Methodology of Selecting the Optimal Receptor to Create an Electrochemical Immunosensor for Equine Arteritis Virus Protein Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Hybridoma Establishment and Antibody Production
2.3. Isolation of Protein
2.3.1. Protein for the Pathogen Equine Arteritis Virus (EVA-N-HisTAG)
2.3.2. Protein for the Pathogen Streptococcus Equi (SeM-HisTAG)
2.3.3. Protein for the Pathogen Equine Herpesvirus (SUMO-EHV-1 gG)
2.4. Apparatus
2.5. Immunosensor Fabrication
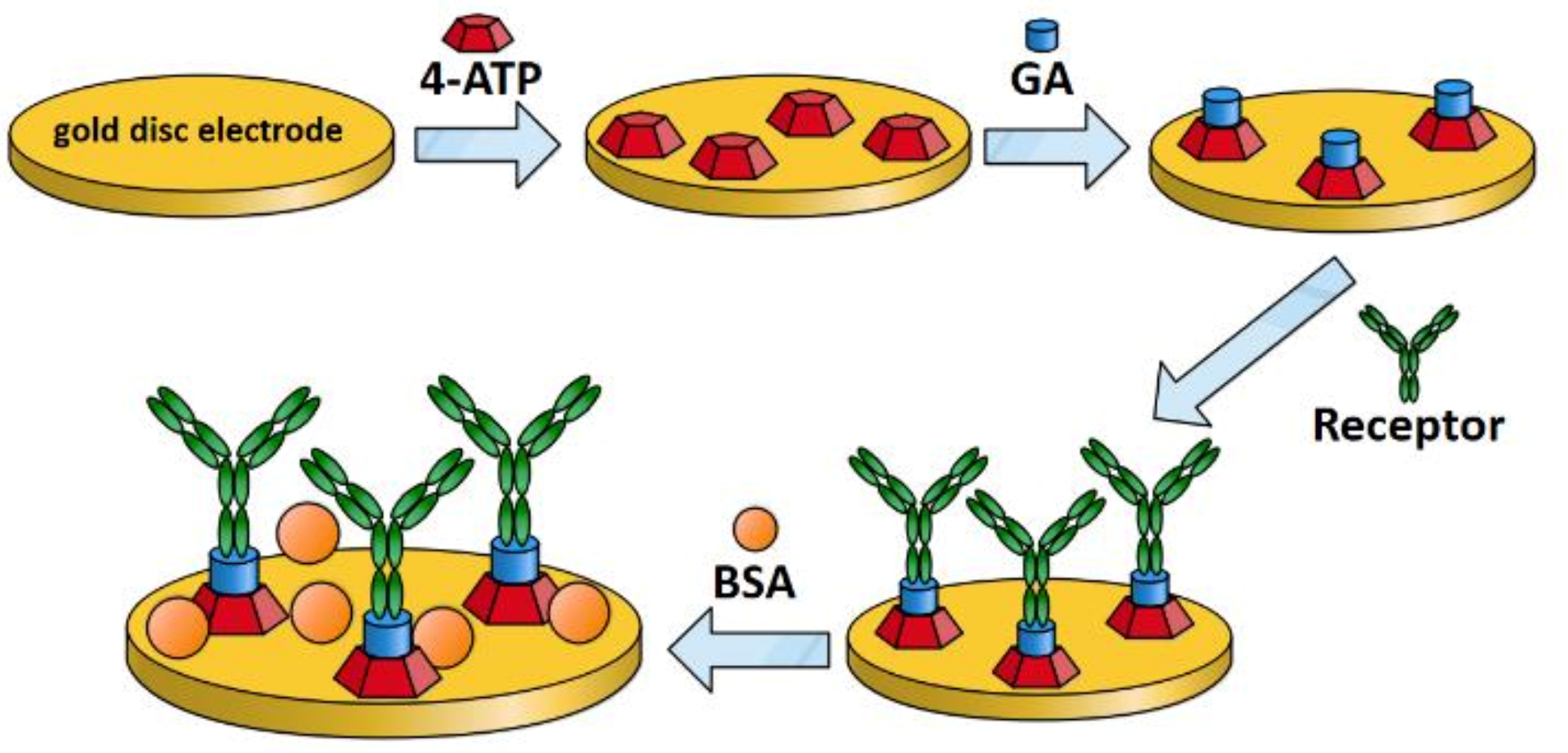
2.5.1. Preparation and Modification of Gold Disc Electrodes
2.5.2. QC Sensors
2.5.3. Electrochemical Experiments
2.6. Quartz Crystal Microbalance Measurements
2.7. Selection of Antibodies
2.8. Characteristics of the Immunosensor
3. Results
3.1. Characterization of Immunosensor
3.2. Determination of the Operating Parameters of the Immunosensor
3.2.1. Selection of Antibodies: Cross-Reactivity Tests
3.2.2. Accurate Evaluation of the Parameters of the Selected Receptor
3.2.3. Stability of Biosensor and Optimization of Storage Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tatem, A.J.; Rogers, D.J.; Hay, S.I. Global Transport Networks and Infectious Disease Spread. Adv. Parasitol. 2006, 62, 293–343. [Google Scholar] [CrossRef]
- Drexler, M.; Institute of Medicine (US). What You Need to Know about Infectious Disease; National Academies Press (US): Washington, DC, USA, 2010. [Google Scholar]
- Carossino, M.; Loynachan, A.T.; Canisso, I.F.; Cook, R.F.; Campos, J.R.; Nam, B.; Go, Y.Y.; Squires, E.L.; Troedsson, M.H.T.; Swerczek, T.; et al. Equine Arteritis Virus Has Specific Tropism for Stromal Cells and CD8+ T and CD21+ B Lymphocytes but Not for Glandular Epithelium at the Primary Site of Persistent Infection in the Stallion Reproductive Tract. J. Virol. 2017, 91, e00418-17. [Google Scholar] [CrossRef] [Green Version]
- Timoney, P.J.; McCollum, W.H.; Roberts, A.W.; McDonald, M.J. Status of Equine Viral Arteritis in Kentucky, 1985. J. Am. Vet. Med. Assoc. 1987, 191, 36–39. [Google Scholar] [PubMed]
- Monreal, L.; Villatoro, A.J.; Hooghuis, H.; Ros, I.; Timoney, P.J. Clinical Features of the 1992 Outbreak of Equine Viral Arteritis in Spain. Equine Vet. J. 1995, 27, 301–304. [Google Scholar] [CrossRef]
- Wood, J.; Chirnside, E.; Mumford, J.; Higgins, A. First Recorded Outbreak of Equine Viral Arteritis in the United Kingdom. Vet. Rec. 1994, 136, 381. [Google Scholar] [CrossRef]
- Holyoak, G.R.; Balasuriya, U.B.R.; Broaddus, C.C.; Timoney, P.J. Equine Viral Arteritis: Current Status and Prevention. Theriogenology 2008, 70, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Miszczak, F.; Legrand, L.; Balasuriya, U.B.R.; Ferry-Abitbol, B.; Zhang, J.; Hans, A.; Fortier, G.; Pronost, S.; Vabret, A. Emergence of Novel Equine Arteritis Virus (EAV) Variants during Persistent Infection in the Stallion: Origin of the 2007 French EAV Outbreak Was Linked to an EAV Strain Present in the Semen of a Persistently Infected Carrier Stallion. Virology 2012, 423, 165–174. [Google Scholar] [CrossRef] [Green Version]
- Żychska, M.; Rakowska, A.; Bereznowski, A.; Witkowski, L. z Samodzielnej Pracowni Epidemiologii i Ekonomiki Weterynaryjnej Wydziału Medycyny Weterynaryjnej w Warszawie. Życie Weter. 2019, 94, 607–609. [Google Scholar]
- Timoney, P.J.; McCollum, W.H. Equine Viral Arteritis. Vet. Clin. N. Am. Equine Pract. 1993, 9, 295–309. [Google Scholar] [CrossRef]
- Ruiz-Sáenz, J. Equine Viral Arteritis: Epidemiological and Intervention Perspectives. Rev. Colomb. Cienc. Pecu. 2010, 23, 501–512. [Google Scholar]
- Timoney, P. Factors Influencing the International Spread of Equine Diseases. Vet. Clin. N. Am. Equine Pract. 2001, 16, 537–551. [Google Scholar] [CrossRef]
- Balasuriya, U.B.R.; Sarkar, S.; Carossino, M.; Go, Y.Y.; Chelvarajan, L.; Cook, R.F.; Loynachan, A.T.; Timoney, P.J.; Bailey, E. Host Factors That Contribute to Equine Arteritis Virus Persistence in the Stallion: An Update. J. Equine Vet. Sci. 2016, 43, S11–S17. [Google Scholar] [CrossRef] [Green Version]
- Timoney, P.J. The Increasing Significance of International Trade in Equids and Its Influence on the Spread of Infectious Diseases. Ann. N. Y. Acad. Sci. 2000, 916, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Garber, L. Eva: Equine Viral Arteritis and The U.S. Horse Industry. Miscellaneous Publications; United States Department of Agriculture, National Animal Health Monitoring System: 2000. Available online: https://ageconsearch.umn.edu/record/32749/ (accessed on 30 August 2021).
- Lazić, S.; Lupulović, D.; Gaudaire, D.; Petrovic, T.; Lazić, G.; Hans, A. Serological Evidence of Equine Arteritis Virus Infection and Phylogenetic Analysis of Viral Isolates in Semen of Stallions from Serbia. BMC Vet. Res. 2017, 13, 316. [Google Scholar] [CrossRef] [Green Version]
- Hedges, J.F.; Balasuriya, U.B.; Timoney, P.J.; McCollum, W.H.; MacLachlan, N.J. Genetic Divergence with Emergence of Novel Phenotypic Variants of Equine Arteritis Virus during Persistent Infection of Stallions. J. Virol. 1999, 73, 3672–3681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chirnside, E.D.; Francis, P.M.; de Vries, A.A.; Sinclair, R.; Mumford, J.A. Development and Evaluation of an ELISA Using Recombinant Fusion Protein to Detect the Presence of Host Antibody to Equine Arteritis Virus. J. Virol. Methods 1995, 54, 1–13. [Google Scholar] [CrossRef]
- Cho, H.J.; Entz, S.C.; Deregt, D.; Jordan, L.T.; Timoney, P.J.; McCollum, W.H. Detection of Antibodies to Equine Arteritis Virus by a Monoclonal Antibody-Based Blocking ELISA. Can. J. Vet. Res. 2000, 64, 38–43. [Google Scholar]
- Severgnini, M.; Cremonesi, P.; Consolandi, C.; Bellis, G.; Castiglioni, B. Advances in DNA Microarray Technology for the Detection of Foodborne Pathogens. Food Bioprocess Technol. 2011, 6, 936–953. [Google Scholar] [CrossRef]
- Ward, S.; Lindsley, A.; Courter, J.; Assa’ad, A. Clinical Testing for COVID-19. J. Allergy Clin. Immunol. 2020, 146, 23–34. [Google Scholar] [CrossRef]
- Velusamy, V.; Arshak, K.; Korostynska, O.; Oliwa, K.; Adley, C. An Overview of Foodborne Pathogen Detection: In the Perspective of Biosensors. Biotechnol. Adv. 2010, 28, 232–254. [Google Scholar] [CrossRef]
- Ahmed, A.; Rushworth, J.V.; Hirst, N.A.; Millner, P.A. Biosensors for Whole-Cell Bacterial Detection. Clin. Microbiol. Rev. 2014, 27, 631–646. [Google Scholar] [CrossRef] [Green Version]
- Legrand, L.; Pitel, P.H.; Fortier, G.; Pronost, S.; Vabret, A. Testing for Antibodies to Equine Arteritis Virus. Vet. Rec. 2007, 161, 599–600. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, A.; Koudelkova, Z.; Sedlackova, E.; Richtera, L.; Adam, V. Review—Electrochemical Sensors and Biosensors for Determination of Mercury Ions. J. Electrochem. Soc. 2018, 165, B824–B834. [Google Scholar] [CrossRef]
- Singhal, C.; Khanuja, M.; Chaudhary, N.; Pundir, C.S.; Narang, J. Detection of Chikungunya Virus DNA Using Two-Dimensional MoS2 Nanosheets Based Disposable Biosensor. Sci. Rep. 2018, 8, 7734. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, P. Biosensors and Their Applications—A Review. J. Oral Biol. Craniofac. Res. 2016, 6, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Contreras-Naranjo, J.E.; Aguilar, O. Suppressing Non-Specific Binding of Proteins onto Electrode Surfaces in the Development of Electrochemical Immunosensors. Biosensors 2019, 9, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, X.; Zhang, X.; Xu, H. Establishment of Hybridoma Cell Lines Producing Monoclonal Antibodies against Hepatitis B Virus Surface Antigens (a, d, and r) and Development of Sensitive ELISA Diagnostic Test. Hybridoma 2001, 20, 47–52. [Google Scholar] [CrossRef]
- Shulman, M.; Wilde, C.D.; Köhler, G. A Better Cell Line for Making Hybridomas Secreting Specific Antibodies. Nature 1978, 276, 269–270. [Google Scholar] [CrossRef] [PubMed]
- Białobrzeska, W.; Dziąbowska, K.; Lisowska, M.; Mohtar, M.A.; Muller, P.; Vojtesek, B.; Krejcir, R.; O’Neill, R.; Hupp, T.R.; Malinowska, N.; et al. An Ultrasensitive Biosensor for Detection of Femtogram Levels of the Cancer Antigen AGR2 Using Monoclonal Antibody Modified Screen-Printed Gold Electrodes. Biosensors 2021, 11, 184. [Google Scholar] [CrossRef]
- Białobrzeska, W.; Firganek, D.; Czerkies, M.; Lipniacki, T.; Skwarecka, M.; Dziąbowska, K.; Cebula, Z.; Malinowska, N.; Bigus, D.; Bięga, E.; et al. Electrochemical Immunosensors Based on Screen-Printed Gold and Glassy Carbon Electrodes: Comparison of Performance for Respiratory Syncytial Virus Detection. Biosensors 2020, 10, 175. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of Effective Capacitance and Film Thickness from Constant-Phase-Element Parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Ryl, J.; Wysocka, J.; Cieslik, M.; Krakowiak, S.; Slepski, P. Galvanostatic Impedance Measurements for the Efficient Adsorption Isotherm Construction in Corrosion Inhibitor Studies. Electrochem. Commun 2013, 27, 42–45. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, R.; Li, X.-Z.; Wang, L.; Huang, X.-J. Layer-by-Layer Assembly and Electrochemical Study of a 4-Aminothiophenol and Ytterbium(Iii) Trifluoromethanesulfonate Hydrate Film on a Gold Electrode. Analyst 2011, 136, 3997. [Google Scholar] [CrossRef]
- Wang, M.; Wang, L.; Wang, G.; Ji, X.; Bai, Y.; Li, T.; Gong, S.; Li, J. Application of Impedance Spectroscopy for Monitoring Colloid Au-Enhanced Antibody Immobilization and Antibody-Antigen Reactions. Biosens. Bioelectron. 2004, 19, 575–582. [Google Scholar] [CrossRef]
- Kaur, S.; Kaur, I. Self-Assembly of p-Aminothiophenol on Gold Surface: Application for Impedimetric and Potentiometric Sensing of Cobalt (II) Ions—A Comparative Study. Electroanalysis 2019, 31, 2507–2517. [Google Scholar] [CrossRef]
- Rosario-Castro, B.I.; Fachini, E.R.; Hernández, J.; Pérez-Davis, M.E.; Cabrera, C.R. Electrochemical and Surface Characterization of 4-Aminothiophenol Adsorption at Polycrystalline Platinum Electrodes. Langmuir 2006, 22, 6102–6108. [Google Scholar] [CrossRef] [PubMed]
- Medina, S.C.; Farinha, A.S.F.; Emwas, A.-H.; Tabatabai, A.; Leiknes, T. A Fundamental Study of Adsorption Kinetics of Surfactants onto Metal Oxides Using Quartz Crystal Microbalance with Dissipation (QCM-D). Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124237. [Google Scholar] [CrossRef]
- 10 MHZ Quartz Sensors Box for Liquid Biosensing—10 Pieces. Available online: https://store.openqcm.com/10-mhz-quartz-sensors-box-for-liquid-biosensing-10-pieces.html (accessed on 28 May 2021).
- Ryl, J.; Wysocka, J.; Cieslik, M.; Gerengi, H.; Ossowski, T.; Krakowiak, S.; Niedzialkowski, P. Understanding the Origin of High Corrosion Inhibition Efficiency of Bee Products towards Aluminium Alloys in Alkaline Environments. Electrochim. Acta 2019, 304, 263–274. [Google Scholar] [CrossRef]
- Cebula, Z.; Żołędowska, S.; Dziąbowska, K.; Skwarecka, M.; Malinowska, N.; Białobrzeska, W.; Czaczyk, E.; Siuzdak, K.; Sawczak, M.; Bogdanowicz, R.; et al. Detection of the Plant Pathogen Pseudomonas Syringae Pv. Lachrymans on Antibody-Modified Gold Electrodes by Electrochemical Impedance Spectroscopy. Sensors 2019, 19, 5411. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, L.R.; Gunasekaran, G. Corrosion Inhibition of Mild Steel by Plant Extract in Dilute HCl Medium. Corros. Sci. 2007, 49, 1143–1161. [Google Scholar] [CrossRef]
- Noor, E.A. Potential of Aqueous Extract of Hibiscus Sabdariffa Leaves for Inhibiting the Corrosion of Aluminum in Alkaline Solutions. J. Appl. Electrochem. 2009, 39, 1465–1475. [Google Scholar] [CrossRef]





| Stage | Rct/Ω | Q/μF × s(1−n) | n/− | Chi-Square |
|---|---|---|---|---|
| Bare | 298.93 | 59.40 | 0.38 | 2 × 10−3 |
| 4-ATP | 78.06 | 7.30 | 0.64 | 1 × 10−4 |
| GA | 805.80 | 0.79 | 0.88 | 3 × 10−4 |
| Receptor | 1008.00 | 0.80 | 0.87 | 2 × 10−4 |
| BSA | 1538.00 | 0.71 | 0.87 | 3 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brodowski, M.; Kowalski, M.; Białobrzeska, W.; Pałka, K.; Walkusz, R.; Roguszczak, J.; Łęga, T.; Sosnowska, M.; Biedulska, M.; Kurzawa, J.K.; et al. Methodology of Selecting the Optimal Receptor to Create an Electrochemical Immunosensor for Equine Arteritis Virus Protein Detection. Chemosensors 2021, 9, 265. https://doi.org/10.3390/chemosensors9090265
Brodowski M, Kowalski M, Białobrzeska W, Pałka K, Walkusz R, Roguszczak J, Łęga T, Sosnowska M, Biedulska M, Kurzawa JK, et al. Methodology of Selecting the Optimal Receptor to Create an Electrochemical Immunosensor for Equine Arteritis Virus Protein Detection. Chemosensors. 2021; 9(9):265. https://doi.org/10.3390/chemosensors9090265
Chicago/Turabian StyleBrodowski, Mateusz, Marcin Kowalski, Wioleta Białobrzeska, Katarzyna Pałka, Rafał Walkusz, Justyna Roguszczak, Tomasz Łęga, Marta Sosnowska, Małgorzata Biedulska, Joanna Kreczko Kurzawa, and et al. 2021. "Methodology of Selecting the Optimal Receptor to Create an Electrochemical Immunosensor for Equine Arteritis Virus Protein Detection" Chemosensors 9, no. 9: 265. https://doi.org/10.3390/chemosensors9090265
APA StyleBrodowski, M., Kowalski, M., Białobrzeska, W., Pałka, K., Walkusz, R., Roguszczak, J., Łęga, T., Sosnowska, M., Biedulska, M., Kurzawa, J. K., Bięga, E., Wysocka, J., Lisowska, M., Niedźwiedzka, K., Lipiński, T., Żołędowska, S., & Nidzworski, D. (2021). Methodology of Selecting the Optimal Receptor to Create an Electrochemical Immunosensor for Equine Arteritis Virus Protein Detection. Chemosensors, 9(9), 265. https://doi.org/10.3390/chemosensors9090265






