Sex Differences in Spironolactone and the Active Metabolite Canrenone Concentrations and Adherence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Measurements
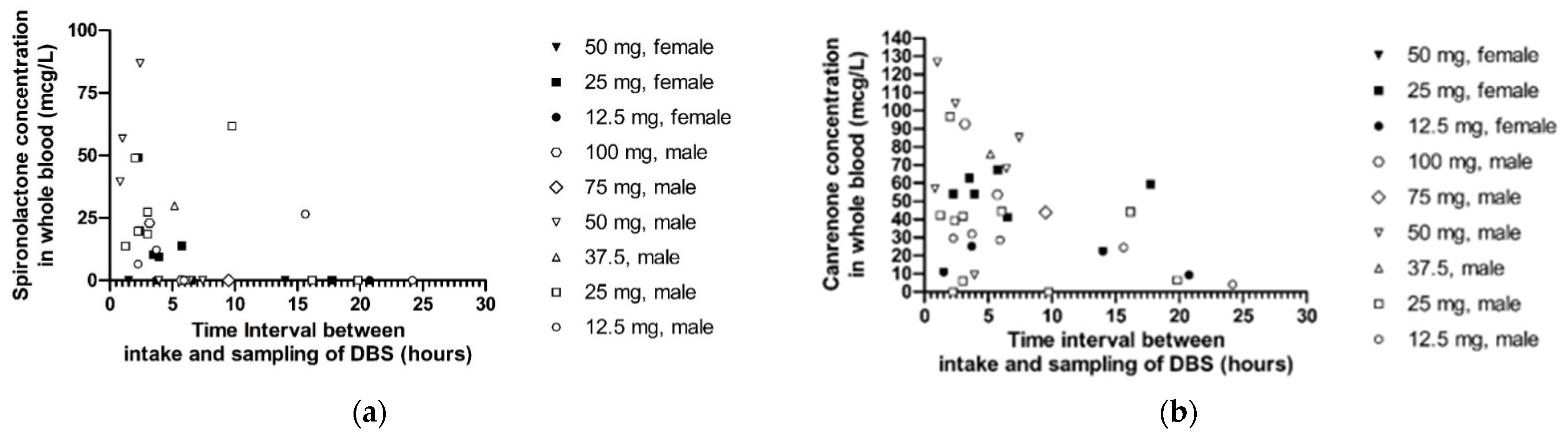
2.3.1. Sampling Method
2.3.2. Adherence
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. Difference in Spironolactone and Canrenone Concentrations between Sexes
3.3. Antihypertensive Drug Adherence between Sexes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Judd, E.; Calhoun, D.A. Apparent and true resistant hypertension: Definition, prevalence and outcomes. J. Hum. Hypertens. 2014, 28, 463–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, S.D.; Akhras, K.S.; Zhang, S.; Rozinsky, M.; Nalysnyk, L. Discontinuation of antihypertensive drugs due to adverse events: A systematic review and meta-analysis. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2001, 21, 940–953. [Google Scholar] [CrossRef]
- Eugene, A.R. Metoprolol Dose Equivalence in Adult Men and Women Based on Gender Differences: Pharmacokinetic Modeling and Simulations. Med. Sci. 2016, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Peeters, L.E.J.; Feyz, L.; Boersma, E.; Daemen, J.; van Gelder, T.; Koch, B.C.P.; Versmissen, J. Clinical Applicability of Monitoring Antihypertensive Drug Levels in Blood. Hypertension 2020, 76, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Di Giosia, P.; Giorgini, P.; Stamerra, C.A.; Petrarca, M.; Ferri, C.; Sahebkar, A. Gender Differences in Epidemiology, Pathophysiology, and Treatment of Hypertension. Curr. Atheroscler. Rep. 2018, 20, 13. [Google Scholar] [CrossRef] [PubMed]
- Ueno, K.; Sato, H. Sex-related differences in pharmacokinetics and pharmacodynamics of anti-hypertensive drugs. Hypertens. Res. 2011, 35, 245–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rydberg, D.M.; Mejyr, S.; Loikas, D.; Schenck-Gustafsson, K.; von Euler, M.; Malmström, R.E. Sex differences in spontaneous reports on adverse drug events for common antihypertensive drugs. Eur. J. Clin. Pharmacol. 2018, 74, 1165–1173. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.; Patel, P.; Štrauch, B.; Lai, F.Y.; Akbarov, A.; Marešová, V.; White, C.M.; Petrak, O.; Gulsin, G.; Patel, V.; et al. Risk Factors for Nonadherence to Antihypertensive Treatment. Hypertension 2017, 69, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Gebreyohannes, E.A.; Bhagavathula, A.S.; Abebe, T.B.; Tefera, Y.G.; Abegaz, T.M. Adverse effects and non-adherence to antihypertensive medications in University of Gondar Comprehensive Specialized Hospital. Clin. Hypertens. 2019, 25, 1. [Google Scholar] [CrossRef] [PubMed]
- Cabaleiro, T.; Román, M.; Ochoa, D.; Talegón, M.; Prieto-Pérez, R.; Wojnicz, A.; López-Rodríguez, R.; Novalbos, J.; Abad-Santos, F. Evaluation of the Relationship between Sex, Polymorphisms in CYP2C8 and CYP2C9, and Pharmacokinetics of Angiotensin Receptor Blockers. Drug Metab. Dispos. 2012, 41, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Oliveras, A.; De La Sierra, A. Resistant hypertension: Patient characteristics, risk factors, co-morbidities and outcomes. J. Hum. Hypertens. 2013, 28, 213–217. [Google Scholar] [CrossRef]
- Peeters, L.; Feyz, L.; Hameli, E.; Zwart, T.; Bahmany, S.; Daemen, J.; Van Gelder, T.; Versmissen, J.; Koch, B.C.P. Clinical Validation of a Dried Blood Spot Assay for 8 Antihypertensive Drugs and 4 Active Metabolites. Ther. Drug Monit. 2020, 42, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Sica, D.A. Pharmacokinetics and Pharmacodynamics of Mineralocorticoid Blocking Agents and their Effects on Potassium Homeostasis. Hear. Fail. Rev. 2005, 10, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Luzier, A.B.; Killian, A.; Wilton, J.H.; Wilson, M.F.; Forrest, A.; Kazierad, D.J. Gender-related effects on metoprolol pharmacokinetics and pharmacodynamics in healthy volunteers. Clin. Pharmacol. Ther. 1999, 66, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Abshagen, U.; Rennekamp, H.; Luszpinski, G. Pharmacokinetics of spironolactone in man. Naunyn-Schmiedebergs Arch. Fur Exp. Pathol. Pharmakol. 1976, 296, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Sportiello, L.; Rafaniello, C.; Sullo, M.G.; Nica, M.; Scavone, C.; Bernardi, F.F.; Colombo, D.M.; Rossi, F. No substantial gender differences in suspected adverse reactions to ACE inhibitors and ARBs: Results from spontaneous reporting system in Campania Region. Expert Opin. Drug Saf. 2016, 15, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Tedla, Y.G.; Bautista, L.E. Drug Side Effect Symptoms and Adherence to Antihypertensive Medication. Am. J. Hypertens. 2016, 29, 772–779. [Google Scholar] [CrossRef]
- Gold, E.B. The Timing of the Age at Which Natural Menopause Occurs. Obstet. Gynecol. Clin. North Am. 2011, 38, 425–440. [Google Scholar] [CrossRef] [Green Version]
- Coylewright, M.; Reckelhoff, J.F.; Ouyang, P. Menopause and hypertension: An age-old debate. Hypertension 2008, 51, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Barton, M.; Meyer, M.R. Postmenopausal hypertension: Mechanisms and therapy. Hypertension 2009, 54, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Maas, A.H.E.M.; Franke, H.R. Women’s health in menopause with a focus on hypertension. Neth. Hear. J. 2009, 17, 68–72. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | All (N = 54) | Female (N = 21) | Male (N = 33) | p-Value |
|---|---|---|---|---|
| Age, y, mean ± SD | 58.2 ± 10.5 | 57.4 ± 10.7 | 58.8 ± 10.5 | 0.64 |
| BMI, kg/m2, mean ± SD | 30.9 ± 6.2 | 32.7 ± 7.4 | 29.8 ± 5.1 | 0.10 |
| Creatinine, μmol/L, mean ± SD | 96.2 ± 36.2 | 75.5 ± 21.3 | 109.4 ± 37.7 | <0.01 * |
| CKD-EPI eGFR, mL/min/1.73 m2, median (IQR) | 76.5 (61.0–87.5) | 80.0 (64.5–90.0) | 76.0 (53.5–86.5) | 0.28 |
| Used antihypertensive drugs, mean ± SD | 4.6 ± 0.9 | 4.5 ± 1.0 | 4.6 ± 0.9 | 0.14 |
| Spironolactone dose, mg, mean ± SD | 37.7 ± 23.4 | 39.3 ± 24.1 | 36.7 ± 23.1 | 0.70 |
| Comorbidity, N (%) | ||||
| Diabetes | 16 (29.6) | 4 (19.1) | 12 (36.4) | 0.18 |
| Stroke | 6 (11.1) | 2 (9.5) | 4 (12.1) | 0.77 |
| Coronary artery disease | 7 (13.0) | 3 (14.3) | 4 (12.1) | 0.82 |
| Hypercholesterolemia | 16 (29.6) | 9 (42.9) | 7 (21.2) | 0.09 |
| Heart failure | 3 (5.6) | 0 (0.0) | 3 (9.1) | 0.16 |
| Asthma/COPD | 3 (5.6) | 2 (9.5) | 1 (3.0) | 0.32 |
| Peripheral vascular disease | 2 (3.7) | 1 (4.8) | 1 (3.0) | 0.75 |
| Aneurysm | 1 (1.9) | 0 (0.0) | 1 (3.0) | 0.43 |
| Atrial fibrillation | 2 (3.7) | 1 (4.8) | 1 (3.0) | 0.75 |
| None | 16 (30.0) | 6 (28.6) | 10 (30.3) | 0.89 |
| Characteristic | All (N = 35) | Female (N = 10) | Male (N = 25) | p-Value |
|---|---|---|---|---|
| Age, y, mean ± SD | 58.8 ± 11.4 | 60.3 ± 11.9 | 58.2 ± 11.4 | 0.64 |
| BMI, kg/m2, mean ± SD | 30.2 ± 5.6 | 30.2 ± 6.5 | 30.2 ± 5.3 | 0.99 |
| Creatinine, μmol/L, mean ± SD | 98.9 ± 36.7 | 73.8 ± 12.0 | 108.9 ± 38.6 | <0.001 * |
| CKD-EPI eGFR, mL/min/1.73m2, median (IQR) | 76.0 (62.0–84.0) | 74.5 (65.8–82.3) | 77.0 (53.0–86.5) | 0.28 |
| Prescribed antihypertensive drugs, mean ± SD | 4.4 ± 0.9 | 4.5 ± 1.0 | 4.4 ± 0.9 | 0.77 |
| Spironolactone dose, mg, mean ± SD | 34.3 ± 22.3 | 27.5 ± 12.9 | 37.0 ± 24.9 | 0.15 |
| Comorbidity, N (%) | ||||
| Diabetes | 12 (34.3) | 2 (20.0) | 10 (40.0) | 0.26 |
| Stroke | 4 (11.4) | 1 (10.0) | 3 (12.0) | 0.87 |
| Coronary artery disease | 6 (17.1) | 2 (20.0) | 4 (16.0) | 0.78 |
| Hypercholesterolemia | 11 (31.4) | 5 (50.0) | 6 (24.0) | 0.13 |
| Heart failure | 3 (8.6) | 0 (0.0) | 3 (12.0) | 0.25 |
| Asthma/COPD | 3 (8.6) | 2 (20.0) | 1 (4.0) | 0.13 |
| Peripheral vascular disease | 1 (2.9) | 1 (10.0) | 0 (0.0) | 0.11 |
| Atrial fibrillation | 2 (5.7) | 1 (10.0) | 1 (4.0) | 0.49 |
| None | 10 (28.6) | 3 (30.0) | 7 (28.0) | 0.91 |
| Spironolactone Whole Blood Concentration | Canrenone Whole Blood Concentration | |||||
|---|---|---|---|---|---|---|
| B (SE B) | β | p-Value | B (SE B) | β | p-Value | |
| Sex (ref = male) | −10.23 (7.92) | −0.214 | 0.206 | 1.24 (10.96) | 0.133 | 0.911 |
| Dose | −0.08 (0.17) | −0.076 | 0.656 | 0.49 (0.23) | 0.343 | 0.042 * |
| Difference in time between intake and sampling DBS | −1.27 (0.58) | −0.369 | 0.034 * | −1.52 (0.80) | −0.304 | 0.065 |
| N | 35 | 35 | ||||
| R square | 0.106 | 0.181 | ||||
| F-test | 2.349 | 0.092 | 3.503 | 0.027 * | ||
| Adherence Spironolactone | N (%) | ||
|---|---|---|---|
| Female (N = 21) | Male (N = 33) | p-Value | |
| Adherent | 13 (61.9) | 28 (84.9) | 0.100 |
| Non-adherent | 8 (38.1) | 5 (15.2) | |
| Adherence Other * | N (%) | ||
| Female (N = 21) | Male (N = 33) | p-Value | |
| Adherent | 12 (57.1) | 27 (81.8) | 0.054 |
| Partially adherent | 4 (19.1) | 5 (15.2) | |
| Non-adherent | 5 (23.8) | 1 (3.0) | |
| N (%) | |||
| Adherence Other * | Adherence Spironolactone | p-Value | |
| Adherent | Non-adherent | ≤0.001 | |
| Adherent | 34 (82.3) | 5 (38.5) | |
| Partially adherent | 6 (14.6) | 3 (23.1) | |
| Non-adherent | 1 (2.4) | 5 (38.5) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peeters, L.E.J.; Tjong, L.K.; Rietdijk, W.J.R.; van Gelder, T.; Koch, B.C.P.; Versmissen, J. Sex Differences in Spironolactone and the Active Metabolite Canrenone Concentrations and Adherence. Biomedicines 2022, 10, 137. https://doi.org/10.3390/biomedicines10010137
Peeters LEJ, Tjong LK, Rietdijk WJR, van Gelder T, Koch BCP, Versmissen J. Sex Differences in Spironolactone and the Active Metabolite Canrenone Concentrations and Adherence. Biomedicines. 2022; 10(1):137. https://doi.org/10.3390/biomedicines10010137
Chicago/Turabian StylePeeters, Laura E. J., Leonardien K. Tjong, Wim J. R. Rietdijk, Teun van Gelder, Birgit C. P. Koch, and Jorie Versmissen. 2022. "Sex Differences in Spironolactone and the Active Metabolite Canrenone Concentrations and Adherence" Biomedicines 10, no. 1: 137. https://doi.org/10.3390/biomedicines10010137
APA StylePeeters, L. E. J., Tjong, L. K., Rietdijk, W. J. R., van Gelder, T., Koch, B. C. P., & Versmissen, J. (2022). Sex Differences in Spironolactone and the Active Metabolite Canrenone Concentrations and Adherence. Biomedicines, 10(1), 137. https://doi.org/10.3390/biomedicines10010137






