Retrograde Analysis of Calcium Signaling by CaMPARI2 Shows Cytosolic Calcium in Chondrocytes Is Unaffected by Parabolic Flights
Abstract
:1. Introduction
1.1. Genetically Encoded Ca2+ Indicators
1.2. CaMPARI
1.3. Chondrocytes and Histamine
2. Materials and Methods
2.1. Vector Construction
2.2. Cell Culture and Treatment
2.3. Chondrocyte Transfection
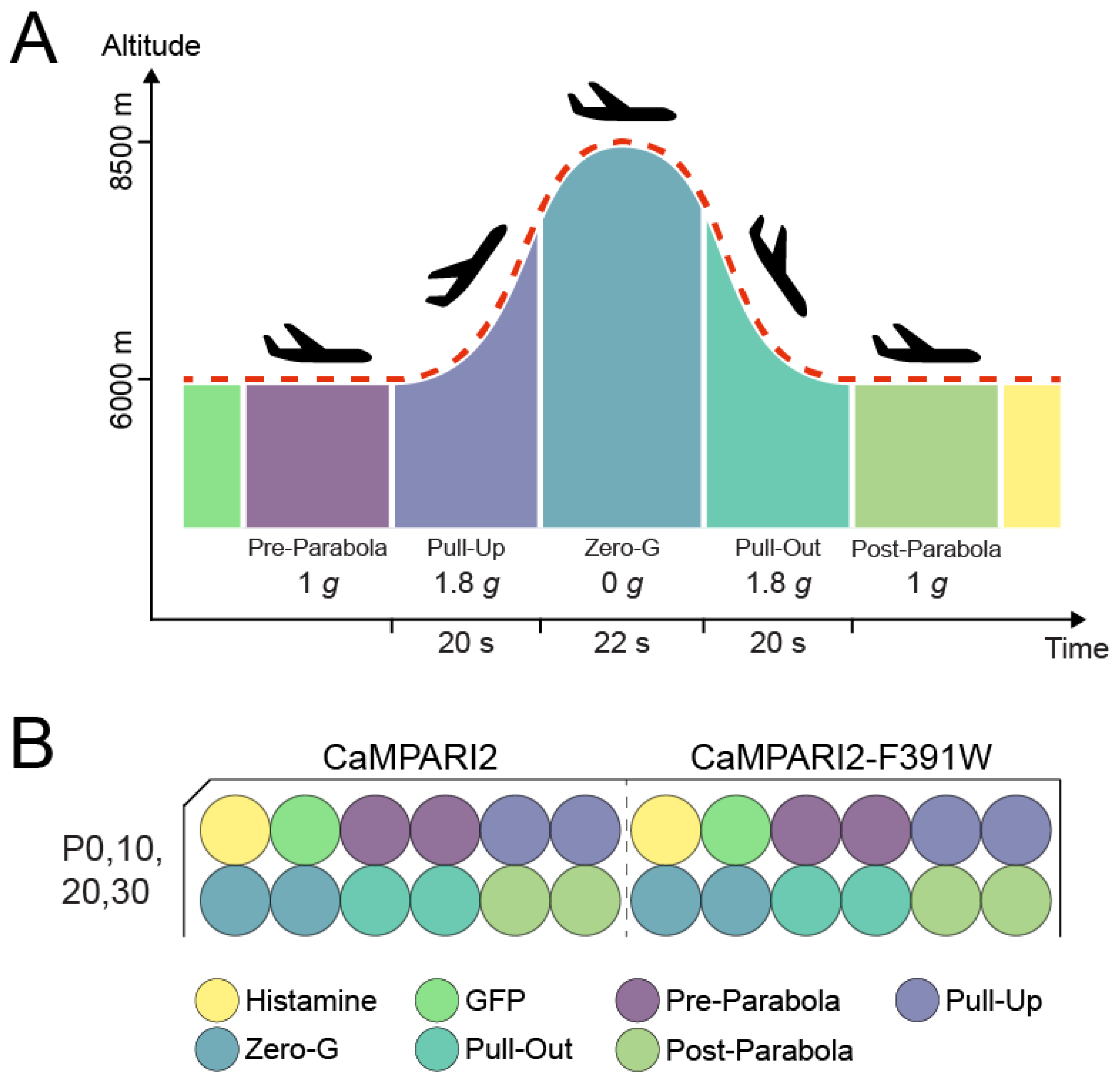
2.4. Parabolic Flight Experimental Design
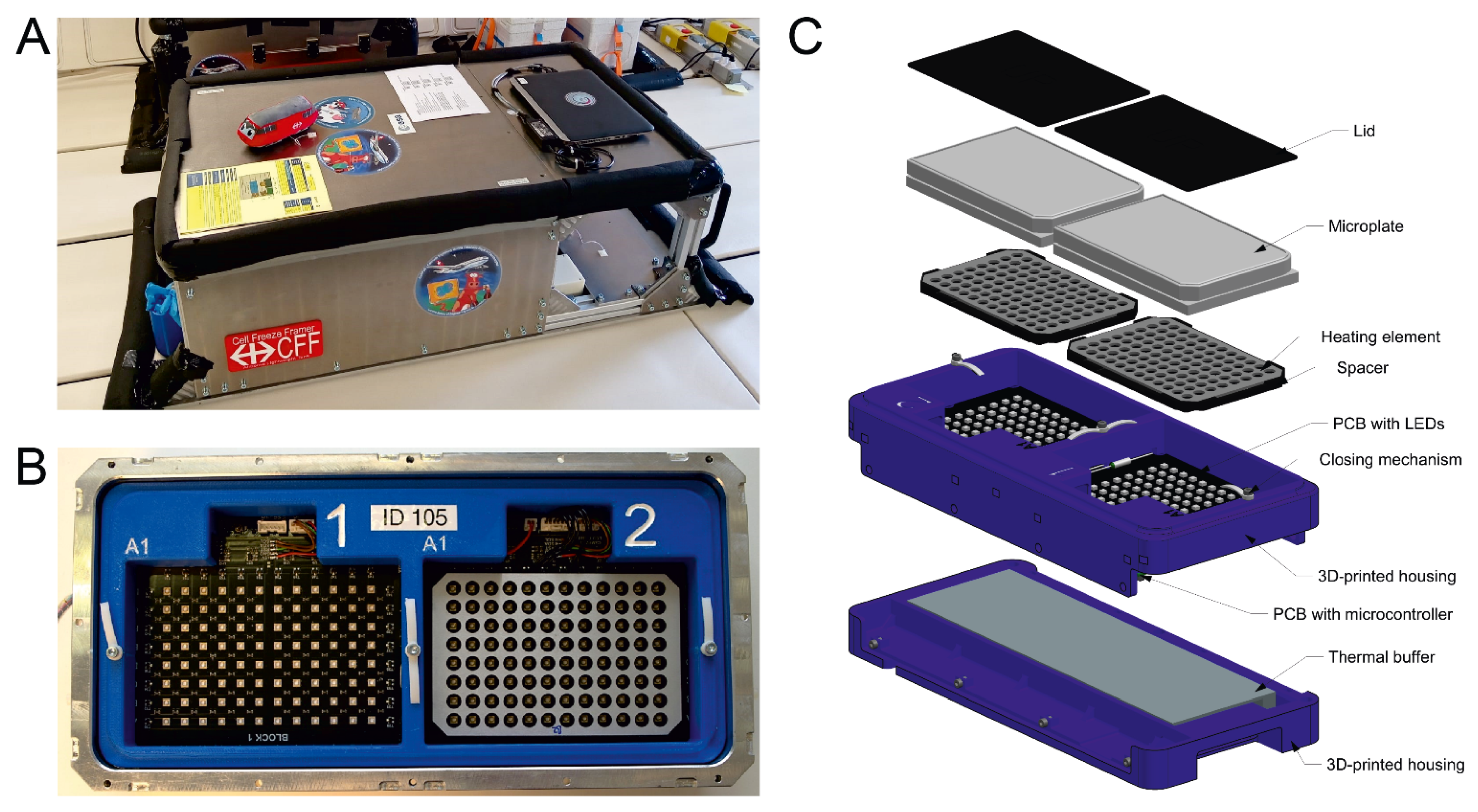
2.5. Parabolic Flight Hardware
2.6. Thermal Management
2.7. Before the Flight
2.8. During the Flight
2.9. After the Flight
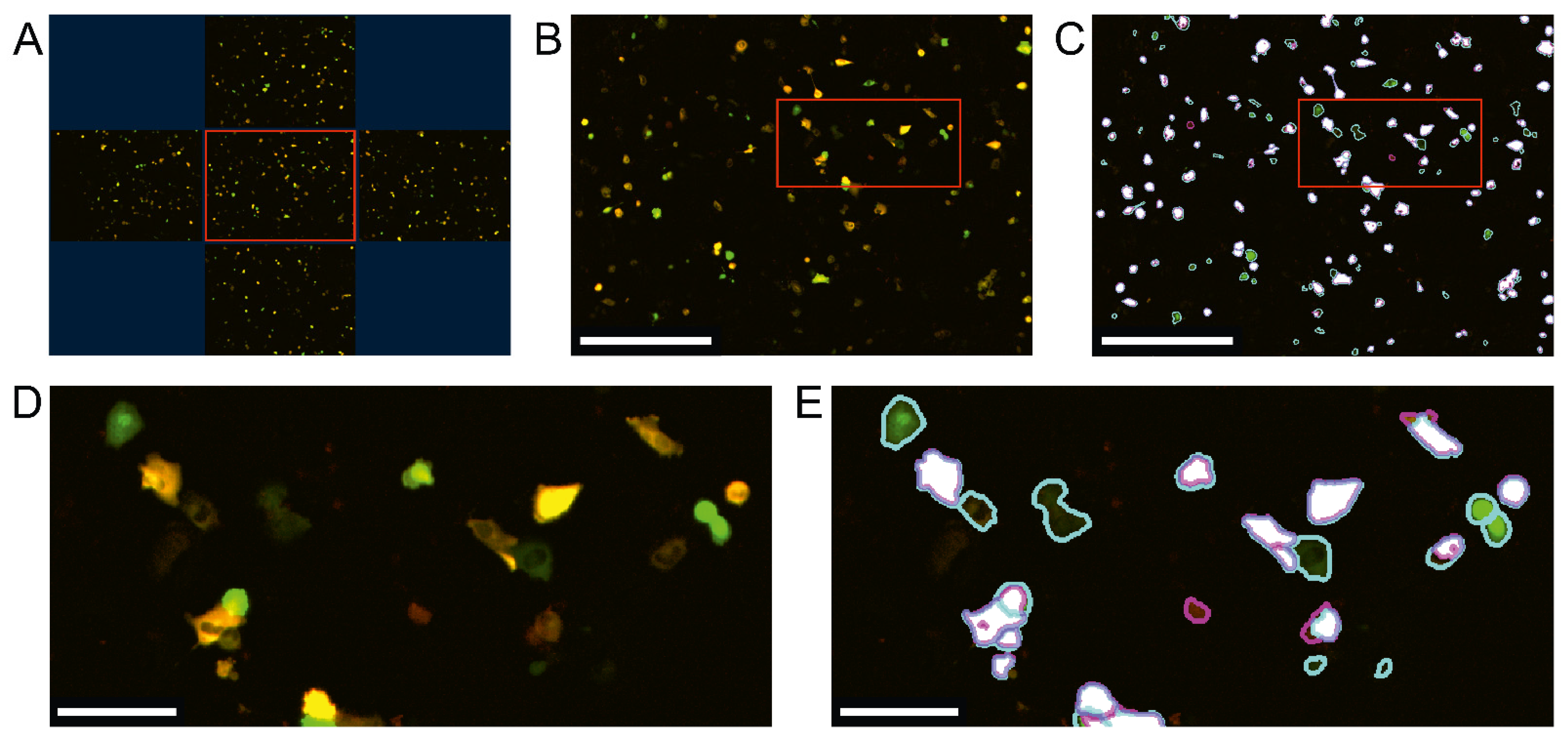
2.10. Image Analysis and Statistics
3. Results
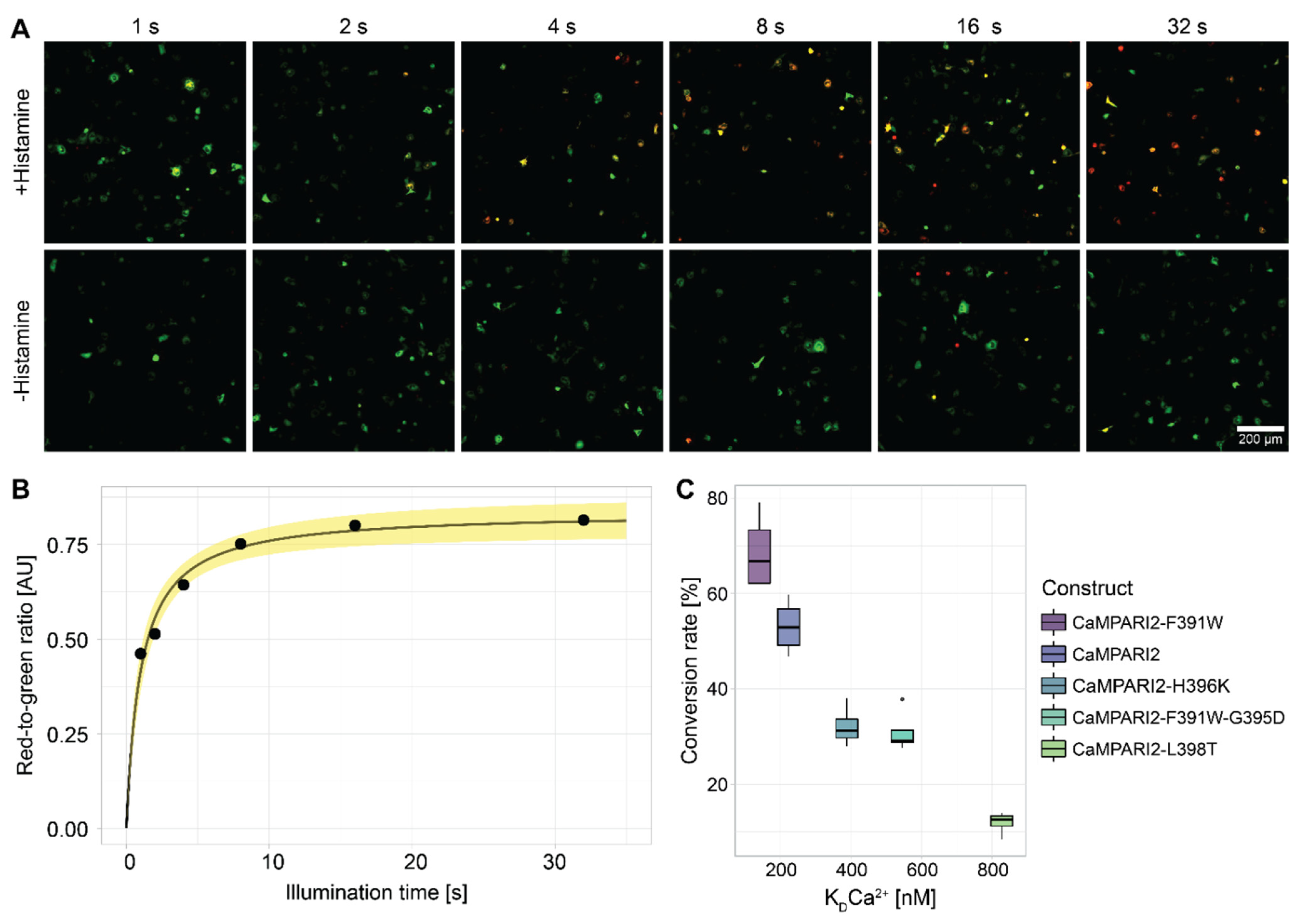
3.1. Selection of CaMPARI2-Constructs
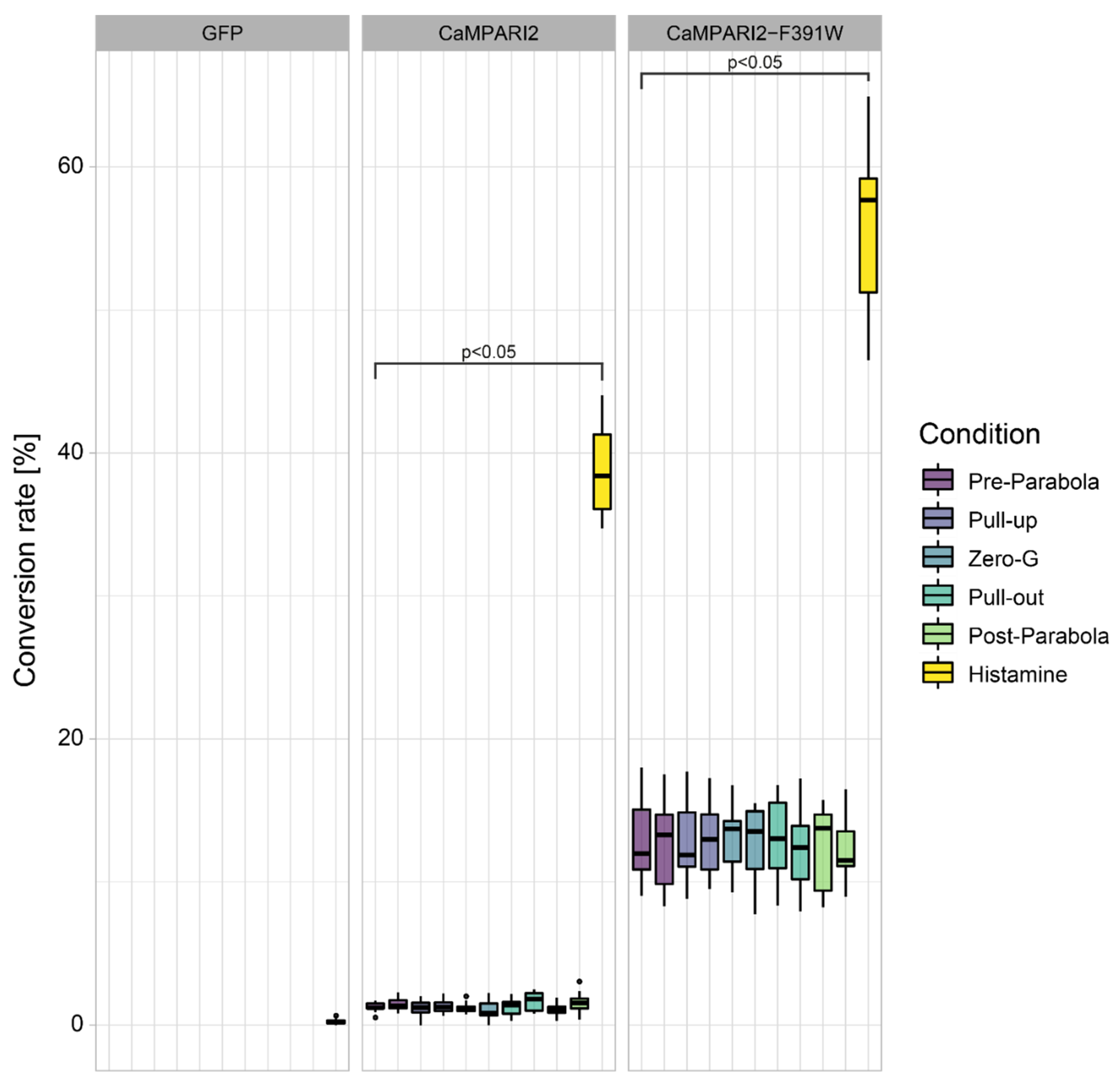
3.2. Gravi-Elicited Ca2+ Elevation Changes during a Parabolic Flight
3.3. Construct and Flight-Specific Conversion Rate
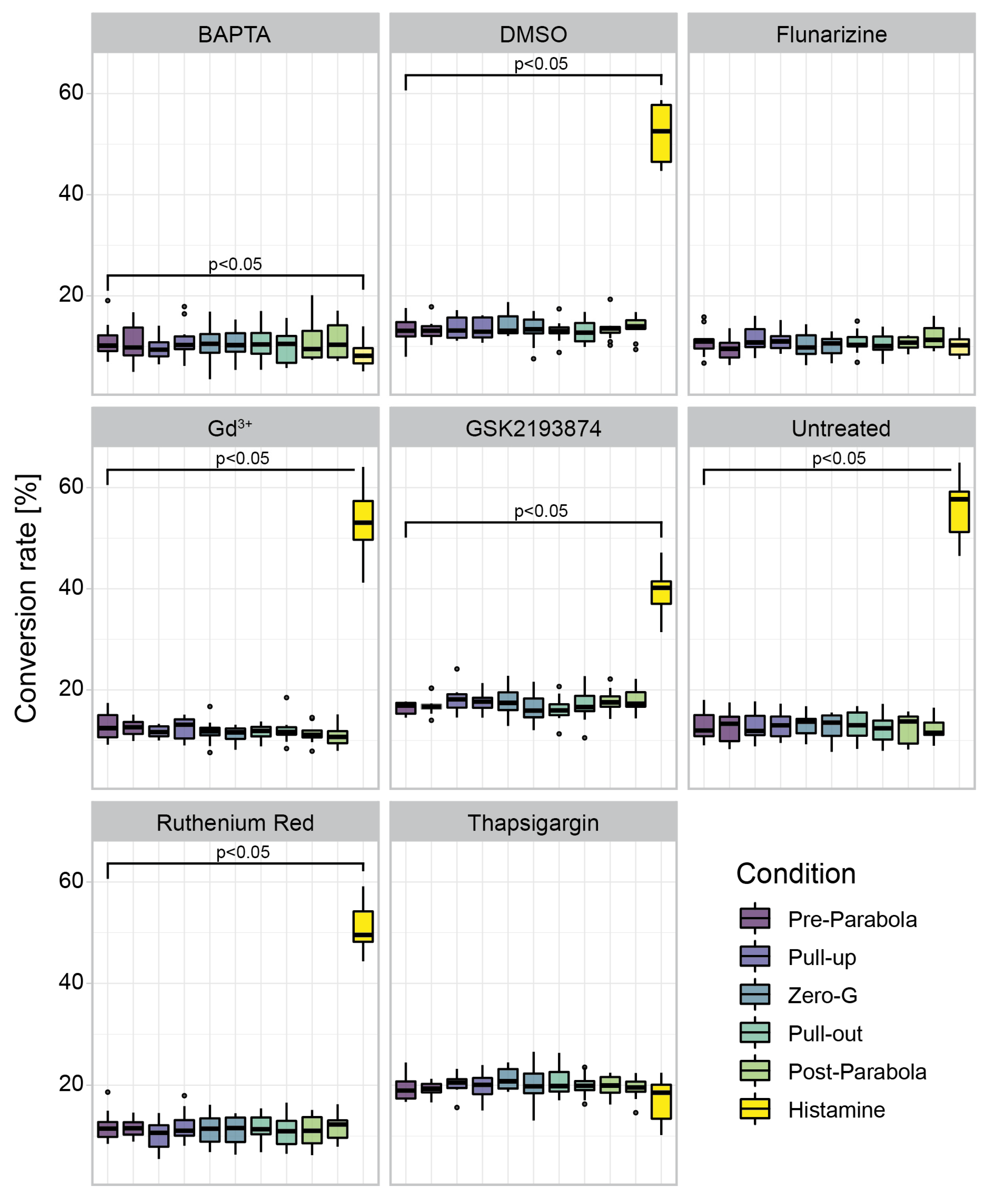
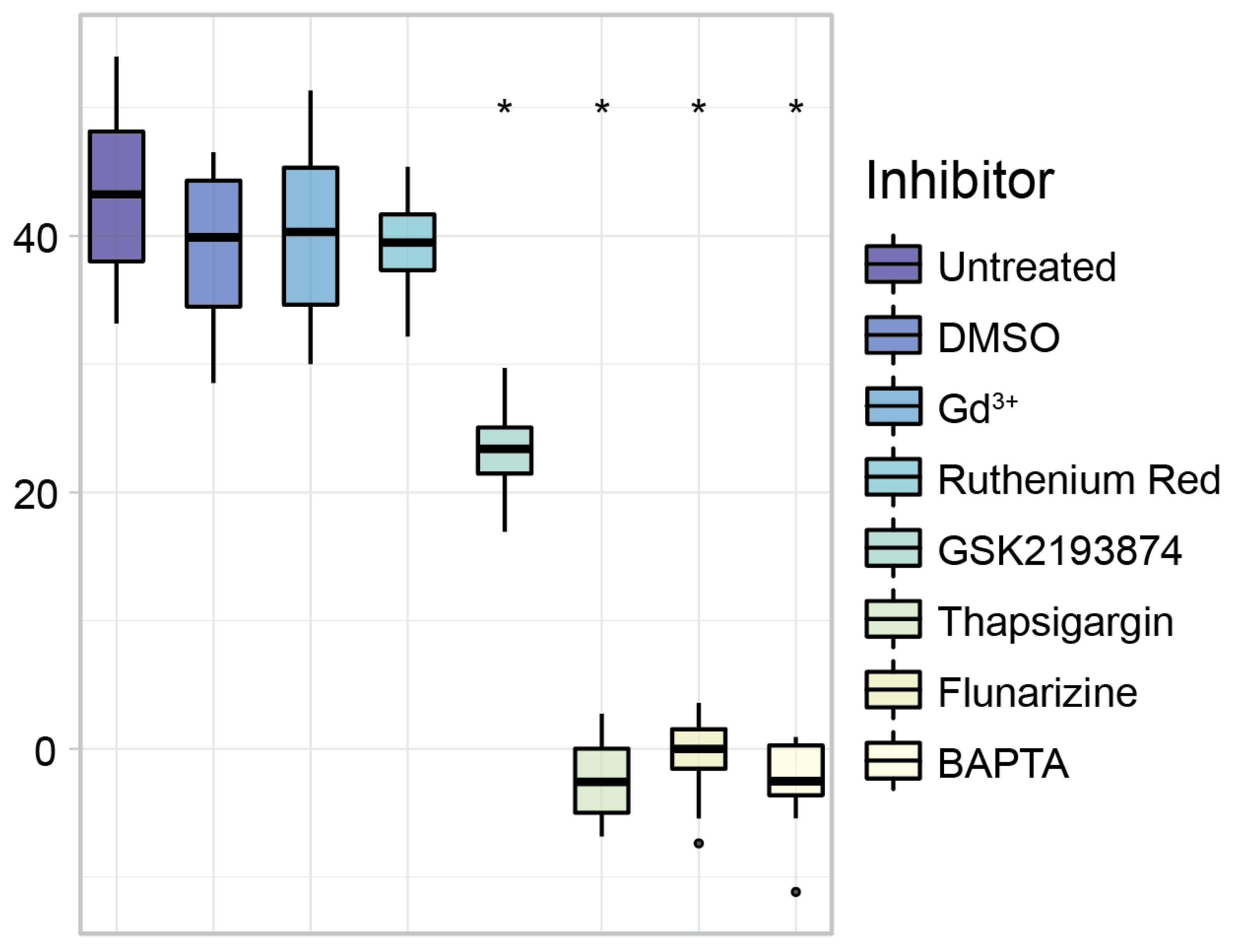
3.4. Inhibitor Specific Conversion Rate
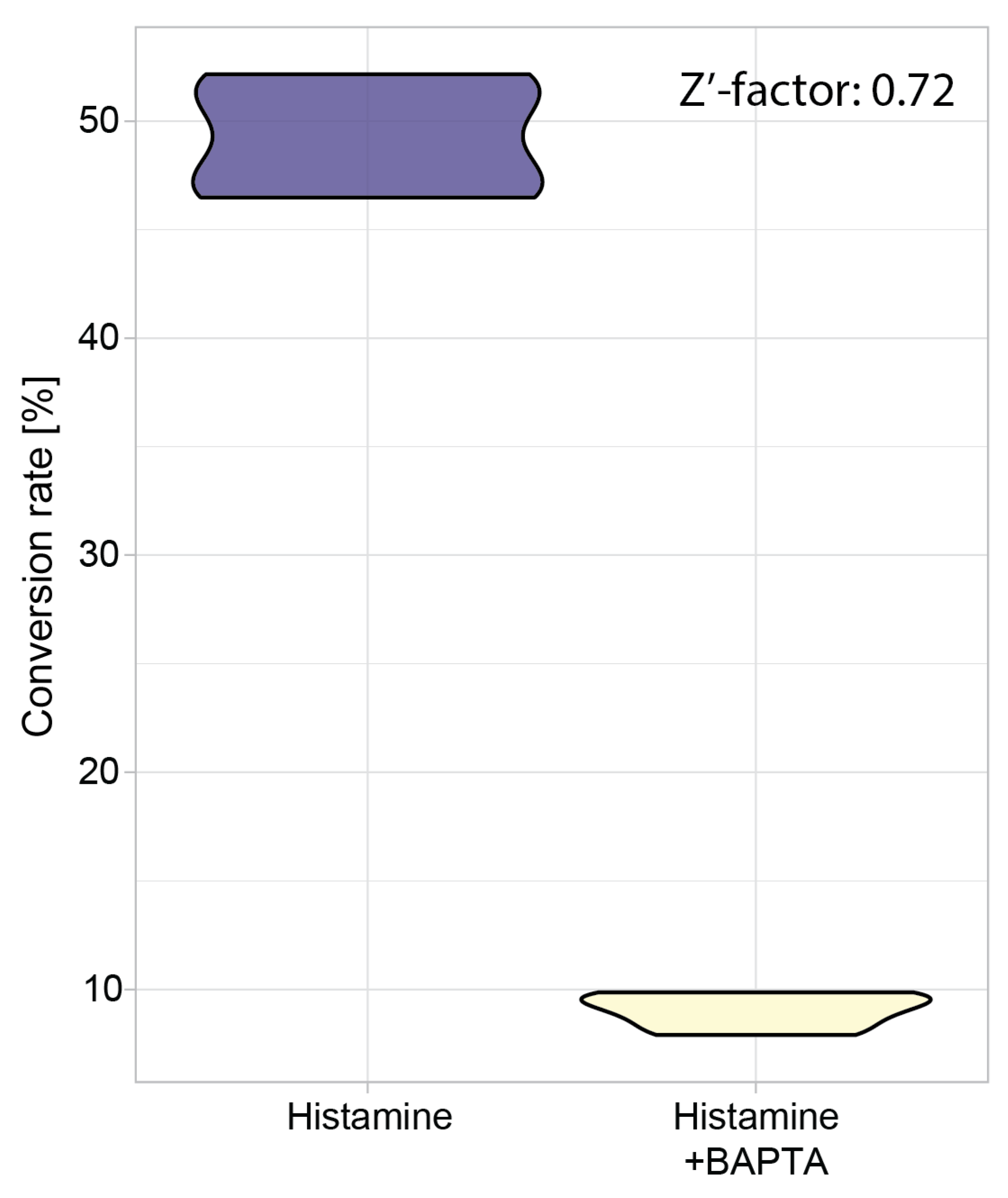
3.5. Z’-Factor Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hardingham, G.E.; Bading, H. Calcium as a Versatile Second Messenger in the Control of Gene Expression. Microsc. Res. Tech. 1999, 46, 348–355. [Google Scholar] [CrossRef]
- Brini, M.; Calì, T.; Ottolini, D.; Carafoli, E. Intracellular Calcium Homeostasis and Signaling. In Metallomics and the Cell. Metal Ions in Life Sciences; Banci, L., Ed.; Springer: Dordrecht, The Netherlands, 2013; Volume 12, pp. 119–168. [Google Scholar] [CrossRef]
- Kekenes-Huskey, P.M.; Metzger, V.T.; Grant, B.J.; Andrew McCammon, J. Calcium Binding and Allosteric Signaling Mechanisms for the Sarcoplasmic Reticulum Ca2+ ATPase. Protein Sci. 2012, 21, 1429–1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lane, J.M.; Russell, L.; Khan, S.N. Osteoporosis. Clin. Orthop. Relat. Res. 2000, 372, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Kulasiri, D.; Samarasinghe, S. Ca2+ Dysregulation in the Endoplasmic Reticulum Related to Alzheimer’s Disease: A Review on Experimental Progress and Computational Modeling. Biosystems 2015, 134, 1–15. [Google Scholar] [CrossRef]
- Lee, E.H. Ca2+ Channels and Skeletal Muscle Diseases. Prog. Biophys. Mol. Biol. 2010, 103, 35–43. [Google Scholar] [CrossRef]
- Bruton, J.; Cheng, A.J.; Westerblad, H. Measuring Ca2+ in Living Cells. In Calcium Signaling. Advances in Experimental Medicine and Biology; Islam, M., Ed.; Springer: Cham, Switzerland, 2020; Volume 1131, pp. 7–26. [Google Scholar] [CrossRef]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A New Generation of Ca2+ Indicators with Greatly Improved Fluorescence Properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar] [CrossRef]
- Zhao, Y.; Araki, S.; Wu, J.; Teramoto, T.; Chang, Y.-F.; Nakano, M.; Abdelfattah, A.S.; Fujiwara, M.; Ishihara, T.; Nagai, T.; et al. An Expanded Palette of Genetically Encoded Ca2+ Indicators. Science 2011, 333, 1888–1891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.-W.; Wardill, T.J.; Sun, Y.; Pulver, S.R.; Renninger, S.L.; Baohan, A.; Schreiter, E.R.; Kerr, R.A.; Orger, M.B.; Jayaraman, V.; et al. Ultrasensitive Fluorescent Proteins for Imaging Neuronal Activity. Nature 2013, 499, 295–300. [Google Scholar] [CrossRef] [Green Version]
- Keinath, N.F.; Waadt, R.; Brugman, R.; Schroeder, J.I.; Grossmann, G.; Schumacher, K.; Krebs, M. Live Cell Imaging with R-GECO1 Sheds Light on Flg22- and Chitin-Induced Transient [Ca2+]Cyt Patterns in Arabidopsis. Mol. Plant 2015, 8, 1188–1200. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, E.A.; Campbell, R.E.; Lin, J.Y.; Lin, M.Z.; Miyawaki, A.; Palmer, A.E.; Shu, X.; Zhang, J.; Tsien, R.Y. The Growing and Glowing Toolbox of Fluorescent and Photoactive Proteins. Trends Biochem. Sci. 2017, 42, 111–129. [Google Scholar] [CrossRef] [Green Version]
- Fosque, B.F.; Sun, Y.; Dana, H.; Yang, C.-T.; Ohyama, T.; Tadross, M.R.; Patel, R.; Zlatic, M.; Kim, D.S.; Ahrens, M.B.; et al. Labeling of Active Neural Circuits in Vivo with Designed Calcium Integrators. Science 2015, 347, 755–760. [Google Scholar] [CrossRef]
- Moeyaert, B.; Holt, G.; Madangopal, R.; Perez-Alvarez, A.; Fearey, B.C.; Trojanowski, N.F.; Ledderose, J.; Zolnik, T.A.; Das, A.; Patel, D.; et al. Improved Methods for Marking Active Neuron Populations. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Zolnik, T.A.; Sha, F.; Johenning, F.W.; Schreiter, E.R.; Looger, L.L.; Larkum, M.E.; Sachdev, R.N.S. All-Optical Functional Synaptic Connectivity Mapping in Acute Brain Slices Using the Calcium Integrator CaMPARI. J. Physiol. 2017, 595, 1465–1477. [Google Scholar] [CrossRef] [Green Version]
- Wuest, S.L.; Gantenbein, B.; Ille, F.; Egli, M. Electrophysiological Experiments in Microgravity: Lessons Learned and Future Challenges. NPJ Microgravity 2018, 4, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kohn, F.P.M. High Throughput Fluorescent Screening of Membrane Potential and Intracellular Calcium Concentration Under Variable Gravity Conditions. Microgravity Sci. Technol. 2013, 25, 113–120. [Google Scholar] [CrossRef]
- Ditengou, F.; Legué, V.; Brugman, R.; Pereda-Loth, V.; Schültze, E.; Tisseyre, L.; Rapp, K.; Oltmann, H.; Feldmann, S.; Cordes, P.; et al. Use of FLUMIAS to Reveal Dynamic Cellular Changes Initiated by Statolith Movement in Arabidopsis Thaliana Root Cells: First Observations from Parabolic Flight Campaign. In Workshop “Human Spaceflight and Weightlessness Science”; CNES: Toulouse, France, 2018. [Google Scholar]
- Corydon, T.J.; Kopp, S.; Wehland, M.; Braun, M.; Schütte, A.; Mayer, T.; Hülsing, T.; Oltmann, H.; Schmitz, B.; Hemmersbach, R.; et al. Alterations of the Cytoskeleton in Human Cells in Space Proved by Life-Cell Imaging. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiel, C.S.; Tauber, S.; Seebacher, C.; Schropp, M.; Uhl, R.; Lauber, B.; Polzer, J.; Neelam, S.; Zhang, Y.; Ullrich, O. Real-Time 3D High-Resolution Microscopy of Human Cells on the International Space Station. Int. J. Mol. Sci. 2019, 20, 2033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauslage, J.; Abbrecht, M.; Hanke, L.; Hemmersbach, R.; Koch, C.; Hanke, W.; Kohn, F.P.M. Cytosolic Calcium Concentration Changes in Neuronal Cells Under Clinorotation and in Parabolic Flight Missions. Microgravity Sci. Technol. 2016, 28, 633–638. [Google Scholar] [CrossRef]
- Wüest, S.L.; Cerretti, G.; Wadsworth, J.L.; Follonier, C.; Rattenbacher-Kiser, K.F.; Bradley, T.; Egli, M.; Ille, F. Cytosolic Calcium and Membrane Potential in Articular Chondrocytes during Parabolic Flight. Acta Astronaut. in revision.
- Clark, R.B.; Schmidt, T.A.; Sachse, F.B.; Boyle, D.; Firestein, G.S.; Giles, W.R. Cellular Electrophysiological Principles That Modulate Secretion from Synovial Fibroblasts. J. Physiol. 2017, 595, 635–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mobasheri, A.; Batt, M. An Update on the Pathophysiology of Osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Shen, J.; Zhao, W.; Wang, T.; Han, L.; Hamilton, J.L.; Im, H.J. Osteoarthritis: Toward a Comprehensive Understanding of Pathological Mechanism. Bone Res. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.M.G.; Beier, F.; Pest, M.A. Recent Developments in Emerging Therapeutic Targets of Osteoarthritis. Curr. Opin. Rheumatol. 2017, 29, 96–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugita, S.; Hosaka, Y.; Okada, K.; Mori, D.; Yano, F.; Kobayashi, H.; Taniguchi, Y.; Mori, Y.; Okuma, T.; Chang, S.H.; et al. Transcription Factor Hes1 Modulates Osteoarthritis Development in Cooperation with Calcium/Calmodulin-Dependent Protein Kinase 2. Proc. Natl. Acad. Sci. USA 2015, 112, 3080–3085. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Jin, Z.; Zhang, H.; Piao, S.; Lu, J.; Bai, L. The Transient Receptor Potential Channel, Vanilloid 5, Induces Chondrocyte Apoptosis via Ca2+ CaMKII-Dependent MAPK and Akt/ MTOR Pathways in a Rat Osteoarthritis Model. Cell. Physiol. Biochem. 2018, 51, 2309–2323. [Google Scholar] [CrossRef]
- Yao, W.; Han, Q.; Wang, L.; Niu, Z. Ropivacaine Relieves Pain and Prevents Chondrocyte Degradation Probably through Calcineurin/NFAT1 Signaling Pathway in Osteoarthritis Rats. Eur. J. Pharmacol. 2018, 818, 518–524. [Google Scholar] [CrossRef]
- Partsch, G.; Schwagerl, W.; Eberl, R. Untersuchungen über Histamin bei rheumatischen Erkrankungen. Z. Für Rheumatol. 1982, 41, 19–22. [Google Scholar]
- Tetlow, L.C.; Woolley, D.E. Histamine stimulates the proliferation of human articular chondrocytes in vitro and is expressed by chondrocytes in osteoarthritic cartilage. Ann. Rheum. Dis. 2003, 62, 991–994. [Google Scholar] [CrossRef] [Green Version]
- Tetlow, L.C.; Woolley, D.E. Histamine, histamine receptors (H1 and H2), and histidine decarboxylase expression by chondrocytes of osteoarthritic cartilage: An immunohistochemical study. Rheumatol. Int. 2005, 26, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Freemont, A.J.; Hampson, V.; Tilman, R.; Goupille, P.; Taiwo, Y.; Hoyland, J.A. Gene expression of matrix metalloproteinases 1, 3, and 9 by chondrocytes in osteoarthritic human knee articular cartilage is zone and grade specific. Ann. Rheum. Dis. 1997, 56, 542–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tetlow, L.C.; Adlam, D.J.; Woolley, D.E. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: Associations with degenerative changes. Arthritis Rheum. 2001, 44, 585–594. [Google Scholar] [CrossRef]
- Gong, X.; Li, G.; Huang, Y.; Fu, Z.; Song, X.; Chen, C.; Yang, L. Synergistically Regulated Spontaneous Calcium Signaling Is Attributed to Cartilaginous Extracellular Matrix Metabolism. J. Cell. Physiol. 2019, 234, 9711–9722. [Google Scholar] [CrossRef]
- Matta, C.; Fodor, J.; Szíjgyártó, Z.; Juhász, T.; Gergely, P.; Csernoch, L.; Zákány, R. Cytosolic Free Ca2+ Concentration Exhibits a Characteristic Temporal Pattern during in Vitro Cartilage Differentiation: A Possible Regulatory Role of Calcineurin in Ca-Signalling of Chondrogenic Cells. Cell Calcium 2008, 44, 310–323. [Google Scholar] [CrossRef] [Green Version]
- Lv, M.; Zhou, Y.; Polson, S.W.; Wan, L.Q.; Wang, M.; Han, L.; Wang, L.; Lu, X.L. Identification of Chondrocyte Genes and Signaling Pathways in Response to Acute Joint Inflammation. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Suzuki, Y.; Yamamura, H.; Imaizumi, Y.; Clark, R.B.; Giles, W.R. K+ and Ca2+ Channels Regulate Ca2+ Signaling in Chondrocytes: An Illustrated Review. Cells 2020, 9, 1577. [Google Scholar] [CrossRef]
- Yellowley, C.E.; Hancox, J.C.; Donahue, H.J. Effects of Cell Swelling on Intracellular Calcium and Membrane Currents in Bovine Articular Chondrocytes. J. Cell. Biochem. 2002, 86, 290–301. [Google Scholar] [CrossRef]
- Dascalu, A.; Korenstein, R.; Oron, Y.; Nevo, Z. A Hyperosmotic Stimulus Regulates Intracellular PH, Calcium, and S-100 Protein Levels in Avian Chondrocytes. Biochem. Biophys. Res. Commun. 1996, 227, 368–373. [Google Scholar] [CrossRef]
- Erickson, G.R.; Alexopoulos, L.G.; Guilak, F. Hyper-Osmotic Stress Induces Volume Change and Calcium Transients in Chondrocytes by Transmembrane, Phospholipid, and G-Protein Pathways. J. Biomech. 2001, 34, 1527–1535. [Google Scholar] [CrossRef]
- Sánchez, J.C.; Danks, T.A.; Wilkins, R.J. Mechanisms Involved in the Increase in Intracellular Calcium Following Hypotonic Shock in Bovine Articular Chondrocytes. Gen. Physiol. Biophys 2003, 22, 487–500. [Google Scholar]
- Sánchez, J.C.; Wilkins, R.J. Changes in Intracellular Calcium Concentration in Response to Hypertonicity in Bovine Articular Chondrocytes. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2004, 137, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Chao, P.H.G.; West, A.C.; Hung, C.T. Chondrocyte Intracellular Calcium, Cytoskeletal Organization, and Gene Expression Responses to Dynamic Osmotic Loading. Am. J. Physiol.-Cell Physiol. 2006, 291. [Google Scholar] [CrossRef]
- Phan, M.N.; Leddy, H.A.; Votta, B.J.; Kumar, S.; Levy, D.S.; Lipshutz, D.B.; Suk, H.L.; Liedtke, W.; Guilak, F. Functional Characterization of TRPV4 as an Osmotically Sensitive Ion Channel in Porcine Articular Chondrocytes. Arthritis Rheum. 2009, 60, 3028–3037. [Google Scholar] [CrossRef] [Green Version]
- O’Conor, C.J.; Leddy, H.A.; Benefield, H.C.; Liedtke, W.B.; Guilak, F. TRPV4-Mediated Mechanotransduction Regulates the Metabolic Response of Chondrocytes to Dynamic Loading. Proc. Natl. Acad. Sci. USA 2014, 111, 1316–1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yellowley, C.E.; Jacobs, C.R.; Li, Z.; Zhou, Z.; Donahue, H.J. Effects of Fluid Flow on Intracellular Calcium in Bovine Articular Chondrocytes. Am. J. Physiol.-Cell Physiol. 1997, 273, C30–C36. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Leddy, H.A.; Chen, Y.; Lee, S.H.; Zelenski, N.A.; Mcnulty, A.L.; Wu, J.; Beicker, K.N.; Coles, J.; Zauscher, S.; et al. Synergy between Piezo1 and Piezo2 Channels Confers High-Strain Mechanosensitivity to Articular Cartilage. Proc. Natl. Acad. Sci. USA 2014, 111, E5114–E5122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Andrea, P.; Vittur, F. Propagation of Intercellular Ca2+ Waves in Mechanically Stimulated Articular Chondrocytes. FEBS Lett. 1997, 400, 58–64. [Google Scholar] [CrossRef] [Green Version]
- Guilak, F.; Zell, R.A.; Erickson, G.R.; Grande, D.A.; Rubin, C.T.; McLeod, K.J.; Donahue, H.J. Mechanically Induced Calcium Waves in Articular Chondrocytes Are Inhibited by Gadolinium and Amiloride. J. Orthop. Res. 1999, 17, 421–429. [Google Scholar] [CrossRef]
- Ohashi, T.; Hagiwara, M.; Bader, D.L.; Knight, M.M. Intracellular Mechanics and Mechanotransduction Associated with Chondrocyte Deformation during Pipette Aspiration. Biorheology 2006, 43, 201–214. [Google Scholar]
- Burks, R.; Daniel, D.; Losse, G. The Effect of Continuous Passive Motion on Anterior Cruciate Ligament Reconstruction Stability. Am. J. Sports Med. 1984, 12, 323–327. [Google Scholar] [CrossRef]
- Grimm, D.; Grosse, J.; Wehland, M.; Mann, V.; Reseland, J.E.; Sundaresan, A.; Corydon, T.J. The Impact of Microgravity on Bone in Humans. Bone 2016, 87. [Google Scholar] [CrossRef]
- Han, S.K.; Wouters, W.; Clark, A.; Herzog, W. Mechanically Induced Calcium Signaling in Chondrocytes in Situ. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2012, 30, 475–481. [Google Scholar] [CrossRef]
- Goldring, M.B. Immortalization of Human Articular Chondrocytes for Generation of Stable, Differentiated Cell Lines. In Cartilage and Osteoarthritis. Methods in Molecular Medicine; Sabatini, M., Pastoureau, P., De Ceuninck, F., Eds.; Humana Press: Totowa, NJ, USA, 2004; Volume 100, pp. 23–35. [Google Scholar] [CrossRef]
- Goldring, M.B.; Birkhead, J.R.; Suen, L.-F.; Yamin, R.; Mizuno, S.; Glowacki, J.; Arbiser, J.L.; Apperleyll, J.F. Interleukin-1 Beta-Modulated Gene Expression in Immortalized Human Chondrocytes. J. Clin. Investig. 1994, 94, 2307–2316. [Google Scholar] [CrossRef]
- Böhmer, M.; Schleiff, E. Microgravity Research in Plants. EMBO Rep. 2019, 20, e48541. [Google Scholar] [CrossRef]
- Wuest, S.L.; Arnold, J.; Gander, S.; Zumbühl, C.; Jost, C.; Giger-Lange, C.; Cerretti, G.; Caliò, M.; Rattenbacher-Kiser, K.; Follonier, C.; et al. Microtubules and Vimentin Fiber Stability during Parabolic Flights. Microgravity Sci. Technol. 2020, 32, 921–933. [Google Scholar] [CrossRef]
- Wright, M.; Jobanputra, P.; Bavington, C.; Salter, D.M.; Nuki, G. Effects of Intermittent Pressure-Induced Strain on the Electrophysiology of Cultured Human Chondrocytes: Evidence for the Presence of Stretch-Activated Membrane Ion Channels. Clin. Sci. 1996, 90, 61–71. [Google Scholar] [CrossRef]
- Millward-Sadler, S.J.; Wright, M.O.; Lee, H.S.; Caldwell, H.; Nuki, G.; Salter, D.M. Altered Electrophysiological Responses to Mechanical Stimulation and Abnormal Signalling through A5β1 Integrin in Chondrocytes from Osteoarthritic Cartilage. Osteoarthr. Cartil. 2000, 8, 272–278. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.Q.; Chen, Q. Mechanoregulation of Chondrocyte Proliferation, Maturation, and Hypertrophy: Ion-Channel Dependent Transduction of Matrix Deformation Signals. Exp. Cell Res. 2000, 256, 383–391. [Google Scholar] [CrossRef]
- van Nueten, J.M.; Janssen, P.A.J. Comparative Study of the Effects of Flunarizine and Cinnarizine on Smooth Muscles and Cardiac Tissues. Arch. Int. Pharmacodyn. Ther. 1973, 204, 37–55. [Google Scholar] [PubMed]
- Awouters, F.; Niemegers, C.J.; Janssen, P.A. Cinnarizine and Flunarizine, Potent Inhibitors of Anaphylactic Shock in Guinea-Pigs. Arch. Int. Pharmacodyn. Ther. 1975, 217, 38–43. [Google Scholar] [PubMed]
- Vincent, F.; Duncton, M.A.J. TRPV4 Agonists and Antagonists. Curr. Top. Med. Chem. 2011, 11, 2216–2226. [Google Scholar] [CrossRef] [PubMed]
- Cheung, M.; Bao, W.; Behm, D.J.; Brooks, C.A.; Bury, M.J.; Dowdell, S.E.; Eidam, H.S.; Fox, R.M.; Goodman, K.B.; Holt, D.A.; et al. Discovery of GSK2193874: An Orally Active, Potent, and Selective Blocker of Transient Receptor Potential Vanilloid 4. ACS Med. Chem. Lett. 2017, 8, 549–554. [Google Scholar] [CrossRef] [Green Version]
- Tsien, R.Y. A Non-Disruptive Technique for Loading Calcium Buffers and Indicators into Cells. Nature 1981, 290, 527–528. [Google Scholar] [CrossRef]
- Star, A.M.; Iannotti, J.P.; Brighton, C.T.; Armstrong, P.F. Cytosolic Calcium Concentration in Bovine Growth Plate Chondrocytes. J. Orthop. Res. 1987, 5, 122–127. [Google Scholar] [CrossRef]
- Groden, D.L.; Guan, Z.; Stokes, B.T. Determination of Fura-2 Dissociation Constants Following Adjustment of the Apparent Ca-EGTA Association Constant for Temperature and Ionic Strength. Cell Calcium 1991, 12, 279–287. [Google Scholar] [CrossRef]
- Zhang, J.H.; Chung, T.D.Y.; Oldenburg, K.R. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J. Biomol. Screen. 1999, 4, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, X.B.; Yang, S.Z.; Li, S.G.; Jiang, P.D.; Lin, Z.H. The Effects of Simulated Microgravity on Cultured Chicken Embryonic Chondrocytes. Adv. Space Res. 2003, 32, 1577–1583. [Google Scholar] [CrossRef]
- Horwitz, E.R.; Higgins, T.M.; Harvey, B.J. Histamine-Induced Cytosolic Calcium Increase in Porcine Articular Chondrocytes. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 1996, 1313, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Becker, D.; Blase, C.; Bereiter-Hahn, J.; Jendrach, M. TRPV4 Exhibits a Functional Role in Cell-Volume Regulation. J. Cell Sci. 2005, 118, 2435–2440. [Google Scholar] [CrossRef] [Green Version]
- Cho, K.J.; Park, E.Y.; Kim, H.S.; Koh, J.S.; Kim, J.C. Expression of Transient Receptor Potential Vanilloid 4 and Effects of Ruthenium Red on Detrusor Overactivity Associated with Bladder Outlet Obstruction in Rats. World J. Urol. 2014, 32, 677–682. [Google Scholar] [CrossRef]
- Caldwell, R.A.; Clemo, H.F.; Baumgarten, C.M. Using Gadolinium to Identify Stretch-Activated Channels: Technical Considerations. Am. J. Physiol.-Cell Physiol. 1998, 275, C619–C621. [Google Scholar] [CrossRef] [PubMed]
- Rogers, T.B.; Inesi, G.; Wade, R.; Lederer, W.J. Use of Thapsigargin to Study Ca2+ Homeostasis in Cardiac Cells. Biosci. Rep. 1995, 15, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.L.E. The Endoplasmic Reticulum and Calcium Storage. BioEssays 1990, 12, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Holmes, B.; Brogden, R.N.; Heel, R.C.; Speight, T.M.; Avery, G.S. Flunarizine A Review of Its Pharmacodynamic and Pharmacokinetic Properties and Therapeutic Use. Drugs 1984, 27, 6–44. [Google Scholar] [CrossRef] [PubMed]

| Drug | Concentration | Solvent | Action | Reference |
|---|---|---|---|---|
| Untreated | - | - | - | |
| DMSO | 0.1% | - | DMSO control | |
| Gadolinium (III) chloride (Gd3+) | 10 µM | H2O | Blocker of extracellular calcium (Ca2+) entry | [38,40,41,46,49,59,60,61] |
| Thapsigargin | 1 µM | 0.1% DMSO | Blocker of SERCA (sarco/endoplasmic reticulum Ca2+-ATPase) | [47] |
| Flunarizine | 50 µM | 0.1% DMSO | Blocker of Ca2+ entry and histamine H1 | [62,63] |
| Ruthenium red | 10 µM | H2O | Blocker of CatSper1, KCNK3, RyR1, RyR2, RyR3, TRPM6, TRPM8, TRPV1, TRPV2, TRPV3, TRPV4, TRPV5, TRPV6, TRPA1, CALHM1, TRPP3, PIEZO | [64] |
| GSK2193874 | 10 µM | 0.1% DMSO | Selective blocker of TRPV4 | [65] |
| BAPTA-AM | 10 µM | 0.1% DMSO | Cell-permeable Ca2+-chelator | [66] |
| Metric | Definition |
|---|---|
| Conversion rate | The proportion of the green fluorescent cells that also display a red fluorescence (photoconversion) |
| Red objects mean fluorescence intensity | The mean red intensity of all cells |
| Merged objects red mean intensity | The mean red intensity of all cells displaying green and red fluorescence |
| Red-integrated intensity per well | The red intensity per well divided by the number of cells per well that display green and red fluorescence |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammer, A.; Cerretti, G.; Ricciardi, D.A.; Schiffmann, D.; Maranda, S.; Kummer, R.; Zumbühl, C.; Rattenbacher-Kiser, K.F.; von Arx, S.; Ammann, S.; et al. Retrograde Analysis of Calcium Signaling by CaMPARI2 Shows Cytosolic Calcium in Chondrocytes Is Unaffected by Parabolic Flights. Biomedicines 2022, 10, 138. https://doi.org/10.3390/biomedicines10010138
Hammer A, Cerretti G, Ricciardi DA, Schiffmann D, Maranda S, Kummer R, Zumbühl C, Rattenbacher-Kiser KF, von Arx S, Ammann S, et al. Retrograde Analysis of Calcium Signaling by CaMPARI2 Shows Cytosolic Calcium in Chondrocytes Is Unaffected by Parabolic Flights. Biomedicines. 2022; 10(1):138. https://doi.org/10.3390/biomedicines10010138
Chicago/Turabian StyleHammer, Andreas, Geraldine Cerretti, Dario A. Ricciardi, David Schiffmann, Simon Maranda, Raphael Kummer, Christoph Zumbühl, Karin F. Rattenbacher-Kiser, Silvan von Arx, Sebastian Ammann, and et al. 2022. "Retrograde Analysis of Calcium Signaling by CaMPARI2 Shows Cytosolic Calcium in Chondrocytes Is Unaffected by Parabolic Flights" Biomedicines 10, no. 1: 138. https://doi.org/10.3390/biomedicines10010138
APA StyleHammer, A., Cerretti, G., Ricciardi, D. A., Schiffmann, D., Maranda, S., Kummer, R., Zumbühl, C., Rattenbacher-Kiser, K. F., von Arx, S., Ammann, S., Strobl, F., Berkane, R., Stolz, A., Stelzer, E. H. K., Egli, M., Schleiff, E., Wuest, S. L., & Böhmer, M. (2022). Retrograde Analysis of Calcium Signaling by CaMPARI2 Shows Cytosolic Calcium in Chondrocytes Is Unaffected by Parabolic Flights. Biomedicines, 10(1), 138. https://doi.org/10.3390/biomedicines10010138







