Revertant Mosaicism in Epidermolysis Bullosa
Abstract
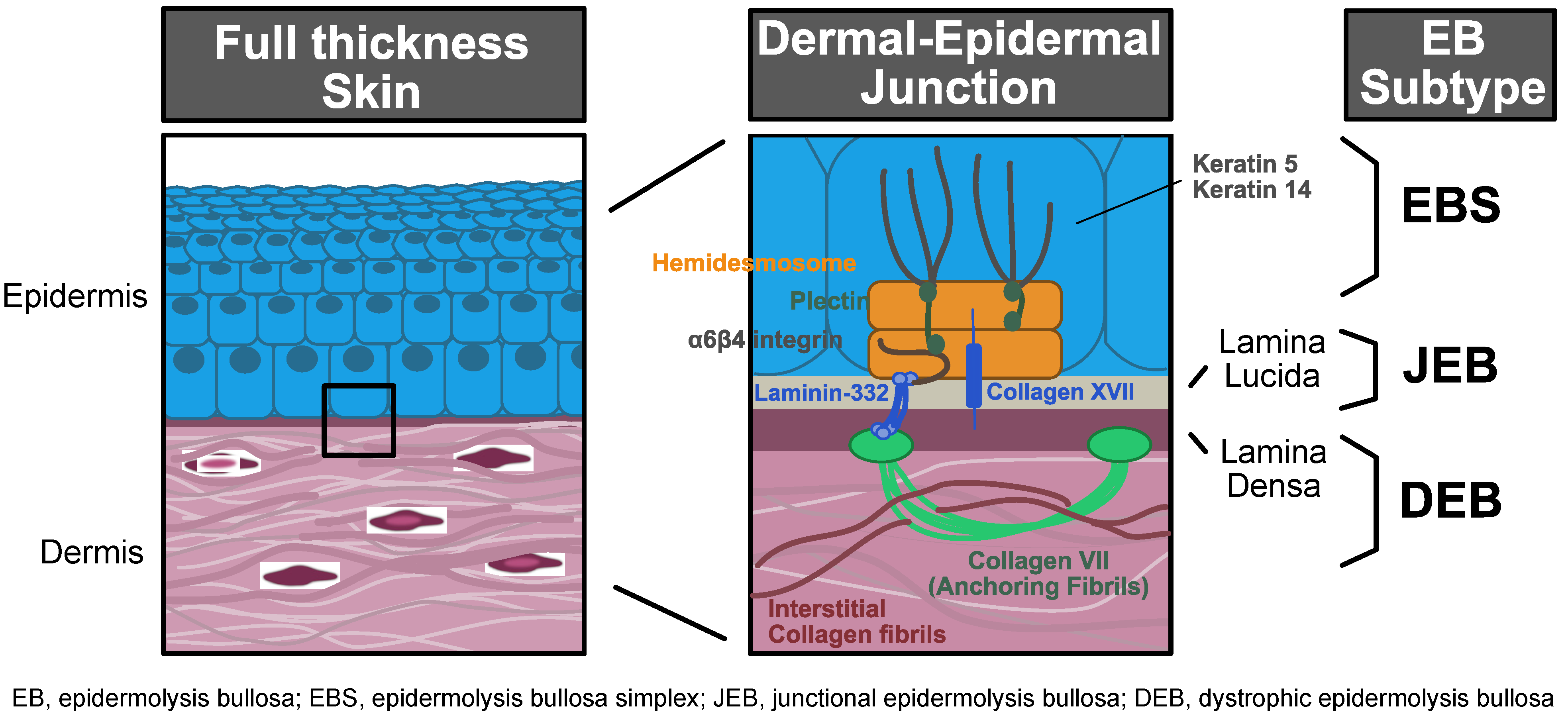
1. Introduction
2. Discovery of Revertant Mosaicism
3. Characterization of RM in EB
4. Therapeutic Applications of RM in EB
5. Future Steps to Expand Therapeutic Translation of RM
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EB | Epidermolysis bullosa |
| EBS | EB simplex |
| JEB | Junctional epidermolysis bullosa |
| DEB | Dystrophic epidermolysis bullosa |
| RDEB | Recessive dystrophic epidermolysis bullosa |
| MSC | Mesenchymal stromal cells |
| AlloHCT | Allogeneic hematopoietic cell transplant |
| RM | Revertant mosaicism |
| SNP | Single nucleotide polymorphism |
| TREx | Targeted RNA expression |
| SMRT | Single molecule real-time |
| iPSC | Induced pluripotent stem cell |
| CEA | Cultured epidermal autografts |
| IWC | Icthyosis with confetti |
References
- Patrizi, A.; Neri, I.; El Hachem, M.; Ravaioli, G.M.; Technau-Hafsi, K.; Has, C. Genetic blistering diseases. In Atlas of Dermatology, Dermatopathology and Venereology; Smoller, B., Bagherani, N., Eds.; Springer: Cham, Switzerland, 2022; pp. 465–494. [Google Scholar]
- Feinstein, J.A.; Jambal, P.; Peoples, K.; Lucky, A.W.; Khuu, P.; Tang, J.Y.; Lara-Corrales, I.; Pope, E.; Wiss, K.; Hook, K.P.; et al. Assessment of the Timing of Milestone Clinical Events in Patients With Epidermolysis Bullosa From North America. JAMA Dermatol. 2019, 155, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Bruckner, A.L.; Losow, M.; Wisk, J.; Patel, N.; Reha, A.; Lagast, H.; Gault, J.; Gershkowitz, J.; Kopelan, B.; Hund, M.; et al. The challenges of living with and managing epidermolysis bullosa: Insights from patients and caregivers. Orphanet J. Rare Dis. 2020, 15, 1. [Google Scholar] [CrossRef]
- Conget, P.; Rodriguez, F.; Kramer, S.; Allers, C.; Simon, V.; Palisson, F.; Gonzalez, S.; Yubero, M.J. Replenishment of type VII collagen and re-epithelialization of chronically ulcerated skin after intradermal administration of allogeneic mesenchymal stromal cells in two patients with recessive dystrophic epidermolysis bullosa. Cytotherapy 2010, 12, 429–431. [Google Scholar] [CrossRef] [PubMed]
- Petrof, G.; Martinez-Queipo, M.; Mellerio, J.E.; Kemp, P.; McGrath, J.A. Fibroblast cell therapy enhances initial healing in recessive dystrophic epidermolysis bullosa wounds: Results of a randomized, vehicle-controlled trial. Br. J. Dermatol. 2013, 169, 1025–1033. [Google Scholar] [CrossRef]
- Kopp, J.; Horch, R.E.; Stachel, K.D.; Holter, W.; Kandler, M.A.; Hertzberg, H.; Rascher, W.; Campean, V.; Carbon, R.; Schneider, H. Hematopoietic stem cell transplantation and subsequent 80% skin exchange by grafts from the same donor in a patient with Herlitz disease. Transplantation 2005, 79, 255–256. [Google Scholar] [CrossRef]
- Wagner, J.E.; Ishida-Yamamoto, A.; McGrath, J.A.; Hordinsky, M.; Keene, D.R.; Woodley, D.T.; Chen, M.; Riddle, M.J.; Osborn, M.J.; Lund, T.; et al. Bone marrow transplantation for recessive dystrophic epidermolysis bullosa. N. Engl. J. Med. 2010, 363, 629–639. [Google Scholar] [CrossRef]
- Ebens, C.L.; McGrath, J.A.; Tamai, K.; Hovnanian, A.; Wagner, J.E.; Riddle, M.J.; Keene, D.R.; DeFor, T.E.; Tryon, R.; Chen, M.; et al. Bone marrow transplant with post-transplant cyclophosphamide for recessive dystrophic epidermolysis bullosa expands the related donor pool and permits tolerance of nonhaematopoietic cellular grafts. Br. J. Dermatol. 2019, 181, 1238–1246. [Google Scholar] [CrossRef]
- Ebens, C.L.; McGrath, J.A.; Riedl, J.A.; Keith, A.R.; Lilja, G.; Rusch, S.; Keene, D.R.; Tufa, S.F.; Riddle, M.J.; Shanley, R.; et al. Immune tolerance of allogeneic haematopoietic cell transplantation supports donor epidermal grafting of recessive dystrophic epidermolysis bullosa chronic wounds. Br. J. Dermatol. 2021, 184, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Siprashvili, Z.; Nguyen, N.T.; Gorell, E.S.; Loutit, K.; Khuu, P.; Furukawa, L.K.; Lorenz, H.P.; Leung, T.H.; Keene, D.R.; Rieger, K.E.; et al. Safety and Wound Outcomes Following Genetically Corrected Autologous Epidermal Grafts in Patients with Recessive Dystrophic Epidermolysis Bullosa. JAMA 2016, 316, 1808–1817. [Google Scholar] [CrossRef]
- Vanden Oever, M.; Twaroski, K.; Osborn, M.J.; Wagner, J.E.; Tolar, J. Inside out: Regenerative medicine for recessive dystrophic epidermolysis bullosa. Pediatr. Res. 2018, 83, 318–324. [Google Scholar] [CrossRef]
- Yang, T.P.; Stout, J.T.; Konecki, D.S.; Patel, P.I.; Alford, R.L.; Caskey, C.T. Spontaneous reversion of novel Lesch-Nyhan mutation by HPRT gene rearrangement. Somat. Cell Mol. Genet. 1988, 14, 293–303. [Google Scholar] [CrossRef]
- Jonkman, M.F.; Scheffer, H.; Stulp, R.; Pas, H.H.; Nijenhuis, M.; Heeres, K.; Owaribe, K.; Pulkkinen, L.; Uitto, J. Revertant mosaicism in epidermolysis bullosa caused by mitotic gene conversion. Cell 1997, 88, 543–551. [Google Scholar] [CrossRef]
- Wada, T.; Candotti, F. Somatic mosaicism in primary immune deficiencies. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 510–514. [Google Scholar] [CrossRef]
- Darling, T.N.; Yee, C.; Bauer, J.W.; Hintner, H.; Yancey, K.B. Revertant mosaicism: Partial correction of a germ-line mutation in COL17A1 by a frame-restoring mutation. J. Clin. Investig. 1999, 103, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.J.; Morley, S.M.; McLean, W.H. Novel mechanism of revertant mosaicism in Dowling-Meara epidermolysis bullosa simplex. J. Investig. Dermatol. 2004, 122, 73–77. [Google Scholar] [CrossRef]
- Pasmooij, A.M.; Pas, H.H.; Deviaene, F.C.; Nijenhuis, M.; Jonkman, M.F. Multiple correcting COL17A1 mutations in patients with revertant mosaicism of epidermolysis bullosa. Am. J. Hum. Genet. 2005, 77, 727–740. [Google Scholar] [CrossRef][Green Version]
- Almaani, N.; Nagy, N.; Liu, L.; Dopping-Hepenstal, P.J.; Lai-Cheong, J.E.; Clements, S.E.; Techanukul, T.; Tanaka, A.; Mellerio, J.E.; McGrath, J.A. Revertant mosaicism in recessive dystrophic epidermolysis bullosa. J. Investig. Dermatol. 2010, 130, 1937–1940. [Google Scholar] [CrossRef] [PubMed]
- Lai-Cheong, J.E.; McGrath, J.A.; Uitto, J. Revertant mosaicism in skin: Natural gene therapy. Trends Mol. Med. 2011, 17, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Pasmooij, A.M.; Pas, H.H.; Bolling, M.C.; Jonkman, M.F. Revertant mosaicism in junctional epidermolysis bullosa due to multiple correcting second-site mutations in LAMB3. J. Clin. Investig. 2007, 117, 1240–1248. [Google Scholar] [CrossRef]
- Jonkman, M.F.; Pasmooij, A.M. Revertant mosaicism--patchwork in the skin. N. Engl. J. Med. 2009, 360, 1680–1682. [Google Scholar] [CrossRef]
- Kowalewski, C.; Bremer, J.; Gostynski, A.; Wertheim-Tysarowska, K.; Wozniak, K.; Bal, J.; Jonkman, M.F.; Pasmooij, A.M. Amelioration of junctional epidermolysis bullosa due to exon skipping. Br. J. Dermatol. 2016, 174, 1375–1379. [Google Scholar] [CrossRef]
- Pasmooij, A.M.; Garcia, M.; Escamez, M.J.; Nijenhuis, A.M.; Azon, A.; Cuadrado-Corrales, N.; Jonkman, M.F.; Del Rio, M. Revertant mosaicism due to a second-site mutation in COL7A1 in a patient with recessive dystrophic epidermolysis bullosa. J. Investig. Dermatol. 2010, 130, 2407–2411. [Google Scholar] [CrossRef]
- Kiritsi, D.; Garcia, M.; Brander, R.; Has, C.; Meijer, R.; Jose Escamez, M.; Kohlhase, J.; van den Akker, P.C.; Scheffer, H.; Jonkman, M.F.; et al. Mechanisms of natural gene therapy in dystrophic epidermolysis bullosa. J. Investig. Dermatol. 2014, 134, 2097–2104. [Google Scholar] [CrossRef] [PubMed]
- Tolar, J.; McGrath, J.A.; Xia, L.; Riddle, M.J.; Lees, C.J.; Eide, C.; Keene, D.R.; Liu, L.; Osborn, M.J.; Lund, T.C.; et al. Patient-specific naturally gene-reverted induced pluripotent stem cells in recessive dystrophic epidermolysis bullosa. J. Investig. Dermatol. 2014, 134, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Al Aboud, K.; Al Hawsawi, K.; Ramesh, V. Kindler syndrome: Two additional features. Dermatol. Online J. 2003, 9, 20. [Google Scholar] [CrossRef]
- Lai-Cheong, J.E.; Moss, C.; Parsons, M.; Almaani, N.; McGrath, J.A. Revertant mosaicism in Kindler syndrome. J. Investig. Dermatol. 2012, 132, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Kiritsi, D.; He, Y.; Pasmooij, A.M.; Onder, M.; Happle, R.; Jonkman, M.F.; Bruckner-Tuderman, L.; Has, C. Revertant mosaicism in a human skin fragility disorder results from slipped mispairing and mitotic recombination. J. Clin. Investig. 2012, 122, 1742–1746. [Google Scholar] [CrossRef] [PubMed]
- Pasmooij, A.M.; Jonkman, M.F.; Uitto, J. Revertant mosaicism in heritable skin diseases: Mechanisms of natural gene therapy. Discov. Med. 2012, 14, 167–179. [Google Scholar]
- Lim, Y.H.; Fisher, J.M.; Choate, K.A. Revertant mosaicism in genodermatoses. Cell. Mol. Life Sci. 2017, 74, 2229–2238. [Google Scholar] [CrossRef] [PubMed]
- Pasmooij, A.M.; Nijenhuis, M.; Brander, R.; Jonkman, M.F. Natural gene therapy may occur in all patients with generalized non-Herlitz junctional epidermolysis bullosa with COL17A1 mutations. J. Investig. Dermatol. 2012, 132, 1374–1383. [Google Scholar] [CrossRef]
- Schuilenga-Hut, P.H.; Scheffer, H.; Pas, H.H.; Nijenhuis, M.; Buys, C.H.; Jonkman, M.F. Partial revertant mosaicism of keratin 14 in a patient with recessive epidermolysis bullosa simplex. J. Investig. Dermatol. 2002, 118, 626–630. [Google Scholar] [CrossRef]
- van den Akker, P.C.; Nijenhuis, M.; Meijer, G.; Hofstra, R.M.; Jonkman, M.F.; Pasmooij, A.M. Natural gene therapy in dystrophic epidermolysis bullosa. Arch. Dermatol. 2012, 148, 213–216. [Google Scholar] [CrossRef]
- Twaroski, K.; Eide, C.; Riddle, M.J.; Xia, L.; Lees, C.J.; Chen, W.; Mathews, W.; Keene, D.R.; McGrath, J.A.; Tolar, J. Revertant mosaic fibroblasts in recessive dystrophic epidermolysis bullosa. Br. J. Dermatol. 2019, 181, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Stark, R.; Grzelak, M.; Hadfield, J. RNA sequencing: The teenage years. Nat. Rev. Genet. 2019, 20, 631–656. [Google Scholar] [CrossRef] [PubMed]
- Gostynski, A.; Deviaene, F.C.; Pasmooij, A.M.; Pas, H.H.; Jonkman, M.F. Adhesive stripping to remove epidermis in junctional epidermolysis bullosa for revertant cell therapy. Br. J. Dermatol. 2009, 161, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Gostynski, A.; Pasmooij, A.M.; Jonkman, M.F. Successful therapeutic transplantation of revertant skin in epidermolysis bullosa. J. Am. Acad. Dermatol. 2014, 70, 98–101. [Google Scholar] [CrossRef]
- Umegaki-Arao, N.; Pasmooij, A.M.; Itoh, M.; Cerise, J.E.; Guo, Z.; Levy, B.; Gostynski, A.; Rothman, L.R.; Jonkman, M.F.; Christiano, A.M. Induced pluripotent stem cells from human revertant keratinocytes for the treatment of epidermolysis bullosa. Sci. Transl. Med. 2014, 6, 264ra164. [Google Scholar] [CrossRef]
- Tolar, J.; McGrath, J.A. The three-body problem of therapy with induced pluripotent stem cells. Genome Med. 2015, 7, 15. [Google Scholar] [CrossRef][Green Version]
- Shinkuma, S.; Sawamura, D.; Fujita, Y.; Kawasaki, H.; Nakamura, H.; Inoie, M.; Nishie, W.; Shimizu, H. Long-term follow-up of cultured epidermal autograft in a patient with recessive dystrophic epidermolysis bullosa. Acta Derm. Venereol. 2014, 94, 98–99. [Google Scholar] [CrossRef][Green Version]
- Matsumura, W.; Fujita, Y.; Shinkuma, S.; Suzuki, S.; Yokoshiki, S.; Goto, H.; Hayashi, H.; Ono, K.; Inoie, M.; Takashima, S.; et al. Cultured Epidermal Autografts from Clinically Revertant Skin as a Potential Wound Treatment for Recessive Dystrophic Epidermolysis Bullosa. J. Investig. Dermatol. 2019, 139, 2115–2124.e11. [Google Scholar] [CrossRef]
- Yoshihara, M.; Oguchi, A.; Murakawa, Y. Genomic Instability of iPSCs and Challenges in Their Clinical Applications. Adv. Exp. Med. Biol. 2019, 1201, 23–47. [Google Scholar] [PubMed]
- Tolar, J.; Wagner, J.E. Allogeneic blood and bone marrow cells for the treatment of severe epidermolysis bullosa: Repair of the extracellular matrix. Lancet 2013, 382, 1214–1223. [Google Scholar] [CrossRef]
- Geyer, M.B.; Radhakrishnan, K.; Giller, R.; Umegaki, N.; Harel, S.; Kiuru, M.; Morel, K.D.; LeBoeuf, N.; Kandel, J.; Bruckner, A.; et al. Reduced Toxicity Conditioning and Allogeneic Hematopoietic Progenitor Cell Transplantation for Recessive Dystrophic Epidermolysis Bullosa. J. Pediatrics 2015, 167, 765–769.e1. [Google Scholar] [CrossRef] [PubMed]
- van den Akker, P.C.; Pasmooij, A.M.G.; Joenje, H.; Hofstra, R.M.W.; Te Meerman, G.J.; Jonkman, M.F. A “late-but-fitter revertant cell” explains the high frequency of revertant mosaicism in epidermolysis bullosa. PLoS ONE 2018, 13, e0192994. [Google Scholar] [CrossRef] [PubMed]
- Revy, P.; Kannengiesser, C.; Fischer, A. Somatic genetic rescue in Mendelian haematopoietic diseases. Nat. Rev. Genet. 2019, 20, 582–598. [Google Scholar] [CrossRef]



| Age at Diagnosis of RM, Sex | Disease Causing Alleles 1 | Partial or Complete Reversion | Reversion Mechanism | Size, Location, and Stability of Revertant Patches | Reference(s) |
|---|---|---|---|---|---|
| JEB generalized intermediate (AR, COL17A1 mutations) | |||||
| 28 y/o F | c.1601delA c.3676C > T (p.Arg1226X) | Complete Complete | Gene conversions (n = 3; upper extremities) Second site mutation, c. 3677G > C (left ankle) | Left upper arm, both lower arms, hands, left ankle, right lower leg, back and scalp (10% BSA). Some areas static, others with slow expansion. | Jonkman et al., 1997 [13] |
| Pasmooij et al., 2005 [17] (Pt 2) | |||||
| Jonkman et al., 2009 [21] (Pt 3) | |||||
| Pasmooij et al., 2012 [31] (026-01) | |||||
| 56 y/o F | c.4003delTC (homozygous) | Partial | Second site mutation restoring reading frame lost with original two nucleotide deletion: c.4080insGG | N/A | Darling et al., 1999 [15] |
| 75 y/o M | c.4320insC c.3676C > T (p.Arg1226X) | Complete Partial | Second site mutation in a splice site restoring frameshift: c.4358-1G > A (finger) Back mutation c.3676T > C or gene conversion (arm) | Right middle finger (2 cm2) and arm. Static. | Pasmooij et al., 2005 [17] (Pt 1) |
| Jonkman et al., 2009 [21] (Pt 8) | |||||
| Pasmooij et al., 2012 [31] (093-01) | |||||
| 46 y/o M | c.2237delG (p.Gly746AlafsX53) c.3676C > T (p.Arg1226X) | Complete | Second site mutation c.2263 + 2T > C | Bilat hands and lower arms, left upper arm, forehead, face | Jonkman et al., 2009 [21] (Pt 4) |
| Pasmooij et al., 2012 [31] (035-02) | |||||
| 48 y/o M | c.2237delG (p.Gly746AlafsX53) c.3676C > T (p.Arg1226X) | Complete | Second site mutations: c.2237insG (or gene conversion), c.2263 + 2T > C, Del14(2259–2263 + 9) | N/D | Jonkman et al., 2009 [21] (Pt 5) |
| 46 y/o M | c.2237delG (p.Gly746AlafsX53) (homozygous) | Complete | Second site mutations resulting in exon 30 skipping (location of disease-causing mutation): c.2238C > T, c.2227 + 153_2336-318del (large deletion, additionally skipping exon 31) | Wrist | Jonkman et al., 2009 [21] (Pt 13) |
| Pasmooij et al., 2012 [31] (208-01) | |||||
| 59 y/o F | c.2237delG (p.Gly746AlafsX53) (homozygous) | Complete | Second site mutation resulting in skipping of exon 30 (location of disease-causing mutation): c.2263_2T > C | Hands, arms, and back | Pasmooij et al., 2012 [31] (025-01) |
| 48 y/o M | c.2237delG (p.Gly746AlafsX53) c.3676C > T (p.Arg1226X) | Complete | Second site mutations resulting in exon 30 skipping (location of disease-causing mutation): c.2228-101_2263 + 70delins15 (indel), c.2259_2263 + 9del, c.2263 + 2T > C | Bilat knees and hands, patches on right upper leg | Pasmooij et al., 2012 [31] (035-01) |
| 21 y/o F | c.3487G > T (p.Glu1163Ter) c.1490_1491delinsT (p.Ala497Valfs*23) | Complete | Second site mutation in splice site resulting in exon 49 skipping (location of disease-causing mutation): c.3419-1G > T | Right lower arm | Kowalewski et al., 2016 [22] |
| EBS (AR, KRT14) | |||||
| 67 y/o F | c.526-2A > C (homozygous) | Partial | Second site mutations disrupting splice site acceptor created by original mutation: c.528T > G, c.529del6 (identified not in DNA but in mRNA) | N/A | Schuilenga-Hut et al., 2002 [32] |
| EBS (AD, KRT14) | |||||
| Early 20′s F | c.373C > T (p.Arg125Cys) | Complete | Second site mutation creating a premature termination codon nullifying the downstream dominant negative allele: c.242insG | Trunk blistering resolved over teen years. Extension with time. | Smith et al., 2004 [16] |
| JEB generalized intermediate (AR, LAMB3) | |||||
| 46 y/o M | c.628G > A (p.Glu210Lys) c.1903C > T (p.Arg635X) | Complete | Multiple second site mutations: c.628 + 42G > A, c.596G > C | Left lower leg. Extension with time. | Pasmooij et al., 2007 [20] (078-01) |
| 63 y/o M | c.628G > A (homozygous) | Complete | Multiple second site mutations: c.565-3T > C, c.619A > C, p.Lys207Gln, c.629-1G > A | Arm, shoulder, chest. Static. | Pasmooij et al., 2007 [20] (029-01) |
| RDEB generalized severe (AR, COL7A1) | |||||
| 41 y/o M | c.1732C > T (p.Arg578X) c.7786delG (p.Gly2593fsX4) | Complete | Intragenic crossover somewhere between the two mutations yielding one normal allele and one with c.7786delG mutation (Left wrist) | Left wrist, right shin (up to 8 × 5 cm). Static. | Almaani et al., 2010 [18] |
| 42 y/o F | c.6527insC (homozygous) | Complete | Second site mutation correcting the reading frame of the original mutation: c.6528delT | Left forearm (8 × 4.5 cm). Static. | Pasmooij et al., 2010 [23] |
| 21 y/o M | c.6508C > T (p.Gln2170X) (homozygous) | Complete | Second site mutation restoring nonsense codon created by original mutation: p.6510G > T (p.Gln2170Tyr) | Patch on right lateral neck (2.5 × 3 cm). Static. | Van den Akker et al., 2012 [33] (EB024) |
| 63 y/o M | c.425A > G c.8206G > A (p.Glu2736Lys) | Complete | Mitotic recombination resulting in loss of original c.425A > G mutation (which had caused altered splicing and a premature termination codon), but noted LOH of neighboring SNP c.2945A > G (p.Pro939Pro) thus deemed not to result from a back mutation | Left lower leg (two 3 × 3 cm patches). Static. | Kiritsi et al., 2014 [24] #2 |
| 21 y/o | c.2142A > G c.6527dupC (p.Gly2177Trpfs*113) | Complete | Second site mutation restoring splice site affected by original c.2144A > G mutation | Dorsum of right hand (7 × 3 cm) | Kiritsi et al., 2014 [24] #3 |
| 22 y/o | c.884delG c.6527dupC (p.Gly2177Trpfs*113) | Complete | Back mutation or mitotic recombination (unable to further differentiate) resulting in loss of original c.884delG mutation | Right lower arm (7 × 4 cm). Noted at 14 years of age. | Kiritsi et al., 2014 [24] #4 |
| 37 y/o | c.425A > G (homozygous) | Complete | Second site mutation restoring normal splice site caused by original c.425A > G mutation: c.426 + 3G > A | Lateral lower leg (4 × 4 cm). Static. | Kiritsi et al., 2014 [24] #5 |
| 17 y/o | c.425A > G c.1837C > T (p.Arg613*) | Complete | Mitotic recombination suggested as both original mutations detected and no additional mutations detected (presume recombination event placed original mutations on 1 allele with other allele without mutations) | Right hand | Kiritsi et al., 2014 [24] #6 |
| 12 y/o | c.4894C > T (p.Arg1632*) c.6176A > G (p.Glu2059Gly) | Complete | Back mutation/mitotic recombination (unable to further differentiate) resulting in loss of original c.6176A > G mutation | Back (10 × 5 cm), Lateral right leg (5 × 3 cm). Static. | Kiritsi et al., 2014 [24] #7 |
| Birth M | c.3840delC (p.Thr1280Thrfs*44) c.6751-2A > G (IVS85-sA > G) | Complete | Uncertain mechanism resulting in the retention of original c.3840delC mutation but skipping of exon 86 (3 outcomes: 6 bp skipped, 10 bp skipped and entire exon 86 skipped) downstream of c.6751-2A > G exon 85 acceptor splice site mutation | Pubic region (7 × 11 cm). Static. | Tolar et al., 2014 [25] |
| Kindler syndrome (AR, FERMT1) | |||||
| 22 y/o M | N/D | Complete | N/D (Normal skin structure of RM patch on biopsy) | Dorsal feet, left palm (4 cm), neck. Static. | Al Aboud et al., 2003 [26] |
| Birth M | c.676dupC (p.Gln226fsX17) (homozygous) | Complete | Transcriptional slippage or RNA editing: Loss of extra cytosine in mRNA despite genomic DNA still containing the mutation | Right hand. Static. | Lai-Cheong et al., 2012 [27] |
| 29 y/o M | c.456dupA (p.Asp153ArgfsX4) (homozygous) | Complete | Back mutation resulting in loss of the adenosine duplication and restoration of the reading frame on a single allele | Innumerable lesions of the entire integument (several mm2 to 15 cm2) | Kiritsi et al., 2012 [28] (P1) |
| 24 y/o F | c.676dupC (p.Gln226ProfsX17) (homozygous) | Complete | Back mutation resulting in loss of the cytosine duplication and restoration of the reading frame on a single allele | Hands, lower legs (0.5 cm2 to 2 cm2) | Kiritsi et al., 2012 [28] (P2) |
| 17 y/o F | c.676dupC (p.Gln226ProfsX17) (homozygous) | Complete | N/D, authors presume heterozygous back mutation restoring reading frame | Hands (0.5 cm2 to 3 cm2) | Kiritsi et al., 2012 [28] (P3) |
| 21 y/o F | c.676dupC (p.Gln226ProfsX17) (homozygous) | Complete | N/D, authors presume heterozygous back mutation restoring reading frame | Hands, neck, legs (0.5 cm2 to 3 cm2) | Kiritsi et al., 2012 [28] (P4) |
| 11 y/o F | c.676dupC (p.Gln226ProfsX17) (homozygous) | Complete | N/D, authors presume heterozygous back mutation restoring reading frame | Hands, lower legs (0.5 cm2 to 2 cm2) | Kiritsi et al., 2012 [28] (P5) |
| 9 y/o M | c.676dupC (p.Gln226ProfsX17) c.1677G > A (p.Trp559X) | Complete | N/D, authors presume heterozygous back mutation restoring reading frame | Hands, arms, legs (several mm2 to 1 cm2) | Kiritsi et al., 2012 [28] (P6) |
| DDEB (AD, COL7A1) | |||||
| 23 y/o | c.6127G > A (p.Gly2043Arg) | Complete | Back mutation/mitotic recombination restoring normal sequence: c.6127C > A | Right arm (3 × 3 cm) | Kiritsi et al., 2014 [24] #1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meyer-Mueller, C.; Osborn, M.J.; Tolar, J.; Boull, C.; Ebens, C.L. Revertant Mosaicism in Epidermolysis Bullosa. Biomedicines 2022, 10, 114. https://doi.org/10.3390/biomedicines10010114
Meyer-Mueller C, Osborn MJ, Tolar J, Boull C, Ebens CL. Revertant Mosaicism in Epidermolysis Bullosa. Biomedicines. 2022; 10(1):114. https://doi.org/10.3390/biomedicines10010114
Chicago/Turabian StyleMeyer-Mueller, Cameron, Mark J. Osborn, Jakub Tolar, Christina Boull, and Christen L. Ebens. 2022. "Revertant Mosaicism in Epidermolysis Bullosa" Biomedicines 10, no. 1: 114. https://doi.org/10.3390/biomedicines10010114
APA StyleMeyer-Mueller, C., Osborn, M. J., Tolar, J., Boull, C., & Ebens, C. L. (2022). Revertant Mosaicism in Epidermolysis Bullosa. Biomedicines, 10(1), 114. https://doi.org/10.3390/biomedicines10010114







