Red Cell Distribution Width and Mortality in Patients Undergoing Percutaneous Coronary Intervention
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients Selection
2.2. Data Sources
2.3. Clinical Attributes and Outcomes Assessment
2.4. Statistical Analyses
3. Results
3.1. Study Subjects
3.2. Mortality Outcomes
3.3. Multivariable Logistic Regression Analysis
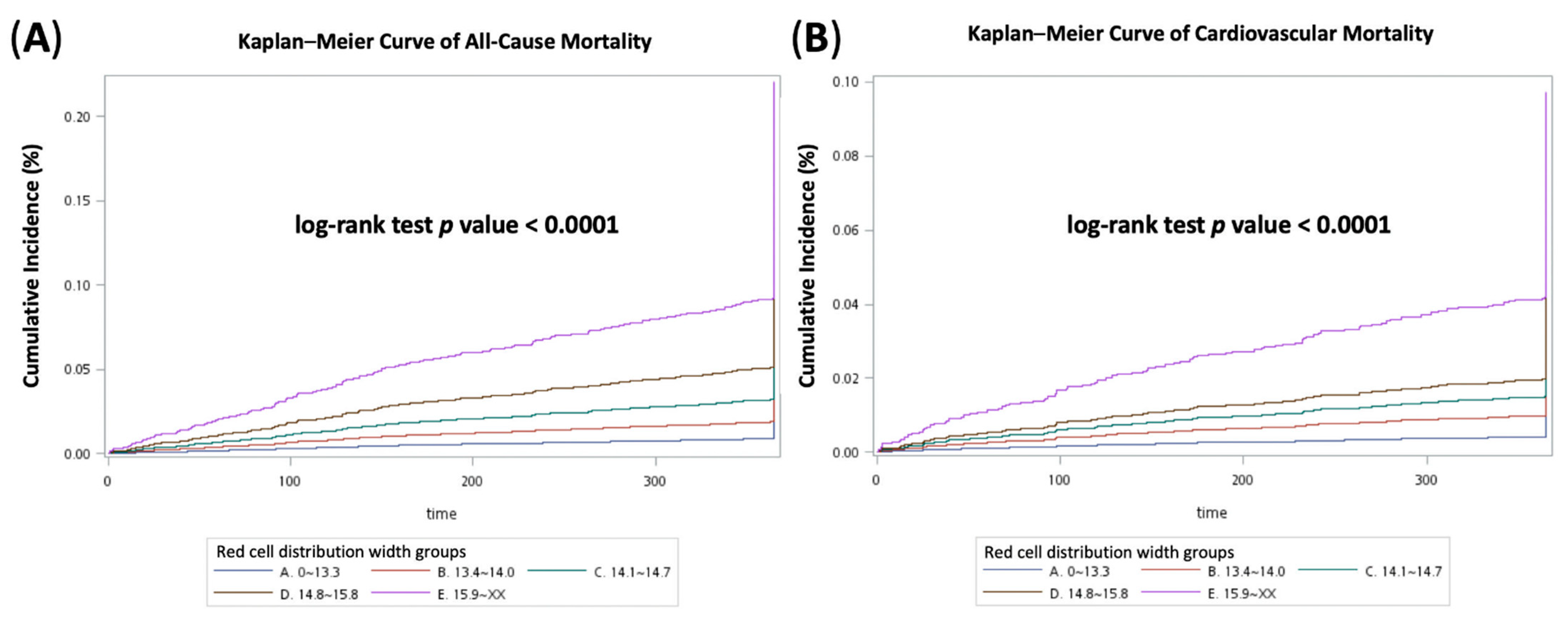
3.4. Cox Proportional Hazards Regression Analysis and Kaplan–Meier Curves
3.5. Prediction Model and ROC Curves
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoffmann, J.J.; Nabbe, K.C.; van den Broek, N.M. Effect of age and gender on reference intervals of red blood cell distribution width (RDW) and mean red cell volume (MCV). Clin. Chem. Lab. Med. 2015, 53, 2015–2019. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Plebani, M. Red blood cell distribution width (RDW) and human pathology. One size fits all. Clin. Chem. Lab. Med. 2014, 52, 1247–1249. [Google Scholar] [CrossRef] [PubMed]
- Evans, T.C.; Jehle, D. The red blood cell distribution width. J. Emerg. Med. 1991, 9 (Suppl. 1), 71–74. [Google Scholar] [CrossRef]
- Lippi, G.; Pavesi, F.; Bardi, M.; Pipitone, S. Lack of harmonization of red blood cell distribution width (RDW). Evaluation of four hematological analyzers. Clin. Biochem. 2014, 47, 1100–1103. [Google Scholar] [CrossRef]
- Ford, J. Red blood cell morphology. Int. J. Lab. Hematol. 2013, 35, 351–357. [Google Scholar] [CrossRef]
- Lippi, G.; Turcato, G.; Cervellin, G.; Sanchis-Gomar, F. Red blood cell distribution width in heart failure: A narrative review. World J. Cardiol. 2018, 10, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.-D. Red blood cell distribution width: A promising index for estimating activity of autoimmune disease. J. Lab. Precis. Med. 2016, 1, 62–68. [Google Scholar] [CrossRef]
- Montagnana, M.; Danese, E. Red cell distribution width and cancer. Ann. Transl. Med. 2016, 4, 399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uyarel, H.; Isik, T.; Ayhan, E.; Ergelen, M. Red cell distrubition width (RDW): A novel risk factor for cardiovascular disease. Int. J. Cardiol. 2012, 154, 351–352. [Google Scholar] [CrossRef] [PubMed]
- Bazick, H.S.; Chang, D.; Mahadevappa, K.; Gibbons, F.K.; Christopher, K.B. Red cell distribution width and all-cause mortality in critically ill patients. Crit. Care Med. 2011, 39, 1913–1921. [Google Scholar] [CrossRef] [Green Version]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D.; Thygesen, K.; Alpert, J.S.; White, H.D.; Jaffe, A.S.; et al. Third universal definition of myocardial infarction. Eur. Heart J. 2012, 33, 2551–2567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fihn, S.D.; Blankenship, J.C.; Alexander, K.P.; Bittl, J.A.; Byrne, J.G.; Fletcher, B.J.; Fonarow, G.C.; Lange, R.A.; Levine, G.N.; Maddox, T.M.; et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2014, 130, 1749–1767. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latif, A.; Ahsan, M.J.; Lateef, N.; Kapoor, V.; Fazeel, H.M.; Razzaq, F.; Iftikhar, A.; Ashfaq, M.Z.; Anwer, F.; Mirza, M.; et al. Prognostic Impact of Red Cell Distribution Width on the Development of Contrast Induced Nephropathy, Major Adverse Cardiac Events, and Mortality in Coronary Artery Disease Patients Undergoing Percutaneous Coronary Intervention. Curr. Cardiol. Rev. 2021. [Google Scholar] [CrossRef]
- Bao, D.; Luo, G.; Kan, F.; Wang, X.; Luo, J.; Jiang, C. Prognostic value of red cell distribution width in patients undergoing percutaneous coronary intervention: A meta-analysis. BMJ Open 2020, 10, e033378. [Google Scholar] [CrossRef]
- Su, C.; Liao, L.Z.; Song, Y.; Xu, Z.W.; Mei, W.Y. The role of red blood cell distribution width in mortality and cardiovascular risk among patients with coronary artery diseases: A systematic review and meta-analysis. J. Thorac Dis. 2014, 6, 1429–1440. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; Hu, Z.D.; Liu, S.J.; Sun, Y.; Qin, Q.; Qin, B.D.; Zhang, W.W.; Zhang, J.R.; Zhong, R.Q.; Deng, A.M. Prognostic value of red blood cell distribution width for patients with heart failure: A systematic review and meta-analysis of cohort studies. PLoS ONE 2014, 9, e104861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.F.; Hsiao, W.C. Does universal health insurance make health care unaffordable? Lessons from Taiwan. Health Aff. 2003, 22, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Chiang, T.L. Taiwan’s 1995 health care reform. Health Policy 1997, 39, 225–239. [Google Scholar] [CrossRef]
- Elixhauser, A.; Steiner, C.; Harris, D.R.; Coffey, R.M. Comorbidity measures for use with administrative data. Med. Care 1998, 36, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Pilling, L.C.; Atkins, J.L.; Kuchel, G.A.; Ferrucci, L.; Melzer, D. Red cell distribution width and common disease onsets in 240,477 healthy volunteers followed for up to 9 years. PLoS ONE 2018, 13, e0203504. [Google Scholar] [CrossRef] [Green Version]
- Hou, H.; Sun, T.; Li, C.; Li, Y.; Guo, Z.; Wang, W.; Li, D. An overall and dose-response meta-analysis of red blood cell distribution width and CVD outcomes. Sci. Rep. 2017, 7, 43420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drakopoulou, M.; Toutouzas, K.; Stefanadi, E.; Tsiamis, E.; Tousoulis, D.; Stefanadis, C. Association of inflammatory markers with angiographic severity and extent of coronary artery disease. Atherosclerosis 2009, 206, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Arbel, Y.; Birati, E.Y.; Finkelstein, A.; Halkin, A.; Berliner, S.; Katz, B.Z.; Revivo, M.; Saranga, H.; Herz, I.; Keren, G.; et al. Red blood cell distribution width and 3-year outcome in patients undergoing cardiac catheterization. J. Thromb. Thrombolysis 2014, 37, 469–474. [Google Scholar] [CrossRef]
- Maluf, C.B.; Barreto, S.M.; Giatti, L.; Ribeiro, A.L.; Vidigal, P.G.; Azevedo, D.R.M.; Griep, R.H.; Matos, S.M.A.; Ji, C.; Cappuccio, F.P.; et al. Association between C reactive protein and all-cause mortality in the ELSA-Brasil cohort. J. Epidemiol. Community Health 2020, 74, 421–427. [Google Scholar] [CrossRef] [Green Version]
- Lindmark, E.; Diderholm, E.; Wallentin, L.; Siegbahn, A. Relationship between interleukin 6 and mortality in patients with unstable coronary artery disease: Effects of an early invasive or noninvasive strategy. JAMA 2001, 286, 2107–2113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvagno, G.L.; Sanchis-Gomar, F.; Picanza, A.; Lippi, G. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit. Rev. Clin. Lab. Sci. 2015, 52, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.V.; Semba, R.D.; Ferrucci, L.; Newman, A.B.; Fried, L.P.; Wallace, R.B.; Bandinelli, S.; Phillips, C.S.; Yu, B.; Connelly, S.; et al. Red cell distribution width and mortality in older adults: A meta-analysis. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Aihemaiti, G.; Cheng, B.; Li, X. Red Blood Cell Distribution Width Is Associated with All-Cause Mortality in Critically Ill Patients with Cardiogenic Shock. Med. Sci. Monit. 2019, 25, 7005–7015. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Hu, J.; Jiang, L.; Zhang, M. Prognostic Value of Red Blood Cell Distribution Width in Non-Cardiovascular Critically or Acutely Patients: A Systematic Review. PLoS ONE 2016, 11, e0167000. [Google Scholar] [CrossRef]
- Shao, Q.; Li, L.; Li, G.; Liu, T. Prognostic value of red blood cell distribution width in heart failure patients: A meta-analysis. Int. J. Cardiol. 2015, 179, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, J.; Lin, Y.; Yang, H.; Cao, S. Association Between Red Blood Cell Distribution Width and All-cause Mortality in Chronic Kidney Disease Patients: A Systematic Review and Meta-analysis. Arch. Med. Res. 2017, 48, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.P.; Kang, K.; Lin, Q.; Hai, J. Prognostic Significance of Preoperative Systemic Cellular Inflammatory Markers in Gliomas: A Systematic Review and Meta-Analysis. Clin. Transl. Sci. 2020, 13, 179–188. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X.; Lu, Z.; Zhang, L.; Dai, T. Prognostic significance of red blood cell distribution width in gastrointestinal cancers: A meta-analysis. Medicine 2020, 99, e19588. [Google Scholar] [CrossRef]
- Xu, W.Y.; Yang, X.B.; Wang, W.Q.; Bai, Y.; Long, J.Y.; Lin, J.Z.; Xiong, J.P.; Zheng, Y.C.; He, X.D.; Zhao, H.T.; et al. Prognostic impact of the red cell distribution width in esophageal cancer patients: A systematic review and meta-analysis. World J. Gastroenterol. 2018, 24, 2120–2129. [Google Scholar] [CrossRef]
- Uyarel, H.; Ergelen, M.; Cicek, G.; Kaya, M.G.; Ayhan, E.; Turkkan, C.; Yıldırım, E.; Kırbas, V.; Onturk, E.T.; Erer, H.B.; et al. Red cell distribution width as a novel prognostic marker in patients undergoing primary angioplasty for acute myocardial infarction. Coron. Artery Dis. 2011, 22, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.T.; Zheng, Y.Y.; Hou, X.G.; Yang, Y.; Ma, X.; Ma, Y.T.; Xie, X. Red blood cell distribution width as long-term prognostic markers in patients with coronary artery disease undergoing percutaneous coronary intervention. Lipids Health Dis. 2019, 18, 140. [Google Scholar] [CrossRef] [Green Version]
- Zhao, N.; Mi, L.; Liu, X.; Pan, S.; Xu, J.; Xia, D.; Liu, Z.; Zhang, Y.; Xiang, Y.; Yuan, Z.; et al. Combined Value of Red Blood Cell Distribution Width and Global Registry of Acute Coronary Events Risk Score for Predicting Cardiovascular Events in Patients with Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention. PLoS ONE 2015, 10, e0140532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.Y.; Li, X.F.; Lin, M.Q.; Xu, J.H.; Yan, H.; Zhang, Z.M.; Wang, S.Y.; Chen, H.C.; Chen, X.N.; Lin, K.Y.; et al. Association between red blood cell distribution width and long-term mortality among patients undergoing percutaneous coronary intervention with previous history of cancer. Biomarkers 2020, 25, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Dai, C.; Xu, K.; Wu, M. Predictive value of neutrophil to lymphocyte ratio and red cell distribution width on death for ST segment elevation myocardial infarction. Sci. Rep. 2021, 11, 11506. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.J.; Liu, J.L.; Pan, N.N.; Wan, J.; Lan, X.; Cui, S.J.; Yu, H.J. The Predictive Value of Red Cell Distribution Width and Red Cell Distribution Width to Erythrocyte Count Ratio for Adverse Cardiovascular Events During the Hospitalization of Patients of ST-segment Elevation Myocardial Infarction. Clin. Lab. 2020, 66. [Google Scholar] [CrossRef] [PubMed]
- Bozorgi, A.; Khaki, S.; Mortazavi, S.H.; Sadeghian, S.; Khoshnevis, M.; Tofighi, S.; Khaki, A. Effect of Baseline Red Blood Cell Distribution Width on Short- and Intermediate-term Mortality of Patients under Primary Percutaneous Coronary Intervention: A Survival Analysis. Crit. Pathw. Cardiol. 2016, 15, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhao, X.; Li, S. Serum Procalcitonin Level and Mortality Risk in Critically ill Patients with Ventilator-Associated Pneumonia. Cell. Physiol. Biochem. 2015, 37, 1967–1972. [Google Scholar] [CrossRef] [PubMed]

| Groups 1–5 of RDW (%) | All | Group 1 ≤13.3 | Group 2 13.4–14.0 | Group 3 14.1–14.7 | Group 4 14.8–15.8 | Group 5 ≥15.9 | p |
|---|---|---|---|---|---|---|---|
| Number of patients | 10669 | 5787 | 2246 | 1133 | 779 | 724 | |
| Age, years | 65.4 (12.1) | 63.3 (11.6) | 67 (12.1) | 68.1 (12.3) | 69.4 (11.8) | 69.1 (12.3) | <0.001 |
| Male | 8382 (78.6) | 4742 (81.9) | 1746 (77.7) | 838 (74) | 550 (70.6) | 506 (69.9) | <0.001 |
| BMI, kg/m2 | 26 (10.6) | 26.3 (10.8) | 26.2 (11.7) | 25.8 (9.4) | 25.1 (11.3) | 24.4 (4.2) | <0.001 |
| Total number of stents | 1.3 (1) | 1.3 (1) | 1.3 (1) | 1.3 (1) | 1.3 (1) | 1.3 (1.1) | 0.057 |
| Hemoglobin, gm/dL | 13.5 (2.2) | 14.2 (1.8) | 13.6 (2.1) | 12.7 (2.2) | 12 (2.3) | 11.1 (2.3) | <0.001 |
| eGFR, mL/min/1.73 m2 | 70.3 (29.9) | 77.6 (25.4) | 69.2 (28.5) | 59.3 (32.8) | 56 (34.9) | 51.3 (35.1) | <0.001 |
| WBC, 103/mm3 | 6.6 (9.8) | 6.3 (9) | 6.7 (9.7) | 6.6 (9.9) | 7.2 (11.1) | 8.3 (13.4) | <0.001 |
| Total cholesterol, mg/dL | 172.2 (41.9) | 174.3 (40.2) | 174.6 (40.4) | 171.8 (42.1) | 167 (50.8) | 155.2 (43.1) | <0.001 |
| Triglyceride, mg/dL | 147.4 (107.5) | 150 (99) | 149.5 (124.5) | 143 (99.5) | 143.1 (136) | 132.8 (89.8) | <0.001 |
| Risk factors | |||||||
| Hypertension | 7276 (68.2) | 3782 (65.4) | 1565 (69.7) | 802 (70.8) | 589 (75.6) | 538 (74.3) | <0.001 |
| Hyperlipidemia | 5628 (52.8) | 3158 (54.6) | 1212 (54) | 567 (50) | 376 (48.3) | 315 (43.5) | <0.001 |
| Diabetes mellitus | 4373 (41) | 2186 (37.8) | 899 (40) | 523 (46.2) | 377 (48.4) | 388 (53.6) | <0.001 |
| Current smoker | 721 (6.8) | 394 (6.8) | 177 (7.9) | 71 (6.3) | 47 (6) | 32 (4.4) | 0.0183 |
| Medical history | |||||||
| Previous MI | 1221 (11.4) | 608 (10.5) | 245 (10.9) | 154 (13.6) | 99 (12.7) | 115 (15.9) | <0.001 |
| ischemic stroke | 980 (9.2) | 452 (7.8) | 229 (10.2) | 117 (10.3) | 99 (12.7) | 83 (11.5) | <0.001 |
| PVD | 631 (5.9) | 253 (4.4) | 118 (5.3) | 110 (9.7) | 83 (10.7) | 67 (9.3) | <0.001 |
| CPD | 1342 (12.6) | 589 (10.2) | 307 (13.7) | 179 (15.8) | 149 (19.1) | 118 (16.3) | <0.001 |
| Previous PCI | 2379 (22.3) | 1213 (21) | 516 (23) | 304 (26.8) | 179 (23) | 167 (23.1) | 0.004 |
| Previous CABG | 423 (4) | 165 (2.9) | 83 (3.7) | 65 (5.7) | 51 (6.5) | 59 (8.1) | <0.001 |
| Diagnosis | <0.001 | ||||||
| ST-elevation MI | 1470 (13.8) | 782 (13.5) | 318 (14.2) | 167 (14.7) | 91 (11.7) | 112 (15.5) | |
| Non-ST elevation MI | 1369 (12.8) | 655 (11.3) | 274 (12.2) | 163 (14.4) | 133 (17.1) | 144 (19.9) | |
| Unstable angina | 222 (2.1) | 96 (1.7) | 51 (2.3) | 31 (2.7) | 22 (2.8) | 22 (3) | |
| Stable angina | 7608 (71.3) | 4254 (73.5) | 1603 (71.4) | 772 (68.1) | 533 (68.4) | 446 (61.6) | |
| Type of intervention | <0.001 | ||||||
| Angioplasty only | 2121 (19.9) | 1128 (19.5) | 432 (19.2) | 242 (21.4) | 155 (19.9) | 164 (22.7) | |
| BMS | 1878 (17.6) | 846 (14.6) | 431 (19.2) | 243 (21.4) | 178 (22.8) | 180 (24.9) | |
| DES | 6338 (59.4) | 3643 (63) | 1313 (58.5) | 607 (53.6) | 422 (54.2) | 353 (48.8) | |
| Both BMS and DES | 332 (3.1) | 170 (2.9) | 70 (3.1) | 41 (3.6) | 24 (3.1) | 27 (3.7) | |
| Medications | |||||||
| Aspirin | 5818 (54.5) | 3185 (55) | 1240 (55.2) | 608 (53.7) | 416 (53.4) | 369 (51) | 0.243 |
| Clopidogrel | 7583 (71.1) | 4116 (71.1) | 1589 (70.7) | 795 (70.2) | 560 (71.9) | 523 (72.2) | 0.861 |
| Ticagrelor | 1162 (10.9) | 701 (12.1) | 259 (11.5) | 93 (8.2) | 63 (8.1) | 46 (6.4) | <0.001 |
| Nitrates | 3397 (31.8) | 1846 (31.9) | 733 (32.6) | 325 (28.7) | 259 (33.2) | 234 (32.3) | 0.156 |
| Amiodarone | 435 (4.1) | 127 (2.2) | 96 (4.3) | 71 (6.3) | 69 (8.9) | 72 (9.9) | <0.001 |
| Beta-blockers | 3858 (36.2) | 2096 (36.2) | 814 (36.2) | 399 (35.2) | 270 (34.7) | 279 (38.5) | 0.561 |
| Diltiazem | 426 (4) | 239 (4.1) | 96 (4.3) | 52 (4.6) | 21 (2.7) | 18 (2.5) | 0.050 |
| Dihydropyridine CCBs | 1564 (14.7) | 758 (13.1) | 366 (16.3) | 186 (16.4) | 142 (18.2) | 112 (15.5) | <0.001 |
| ACEIs | 787 (7.4) | 402 (6.9) | 165 (7.3) | 81 (7.1) | 69 (8.9) | 70 (9.7) | 0.045 |
| ARBs | 2722 (25.5) | 1476 (25.5) | 615 (27.4) | 260 (22.9) | 195 (25) | 176 (24.3) | 0.069 |
| Loop diuretics | 1128 (10.6) | 383 (6.6) | 244 (10.9) | 188 (16.6) | 137 (17.6) | 176 (24.3) | <0.001 |
| Spironolactone | 513 (4.8) | 179 (3.1) | 115 (5.1) | 76 (6.7) | 56 (7.2) | 87 (12) | <0.001 |
| Statins | 4125 (38.7) | 2343 (40.5) | 896 (39.9) | 385 (34) | 279 (35.8) | 222 (30.7) | <0.001 |
| OADs | 1819 (17) | 933 (16.1) | 360 (16) | 211 (18.6) | 158 (20.3) | 157 (21.7) | <0.001 |
| Insulin | 281 (2.6) | 94 (1.6) | 56 (2.5) | 39 (3.4) | 37 (4.7) | 55 (7.6) | <0.001 |
| PPIs | 1992 (18.7) | 852 (14.7) | 427 (19) | 258 (22.8) | 195 (25) | 260 (35.9) | <0.001 |
| H2-blockers | 407 (3.8) | 197 (3.4) | 91 (4.1) | 57 (5) | 28 (3.6) | 34 (4.7) | 0.056 |
| Groups of RDW (%) | Group 1 ≤13.3 | Group 2 13.4–14.0 | Group 3 14.1–14.7 | Group 4 14.8–15.8 | Group 5 ≥15.9 | p |
|---|---|---|---|---|---|---|
| Number of patients | 5787 | 2246 | 1133 | 779 | 724 | |
| 6-month all-cause mortality | 71 (1.23) | 65 (2.89) | 51 (4.50) | 60 (7.70) | 104 (14.36) | <0.001 |
| 6-month CV mortality | 35 (0.60) | 29 (1.29) | 24 (2.12) | 19 (2.44) | 45 (6.22) | <0.001 |
| 1-year all-cause mortality | 132 (2.28) | 110 (4.90) | 93 (8.21) | 102 (13.09) | 170 (23.48) | <0.001 |
| 1-year CV mortality | 58 (1.00) | 53 (2.36) | 40 (3.53) | 36 (4.62) | 70 (9.67) | <0.001 |
| Unadjusted OR | 95% CI | p for Trend | Adjusted OR | 95% CI | p for Trend | |
|---|---|---|---|---|---|---|
| 6-month all-cause mortality | ||||||
| Group 1 RDW ≤13.3 | 1.000 | - | 0.005 | 1.000 | - | 0.004 |
| Group 2 RDW 13.4–14.0 | 2.399 | 1.707–3.370 | 1.554 | 1.090–2.216 | ||
| Group 3 RDW 14.1–14.7 | 3.794 | 2.632–5.468 | 1.608 | 1.089–2.375 | ||
| Group 4 RDW 14.8–15.8 | 6.716 | 4.722–9.553 | 2.245 | 1.523–3.310 | ||
| Group 5 RDW ≥15.9 | 13.501 | 9.874–18.461 | 3.241 | 2.255–4.657 | ||
| 6-month CV mortality | ||||||
| Group 1 RDW ≤13.3 | 1.000 | - | 0.004 | 1.000 | - | 0.022 |
| Group 2 RDW 13.4–14.0 | 2.150 | 1.311–3.525 | 1.423 | 0.856–2.365 | ||
| Group 3 RDW 14.1–14.7 | 3.556 | 2.107–6.002 | 1.605 | 0.925–2.784 | ||
| Group 4 RDW 14.8–15.8 | 4.108 | 2.338–7.218 | 1.441 | 0.786–2.642 | ||
| Group 5 RDW ≥15.9 | 10.893 | 6.954–17.062 | 3.058 | 1.821–5.137 | ||
| 1-year all-cause mortality | ||||||
| Group 1 RDW ≤13.3 | 1.000 | - | 0.004 | 1.000 | - | <0.001 |
| Group 2 RDW 13.4–14.0 | 2.206 | 1.705–2.855 | 1.386 | 1.055–1.822 | ||
| Group 3 RDW 14.1–14.7 | 3.831 | 2.914–5.036 | 1.589 | 1.180–2.138 | ||
| Group 4 RDW 14.8–15.8 | 6.455 | 4.925–8.459 | 2.090 | 1.543–2.830 | ||
| Group 5 RDW ≥15.9 | 13.146 | 10.305–16.771 | 3.192 | 2.398–4.248 | ||
| 1-year CV mortality | ||||||
| Group 1 RDW ≤13.3 | 1.000 | - | 0.006 | 1.000 | - | 0.015 |
| Group 2 RDW 13.4–14.0 | 2.387 | 1.640–3.476 | 1.555 | 1.053–2.295 | ||
| Group 3 RDW 14.1–14.7 | 3.615 | 2.404–5.436 | 1.585 | 1.027–2.447 | ||
| Group 4 RDW 14.8–15.8 | 4.786 | 3.136–7.305 | 1.623 | 1.023–2.574 | ||
| Group 5 RDW ≥15.9 | 10.572 | 7.396–15.113 | 2.850 | 1.883–4.312 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, M.-T.; Lai, C.-L.; Wang, T.-C.; Lin, J.-W.; Ho, Y.-L.; Chan, K.A. Red Cell Distribution Width and Mortality in Patients Undergoing Percutaneous Coronary Intervention. Biomedicines 2022, 10, 45. https://doi.org/10.3390/biomedicines10010045
Liao M-T, Lai C-L, Wang T-C, Lin J-W, Ho Y-L, Chan KA. Red Cell Distribution Width and Mortality in Patients Undergoing Percutaneous Coronary Intervention. Biomedicines. 2022; 10(1):45. https://doi.org/10.3390/biomedicines10010045
Chicago/Turabian StyleLiao, Min-Tsun, Chao-Lun Lai, Ting-Chuan Wang, Jou-Wei Lin, Yi-Lwun Ho, and K. Arnold Chan. 2022. "Red Cell Distribution Width and Mortality in Patients Undergoing Percutaneous Coronary Intervention" Biomedicines 10, no. 1: 45. https://doi.org/10.3390/biomedicines10010045
APA StyleLiao, M.-T., Lai, C.-L., Wang, T.-C., Lin, J.-W., Ho, Y.-L., & Chan, K. A. (2022). Red Cell Distribution Width and Mortality in Patients Undergoing Percutaneous Coronary Intervention. Biomedicines, 10(1), 45. https://doi.org/10.3390/biomedicines10010045






