Biosensors for Detection and Monitoring of Joint Infections
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Selection
2.1.1. Inclusion Criteria
2.1.2. Exclusion Criteria
2.2. Search
2.3. Data Collection Process
2.4. Data Items
2.5. Study Risk of Bias Assessment
3. Results
3.1. Search results
3.2. Study Characteristics
3.3. Use of Biosensors in the Diagnosis of JI
| Author and Year | Journal Name | Type of Study and Level of Evidence | Sample Size | Joint Involved | Primary Surgery/Revision |
|---|---|---|---|---|---|
| Jacovides et al., 2012 [18] | The Jurnal of Bone and Joint Surgery | Diagnostic study, LOE III | 341 (133 men) | Knee or Hip | PJI |
| Rasouli et al., 2012 [23] | The Journal of arthroplasty | Prospective observational study, LOE III | 65 (33 men) | Knee | PJI |
| Chang et al., 2014 [24] | Lab on a Chip | Laboratory study | 9 (6 men) | Not specified | PJI |
| Fargašová et al., 2017 [25] | Clinical Orthopaedics and Related Research | Laboratory study | 4 (sex not specified) | Knee | PJI |
| Author and Year | Type of Bacteria | Biosensor | Clinical Specimen | Characteristics of the Biosensor | Advantages |
|---|---|---|---|---|---|
| Jacovides et al., 2012 [18] | Candida spp., Streptococcus spp., Treponema spp., Peptostreptococcus spp., corynebacterium spp., Enterococcus spp., Staphylococcus spp. | Ibis T5000 biosensor (PCR and Mass spectrometry) | Joint fluid and/or tissue specimens | The Ibis technique is based on the principle that microbial organisms have genomes containing sets of shared genes at various taxonomic levels and that can provide targets for detection and speciation. The broadest range primers are designed to amplify a product from an entire domain of microbial life. In contrast, more specific primers are designed to identify genera and species in major pathogenic groups, as well as genes that determine antibiotic resistance. The presence of one or more organisms, the presence of staphylococci and/or streptococci, and a confidence level of >0.7 for the identification of any organism had the most significant sensitivity for PJI Limitations: The sensitivity of Ibis is not high as that of conventional PCR | The Ibis looks to be a viable tool for identifying organisms in periprosthetic joint infection that is culture-negative. Its great sensitivity prevents its use as a diagnostic tool for periprosthetic joint infection at this time, as it appears to be capable of detecting organisms that are not linked with clinically significant illness. Nonetheless, we feel these data point to the complex biology of periprosthetic joint infection, and we believe they constitute true-positive results. |
| Rasouli et al., 2012 [23] | Candida spp., Enterococcus spp., Staphylococcus spp. | Ibis T5000 biosensor (PCR and Mass spectrometry) | Joint fluid | Ibis identified a pathogen with a confidence level of 0.7 or higher in a total of 36 cases. Limitations: The sensitivity of Ibis is not high as that of conventional PCR | The Ibis T5000 universal biosensor is a promising technology that has been utilized to identify a wide range of pathogens in sepsis. It has the potential to overcome the limitations of the PCR approach for PJI diagnosis. Furthermore, pan-genomic amplification may allow Ibis to detect infecting organisms that would otherwise be missed by traditional PCR. |
| Chang et al., 2014 [24] | Staphylococcus spp., Enterobacter spp. and Acynetobacter spp. | Integrated microfluidic chip (EIS) | Joint fluid | Biosensors have micro-components for liquid transportation, such as normally closed valves and pneumatically driven micro-pumps, so that samples and reagents can be regulated automatically by the integrated control system. | A new microfluidic system was developed for rapid and accurate diagnosis of PJI instead of the conventional methods for PJI diagnosis |
| Fargašová et al., 2017 [25] | Staphylococcus aureus and Streptococcus pyogenes | Magnetically assisted surface-enhanced Raman spectroscopy (MA-SERS) | Joint fluid | MA-SERS uses streptavidin-modified magnetic nanoparticles whose surface is functionalized with suitable biotinylated antibodies and then coated with silver nanoparticles by self-assembly. | The MA-SERS procedure is simple, versatile, inexpensive, and quick to perform. Moreover, it could be a valid alternative to Koch′s culturing or colony counting methods |
3.4. Quality Assessment
4. Discussion
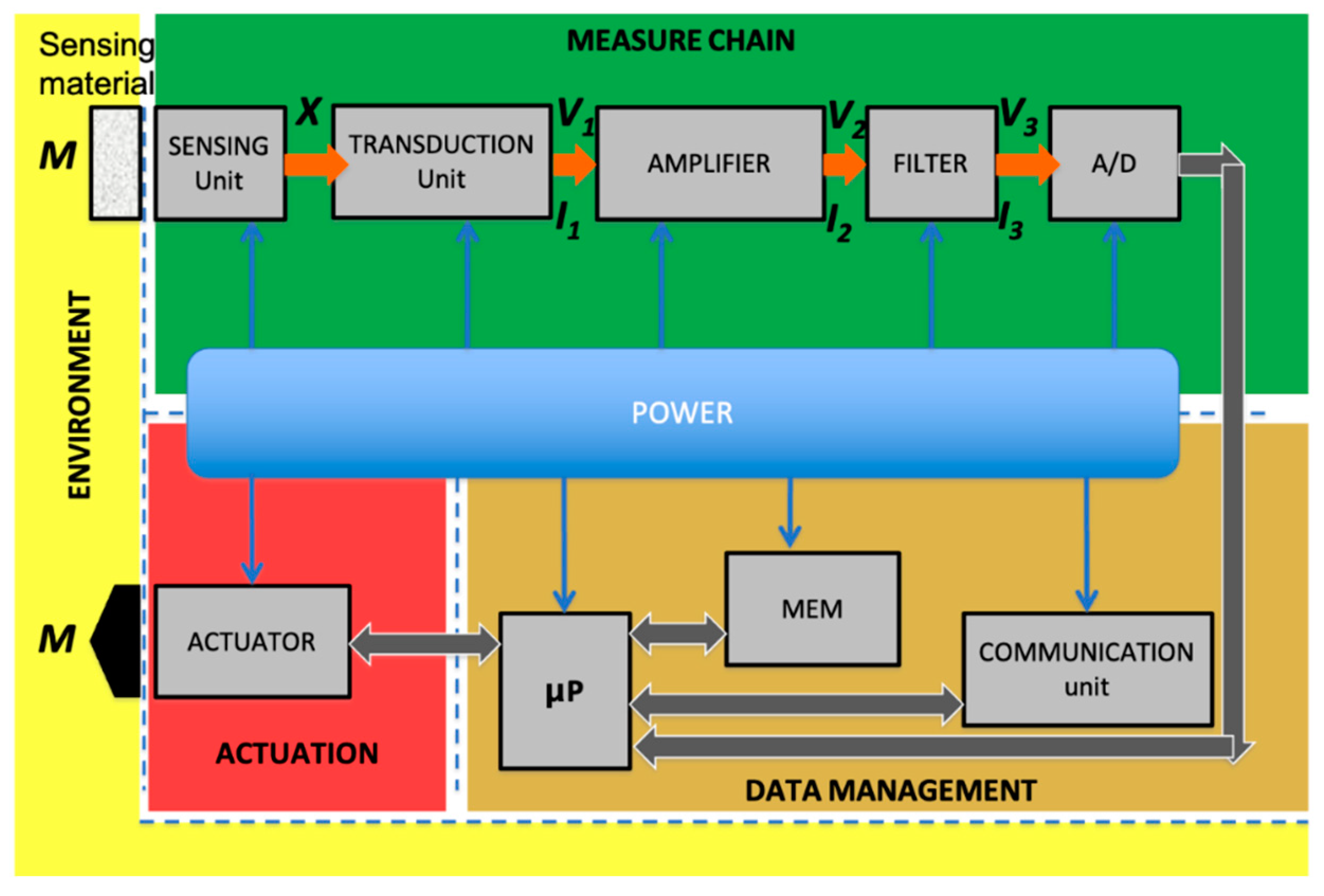
4.1. Sensor Measure Chain
4.2. Working Principles and Transduction Mechanisms
- -
- Electrochemical sensors;
- -
- Optical sensors;
- -
- Conductometric sensors;
- -
- Piezoelectric sensors;
- -
- Surface plasmon resonance sensors.
4.2.1. Electrochemical Sensors
4.2.2. Optical Sensors
4.2.3. Surface Plasmon Resonance Sensors
4.2.4. Conductometric Sensors
4.2.5. Acoustic and Piezoelectric Sensors
4.3. Sensing Materials
4.4. Focus on Joint Infections
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Boselli, E.; Allaouchiche, B. Diffusion in bone tissue of antibiotics. Presse Méd. 1999, 28, 2265–2276. [Google Scholar]
- Zegzulková, K.; Forejtová, Š. Differential diagnosis of monoarthritis. Cas. Lek. Ceskych 2016, 155, 299–304. [Google Scholar]
- García-Arias, M.; Balsa, A.; Mola, E.M. Septic arthritis. Best Pract. Res. Clin. Rheumatol. 2011, 25, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Coakley, G.; Mathews, C.; Field, M.; Jones, A.; Kingsley, G.; Walker, D.; Phillips, M.; Bradish, C.; McLachlan, A.; Mohammed, R.; et al. BSR & BHPR, BOA, RCGP and BSAC guidelines for management of the hot swollen joint in adults. Rheumatology 2006, 45, 1039–1041. [Google Scholar] [CrossRef]
- Mathews, C.J.; Kingsley, G.; Field, M.; Jones, A.; Weston, V.C.; Phillips, M.; Walker, D.; Coakley, G. Management of septic arthritis: A systematic review. Postgrad. Med. J. 2008, 84, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Roerdink, R.L.; Huijbregts, H.J.T.A.M.; Van Lieshout, A.W.T.; Dietvorst, M.; Van Der Zwaard, B.C. The difference between native septic arthritis and prosthetic joint infections: A review of literature. J. Orthop. Surg. 2019, 27, 2309499019860468. [Google Scholar] [CrossRef]
- Singh, J.A.; Yu, S. The burden of septic arthritis on the U.S. inpatient care: A national study. PLoS ONE 2017, 12, e0182577. [Google Scholar] [CrossRef]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of Primary and Revision Hip and Knee Arthroplasty in the United States from 2005 to 2030. J. Bone Joint Surg. Am. 2007, 89, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Petis, S.; Howard, J.L.; Lanting, B.L.; Vasarhelyi, E.M. Surgical approach in primary total hip arthroplasty: Anatomy, technique and clinical outcomes. Can. J. Surg. 2015, 58, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Büttner, M.; Mayer, A.M.; Büchler, B.; Betz, U.; Drees, P.; Susanne, S. Economic analyses of fast-track total hip and knee arthroplasty: A systematic review. Eur. J. Orthop. Surg. Traumatol. 2020, 30, 67–74. [Google Scholar] [CrossRef]
- Yi, P.H.; Cross, M.B.; Moric, M.; Sporer, S.M.; Berger, R.A.; Della Valle, C.J. The 2013 Frank Stinchfield Award: Diagnosis of Infection in the Early Postoperative Period After Total Hip Arthroplasty. Clin. Orthop. Relat. Res. 2014, 472, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Bedair, H.; Ting, N.; Jacovides, C.; Saxena, A.; Moric, M.; Parvizi, J.; Della Valle, C.J. The Mark Coventry Award: Diagnosis of Early Postoperative TKA Infection Using Synovial Fluid Analysis. Clin. Orthop. Relat. Res. 2011, 469, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Luthringer, T.A.; Fillingham, Y.A.; Okroj, K.; Ward, E.J.; Della Valle, C. Periprosthetic Joint Infection After Hip and Knee Arthroplasty: A Review for Emergency Care Providers. Ann. Emerg. Med. 2016, 68, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Della Valle, C.; Parvizi, J.; Bauer, T.; Dicesare, P.E.; Evans, R.P.; Segreti, J.; Spangehl, M.; Watters, W.C.; Keith, M.; Turkelson, C.M.; et al. Diagnosis of Periprosthetic Joint Infections of the Hip and Knee. J. Am. Acad. Orthop. Surg. 2010, 18, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Della Valle, C.J. AAOS Clinical Practice Guideline: Diagnosis and Treatment of Periprosthetic Joint Infections of the Hip and Knee. J. Am. Acad. Orthop. Surg. 2010, 18, 771–772. [Google Scholar] [CrossRef]
- Parvizi, J.; Ghanem, E.; Menashe, S.; Barrack, R.L.; Bauer, T.W. Periprosthetic Infection: What Are the Diagnostic Challenges? J. Bone Joint Surg. Am. 2006, 88 (Suppl. 4), 138–147. [Google Scholar] [CrossRef]
- Zimmerli, W.; Trampuz, A.; Ochsner, P.E. Prosthetic-Joint Infections. N. Engl. J. Med. 2004, 351, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Jacovides, C.L.; Kreft, R.; Adeli, B.; Hozack, B.; Ehrlich, G.D.; Parvizi, J. Successful Identification of Pathogens by Polymerase Chain Reaction (PCR)-Based Electron Spray Ionization Time-of-Flight Mass Spectrometry (ESI-TOF-MS) in Culture-Negative Periprosthetic Joint Infection. J. Bone Joint Surg. Am. 2012, 94, 2247–2254. [Google Scholar] [CrossRef]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar] [CrossRef]
- Longo, U.; Candela, V.; Berton, A.; De Salvatore, S.; Fioravanti, S.; Giannone, L.; Marchetti, A.; De Marinis, M.; Denaro, V. Biosensors for Detection of Biochemical Markers Relevant to Osteoarthritis. Biosensors 2021, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.D.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef]
- Rasouli, M.R.; Harandi, A.A.; Adeli, B.; Purtill, J.J.; Parvizi, J. Revision Total Knee Arthroplasty: Infection Should Be Ruled Out in All Cases. J. Arthroplast. 2012, 27, 1239–1243.e2. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chang, W.-H.; Wang, C.-H.; Lin, C.-L.; Wu, J.-J.; Lee, M.S.; Lee, G.-B. Rapid detection and typing of live bacteria from human joint fluid samples by utilizing an integrated microfluidic system. Biosens. Bioelectron. 2015, 66, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Fargašová, A.; Balzerová, A.; Prucek, R.; Sedláková, M.H.; Bogdanová, K.; Gallo, J.; Kolář, M.; Ranc, V.; Zbořil, R. Detection of Prosthetic Joint Infection Based on Magnetically Assisted Surface Enhanced Raman Spectroscopy. Anal. Chem. 2017, 89, 6598–6607. [Google Scholar] [CrossRef] [PubMed]
- Longo, U.; Forriol, F.; Candela, V.; Tecce, S.; De Salvatore, S.; Altonaga, J.; Wallace, A.; Denaro, V. Arthroscopic Tenotomy of the Long Head of the Biceps Tendon and Section of the Anterior Joint Capsule Produce Moderate Osteoarthritic Changes in an Experimental Sheep Model. Int. J. Environ. Res. Public Health 2021, 18, 7471. [Google Scholar] [CrossRef] [PubMed]
- Papalia, R.; Vespasiani-Gentilucci, U.; Longo, U.G.; Esposito, C.; Zampogna, B.; Incalzi, R.A.; Denaro, V. Advances in management of periprosthetic joint infections: An historical prospective study. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 129–138. [Google Scholar]
- Denaro, V.; Longo, U.G.; Salvatore, G.; Candela, V.; Maffulli, N. Subcutaneous emphysema of the leg after hardware removal and bone allografting for infected non-union of the distal femur. BMC Musculoskelet. Disord. 2017, 18, 351. [Google Scholar] [CrossRef]
- Pacheco, J.G.; Barroso, M.F.; Nouws, H.P.A.; Morais, S.; Delerue-Matos, C. Biosensors. In Current Developments in Biotechnology and Bioengineering: Bioprocesses, Bioreactors and Controls; Elsevier: Amsterdam, The Netherlands, 2017; Chapter 21; pp. 627–648. [Google Scholar]
- D’Amico, A.; Di Natale, C. A contribution on some basic definitions of sensors properties. IEEE Sens. J. 2001, 1, 183–190. [Google Scholar] [CrossRef]
- Falconi, C.; Martinelli, E.; Di Natale, C.; D’Amico, A.; Maloberti, F.; Malcovati, P.; Baschirotto, A.; Stornelli, V.; Ferri, G. Electronic interfaces. Sens. Actuators B Chem. 2007, 121, 295–321. [Google Scholar] [CrossRef]
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef]
- Wang, H.-W.; Bringans, C.; Hickey, A.; Windsor, J.; Kilmartin, P.; Phillips, A. Cyclic Voltammetry in Biological Samples: A Systematic Review of Methods and Techniques Applicable to Clinical Settings. Signals 2021, 2, 138–158. [Google Scholar] [CrossRef]
- Pennazza, G.; Santonico, M.; Vollero, L.; Zompanti, A.; Sabatini, A.; Kumar, N.; Pini, I.; Solano, W.F.Q.; Sarro, L.; D’Amico, A. Advances in the Electronics for Cyclic Voltammetry: The Case of Gas Detection by Using Microfabricated Electrodes. Front. Chem. 2018, 6, 327. [Google Scholar] [CrossRef]
- Ingle, J.D., Jr.; Crouch, S.R. Spectrochemical Analysis; Prentice Hall: Prentice, NJ, USA, 1988. [Google Scholar]
- Jarockyte, G.; Karabanovas, V.; Rotomskis, R.; Mobasheri, A. Multiplexed Nanobiosensors: Current Trends in Early Diagnostics. Sensors 2020, 20, 6890. [Google Scholar] [CrossRef] [PubMed]
- Zappa, D.; Galstyan, V.; Kaur, N.; Arachchige, H.M.M.; Sisman, O.; Comini, E. “Metal oxide-based heterostructures for gas sensors”—A review. Anal. Chim. Acta 2018, 1039, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Hashtroudi, H.; Mackinnon, I.D.; Shafiei, M. Emerging 2D hybrid nanomaterials: Towards enhanced sensitive and selective conductometric gas sensors at room tenperature. J. Mater. Chem. C 2020, 8, 13108–13126. [Google Scholar] [CrossRef]
- Fonollosa, J.; Fernández, L.; Huerta, R.; Gutiérrez-Gálvez, A.; Marco, S. Temperature optimization of metal oxide sensor arrays using Mutual Information. Sens. Actuators B Chem. 2013, 187, 331–339. [Google Scholar] [CrossRef]
- Chen, X.; Cao, M.; Li, Y.; Hu, W.; Wang, P.; Ying, K.; Pan, H. A study of an electronic nose for detection of lung cancer based on a virtual SAW gas sensors array and imaging recognition method. Meas. Sci. Technol. 2005, 16, 1535–1546. [Google Scholar] [CrossRef]
- Bartolazzi, A.; Santonico, M.; Pennazza, G.; Martinelli, E.; Paolesse, R.; D’Amico, A.; Di Natale, C. A sensor array and GC study about VOCs and cancer cells. Sens. Actuators B Chem. 2010, 146, 483–488. [Google Scholar] [CrossRef]
- Pennazza, G.; Marchetti, E.; Santonico, M.; Mantini, G.; Mummolo, S.; Marzo, G.; Paolesse, R.; D’Amico, A.; Di Natale, C. Application of a quartz microbalance based gas sensor array for the study of halitosis. J. Breath Res. 2008, 2, 017009. [Google Scholar] [CrossRef]
- Pennazza, G.; Santonico, M.; Zompanti, A.; Grasso, S.; D’Amico, A. Electronic Interface for a Gas Sensor System Based on 32 MHz QCMs: Design and Calibration. IEEE Sens. J. 2017, 18, 1419–1426. [Google Scholar] [CrossRef]
- O’Sullivan, C.K.; Guilbault, G. Commercial quartz crystal microbalances—Theory and applications. Biosens. Bioelectron. 1999, 14, 663–670. [Google Scholar] [CrossRef]
- Homola, J.; Piliarik, M. Surface Plasmon Resonance Based Sensors; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Steinem, C.; Janshoff, A. (Eds.) Piezoelectric Sensors; Springer Series on Chemical Sensors and Biosensors; Springer: Berlin/Heidelberg, Germany, 2007; Volume 5. [Google Scholar]
- Florinel-Gabriel, B. Chemical Sensors and Biosensors: Fundamentals and Applications; Wiley-Blackwell: Chichester, UK, 2012. [Google Scholar]
- Lalauze, R. (Ed.) Chemical Sensors and Biosensors; Wiley-ISTE: London, UK, 2012. [Google Scholar]
- D’Amico, A.; Di Natale, C.; Falconi, C.; Pennazza, G.; Santonico, M.; Lundstrom, I. Equivalent electric circuits for chemical sensors in the Langmuir regime. Sens. Actuators B Chem. 2017, 238, 214–220. [Google Scholar] [CrossRef]
- Marchetti, E.; Tecco, S.; Santonico, M.; Vernile, C.; Ciciarelli, D.; Tarantino, E.; Marzo, G.; Pennazza, G. Multi-Sensor Approach for the Monitoring of Halitosis Treatment via Lactobacillus brevis (CD2)-Containing Lozenges--A Randomized, Double-Blind Placebo-Controlled Clinical Trial. Sensors 2015, 15, 19583–19596. [Google Scholar] [CrossRef]
- Yun, Y.-H.; Bhattacharya, A.; Watts, N.B.; Schulz, M.J. A Label-Free Electronic Biosensor for Detection of Bone Turnover Markers. Sensors 2009, 9, 7957–7969. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Su, S.; Yu, C.; Gopinath, S.C.B.; Yang, Z. Immunodetection of urinary C-terminal telopeptide fragment of type II col-lagen: An osteoarthritis biomarker analysis. Biotechnol. Appl. Biochem. 2020, 68, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-H.; Shen, C.-Y.; Weng, T.-C.; Lin, P.-H.; Yang, J.-J.; Chen, I.-F.; Kuo, S.-M.; Chang, S.-J.; Tu, Y.-K.; Kao, Y.-H.; et al. Detection of Cartilage Oligomeric Matrix Protein Using a Quartz Crystal Microbalance. Sensors 2010, 10, 11633–11643. [Google Scholar] [CrossRef]
- Ahmad, N.; Colak, B.; Zhang, D.-W.; Gibbs, M.J.; Watkinson, M.; Becer, C.R.; Gautrot, J.E.; Krause, S. Peptide Cross-Linked Poly (Ethylene Glycol) Hydrogel Films as Biosensor Coatings for the Detection of Collagenase. Sensors 2019, 19, 1677. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Lee, M.-H.; Jung, K.-I.; Na, H.Y.; Cha, H.-S.; Ko, E.-M.; Kim, T.J. Detection of antibodies against glucose 6-phosphate isomerase in synovial fluid of rheumatoid arthritis using surface plasmon resonance (BIAcore). Exp. Mol. Med. 2003, 35, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.-T.; Hsieh, W.-H.; Cheng, S.-F.; Jen, C.-P.; Wu, C.-C.; Li, C.-H.; Lee, C.-Y.; Li, W.-Y.; Chau, L.-K.; Chiang, C.-Y.; et al. Integration of fiber optic-particle plasmon resonance biosensor with microfluidic chip. Anal. Chim. Acta 2011, 697, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Chiang, C.Y.; Li, C.H.; Chang, T.C.; Chiang, C.S.; Chau, L.K.; Huang, K.W.; Wu, C.W.; Wang, S.C.; Lyu, S.R. Quanti-fication of tumor necrosis factor-α and matrix metalloproteinases-3 in synovial fluid by a fiber-optic particle plasmon reso-nance sensor. Analyst 2013, 138, 4599–4606. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-Y.; Hsieh, M.-L.; Huang, K.-W.; Chau, L.-K.; Chang, C.-M.; Lyu, S.-R. Fiber-optic particle plasmon resonance sensor for detection of interleukin-1β in synovial fluids. Biosens. Bioelectron. 2010, 26, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Vance, S.A.; Sandros, M.G. Zeptomole Detection of C-Reactive Protein in Serum by a Nanoparticle Amplified Surface Plasmon Resonance Imaging Aptasensor. Sci. Rep. 2014, 4, 5129. [Google Scholar] [CrossRef]
- Hartmann, B.; Marchi, G.; Alberton, P.; Farkas, Z.; Aszodi, A.; Roths, J.; Clausen-Schaumann, H. Early Detection of Cartilage Degeneration: A Comparison of Histology, Fiber Bragg Grating-Based Micro-Indentation, and Atomic Force Microscopy-Based Nano-Indentation. Int. J. Mol. Sci. 2020, 21, 7384. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.C.; Alford, S.C.; Hunter, S.A.; Kannan, D.; Sperberg, R.A.P.; Chang, C.H.; Cochran, J.R. Development of a Protease Biosensor Based on a Dimerization-Dependent Red Fluorescent Protein. ACS Chem. Biol. 2018, 13, 66–72. [Google Scholar] [CrossRef]
- Song, S.Y.; Han, Y.D.; Hong, S.Y.; Kim, K.; Yang, S.S.; Min, B.-H.; Yoon, H.C. Chip-based cartilage oligomeric matrix protein detection in serum and synovial fluid for osteoarthritis diagnosis. Anal. Biochem. 2012, 420, 139–146. [Google Scholar] [CrossRef]
- Park, Y.M.; Kim, S.J.; Lee, K.J.; Yang, S.S.; Min, B.H.; Yoon, H.C. Detection of CTX-II in serum and urine to diagnose osteoar-thritis by using a fluoro-microbeads guiding chip. Biosens. Bioelectron. 2015, 67, 192–199. [Google Scholar] [CrossRef] [PubMed]

| Author | Clearly Stated Aim | Inclusion of Consecutive Patients | Prospective Data Collection | Endpoints Appropriate to Study Aim | Unbiased Assessment of Study Endpoint | Follow-Uup Period Appropriate to Study Aim | <5% Lost to Follow-Up | Prospective Calculation of Study Size | Adequate Control Group | Contemporary Groups | Baseline Equivalence of Groups | Adequate Statistical Analyses | Total Score (…/24) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chang et al., 2015 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 2 | 2 | 2 | 0 | 14 |
| Jacovides et al., 2012 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 2 | 2 | 2 | 2 | 18 |
| Rasouli et al., 2012 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 2 | 2 | 0 | 18 |
| Fargašová et al., 2017 [25] | 2 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 10 |
| Method | Technology | Cost | Where | Performances |
|---|---|---|---|---|
| EIS (Electrochemical Impedance Spectroscopy) [52,53] | Impedance measurement of a system linked to the AC potentials frequency | Medium/Low | Lab/Home | High |
| Cyclic Voltammetry [53] | Measurement of the current that develops in an electrochemical cell applying a triangular potential to the cell | Low | Lab/Home | Medium/High |
| Amperometry [53,54] | Measurement of the current generated on an electrode | Low | Lab/Home | Medium/High |
| QCM (Quartz Crystal Microbalance) [55,56] | Measurement of a mass variation by measuring the change in frequency of a quartz crystal resonator | Medium/Low | Lab | Medium |
| Plasmon Resonance [57,58,59,60,61] | Measurement of light absorption of a metal surface caused by refractive index changes | High | Lab | Very High |
| Fiber Bragg grating (FBG)-based optoelectronic micro-indenter [62] | Measurement of a wavelength shift | High | Lab | Medium |
| Dimerization-dependent red fluorescent protein [63] | Measurement of a fluorescence | High | Lab | High |
| Fluoro-microbeads guiding chips [64] | Measurement of a fluorescence | High | Lab | High |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longo, U.G.; De Salvatore, S.; Zompanti, A.; Di Naro, C.; Grasso, S.; Casciaro, C.; Sabatini, A.; Mazzola, A.; Pennazza, G.; Santonico, M.; et al. Biosensors for Detection and Monitoring of Joint Infections. Chemosensors 2021, 9, 256. https://doi.org/10.3390/chemosensors9090256
Longo UG, De Salvatore S, Zompanti A, Di Naro C, Grasso S, Casciaro C, Sabatini A, Mazzola A, Pennazza G, Santonico M, et al. Biosensors for Detection and Monitoring of Joint Infections. Chemosensors. 2021; 9(9):256. https://doi.org/10.3390/chemosensors9090256
Chicago/Turabian StyleLongo, Umile Giuseppe, Sergio De Salvatore, Alessandro Zompanti, Calogero Di Naro, Simone Grasso, Carlo Casciaro, Anna Sabatini, Alessandro Mazzola, Giorgio Pennazza, Marco Santonico, and et al. 2021. "Biosensors for Detection and Monitoring of Joint Infections" Chemosensors 9, no. 9: 256. https://doi.org/10.3390/chemosensors9090256
APA StyleLongo, U. G., De Salvatore, S., Zompanti, A., Di Naro, C., Grasso, S., Casciaro, C., Sabatini, A., Mazzola, A., Pennazza, G., Santonico, M., & Denaro, V. (2021). Biosensors for Detection and Monitoring of Joint Infections. Chemosensors, 9(9), 256. https://doi.org/10.3390/chemosensors9090256











