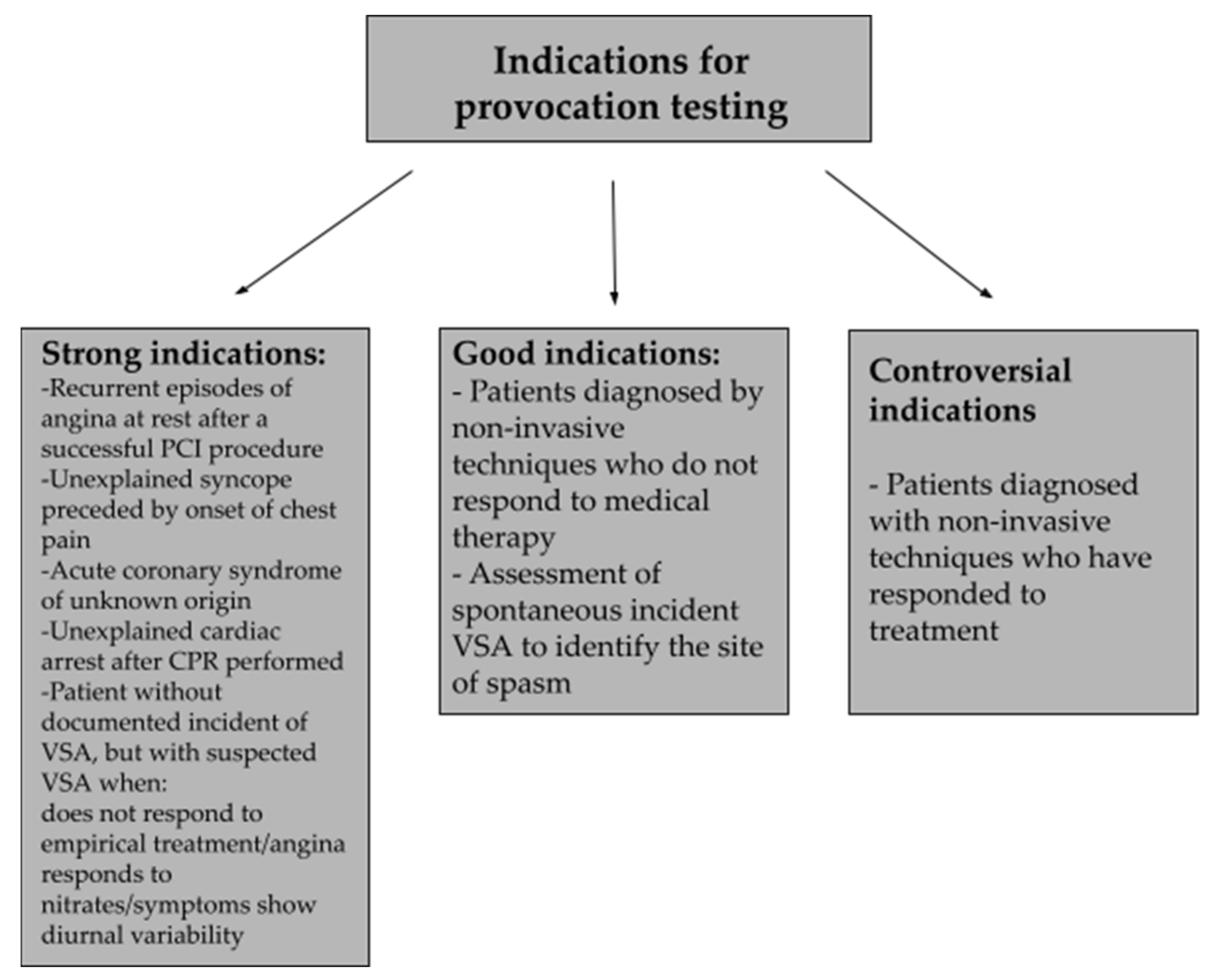
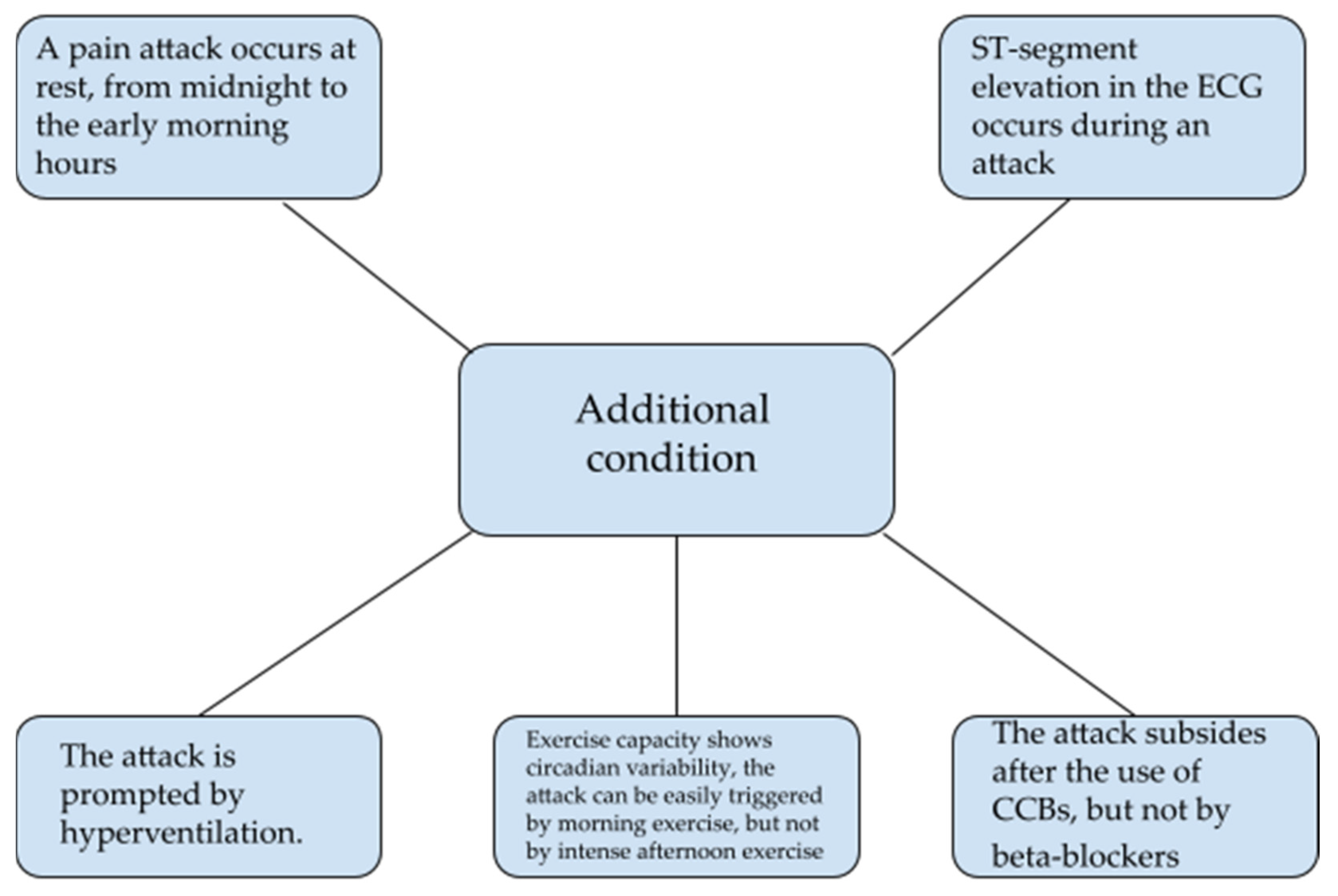
9. Diagnosis of Vasospastic Angina
10. Management of Coronary Artery Spasm
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matta, A.; Bouisset, F.; Lhermusier, T.; Campelo-Parada, F.; Elbaz, M.; Carrié, D.; Roncalli, J. Coronary Artery Spasm: New Insights. J. Interv. Cardiol. 2020, 2020, 5894586. [Google Scholar] [CrossRef] [PubMed]
- Hung, M.-J.; Hu, P.; Hung, M.-Y. Coronary Artery Spasm: Review and Update. Int. J. Med. Sci. 2014, 11, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Prinzmetal, M.; Kennamer, R.; Merliss, R.; Wada, T.; Bor, N. Angina Pectoris I. A Variant Form of Angina Pectoris: Preliminary Report. Am. J. Med. 1959, 27, 375–388. [Google Scholar] [CrossRef]
- Yasue, H.; Mizuno, Y.; Harada, E. Coronary Artery Spasm—Clinical Features, Pathogenesis and Treatment. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2019, 95, 53–66. [Google Scholar] [CrossRef]
- Yasue, H.; Nakagawa, H.; Itoh, T.; Harada, E.; Mizuno, Y. Coronary Artery Spasm--Clinical Features, Diagnosis, Pathogenesis, and Treatment. J. Cardiol. 2008, 51, 2–17. [Google Scholar] [CrossRef]
- Hung, M.-Y.; Kounis, N.G.; Lu, M.-Y.; Hu, P. Myocardial Ischemic Syndromes, Heart Failure Syndromes, Electrocardiographic Abnormalities, Arrhythmic Syndromes and Angiographic Diagnosis of Coronary Artery Spasm: Literature Review. Int. J. Med. Sci. 2020, 17, 1071–1082. [Google Scholar] [CrossRef]
- Kishida, H.; Tada, Y.; Fukuma, N.; Saitoh, T.; Kusama, Y.; Sano, J. Significant Characteristics of Variant Angina Patients with Associated Syncope. Jpn. Heart J. 1996, 37, 317–326. [Google Scholar] [CrossRef][Green Version]
- Yasue, H.; Matsuyama, K.; Matsuyama, K.; Okumura, K.; Morikami, Y.; Ogawa, H. Responses of Angiographically Normal Human Coronary Arteries to Intracoronary Injection of Acetylcholine by Age and Segment. Possible Role of Early Coronary Atherosclerosis. Circulation 1990, 81, 482–490. [Google Scholar] [CrossRef]
- Sugiishi, M.; Takatsu, F. Cigarette Smoking Is a Major Risk Factor for Coronary Spasm. Circulation 1993, 87, 76–79. [Google Scholar] [CrossRef]
- Shimokawa, H. Cellular and Molecular Mechanisms of Coronary Artery Spasm: Lessons from Animal Models. Jpn. Circ. J. 2000, 64, 1–12. [Google Scholar] [CrossRef]
- Itoh, T.; Mizuno, Y.; Harada, E.; Yoshimura, M.; Ogawa, H.; Yasue, H. Coronary Spasm Is Associated with Chronic Low-Grade Inflammation. Circ. J. 2007, 71, 1074–1078. [Google Scholar] [CrossRef] [PubMed]
- Hubert, A.; Seitz, A.; Pereyra, V.M.; Bekeredjian, R.; Sechtem, U.; Ong, P. Coronary Artery Spasm: The Interplay Between Endothelial Dysfunction and Vascular Smooth Muscle Cell Hyperreactivity. Eur. Cardiol. 2020, 15, e12. [Google Scholar] [CrossRef] [PubMed]
- Yasue, H.; Hirai, N.; Mizuno, Y.; Harada, E.; Itoh, T.; Yoshimura, M.; Kugiyama, K.; Ogawa, H. Low-Grade Inflammation, Thrombogenicity, and Atherogenic Lipid Profile in Cigarette Smokers. Circ. J. 2006, 70, 8–13. [Google Scholar] [CrossRef]
- Motoyama, T.; Kawano, H.; Kugiyama, K.; Hirashima, O.; Ohgushi, M.; Yoshimura, M.; Ogawa, H.; Yasue, H. Endothelium-Dependent Vasodilation in the Brachial Artery Is Impaired in Smokers: Effect of Vitamin C. Am. J. Physiol. 1997, 273, H1644–H1650. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, T.; Kawano, H.; Kugiyama, K.; Hirashima, O.; Ohgushi, M.; Tsunoda, R.; Moriyama, Y.; Miyao, Y.; Yoshimura, M.; Ogawa, H.; et al. Vitamin E Administration Improves Impairment of Endothelium-Dependent Vasodilation in Patients with Coronary Spastic Angina. J. Am. Coll. Cardiol. 1998, 32, 1672–1679. [Google Scholar] [CrossRef]
- Kawano, H.; Ogawa, H. Endothelial Dysfunction and Coronary Artery Spasm. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2004, 4, 23–33. [Google Scholar] [CrossRef]
- Chen, C.-C.; Lamping, K.G.; Nuno, D.W.; Barresi, R.; Prouty, S.J.; Lavoie, J.L.; Cribbs, L.L.; England, S.K.; Sigmund, C.D.; Weiss, R.M.; et al. Abnormal Coronary Function in Mice Deficient in Alpha1H T-Type Ca2+ Channels. Science 2003, 302, 1416–1418. [Google Scholar] [CrossRef]
- Kakkar, R.; Ye, B.; Stoller, D.A.; Smelley, M.; Shi, N.-Q.; Galles, K.; Hadhazy, M.; Makielski, J.C.; McNally, E.M. Spontaneous Coronary Vasospasm in KATP Mutant Mice Arises From a Smooth Muscle–Extrinsic Process. Circ. Res. 2006, 98, 682–689. [Google Scholar] [CrossRef]
- Chutkow, W.A.; Pu, J.; Wheeler, M.T.; Wada, T.; Makielski, J.C.; Burant, C.F.; McNally, E.M. Episodic Coronary Artery Vasospasm and Hypertension Develop in the Absence of Sur2 KATP Channels. J. Clin. Investig. 2002, 110, 203–208. [Google Scholar] [CrossRef]
- McFadden, E.P.; Clarke, J.G.; Davies, G.J.; Kaski, J.C.; Haider, A.W.; Maseri, A. Effect of Intracoronary Serotonin on Coronary Vessels in Patients with Stable Angina and Patients with Variant Angina. N. Engl. J. Med. 1991, 324, 648–654. [Google Scholar] [CrossRef]
- Crea, F.; Chierchia, S.; Kaski, J.C.; Davies, G.J.; Margonato, A.; Miran, D.O.; Maseri, A. Provocation of Coronary Spasm by Dopamine in Patients with Active Variant Angina Pectoris. Circulation 1986, 74, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, R.; Bristow, M.R.; Kantrowitz, N.; Baim, D.S.; Harrison, D.C. Histamine Provocation of Clinical Coronary Artery Spasm: Implications Concerning Pathogenesis of Variant Angina Pectoris. Am. Heart J. 1981, 102, 819–822. [Google Scholar] [CrossRef]
- Yasue, H.; Horio, Y.; Nakamura, N.; Fujii, H.; Imoto, N.; Sonoda, R.; Kugiyama, K.; Obata, K.; Morikami, Y.; Kimura, T. Induction of Coronary Artery Spasm by Acetylcholine in Patients with Variant Angina: Possible Role of the Parasympathetic Nervous System in the Pathogenesis of Coronary Artery Spasm. Circulation 1986, 74, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Kusama, Y.; Kodani, E.; Nakagomi, A.; Otsuka, T.; Atarashi, H.; Kishida, H.; Mizuno, K. Variant Angina and Coronary Artery Spasm: The Clinical Spectrum, Pathophysiology, and Management. J. Nippon Med. Sch. 2011, 78, 4–12. [Google Scholar] [CrossRef]
- Kondo, T.; Terada, K. Coronary-Artery Vasospasm. N. Engl. J. Med. 2017, 376, e52. [Google Scholar] [CrossRef]
- Picard, F.; Sayah, N.; Spagnoli, V.; Adjedj, J.; Varenne, O. Vasospastic Angina: A Literature Review of Current Evidence. Arch. Cardiovasc. Dis. 2019, 112, 44–55. [Google Scholar] [CrossRef]
- Rubanyi, G.M. The Role of Endothelium in Cardiovascular Homeostasis and Diseases. J. Cardiovasc. Pharm. 1993, 22 (Suppl. 4), S1–S14. [Google Scholar] [CrossRef]
- Boulanger, C.; Lüscher, T.F. Release of Endothelin from the Porcine Aorta. Inhibition by Endothelium-Derived Nitric Oxide. J. Clin. Investig. 1990, 85, 587–590. [Google Scholar] [CrossRef]
- Takemoto, M.; Egashira, K.; Usui, M.; Numaguchi, K.; Tomita, H.; Tsutsui, H.; Shimokawa, H.; Sueishi, K.; Takeshita, A. Important Role of Tissue Angiotensin-Converting Enzyme Activity in the Pathogenesis of Coronary Vascular and Myocardial Structural Changes Induced by Long-Term Blockade of Nitric Oxide Synthesis in Rats. J. Clin. Investig. 1997, 99, 278–287. [Google Scholar] [CrossRef]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric Oxide: Physiology, Pathophysiology, and Pharmacology. Pharm. Rev. 1991, 43, 109–142. [Google Scholar]
- Kugiyama, K.; Yasue, H.; Okumura, K.; Ogawa, H.; Fujimoto, K.; Nakao, K.; Yoshimura, M.; Motoyama, T.; Inobe, Y.; Kawano, H. Nitric Oxide Activity Is Deficient in Spasm Arteries of Patients with Coronary Spastic Angina. Circulation 1996, 94, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, M.E.; Karas, R.H. The Protective Effects of Estrogen on the Cardiovascular System. N. Engl. J. Med. 1999, 340, 1801–1811. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.J.; Uehata, A.; Gerhard, M.D.; Meredith, I.T.; Knab, S.; Delagrange, D.; Lieberman, E.H.; Ganz, P.; Creager, M.A.; Yeung, A.C. Close Relation of Endothelial Function in the Human Coronary and Peripheral Circulations. J. Am. Coll. Cardiol. 1995, 26, 1235–1241. [Google Scholar] [CrossRef]
- Joannides, R.; Haefeli, W.E.; Linder, L.; Richard, V.; Bakkali, E.H.; Thuillez, C.; Lüscher, T.F. Nitric Oxide Is Responsible for Flow-Dependent Dilatation of Human Peripheral Conduit Arteries in Vivo. Circulation 1995, 91, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Hayyan, M.; Hashim, M.A.; AlNashef, I.M. Superoxide Ion: Generation and Chemical Implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef]
- Diaz, M.N.; Frei, B.; Vita, J.A.; Keaney, J.F. Antioxidants and Atherosclerotic Heart Disease. N. Engl. J. Med. 1997, 337, 408–416. [Google Scholar] [CrossRef]
- Gryglewski, R.J.; Palmer, R.M.; Moncada, S. Superoxide Anion Is Involved in the Breakdown of Endothelium-Derived Vascular Relaxing Factor. Nature 1986, 320, 454–456. [Google Scholar] [CrossRef]
- Nakamura, H.; Nakamura, K.; Yodoi, J. Redox Regulation of Cellular Activation. Annu. Rev. Immunol. 1997, 15, 351–369. [Google Scholar] [CrossRef]
- Miyamoto, S.; Kawano, H.; Sakamoto, T.; Soejima, H.; Kajiwara, I.; Hokamaki, J.; Hirai, N.; Sugiyama, S.; Yoshimura, M.; Yasue, H.; et al. Increased Plasma Levels of Thioredoxin in Patients with Coronary Spastic Angina. Antioxid. Redox Signal. 2004, 6, 75–80. [Google Scholar] [CrossRef]
- Ota, Y.; Kugiyama, K.; Sugiyama, S.; Ohgushi, M.; Matsumura, T.; Doi, H.; Ogata, N.; Oka, H.; Yasue, H. Impairment of Endothelium-Dependent Relaxation of Rabbit Aortas by Cigarette Smoke Extract--Role of Free Radicals and Attenuation by Captopril. Atherosclerosis 1997, 131, 195–202. [Google Scholar] [CrossRef]
- Miwa, K.; Miyagi, Y.; Igawa, A.; Nakagawa, K.; Inoue, H. Vitamin E Deficiency in Variant Angina. Circulation 1996, 94, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Meister, A. Glutathione-Ascorbic Acid Antioxidant System in Animals. J. Biol. Chem. 1994, 269, 9397–9400. [Google Scholar] [CrossRef]
- Kugiyama, K.; Ohgushi, M.; Motoyama, T.; Hirashima, O.; Soejima, H.; Misumi, K.; Yoshimura, M.; Ogawa, H.; Sugiyama, S.; Yasue, H. Intracoronary Infusion of Reduced Glutathione Improves Endothelial Vasomotor Response to Acetylcholine in Human Coronary Circulation. Circulation 1998, 97, 2299–2301. [Google Scholar] [CrossRef] [PubMed]
- Kugiyama, K.; Miyao, Y.; Sakamoto, T.; Kawano, H.; Soejima, H.; Miyamoto, S.; Yoshimura, M.; Ogawa, H.; Sugiyama, S.; Yasue, H. Glutathione Attenuates Coronary Constriction to Acetylcholine in Patients with Coronary Spastic Angina. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H264–H271. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.R.; Kisilevsky, R.; Armstrong, P.W. Prinzmetal’s Angina, Normal Coronary Arteries and Pericarditis. Can Med. Assoc. J. 1978, 119, 36–39. [Google Scholar] [PubMed]
- Kaikita, K.; Ogawa, H.; Yasue, H.; Sakamoto, T.; Suefuji, H.; Sumida, H.; Okumura, K. Soluble P-Selectin Is Released into the Coronary Circulation after Coronary Spasm. Circulation 1995, 92, 1726–1730. [Google Scholar] [CrossRef]
- Jialal, I.; Devaraj, S.; Singh, U. C-Reactive Protein and the Vascular Endothelium. J. Am. Coll. Cardiol. 2006, 47, 1379–1381. [Google Scholar] [CrossRef][Green Version]
- Forman, M.B.; Oates, J.A.; Robertson, D.; Robertson, R.M.; Roberts, L.J.; Virmani, R. Increased Adventitial Mast Cells in a Patient with Coronary Spasm. N. Engl. J. Med. 1985, 313, 1138–1141. [Google Scholar] [CrossRef]
- Ohyama, K.; Matsumoto, Y.; Takanami, K.; Ota, H.; Nishimiya, K.; Sugisawa, J.; Tsuchiya, S.; Amamizu, H.; Uzuka, H.; Suda, A.; et al. Coronary Adventitial and Perivascular Adipose Tissue Inflammation in Patients With Vasospastic Angina. J. Am. Coll. Cardiol. 2018, 71, 414–425. [Google Scholar] [CrossRef]
- Kuga, T.; Shimokawa, H.; Hirakawa, Y.; Kadokami, Y.; Arai, Y.; Fukumoto, Y.; Kuwata, K.; Kozai, T.; Egashira, K.; Takeshita, A. Increased Expression of L-Type Calcium Channels in Vascular Smooth Muscle Cells at Spastic Site in a Porcine Model of Coronary Artery Spasm. J. Cardiovasc. Pharm. 2000, 35, 822–828. [Google Scholar] [CrossRef]
- Nakano, T.; Osanai, T.; Tomita, H.; Sekimata, M.; Homma, Y.; Okumura, K. Enhanced Activity of Variant Phospholipase C-Δ1 Protein (R257H) Detected in Patients With Coronary Artery Spasm. Circulation 2002, 105, 2024–2029. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, H.; Seto, M.; Katsumata, N.; Amano, M.; Kozai, T.; Yamawaki, T.; Kuwata, K.; Kandabashi, T.; Egashira, K.; Ikegaki, I.; et al. Rho-Kinase-Mediated Pathway Induces Enhanced Myosin Light Chain Phosphorylations in a Swine Model of Coronary Artery Spasm. Cardiovasc. Res. 1999, 43, 1029–1039. [Google Scholar] [CrossRef]
- Kandabashi, T.; Shimokawa, H.; Miyata, K.; Kunihiro, I.; Kawano, Y.; Fukata, Y.; Higo, T.; Egashira, K.; Takahashi, S.; Kaibuchi, K.; et al. Inhibition of Myosin Phosphatase by Upregulated Rho-Kinase Plays a Key Role for Coronary Artery Spasm in a Porcine Model With Interleukin-1β. Circulation 2000, 101, 1319–1323. [Google Scholar] [CrossRef] [PubMed]
- Büssemaker, E.; Pistrosch, F.; Förster, S.; Herbrig, K.; Gross, P.; Passauer, J.; Brandes, R.P. Rho Kinase Contributes to Basal Vascular Tone in Humans: Role of Endothelium-Derived Nitric Oxide. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H541–H547. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, H.; Ito, A.; Fukumoto, Y.; Kadokami, T.; Nakaike, R.; Sakata, M.; Takayanagi, T.; Egashira, K.; Takeshita, A. Chronic Treatment with Interleukin-1 Beta Induces Coronary Intimal Lesions and Vasospastic Responses in Pigs in Vivo. The Role of Platelet-Derived Growth Factor. J. Clin. Investig 1996, 97, 769–776. [Google Scholar] [CrossRef]
- Botts, S.R.; Fish, J.E.; Howe, K.L. Dysfunctional Vascular Endothelium as a Driver of Atherosclerosis: Emerging Insights Into Pathogenesis and Treatment. Front. Pharm. 2021, 12, 787541. [Google Scholar] [CrossRef]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharm. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef]
- Frąk, W.; Wojtasińska, A.; Lisińska, W.; Młynarska, E.; Franczyk, B.; Rysz, J. Pathophysiology of Cardiovascular Diseases: New Insights into Molecular Mechanisms of Atherosclerosis, Arterial Hypertension, and Coronary Artery Disease. Biomedicines 2022, 10, 1938. [Google Scholar] [CrossRef]
- Shin, D.I.; Baek, S.H.; Her, S.H.; Han, S.H.; Ahn, Y.; Park, K.-H.; Kim, D.-S.; Yang, T.-H.; Choi, D.-J.; Suh, J.-W.; et al. The 24-Month Prognosis of Patients With Positive or Intermediate Results in the Intracoronary Ergonovine Provocation Test. JACC Cardiovasc. Interv. 2015, 8, 914–923. [Google Scholar] [CrossRef]
- Pellegrini, D.; Konst, R.; van den Oord, S.; Dimitriu-Leen, A.; Mol, J.-Q.; Jansen, T.; Maas, A.; Gehlmann, H.; van Geuns, R.-J.; Elias-Smale, S.; et al. Features of Atherosclerosis in Patients with Angina and No Obstructive Coronary Artery Disease. EuroIntervention 2022, 18, e397–e404. [Google Scholar] [CrossRef]
- Slavich, M.; Patel, R.S. Coronary Artery Spasm: Current Knowledge and Residual Uncertainties. Int. J. Cardiol. Heart Vasc. 2016, 10, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, M.; Miyatake, K.; Tamai, J.; Nakatani, S.; Koyama, J.; Nissen, S.E. Intravascular Ultrasound Detection of Atherosclerosis at the Site of Focal Vasospasm in Angiographically Normal or Minimally Narrowed Coronary Segments. J. Am. Coll. Cardiol. 1994, 23, 352–357. [Google Scholar] [CrossRef]
- Takagi, Y.; Yasuda, S.; Takahashi, J.; Tsunoda, R.; Ogata, Y.; Seki, A.; Sumiyoshi, T.; Matsui, M.; Goto, T.; Tanabe, Y.; et al. Clinical Implications of Provocation Tests for Coronary Artery Spasm: Safety, Arrhythmic Complications, and Prognostic Impact: Multicentre Registry Study of the Japanese Coronary Spasm Association. Eur. Heart J. 2013, 34, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Morita, S.; Mizuno, Y.; Harada, E.; Nakagawa, H.; Morikawa, Y.; Saito, Y.; Katoh, D.; Kashiwagi, Y.; Yoshimura, M.; Murohara, T.; et al. Differences and Interactions between Risk Factors for Coronary Spasm and Atherosclerosis--Smoking, Aging, Inflammation, and Blood Pressure. Intern. Med. 2014, 53, 2663–2670. [Google Scholar] [CrossRef]
- Vidal-Perez, R.; Abou Jokh Casas, C.; Agra-Bermejo, R.M.; Alvarez-Alvarez, B.; Grapsa, J.; Fontes-Carvalho, R.; Rigueiro Veloso, P.; Garcia Acuña, J.M.; Gonzalez-Juanatey, J.R. Myocardial Infarction with Non-Obstructive Coronary Arteries: A Comprehensive Review and Future Research Directions. World J. Cardiol. 2019, 11, 305–315. [Google Scholar] [CrossRef]
- Abbate, R.; Cioni, G.; Ricci, I.; Miranda, M.; Gori, A.M. Thrombosis and Acute Coronary Syndrome. Thromb. Res. 2012, 129, 235–240. [Google Scholar] [CrossRef]
- Lin, C.S.; Penha, P.D.; Zak, F.G.; Lin, J.C. Morphodynamic Interpretation of Acute Coronary Thrombosis, with Special Reference to Volcano-like Eruption of Atheromatous Plaque Caused by Coronary Artery Spasm. Angiology 1988, 39, 535–547. [Google Scholar] [CrossRef]
- Park, H.-C.; Shin, J.H.; Jeong, W.K.; Choi, S.I.; Kim, S.-G. Comparison of Morphologic Findings Obtained by Optical Coherence Tomography in Acute Coronary Syndrome Caused by Vasospasm and Chronic Stable Variant Angina. Int. J. Cardiovasc. Imaging 2015, 31, 229–237. [Google Scholar] [CrossRef]
- Kitano, D.; Takayama, T.; Sudo, M.; Kogo, T.; Kojima, K.; Akutsu, N.; Nishida, T.; Haruta, H.; Fukamachi, D.; Kawano, T.; et al. Angioscopic Differences of Coronary Intima between Diffuse and Focal Coronary Vasospasm: Comparison of Optical Coherence Tomography Findings. J. Cardiol. 2018, 72, 200–207. [Google Scholar] [CrossRef]
- Teragawa, H.; Orita, Y.; Oshita, C.; Uchimura, Y. Intracoronary Thrombogenicity in Patients with Vasospastic Angina: An Observation Using Coronary Angioscopy. Diagnostics 2021, 11, 1632. [Google Scholar] [CrossRef]
- Nishi, T.; Kume, T.; Yamada, R.; Okamoto, H.; Koto, S.; Yamashita, M.; Ueno, M.; Kamisaka, K.; Sasahira, Y.; Enzan, A.; et al. Layered Plaque in Organic Lesions in Patients With Coronary Artery Spasm. J. Am. Heart Assoc. 2022, 11, e024880. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.-Y.; Li, L.-W. ALDH2 Glu504Lys Polymorphism and Susceptibility to Coronary Artery Disease and Myocardial Infarction in East Asians: A Meta-Analysis. Arch. Med. Res. 2014, 45, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, Y.; Hokimoto, S.; Harada, E.; Kinoshita, K.; Yoshimura, M.; Yasue, H. Variant Aldehyde Dehydrogenase 2 (ALDH2*2) in East Asians Interactively Exacerbates Tobacco Smoking Risk for Coronary Spasm—Possible Role of Reactive Aldehydes. Circ. J. 2016, 81, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, K.; Gordon, S. Effects of “Essential AD2” Supplement on Blood Acetaldehyde Levels in Individuals Who Have Aldehyde Dehydrogenase (ALDH2) Deficiency. Am. J. 2019, 26, 583–588. [Google Scholar] [CrossRef]
- Zhang, L.L.; Wang, Y.Q.; Fu, B.; Zhao, S.L.; Kui, Y. Aldehyde Dehydrogenase 2 (ALDH2) Polymorphism Gene and Coronary Artery Disease Risk: A Meta-Analysis. Genet. Mol. Res. 2015, 14, 18503–18514. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Wu, J.; Kim, H.J.; Yang, X.; Geng, H.; Gong, G. ALDH2 Gene G487A Polymorphism and Coronary Artery Disease: A Meta-analysis Including 5644 Participants. J. Cell. Mol. Med. 2018, 22, 1666–1674. [Google Scholar] [CrossRef] [PubMed]
- Popow, M.; Kochanowski, J.; Krakowian, M. Coronary Spasm Secondary to Hypocalcaemia and Hypomagnesaemia. Kardiol. Pol. (Pol. Heart J.) 2015, 73, 57. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Ziccardi, M.; Hatcher, J.D. Prinzmetal Angina. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Swarup, S.; Patibandla, S.; Grossman, S.A. Coronary Artery Vasospasm. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- de Luna, A.B.; Cygankiewicz, I.; Baranchuk, A.; Fiol, M.; Birnbaum, Y.; Nikus, K.; Goldwasser, D.; Garcia-Niebla, J.; Sclarovsky, S.; Wellens, H.; et al. Prinzmetal Angina: ECG Changes and Clinical Considerations: A Consensus Paper. Ann. Noninvasive Electrocardiol. 2014, 19, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Beltrame, J.F.; Crea, F.; Kaski, J.C.; Ogawa, H.; Ong, P.; Sechtem, U.; Shimokawa, H.; Bairey Merz, C.N. Coronary Vasomotion Disorders International Study Group (COVADIS) International Standardization of Diagnostic Criteria for Vasospastic Angina. Eur. Heart J. 2017, 38, 2565–2568. [Google Scholar] [CrossRef]
- Song, J.-K. Coronary Artery Vasospasm. Korean Circ. J. 2018, 48, 767–777. [Google Scholar] [CrossRef]
- Balla, C.; Pavasini, R.; Ferrari, R. Treatment of Angina: Where Are We? Cardiology 2018, 140, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Liu, H.; Yu, D.; Wu, M.; Liu, Q.; Liang, X.; Pang, X.; Chen, K.; Luo, L.; Dong, S. Sympathectomy versus Conventional Treatment for Refractory Coronary Artery Spasm. Coron. Artery Dis. 2019, 30, 418–424. [Google Scholar] [CrossRef] [PubMed]
| References | Study Design | Year | All Patients | Examined Indicator | Conclusions |
|---|---|---|---|---|---|
| Motoyama et al. [14] | Clinical trial | 1997 | 40 | vitamin C | Decreased vitamin C levels in smokers. |
| Motoyama et al. [14] | Clinical trial | 1997 | 40 | TBARS | Increased TBARS levels in smokers. |
| Miyamoto et al. [39] | Clinical trial | 2004 | 170 | thioredoxin | Increased thioredoxin levels in subjects with CSA. |
| Miwa et al. [41] | Clinical trial | 1996 | 103 | vitamin E | Decreased vitamin E levels in subjects with CSA. |
| Authors | Gu et al. [72] | Mizuno et al. [73] | Fujioka et al. [74] | Zhang et al. [75] | Li et al. [76] |
|---|---|---|---|---|---|
| Year | 2013 | 2017 | 2019 | 2015 | 2018 |
| Study design | Meta-analysis | Clinical trial | Clinical trial | Meta-analysis | Meta-analysis |
| All patients | 6762 | 410 | 12 | 8366 | 5644 |
| Aim of the study | Evaluation of the association between the Glu504Lys mutation in the ALDH2 gene and the risk of CAD and myocardial infarction. | Evaluation of the correlation between smoking and the presence of ALDH2 gene mutation and their impact on CAD risk. | Evaluation of the effect of Essential AD2 supplementation by ALDH2-deficient subjects on blood acetaldehyde levels. | Evaluation of the correlation between polymorphisms of ALDH2 and CAD. | The effect of the G487A ALDH2 mutation on the occurrence of CAD. |
| Conclusions | The Glu504Lys mutation in the ALDH2 gene in the Asian population is associated with a high risk of myocardial infarction and CAD. The polymorphism’s correlation with high risk of the above diseases is particularly evident in the Korean and Chinese populations but not in the Japanese population. | The synergism of smoking and ALDH2 gene mutation has a greater impact on CAD risk than either factor alone by increasing the amount of reactive aldehydes. | Daily supplementation of essential AD2 by ALDH2-deficient individuals resulted in lower blood levels of acetaldehyde. | The presence of a dominant A allele in the genotype of individuals with the mutation is associated with a higher risk of CAD. Patients with a mutation in the ALDH2 gene consume significantly less alcohol than patients without the A allele. | The G487A of ALDH2 mutation significantly increases the risk of CAD. |
| Some of the Contraindications to Performing an Intracoronary Provocation Test |
|---|
| Pregnancy |
| Severe hypertension |
| Significant left coronary artery trunk stenosis |
| Advanced heart failure |
| Severe aortic stenosis |
| Medication | Mechanism | Effect |
|---|---|---|
| CCBs | Inhibition of L-type calcium channels of myocytes in vessels | Reduction of vascular resistance and relaxation of coronary arteries |
| nitrates | NO production as a result of transformations occurring in the vessel wall | Vasodilatation |
| statins | Inhibition of RhoA/ROCK pathway, increase in NO activity, decrease in ROS | CAS inhibition |
| aspirin | Blockage of thromboxane A2 production (dose < 100 mg per day) | Vasodilatation |
| Rho-kinase inhibitors | Inhibition of RhoA/ROCK pathway | Decreased contraction of VSMCs |
| nicorandil | Activation of nitrates and potassium channel | Dilation of the coronary arteries |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franczyk, B.; Dybiec, J.; Frąk, W.; Krzemińska, J.; Kućmierz, J.; Młynarska, E.; Szlagor, M.; Wronka, M.; Rysz, J. Cellular Mechanisms of Coronary Artery Spasm. Biomedicines 2022, 10, 2349. https://doi.org/10.3390/biomedicines10102349
Franczyk B, Dybiec J, Frąk W, Krzemińska J, Kućmierz J, Młynarska E, Szlagor M, Wronka M, Rysz J. Cellular Mechanisms of Coronary Artery Spasm. Biomedicines. 2022; 10(10):2349. https://doi.org/10.3390/biomedicines10102349
Chicago/Turabian StyleFranczyk, Beata, Jill Dybiec, Weronika Frąk, Julia Krzemińska, Joanna Kućmierz, Ewelina Młynarska, Magdalena Szlagor, Magdalena Wronka, and Jacek Rysz. 2022. "Cellular Mechanisms of Coronary Artery Spasm" Biomedicines 10, no. 10: 2349. https://doi.org/10.3390/biomedicines10102349
APA StyleFranczyk, B., Dybiec, J., Frąk, W., Krzemińska, J., Kućmierz, J., Młynarska, E., Szlagor, M., Wronka, M., & Rysz, J. (2022). Cellular Mechanisms of Coronary Artery Spasm. Biomedicines, 10(10), 2349. https://doi.org/10.3390/biomedicines10102349







