Application of Metal-Organic Framework-Based Composites for Gas Sensing and Effects of Synthesis Strategies on Gas-Sensitive Performance
Abstract
:1. Introduction
2. MOFs-Semiconducting Metal Oxides Composites
2.1. Sensing Mechanism of SMOx and Limitations
2.2. Selection of Composite Materials with SMOx and MOF
2.3. Development of SMOx@MOF Composites
2.3.1. Detection Using Molecular Sieve Only
2.3.2. The Improvement of Response Rate
2.3.3. Exploration of the Types of Detection Gases
2.3.4. Addition of Other Substances
2.4. MOF-Derived SMOx for Gas Sensors
2.5. Problems to Be Solved
3. MOFs-Carbon Composites
3.1. Advantages and Disadvantages of a Single Material
3.2. The Choice of Carbon-Based Materials and MOF Materials
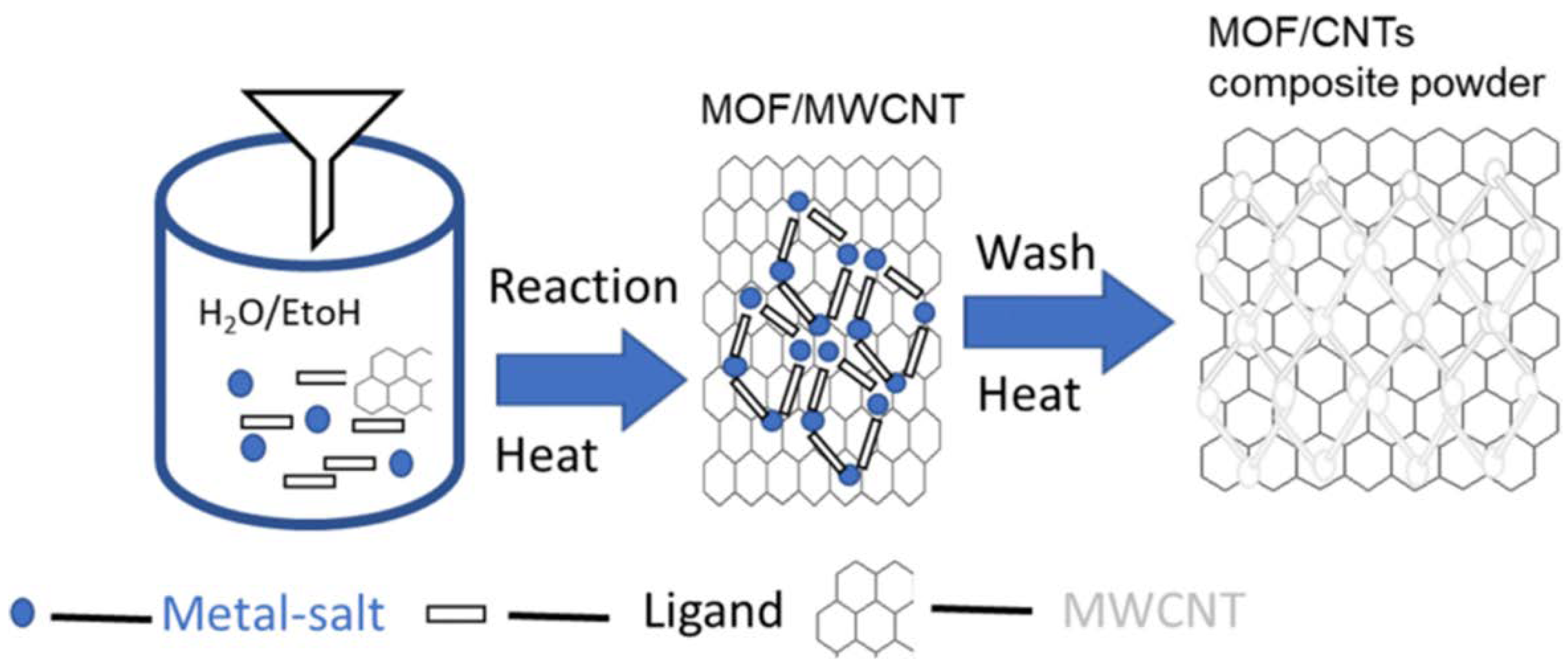
3.3. Preparation Method of MOF/Carbon-Based Composite Materials
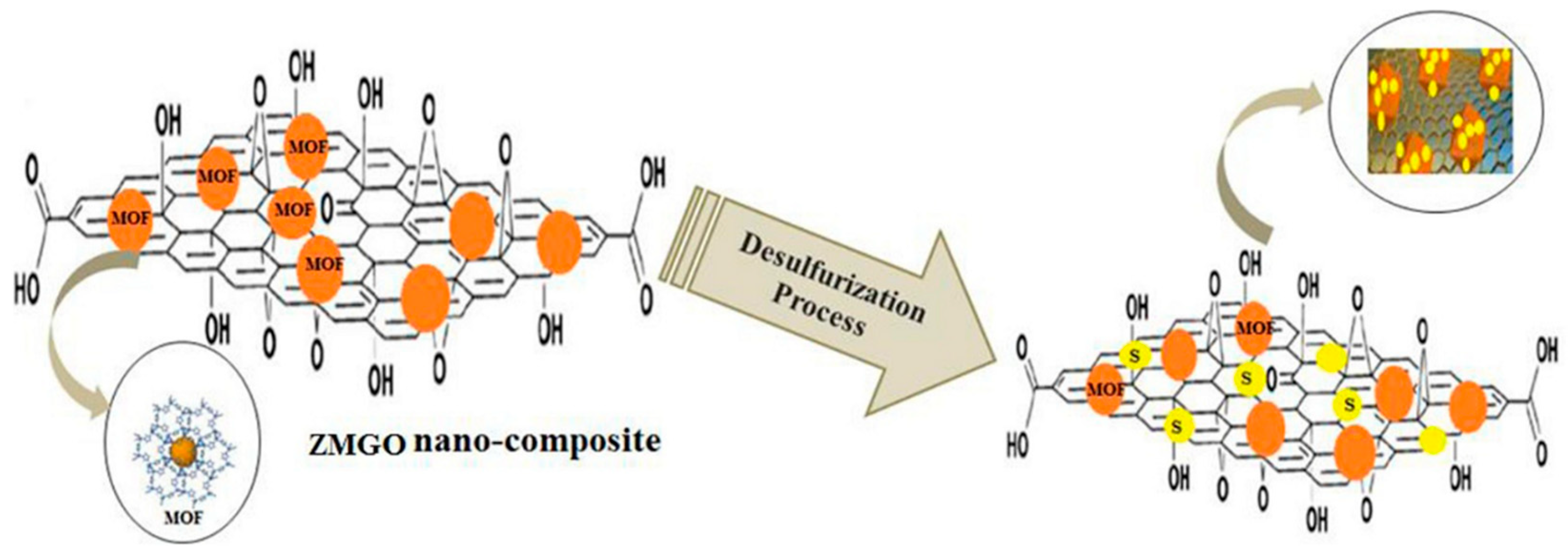
3.4. Current Development of Research in Gas Sensing and Gas Adsorption
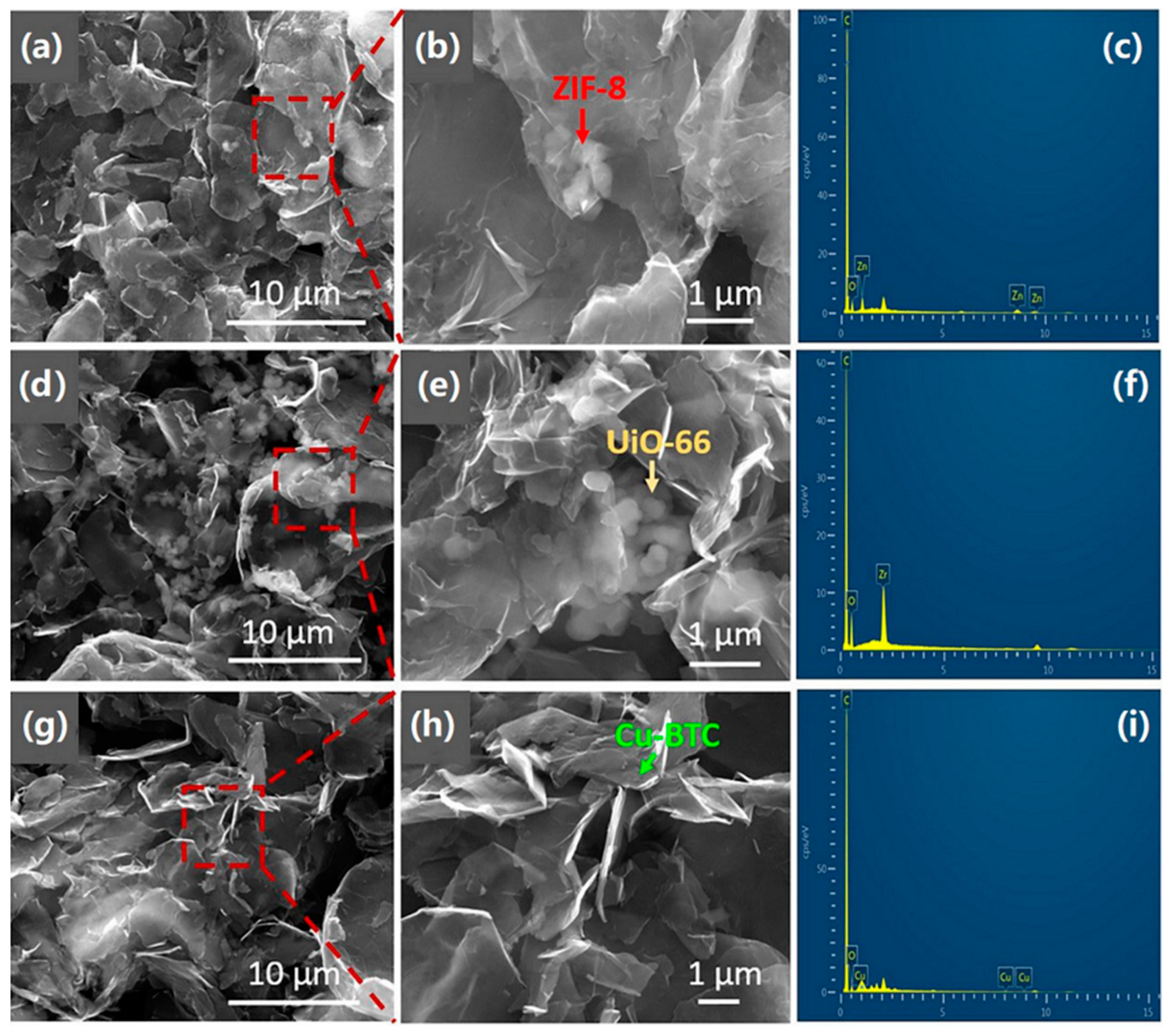
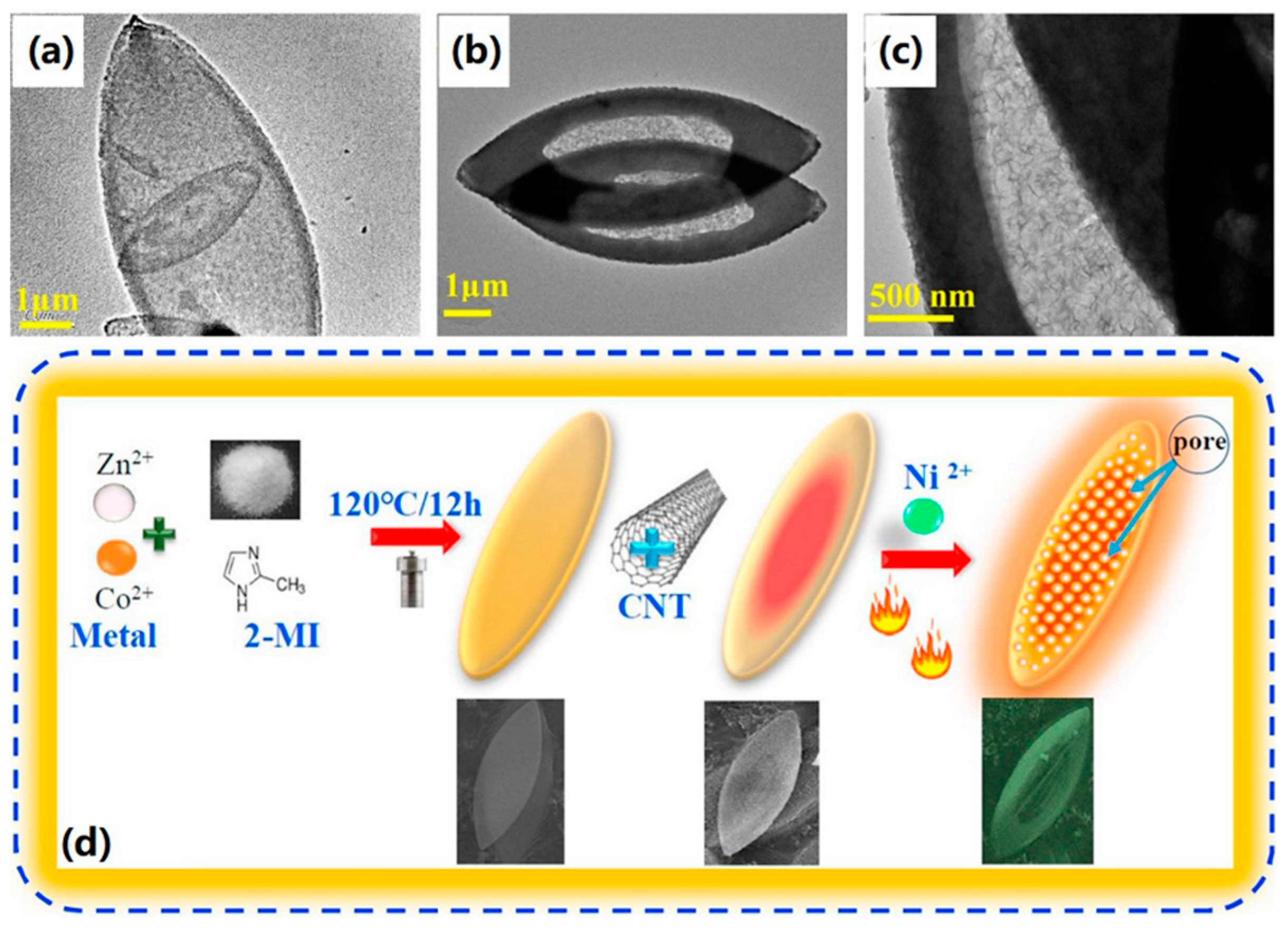
3.4.1. MOF-Carbon Nanotube/Graphene-Based Composites for Gas-Sensitive Sensing Applications
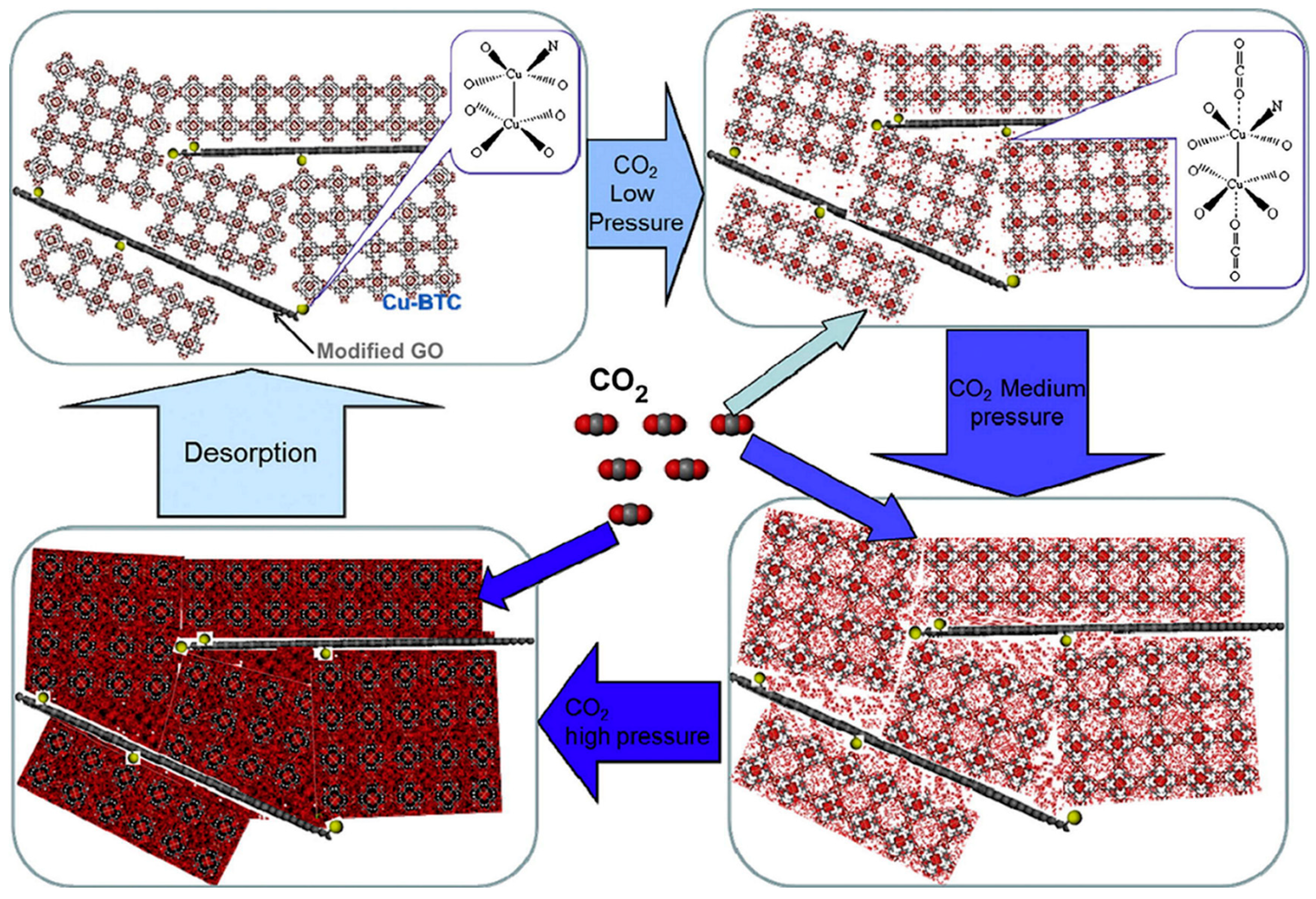
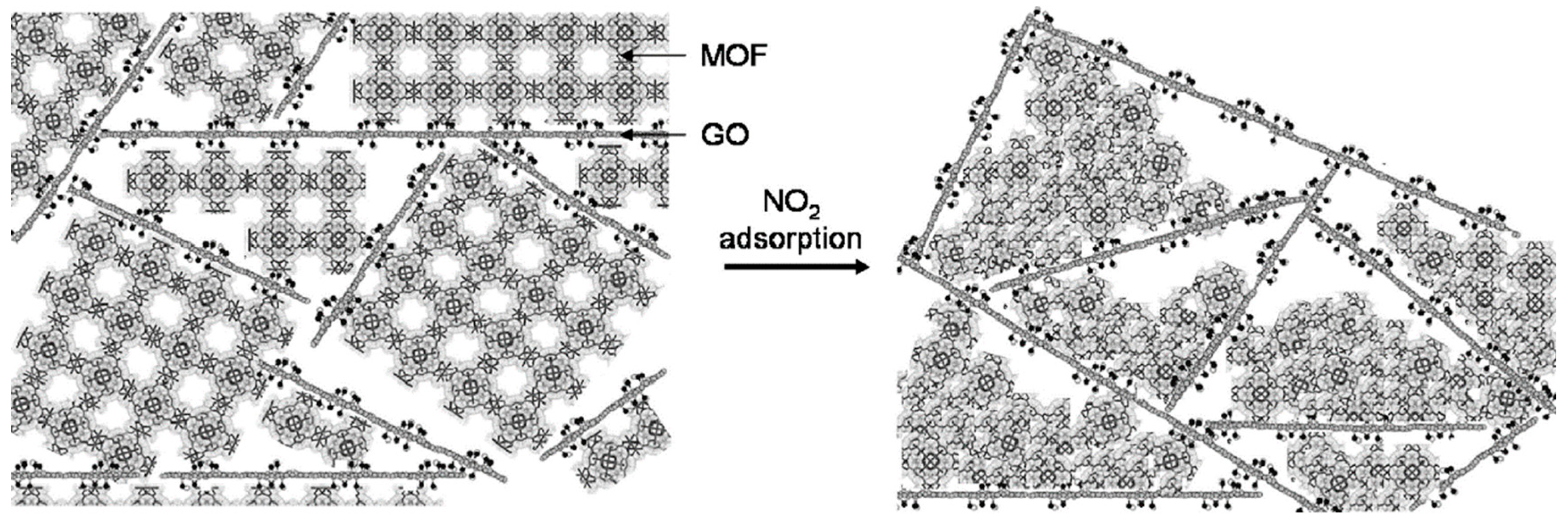
3.4.2. Gas Adsorption of MOF-GO Composites
3.4.3. Future Developments
4. MOFs-Polymer Composites
4.1. Advantages and Disadvantages of Polymers as Gas Sensing Materials
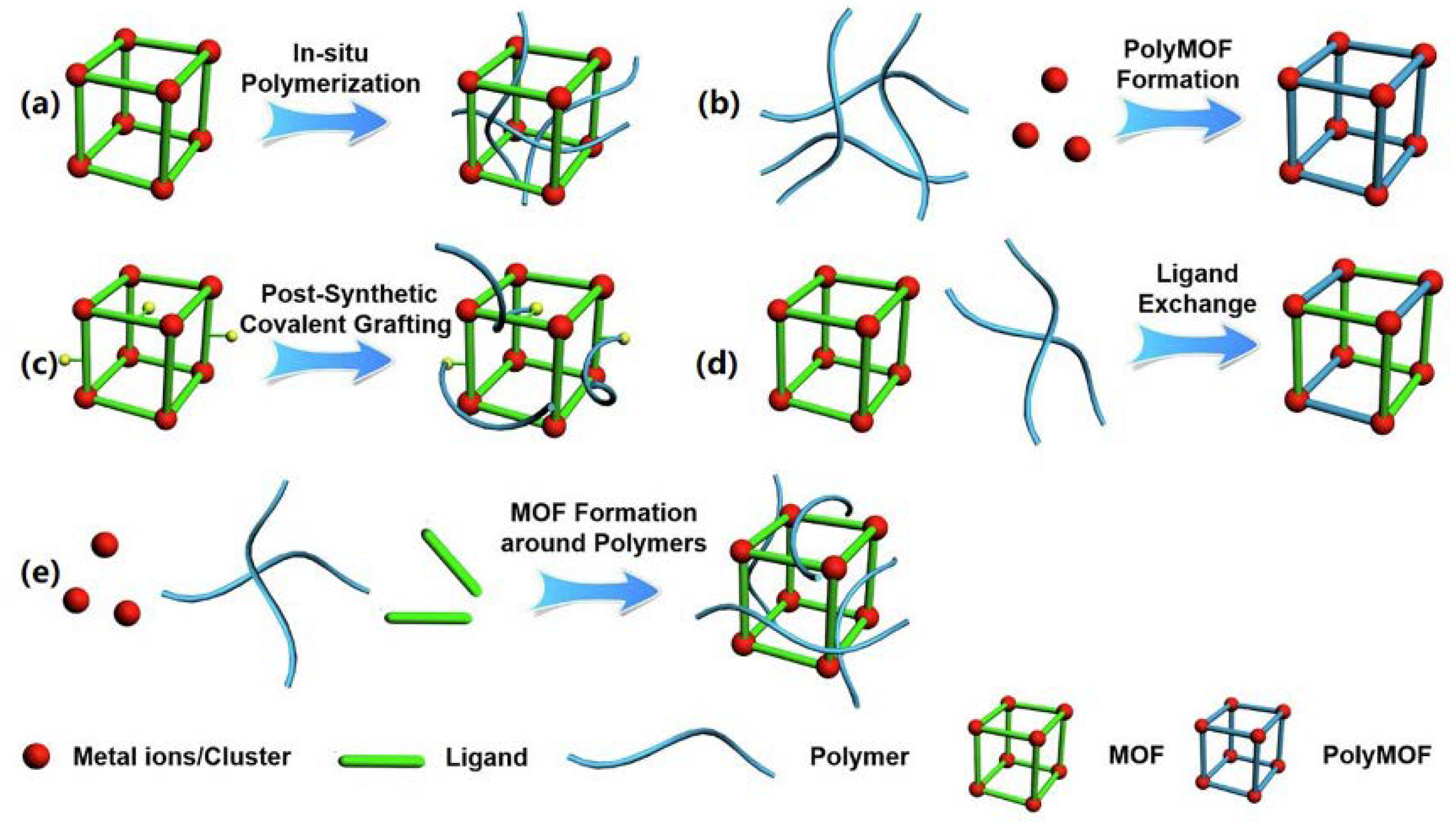
4.2. Comparison of Synthesis Methods of MOF-Polymer Composites and Their Advantages and Disadvantages
4.3. Development of MOF-Polymer Gas-Sensitive Sensing Materials
4.3.1. Blended Films Formed by MOF and Polymers
4.3.2. Application of Composites Prepared by Doping MOF in Polymers for Gas Sensing
4.3.3. Application of Composites Obtained by Doping Polymers in MOF for Gas Sensing and Gas Adsorption
4.3.4. Application of MOF-Polymer Composites for Enhanced CO2 Adsorption
4.3.5. Current Problems
5. Conclusions
6. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiao, L.; Bing, Y.; Wang, Y.; Yu, S.; Liang, Z.; Zeng, Y. Enhanced toluene sensing performances of Pd- loaded SnO2 cubic nanocages with porous nanoparticle-assembled shells. Sens. Actuators B Chem. 2017, 241, 1121–1129. [Google Scholar] [CrossRef]
- Wei, A.; Pan, L.; Huang, W. Recent progress in the ZnO nanostructure-based sensors. Mater. Sci. Eng. B Adv. Funct. Solid State Mater. 2011, 176, 1409–1421. [Google Scholar] [CrossRef]
- Mokoena, T.P.; Swart, H.C.; Motaung, D.E. A review on recent progress of p-type nickel oxide based gas sensors: Future perspectives. J. Alloy. Compd. 2019, 805, 267–294. [Google Scholar] [CrossRef]
- Rydosz, A. The Use of Copper Oxide Thin Films in Gas-Sensing Applications. Coatings 2018, 8, 425. [Google Scholar] [CrossRef] [Green Version]
- Yuliarto, B.; Gumilar, G.; Septiani, N.L.W. SnO2 Nanostructure as Pollutant Gas Sensors: Synthesis, Sensing Performances, and Mechanism. Adv. Mater. Sci. Eng. 2015, 2015, 694823. [Google Scholar] [CrossRef] [Green Version]
- Singkammo, S.; Wisitsoraat, A.; Sriprachuabwong, C.; Tuantranont, A.; Phanichphant, S.; Liewhiran, C. Electrolytically Exfoliated Graphene-Loaded Flame-Made Ni-Doped SnO2 Composite Film for Acetone Sensing. ACS Appl. Mater. Interfaces 2015, 7, 3077–3092. [Google Scholar] [CrossRef]
- Xu, X.L.; Chen, Y.; Ma, S.Y.; Li, W.Q.; Mao, Y.Z. Excellent acetone sensor of La-doped ZnO nanofibers with unique bead-like structures. Sens. Actuators B Chem. 2015, 213, 222–233. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, B.; Sun, H.; Wang, C.; Sun, P.; Li, X.; Hu, X.; Lu, G. Template-free synthesis of hierarchical ZnFe2O4 yolk-shell microspheres for high-sensitivity acetone sensors. Nanoscale 2016, 8, 5446–5453. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Wang, Q.; Yang, Z. Preparation, characterization and acetone sensing properties of Ce-doped SnO2 hollow spheres. Sens. Actuators B Chem. 2012, 173, 839–846. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Guo, H.; Sun, P.; Liu, F.; Liang, X.; Lu, G. Double-Shell Architectures of ZnFe2O4 Nanosheets on ZnO Hollow Spheres for High-Performance Gas Sensors. ACS Appl. Mater. Interfaces 2015, 7, 17811–17818. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Choi, N.-J.; Kang, H.; Jung, M.Y.; Park, J.W.; Park, K.H.; Lee, D.-S. A ppb-level formaldehyde gas sensor based on CuO nanocubes prepared using a polyol process. Sens. Actuators B Chem. 2014, 203, 282–288. [Google Scholar] [CrossRef]
- Yao, M.-S.; Tang, W.-X.; Wang, G.-E.; Nath, B.; Xu, G. MOF Thin Film-Coated Metal Oxide Nanowire Array: Significantly Improved Chemiresistor Sensor Performance. Adv. Mater. 2016, 28, 5229–5234. [Google Scholar] [CrossRef]
- Barsan, N.; Schweizer-Berberich, M.; Gopel, W. Fundamental and practical aspects in the design of nanoscaled SnO2 gas sensors: A status report. Fresenius J. Anal. Chem. 1999, 365, 287–304. [Google Scholar] [CrossRef]
- Jang, S.; Song, S.; Lim, J.H.; Kim, H.S.; Phan, B.T.; Ha, K.-T.; Park, S.; Park, K.H. Application of Various Metal-Organic Frameworks (MOFs) as Catalysts for Air and Water Pollution Environmental Remediation. Catalysts 2020, 10, 195. [Google Scholar] [CrossRef] [Green Version]
- Swager, T.M. Sensor Technologies Empowered by Materials and Molecular Innovations. Angew. Chem. Int. Ed. 2018, 57, 4248–4257. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Yoon, Y.S.; Kim, D.-J. Two-Dimensional Transition Metal Dichalcogenides and Metal Oxide Hybrids for Gas Sensing. ACS Sens. 2018, 3, 2045–2060. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Park, J.; Jang, J.; Lee, H.; Jeong, H.; Cho, K.; Hong, S.; Lee, T. Oxygen environmental and passivation effects on molybdenum disulfide field effect transistors. Nanotechnology 2013, 24, 095202. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.; Majumdar, S. Polymers in sensor applications. Prog. Polym. Sci. 2004, 29, 699–766. [Google Scholar] [CrossRef]
- Lakard, B.; Carquigny, S.; Segut, O.; Patois, T.; Lakard, S. Gas Sensors Based on Electrodeposited Polymers. Metals 2015, 5, 1371–1386. [Google Scholar] [CrossRef] [Green Version]
- Koo, W.-T.; Cha, J.-H.; Jung, J.-W.; Choi, S.-J.; Jang, J.-S.; Kim, D.-H.; Kim, I.-D. Few-Layered WS2 Nanoplates Confined in Co, N-Doped Hollow Carbon Nanocages: Abundant WS2 Edges for Highly Sensitive Gas Sensors. Adv. Funct. Mater. 2018, 28. [Google Scholar] [CrossRef]
- Lei, J.-C.; Zhang, X.; Zhou, Z. Recent advances in MXene: Preparation, properties, and applications. Front. Phys. 2015, 10, 276–286. [Google Scholar] [CrossRef]
- Xin, M.; Li, J.; Ma, Z.; Pan, L.; Shi, Y. MXenes and Their Applications in Wearable Sensors. Front. Chem. 2020, 8, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, D.-X.; Wang, Q.; Bai, J. Amide-functionalized metal-organic frameworks: Syntheses, structures and improved gas storage and separation properties. Coord. Chem. Rev. 2019, 378, 2–16. [Google Scholar] [CrossRef]
- Beheshti, S.; Safarifard, V.; Morsali, A. Isoreticular interpenetrated pillared-layer microporous metal-organic framework as a highly effective catalyst for three-component synthesis of pyrano 2, 3-d pyrimidines. Inorg. Chem. Commun. 2018, 94, 80–84. [Google Scholar] [CrossRef]
- Hu, M.-L.; Safarifard, V.; Doustkhah, E.; Rostamnia, S.; Morsali, A.; Nouruzi, N.; Beheshti, S.; Akhbari, K. Taking organic reactions over metal-organic frameworks as heterogeneous catalysis. Microporous Mesoporous Mater. 2018, 256, 111–127. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, Q.-L.; Zou, R.; Xu, Q. Metal-Organic Frameworks for Energy Applications. Chem 2017, 2, 52–80. [Google Scholar] [CrossRef] [Green Version]
- Koo, W.-T.; Jang, J.-S.; Kim, I.-D. Metal-Organic Frameworks for Chemiresistive Sensors. Chem 2019, 5, 1938–1963. [Google Scholar] [CrossRef]
- Chernikova, V.; Yassine, O.; Shekhah, O.; Eddaoudi, M.; Salama, K.N. Highly sensitive and selective SO2 MOF sensor: The integration of MFM-300 MOF as a sensitive layer on a capacitive interdigitated electrode. J. Mater. Chem. A 2018, 6, 5550–5554. [Google Scholar] [CrossRef] [Green Version]
- Petit, C.; Bandosz, T.J. Engineering the surface of a new class of adsorbents: Metal-organic framework/graphite oxide composites. J. Colloid Interface Sci. 2015, 447, 139–151. [Google Scholar] [CrossRef]
- Kuesgens, P.; Rose, M.; Senkovska, I.; Froede, H.; Henschel, A.; Siegle, S.; Kaskel, S. Characterization of metal-organic frameworks by water adsorption. Microporous Mesoporous Mater. 2009, 120, 325–330. [Google Scholar] [CrossRef]
- Lu, Z.; Xing, H.; Sun, R.; Bai, J.; Zheng, B.; Li, Y. Water Stable Metal-Organic Framework Evolutionally Formed from a Flexible Multidentate Ligand with Acylamide Groups for Selective CO2 Adsorption. Cryst. Growth Des. 2012, 12, 1081–1084. [Google Scholar] [CrossRef]
- Schoenecker, P.M.; Carson, C.G.; Jasuja, H.; Flemming, C.J.J.; Walton, K.S. Effect of Water Adsorption on Retention of Structure and Surface Area of Metal-Organic Frameworks. Ind. Eng. Chem. Res. 2012, 51, 6513–6519. [Google Scholar] [CrossRef]
- Wales, D.J.; Grand, J.; Ting, V.P.; Burke, R.D.; Edler, K.J.; Bowen, C.R.; Mintova, S.; Burrows, A.D. Gas sensing using porous materials for automotive applications. Chem. Soc. Rev. 2015, 44, 4290–4321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, M.G.; Dinca, M. Metal-Organic Frameworks as Active Materials in Electronic Sensor Devices. Sensors 2017, 17, 1108. [Google Scholar] [CrossRef]
- Yi, F.-Y.; Chen, D.; Wu, M.-K.; Han, L.; Jiang, H.-L. Chemical Sensors Based on Metal-Organic Frameworks. Chempluschem 2016, 81, 675–690. [Google Scholar] [CrossRef]
- Yadav, B.C.; Chauhan, K.S.; Singh, S.; Sonker, R.K.; Sikarwar, S.; Kumar, R. Growth and characterization of sol-gel processed rectangular shaped nanostructured ferric oxide thin film followed by humidity and gas sensing. J. Mater. Sci. Mater. Electron. 2017, 28, 5270–5280. [Google Scholar] [CrossRef]
- Campbell, M.G.; Liu, S.F.; Swager, T.M.; Dinca, M. Chemiresistive Sensor Arrays from Conductive 2D Metal-Organic Frameworks. J. Am. Chem. Soc. 2015, 137, 13780–13783. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.K.; Jensen, K.E.; Pivak, P.A.; Mirica, K.A. Direct Self-Assembly of Conductive Nanorods of Metal Organic Frameworks into Chemiresistive Devices on Shrinkable Polymer Films. Chem. Mater. 2016, 28, 5264–5268. [Google Scholar] [CrossRef]
- Chen, E.-X.; Yang, H.; Zhang, J. Zeolitic Imidazolate Framework as Formaldehyde Gas Sensor. Inorg. Chem. 2014, 53, 5411–5413. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Zhao, S.-N.; Zang, S.-Q.; Li, J. Functional metal-organic frameworks as effective sensors of gases and volatile compounds. Chem. Soc. Rev. 2020, 49, 6364–6401. [Google Scholar] [CrossRef] [PubMed]
- Nasalevich, M.A.; van der Veen, M.; Kapteijn, F.; Gascon, J. Metal-organic frameworks as heterogeneous photocatalysts: Advantages and challenges. Crystengcomm 2014, 16, 4919–4926. [Google Scholar] [CrossRef] [Green Version]
- Leong, C.F.; Chan, B.; Faust, T.B.; D’Alessandro, D.M. Controlling charge separation in a novel donor-acceptor metal-organic framework via redox modulation. Chem. Sci. 2014, 5, 4724–4728. [Google Scholar] [CrossRef] [Green Version]
- Narayan, T.C.; Miyakai, T.; Seki, S.; Dinca, M. High Charge Mobility in a Tetrathiafulvalene-Based Microporous Metal-Organic Framework. J. Am. Chem. Soc. 2012, 134, 12932–12935. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Jin, L.; Zhang, R.; Bai, Y.; Zhu, R.; Pang, H. Recent advances in the development of electronically and ionically conductive metal-organic frameworks. Coord. Chem. Rev. 2021, 439, 213915. [Google Scholar] [CrossRef]
- Burtch, N.C.; Jasuja, H.; Walton, K.S. Water Stability and Adsorption in Metal-Organic Frameworks. Chem. Rev. 2014, 114, 10575–10612. [Google Scholar] [CrossRef] [PubMed]
- Howarth, A.J.; Liu, Y.; Li, P.; Li, Z.; Wang, T.C.; Hupp, J.; Farha, O.K. Chemical, thermal and mechanical stabilities of metal-organic frameworks. Nat. Rev. Mater. 2016, 1, 1–15. [Google Scholar] [CrossRef]
- Kung, C.-W.; Han, P.-C.; Chuang, C.-H.; Wu, K.C.W. Electronically conductive metal-organic framework-based materials. APL Mater. 2019, 7, 110902. [Google Scholar] [CrossRef] [Green Version]
- Bradshaw, D.; Garai, A.; Huo, J. Metal-organic framework growth at functional interfaces: Thin films and composites for diverse applications. Chem. Soc. Rev. 2012, 41, 2344–2381. [Google Scholar] [CrossRef] [Green Version]
- Petit, C.; Mendoza, B.; O’Donnell, D.; Bandosz, T.J. Effect of Graphite Features on the Properties of Metal-Organic Framework/Graphite Hybrid Materials Prepared Using an in Situ Process. Langmuir 2011, 27, 10234–10242. [Google Scholar] [CrossRef]
- Ge, L.; Wang, L.; Rudolph, V.; Zhu, Z. Hierarchically structured metal-organic framework/vertically-aligned carbon nanotubes hybrids for CO2 capture. RSC Adv. 2013, 3, 25360–25366. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Yue, D.; Zhang, J.; Wang, J.; Li, B.; Yang, Y.; Cui, Y.; Qian, G. Flexible Metal-Organic Framework-Based Mixed-Matrix Membranes: A New Platform for H2S Sensors. Small 2018, 14, 1801563. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, R.; Zhang, T.; Liu, S.; Fei, T. Zeolitic imidazolate framework-8 (ZIF-8)-coated In2O3 nanofibers as an efficient sensing material for ppb-level NO2 detection. J. Colloid Interface Sci. 2019, 541, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhan, W.; He, Y.; Wang, Y.; Kong, X.; Kuang, Q.; Xie, Z.; Zheng, L. MOF-Templated Synthesis of Porous Co3O4 Concave Nanocubes with High Specific Surface Area and Their Gas Sensing Properties. ACS Appl. Mater. Interfaces 2014, 6, 4186–4195. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; He, Y.; Gong, M.; He, X.; Ning, Z.; Yu, H.; Jiao, Z. MOF-derived synthesis of Co3O4 nanospheres with rich oxygen vacancies for long-term stable and highly selective n-butanol sensing performance. J. Alloy. Compd. 2021, 857, 158205. [Google Scholar] [CrossRef]
- Yin, M.; Liu, S. Preparation of ZnO hollow spheres with different surface roughness and their enhanced gas sensing property. Sens. Actuators B Chem. 2014, 197, 58–65. [Google Scholar] [CrossRef]
- Wei, S.; Zhang, Y.; Zhou, M. Formaldehyde sensing properties of ZnO-based hollow nanofibers. Sens. Rev. 2014, 34, 327–334. [Google Scholar] [CrossRef]
- Li, J.; Fan, H.; Jia, X. Multi layered ZnO Nanosheets with 3D Porous Architectures: Synthesis and Gas Sensing Application. J. Phys. Chem. C 2010, 114, 14684–14691. [Google Scholar] [CrossRef]
- Chen, E.-X.; Fu, H.-R.; Lin, R.; Tan, Y.-X.; Zhang, J. Highly Selective and Sensitive Trimethylamine Gas Sensor Based on Cobalt Imidazolate Framework Material. ACS Appl. Mater. Interfaces 2014, 6, 22871–22875. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Park, K.H.; Lee, H.-K. Transformation of CuO from Cu-MOF Templates and Their Enhanced Sensing Performance for HCHO. Bull. Korean Chem. Soc. 2016, 37, 123–128. [Google Scholar] [CrossRef]
- Le Ouay, B.; Boudot, M.; Kitao, T.; Yanagida, T.; Kitagawa, S.; Uemura, T. Nanostructuration of PEDOT in Porous Coordination Polymers for Tunable Porosity and Conductivity. J. Am. Chem. Soc. 2016, 138, 10088–10091. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.-H.; Kung, C.-W. Metal-Organic Frameworks toward Electrochemical Sensors: Challenges and Opportunities. Electroanalysis 2020, 32, 1885–1895. [Google Scholar] [CrossRef]
- Ahmed, I.; Jhung, S.H. Composites of metal-organic frameworks: Preparation and application in adsorption. Mater. Today 2014, 17, 136–146. [Google Scholar] [CrossRef]
- Zheng, S.; Li, Q.; Xue, H.; Pang, H.; Xu, Q. A highly alkaline-stable metal oxide@metal-organic framework composite for high-performance electrochemical energy storage. Natl. Sci. Rev. 2020, 7, 305–314. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Yan, S.; Deng, D.; Zhang, L.; Liu, R.; Lv, Y. Novel Strategy for Engineering the Metal-Oxide@MOF Core@Shell Architecture and Its Applications in Cataluminescence Sensing. ACS Appl. Mater. Interfaces 2021, 13, 3471–3480. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.R.; Lim, D.-W.; Suh, M.P. Fabrication of metal nanoparticles in metal-organic frameworks. Chem. Soc. Rev. 2013, 42, 1807–1824. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Liu, Y.; Liu, J.; Xiong, Y.; Zheng, J.; Liu, Y.; Tang, Z. CoreShell Noble-Metal@Metal-Organic-Framework Nanoparticles with Highly Selective Sensing Property. Angew. Chem. Int. Ed. 2013, 52, 3741–3745. [Google Scholar] [CrossRef]
- Wei, W.; Liu, Z.; Wei, R.; Han, G.-C.; Liang, C. Synthesis of MOFs/GO composite for corrosion resistance application on carbon steel. RSC Adv. 2020, 10, 29923–29934. [Google Scholar] [CrossRef]
- Lin, L.-C.; Paik, D.; Kim, J. Understanding gas adsorption in MOF-5/graphene oxide composite materials. Phys. Chem. Chem. Phys. 2017, 19, 11639–11644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Ha Thi Hoang, N.; Miller, S.A.; Cohen, S.M. polyMOFs: A Class of Interconvertible Polymer-Metal-Organic-Framework Hybrid Materials. Angew. Chem. Int. Ed. 2015, 54, 6152–6157. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Marcello, M.; Garai, A.; Bradshaw, D. MOF-Polymer Composite Microcapsules Derived from Pickering Emulsions. Adv. Mater. 2013, 25, 2717–2722. [Google Scholar] [CrossRef]
- Fu, Y.-Y.; Yang, C.-X.; Yan, X.-P. Fabrication of ZIF-8@SiO2 Core-Shell Microspheres as the Stationary Phase for High-Performance Liquid Chromatography. Chem. A Eur. J. 2013, 19, 13484–13491. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Liao, H.; Wu, X.; Cao, X. Selective adsorption behaviours of MOFs@SiO2 with different pore sizes and shell thicknesses. J. Solid State Chem. 2020, 292, 121693. [Google Scholar] [CrossRef]
- Aguilera-Sigalat, J.; Bradshaw, D. Synthesis and applications of metal-organic framework-quantum dot (QD@MOF) composites. Coord. Chem. Rev. 2016, 307, 267–291. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Liu, X.; Liu, Y.; Cheng, M.; Liu, Z.; Zeng, G.; Shao, B.; Liang, Q.; Zhang, W.; He, Q.; et al. Application of QD-MOF composites for photocatalysis: Energy production and environmental remediation. Coord. Chem. Rev. 2020, 403. [Google Scholar] [CrossRef]
- Ji, H.; Zeng, W.; Li, Y. Gas sensing mechanisms of metal oxide semiconductors: A focus review. Nanoscale 2019, 11, 22664–22684. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, J.-H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Nair, S.S.; Illyaskutty, N.; Tam, B.; Yazaydin, A.O.; Emmerich, K.; Steudel, A.; Hashem, T.; Schoettner, L.; Woell, C.; Kohler, H.; et al. ZnO@ZIF-8: Gas sensitive core-shell hetero-structures show reduced cross-sensitivity to humidity. Sens. Actuators B Chem. 2020, 304, 127184. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Neri, G.; Pinna, N. Nanostructured Materials for Room-Temperature Gas Sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef]
- Tian, H.; Fan, H.; Li, M.; Ma, L. Zeolitic Imidazolate Framework Coated ZnO Nanorods as Molecular Sieving to Improve Selectivity of Formaldehyde Gas Sensor. ACS Sens. 2016, 1, 243–250. [Google Scholar] [CrossRef]
- Li, W.; Wu, X.; Han, N.; Chen, J.; Qian, X.; Deng, Y.; Tang, W.; Chen, Y. MOF-derived hierarchical hollow ZnO nanocages with enhanced low-concentration VOCs gas-sensing performance. Sens. Actuators B Chem. 2016, 225, 158–166. [Google Scholar] [CrossRef]
- Jimenez-Cadena, G.; Riu, J.; Rius, F.X. Gas sensors based on nanostructured materials. Analyst 2007, 132, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-J.; Choi, Y.-K. Chemical sensors based on nanostructured materials. Sens. Actuators B Chem. 2007, 122, 659–671. [Google Scholar] [CrossRef]
- Meng, Z.; Stolz, R.M.; Mendecki, L.; Mirica, K.A. Electrically-Transduced Chemical Sensors Based on Two Dimensional Nanomaterials. Chem. Rev. 2019, 119, 478–598. [Google Scholar] [CrossRef]
- Ueda, T.; Maeda, T.; Huang, Z.; Higuchi, K.; Izawa, K.; Kamada, K.; Hyodo, T.; Shimizu, Y. Enhancement of methylmercaptan sensing response of WO3 semiconductor gas sensors by gas reactivity and gas diffusivity. Sens. Actuators B Chem. 2018, 273, 826–833. [Google Scholar] [CrossRef]
- Comini, E. Metal oxide nano-crystals for gas sensing. Anal. Chim. Acta 2006, 568, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Khudiar, A.I.; Elttayef, A.K.; Khalaf, M.K.; Oufi, A.M. Fabrication of ZnO@ZIF-8 gas sensors for selective gas detection. Mater. Res. Express 2019, 6, 126450. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, T.; Zhang, Y.; Tang, L.; Guo, Q.; Wang, M.; Xie, C.; Zeng, D. Synthesis of core-shell flower-like WO3@ZIF-71 with enhanced response and selectivity to H2S gas. Solid State Ion. 2020, 350, 115278. [Google Scholar] [CrossRef]
- Drobek, M.; Kim, J.-H.; Bechelany, M.; Vallicari, C.; Julbe, A.; Kim, S.S. MOF-Based Membrane Encapsulated ZnO Nanowires for Enhanced Gas Sensor Selectivity. ACS Appl. Mater. Interfaces 2016, 8, 8323–8328. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xiong, S.; Mao, Z.; Hu, S.; Long, X. A Designed ZnO@ZIF-8 Core-Shell Nanorod Film as a Gas Sensor with Excellent Selectivity for H-2 over CO. Chem. A Eur. J. 2017, 23, 7969–7975. [Google Scholar] [CrossRef] [Green Version]
- Cui, F.; Chen, W.; Jin, L.; Zhang, H.; Jiang, Z.; Song, Z. Fabrication of ZIF-8 encapsulated ZnO microrods with enhanced sensing properties for H-2 detection. J. Mater. Sci. Mater. Electron. 2018, 29, 19697–19709. [Google Scholar] [CrossRef]
- Zhou, T.; Sang, Y.; Wang, X.; Wu, C.; Zeng, D.; Xie, C. Pore size dependent gas-sensing selectivity based on ZnO@ZIF nanorod arrays. Sens. Actuators B Chem. 2018, 258, 1099–1106. [Google Scholar] [CrossRef]
- Dmello, M.E.; Sundaram, N.G.; Kalidindi, S.B. Assembly of ZIF-67 Metal-Organic Framework over Tin Oxide Nanoparticles for Synergistic Chemiresistive CO2 Gas Sensing. Chem. A Eur. J. 2018, 24, 9220–9223. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xiong, S.; Gong, Y.; Gong, Y.; Wu, W.; Mao, Z.; Liu, Q.; Hu, S.; Long, X. MOF-SMO hybrids as a H2S sensor with superior sensitivity and selectivity. Sens. Actuators B Chem. 2019, 292, 32–39. [Google Scholar] [CrossRef]
- Zhou, T.; Sang, Y.; Sun, Y.; Wu, C.; Wang, X.; Tang, X.; Zhang, T.; Wang, H.; Xie, C.; Zeng, D. Gas Adsorption at Metal Sites for Enhancing Gas Sensing Performance of ZnO@ZIF-71 Nanorod Arrays. Langmuir 2019, 35, 3248–3255. [Google Scholar] [CrossRef]
- Yao, M.-S.; Cao, L.-A.; Tang, Y.-X.; Wang, G.-E.; Liu, R.-H.; Kumar, P.N.; Wu, G.-D.; Deng, W.-H.; Hong, W.-J.; Xu, G. Gas transport regulation in a MO/MOF interface for enhanced selective gas detection. J. Mater. Chem. A 2019, 7, 18397–18403. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, S.; Wang, X.; Xie, C.; Zeng, D. Catalytic Activation of Cobalt Doping Sites in ZIF-71-Coated ZnO Nanorod Arrays for Enhancing Gas-Sensing Performance to Acetone. ACS Appl. Mater. Interfaces 2020, 12, 48948–48956. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Ni, Z.; Cote, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matatagui, D.; Sainz-Vidal, A.; Gracia, I.; Figueras, E.; Cane, C.; Saniger, J.M. Chemoresistive gas sensor based on ZIF-8/ZIF-67 nanocrystals. Sens. Actuators B Chem. 2018, 274, 601–608. [Google Scholar] [CrossRef]
- Phan, A.; Doonan, C.J.; Uribe-Romo, F.J.; Knobler, C.B.; O’Keeffe, M.; Yaghi, O.M. Synthesis, Structure, and Carbon Dioxide Capture Properties of Zeolitic Imidazolate Frameworks. Acc. Chem. Res. 2010, 43, 58–67. [Google Scholar] [CrossRef]
- Sun, J.; Sun, L.; Bai, S.; Fu, H.; Guo, J.; Feng, Y.; Luo, R.; Li, D.; Chen, A. Pyrolyzing Co/Zn bimetallic organic framework to form p-n heterojunction of Co3O4/ZnO for detection of formaldehyde. Sens. Actuators B Chem. 2019, 285, 291–301. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Zhang, C.; Meng, Q.; Xu, Z.; Su, P.; Li, Q.; Shen, C.; Fan, Z.; Qin, L.; et al. Transformation of metal-organic frameworks for molecular sieving membranes. Nat. Commun. 2016, 7, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Lively, R.P.; Zhang, C.; Koros, W.J.; Chance, R.R. Investigating the Intrinsic Ethanol/Water Separation Capability of ZIF-8: An Adsorption and Diffusion Study. J. Phys. Chem. C 2013, 117, 7214–7225. [Google Scholar] [CrossRef]
- Yin, H.; Lau, C.Y.; Rozowski, M.; Howard, C.; Xu, Y.; Lai, T.; Dose, M.E.; Lively, R.P.; Lind, M.L. Free-standing ZIF-71/PDMS nanocomposite membranes for the recovery of ethanol and 1-butanol from water through pervaporation. J. Membr. Sci. 2017, 529, 286–292. [Google Scholar] [CrossRef]
- Zhan, W.-W.; Kuang, Q.; Zhou, J.-Z.; Kong, X.-J.; Xie, Z.-X.; Zheng, L.-S. Semiconductor@Metal-Organic Framework Core-Shell Heterostructures: A Case of ZnO@ZIF-8 Nanorods with Selective Photoelectrochemical Response. J. Am. Chem. Soc. 2013, 135, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, W.; Zhu, Y.; Zhao, Q.; Huo, F. Synthesis of MOFs and Their Composite Structures through Sacrificial-Template Strategy. Cryst. Growth Des. 2015, 15, 1017–1021. [Google Scholar] [CrossRef]
- Julien, P.A.; Uzarevic, K.; Katsenis, A.D.; Kimber, S.A.J.; Wang, T.; Farha, O.K.; Zhang, Y.; Casaban, J.; Germann, L.S.; Etter, M.; et al. In Situ Monitoring and Mechanism of the Mechanochemical Formation of a Microporous MOF-74 Framework. J. Am. Chem. Soc. 2016, 138, 2929–2932. [Google Scholar] [CrossRef] [PubMed]
- Flaconneche, B.; Martin, J.; Klopffer, M.H. Permeability, diffusion and solubility of gases in polyethylene, polyamide 11 and poly(vinylidene fluoride). Oil Gas Sci. Technol. Rev. D Ifp Energ. Nouv. 2001, 56, 261–278. [Google Scholar] [CrossRef] [Green Version]
- Ellern, I.; Venkatasubramanian, A.; Lee, J.-H.; Hesketh, P.; Stavila, V.; Robinson, A.; Allendorf, M. HKUST-1 coated piezoresistive microcantilever array for volatile organic compound sensing. Micro Nano Lett. 2013, 8, 766–769. [Google Scholar] [CrossRef]
- Gutierrez, I.; Diaz, E.; Vega, A.; Ordonez, S. Consequences of cavity size and chemical environment on the adsorption properties of isoreticular metal-organic frameworks: An inverse gas chromatography study. J. Chromatogr. A 2013, 1274, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Fu, Z.; Wang, T.; Lei, W.; Sun, P.; Sui, Y.; Zou, B. A rational design of hollow nanocages Ag@CuO-TiO2 for enhanced acetone sensing performance. Sens. Actuators B Chem. 2019, 295, 70–78. [Google Scholar] [CrossRef]
- Saliba, D.; Ammar, M.; Rammal, M.; Al-Ghoul, M.; Hmadeh, M. Crystal Growth of ZIF-8, ZIF-67, and Their Mixed-Metal Derivatives. J. Am. Chem. Soc. 2018, 140, 1812–1823. [Google Scholar] [CrossRef]
- Wang, D.; Li, Z.; Zhou, J.; Fang, H.; He, X.; Jena, P.; Zeng, J.-B.; Wang, W.-N. Simultaneous Detection and Removal of Formaldehyde at Room Temperature: Janus Au@ZnO@ZIF-8 Nanoparticles. Nano Micro Lett. 2018, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, S.; Liu, C.; Luo, R.; Chen, A. Metal organic frameworks-derived sensing material of SnO2/NiO composites for detection of triethylamine. Appl. Surf. Sci. 2018, 437, 304–313. [Google Scholar] [CrossRef]
- Rai, P.; Majhi, S.M.; Yu, Y.T.; Lee, J.H. Noble metal@metal oxide semiconductor core@shell nano-architectures as a new platform for gas sensor applications. RSC Adv. 2015, 5, 76229–76248. [Google Scholar] [CrossRef]
- Jiang, H.-L.; Akita, T.; Ishida, T.; Haruta, M.; Xu, Q. Synergistic Catalysis of Au@Ag Core-Shell Nanoparticles Stabilized on Metal-Organic Framework. J. Am. Chem. Soc. 2011, 133, 1304–1306. [Google Scholar] [CrossRef] [PubMed]
- Koo, W.-T.; Yu, S.; Choi, S.-J.; Jang, J.-S.; Cheong, J.Y.; Kim, I.-D. Nanoscale PdO Catalyst Functionalized Co3O4 Hollow Nanocages Using MOF Templates for Selective Detection of Acetone Molecules in Exhaled Breath. ACS Appl. Mater. Interfaces 2017, 9, 8201–8210. [Google Scholar] [CrossRef] [PubMed]
- Koo, W.-T.; Jang, J.-S.; Choi, S.-J.; Cho, H.-J.; Kim, I.-D. Metal-Organic Framework Templated Catalysts: Dual Sensitization of PdO-ZnO Composite on Hollow SnO2 Nanotubes for Selective Acetone Sensors. ACS Appl. Mater. Interfaces 2017, 9, 18069–18077. [Google Scholar] [CrossRef] [PubMed]
- Koo, W.-T.; Choi, S.-J.; Kim, S.-J.; Jang, J.-S.; Tuller, H.L.; Kim, I.-D. Heterogeneous Sensitization of Metal-Organic Framework Driven Metal@Metal Oxide Complex Catalysts on an Oxide Nanofiber Scaffold Toward Superior Gas Sensors. J. Am. Chem. Soc. 2016, 138, 13431–13437. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Zhu, H.; Wang, G.; Luo, M.; Shen, S.; Ai, C.; Yang, L.; Lin, S.; Zhang, Q.; Gu, L.; et al. Boosting fast energy storage by synergistic engineering of carbon and deficiency. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, X.; Li, Y.; Zhu, R.; Pang, H. Applications of Metal-Organic-Framework-Derived Carbon Materials. Adv. Mater. 2019, 31, 1804740. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Hu, Y.; Liang, Y.; Kong, B.; Zheng, Z.; Zhang, J.; Jiang, S.P.; Zhao, Y.; Wang, H. Graphene oxide/core-shell structured metal-organic framework nano-sandwiches and their derived cobalt/N-doped carbon nanosheets for oxygen reduction reactions. J. Mater. Chem. A 2017, 5, 10182–10189. [Google Scholar] [CrossRef]
- Liu, P.; Gao, S.; Wang, Y.; Huang, Y.; He, W.; Huang, W.; Luo, J. Carbon nanocages with N-doped carbon inner shell and Co/N-doped carbon outer shell as electromagnetic wave absorption materials. Chem. Eng. J. 2020, 381, 122653. [Google Scholar] [CrossRef]
- Varghese, S.S.; Lonkar, S.; Singh, K.K.; Swaminathan, S.; Abdala, A. Recent advances in graphene based gas sensors. Sens. Actuators B Chem. 2015, 218, 160–183. [Google Scholar] [CrossRef]
- Zhang, H.; Fan, L.; Dong, H.; Zhang, P.; Nie, K.; Zhong, J.; Li, Y.; Guo, J.; Sun, X. Spectroscopic Investigation of Plasma-Fluorinated Monolayer Graphene and Application for Gas Sensing. ACS Appl. Mater. Interfaces 2016, 8, 8652–8661. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroeder, V.; Savagatrup, S.; He, M.; Ling, S.; Swager, T.M. Carbon Nanotube Chemical Sensors. Chem. Rev. 2019, 119, 599–663. [Google Scholar] [CrossRef]
- Ingle, N.; Sayyad, P.; Deshmukh, M.; Bodkhe, G.; Mahadik, M.; Al-Gahouari, T.; Shirsat, S.; Shirsat, M.D. A chemiresistive gas sensor for sensitive detection of SO2 employing Ni-MOF modified -OH-SWNTs and -OH-MWNTs. Appl. Phys. A Mater. Sci. Process. 2021, 127. [Google Scholar] [CrossRef]
- Sule, R.; Mishra, A.K. MOFs-carbon hybrid nanocomposites in environmental protection applications. Environ. Sci. Pollut. Res. 2020, 27, 16004–16018. [Google Scholar] [CrossRef]
- Liu, X.-W.; Sun, T.-J.; Hu, J.-L.; Wang, S.-D. Composites of metal-organic frameworks and carbon-based materials: Preparations, functionalities and applications. J. Mater. Chem. A 2016, 4, 3584–3616. [Google Scholar] [CrossRef]
- Travlou, N.A.; Singh, K.; Rodriguez-Castellon, E.; Bandosz, T.J. Cu-BTC MOF-graphene-based hybrid materials as low concentration ammonia sensors. J. Mater. Chem. A 2015, 3, 11417–11429. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, H.; Huang, P.; Xiang, C.; Zou, Y.; Xu, F.; Sun, L. Inducement of nanoscale Cu-BTC on nanocomposite of PPy-rGO and its performance in ammonia sensing. Mater. Res. Bull. 2018, 99, 152–160. [Google Scholar] [CrossRef]
- Bhardwaj, S.K.; Mohanta, G.C.; Sharma, A.L.; Kim, K.-H.; Deep, A. A three-phase copper MOF-graphene-polyaniline composite for effective sensing of ammonia. Anal. Chim. Acta 2018, 1043, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Jafari, N.; Zeinali, S.; Shadmehr, J. Room temperature resistive gas sensor based on ZIF-8/MWCNT/AgNPs nanocomposite for VOCs detection. J. Mater. Sci. Mater. Electron. 2019, 30, 12339–12350. [Google Scholar] [CrossRef]
- Tan, J.; Hussain, S.; Ge, C.; Zhan, M.; Liu, J.; Liu, S.; Liu, G.; Qiao, G. Construction of hierarchical trimetallic organic framework leaf-like nanostructures derived from carbon nanotubes for gas-sensing applications. J. Hazard. Mater. 2020, 400, 123155. [Google Scholar] [CrossRef] [PubMed]
- Ingle, N.; Mane, S.; Sayyad, P.; Bodkhe, G.; Al-Gahouari, T.; Mahadik, M.; Shirsat, S.; Shirsat, M.D. Sulfur Dioxide (SO2) Detection Using Composite of Nickel Benzene Carboxylic (Ni3BTC2) and OH-Functionalized Single Walled Carbon Nanotubes (OH-SWNTs). Front. Mater. 2020, 7, 93. [Google Scholar] [CrossRef]
- Wong, D.; Abuzalat, O.; Mostafa, S.; Park, S.S.; Kim, S. Intense pulsed light-based synthesis of hybrid TiO2-SnO2/MWCNT doped Cu-BTC for room temperature ammonia sensing. J. Mater. Chem. C 2020, 8, 7567–7574. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, M.; Tang, B.; Li, S.; Jiang, L.; Sun, X.; Que, M.; Tao, C.; Wu, Z. Graphene modified Cu-BTC with high stability in water and controllable selective adsorption of various gases. J. Alloy. Compd. 2019, 808, 151721. [Google Scholar] [CrossRef]
- Yang, S.J.; Choi, J.Y.; Chae, H.K.; Cho, J.H.; Nahm, K.S.; Park, C.R. Preparation and Enhanced Hydrostability and Hydrogen Storage Capacity of CNT@MOF-5 Hybrid Composite. Chem. Mater. 2009, 21, 1893–1897. [Google Scholar] [CrossRef]
- Huang, Z.-H.; Liu, G.; Kang, F. Glucose-Promoted Zn-Based Metal-Organic Framework/Graphene Oxide Composites for Hydrogen Sulfide Removal. ACS Appl. Mater. Interfaces 2012, 4, 4942–4947. [Google Scholar] [CrossRef]
- Sofi, F.A.; Majid, K.; Mehraj, O. The visible light driven copper based metal-organic-framework heterojunction:HKUST-1@Ag-Ag3PO4 for plasmon enhanced visible light photocatalysis. J. Alloy. Compd. 2018, 737, 798–808. [Google Scholar] [CrossRef]
- Yu, L.; Liu, Q.; Dai, W.; Tian, N.; Ma, N. Efficient thiophene capture with a hydrophobic Cu-BTC-(n)Br adsorbent in the presence of moisture. Microporous Mesoporous Mater. 2018, 266, 7–13. [Google Scholar] [CrossRef]
- Zhou, C.; Cao, L.; Wei, S.; Zhang, Q.; Chen, L. A first principles study of gas adsorption on charged Cu-BTC. Comput. Theor. Chem. 2011, 976, 153–160. [Google Scholar] [CrossRef]
- Bandosz, T.J.; Petit, C. MOF/graphite oxide hybrid materials: Exploring the new concept of adsorbents and catalysts. Adsorpt. J. Int. Adsorpt. Soc. 2011, 17, 5–16. [Google Scholar] [CrossRef]
- Petit, C.; Huang, L.; Jagiello, J.; Kenvin, J.; Gubbins, K.E.; Bandosz, T.J. Toward Understanding Reactive Adsorption of Ammonia on Cu-MOF/Graphite Oxide Nanocomposites. Langmuir 2011, 27, 13043–13051. [Google Scholar] [CrossRef]
- Petit, C.; Mendoza, B.; Bandosz, T.J. Reactive Adsorption of Ammonia on Cu-Based MOF/Graphene Composites. Langmuir 2010, 26, 15302–15309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, T.; Sholl, D.S. Molecular chemisorption on open metal sites in Cu-3(benzenetricarboxylate)(2): A spatially periodic density functional theory study. J. Chem. Phys. 2010, 133, 094509. [Google Scholar] [CrossRef]
- Peterson, G.W.; Wagner, G.W.; Balboa, A.; Mahle, J.; Sewell, T.; Karwacki, C.J. Ammonia Vapor Removal by Cu-3(BTC)(2) and Its Characterization by MAS NMR. J. Phys. Chem. C 2009, 113, 13906–13917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petit, C.; Burress, J.; Bandosz, T.J. The synthesis and characterization of copper-based metal-organic framework/graphite oxide composites. Carbon 2011, 49, 563–572. [Google Scholar] [CrossRef]
- Lin, Z.; Lv, Z.; Zhou, X.; Xiao, H.; Wu, J.; Li, Z. Postsynthetic Strategy To Prepare ACN@Cu-BTCs with Enhanced Water Vapor Stability and CO2/CH4 Separation Selectivity. Ind. Eng. Chem. Res. 2018, 57, 3765–3772. [Google Scholar] [CrossRef]
- Li, H.; Lin, Z.; Zhou, X.; Wang, X.; Li, Y.; Wang, H.; Li, Z. Ultrafast room temperature synthesis of novel composites Imi@Cu-BTC with improved stability against moisture. Chem. Eng. J. 2017, 307, 537–543. [Google Scholar] [CrossRef]
- Yao, W.; Liu, L.; Wu, X.; Qin, C.; Xie, H.; Su, Z. Polyoxometalates/Active Carbon Thin Separator for Improving Cycle Performance of Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2018, 10, 35911–35918. [Google Scholar] [CrossRef] [PubMed]
- Sakaushi, K.; Eckardt, M.; Lyalin, A.; Taketsugu, T.; Behm, R.J.; Uosaki, K. Microscopic Electrode Processes in the Four-Electron Oxygen Reduction on Highly Active Carbon-Based Electrocatalysts. Acs Catal. 2018, 8, 8162–8176. [Google Scholar] [CrossRef]
- Deng, X.; Shi, W.; Zhong, Y.; Zhou, W.; Liu, M.; Shao, Z. Facile Strategy to Low-Cost Synthesis of Hierarchically Porous, Active Carbon of High Graphitization for Energy Storage. Acs Appl. Mater. Interfaces 2018, 10, 21573–21581. [Google Scholar] [CrossRef] [PubMed]
- Rieter, W.J.; Taylor, K.M.L.; Lin, W. Surface modification and functionalization of nanoscale metal-organic frameworks for controlled release and luminescence sensing. J. Am. Chem. Soc. 2007, 129, 9852–9853. [Google Scholar] [CrossRef] [Green Version]
- Petit, C.; Bandosz, T.J. Synthesis, Characterization, and Ammonia Adsorption Properties of Mesoporous Metal-Organic Framework (MIL(Fe))-Graphite Oxide Composites: Exploring the Limits of Materials Fabrication. Adv. Funct. Mater. 2011, 21, 2108–2117. [Google Scholar] [CrossRef]
- Patel, D.G.; Walton, I.M.; Cox, J.M.; Gleason, C.J.; Butzer, D.R.; Benedict, J.B. Photoresponsive porous materials: The design and synthesis of photochromic diarylethene-based linkers and a metal-organic framework. Chem. Commun. 2014, 50, 2653–2656. [Google Scholar] [CrossRef]
- Zhu, P.; Sumpter, B.G.; Meunier, V. Electronic, Thermal, and Structural Properties of Graphene Oxide Frameworks. J. Phys. Chem. C 2013, 117, 8276–8281. [Google Scholar] [CrossRef]
- Szczesniak, B.; Choma, J.; Jaroniec, M. Ultrahigh benzene adsorption capacity of graphene-MOF composite fabricated via MOF crystallization in 3D mesoporous graphene. Microporous Mesoporous Mater. 2019, 279, 387–394. [Google Scholar] [CrossRef]
- Jabbari, V.; Veleta, J.M.; Zarei-Chaleshtori, M.; Gardea-Torresdey, J.; Villagran, D. Green synthesis of magnetic MOF@GO and MOF@CNT hybrid nanocomposites with high adsorption capacity towards organic pollutants. Chem. Eng. J. 2016, 304, 774–783. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Hou, C.; Zhang, Y.; He, F.; Liu, M.; Li, X. Preparation of graphene nano-sheet bonded PDA/MOF microcapsules with immobilized glucose oxidase as a mimetic multi-enzyme system for electrochemical sensing of glucose. J. Mater. Chem. B 2016, 4, 3695–3702. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Liu, Q.; Liu, J.; Zhang, H.; Li, Z.; Li, R.; Liu, L.; Wang, J. Interfacial growth of a metal-organic framework (UiO-66) on functionalized graphene oxide (GO) as a suitable seawater adsorbent for extraction of uranium(VI). J. Mater. Chem. A 2017, 5, 17933–17942. [Google Scholar] [CrossRef]
- Tang, Y.; Zheng, S.; Xu, Y.; Xiao, X.; Xue, H.; Pang, H. Advanced batteries based on manganese dioxide and its composites. Energy Storage Mater. 2018, 12, 284–309. [Google Scholar] [CrossRef]
- Dong, L.; Chen, M.; Li, J.; Shi, D.; Dong, W.; Li, X.; Bai, Y. Metal-organic framework-graphene oxide composites: A facile method to highly improve the CO2 separation performance of mixed matrix membranes. J. Membr. Sci. 2016, 520, 801–811. [Google Scholar] [CrossRef]
- Banerjee, P.C.; Lobo, D.E.; Middag, R.; Ng, W.K.; Shaibani, M.E.; Majumder, M. Electrochemical Capacitance of Ni-Doped Metal Organic Framework and Reduced Graphene Oxide Composites: More than the Sum of Its Parts. ACS Appl. Mater. Interfaces 2015, 7, 3655–3664. [Google Scholar] [CrossRef] [PubMed]
- Talin, A.A.; Centrone, A.; Ford, A.C.; Foster, M.E.; Stavila, V.; Haney, P.; Kinney, R.A.; Szalai, V.; El Gabaly, F.; Yoon, H.P.; et al. Tunable Electrical Conductivity in Metal-Organic Framework Thin-Film Devices. Science 2014, 343, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.; Bao, Q.; Yang, J.-X.; Loh, K.P. Structure-Directing Role of Graphene in the Synthesis of Metal-Organic Framework Nanowire. J. Am. Chem. Soc. 2010, 132, 14487–14495. [Google Scholar] [CrossRef] [PubMed]
- Allendorf, M.D.; Schwartzberg, A.; Stavila, V.; Talin, A.A. A Roadmap to Implementing Metal-Organic Frameworks in Electronic Devices: Challenges and Critical Directions. Chem. A Eur. J. 2011, 17, 11372–11388. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Xu, J.; Zhang, S.; Zhu, X.; Liu, H.; Hu, J. Interfacial Growth of Metal Organic Framework/Graphite Oxide Composites through Pickering Emulsion and Their CO2 Capture Performance in the Presence of Humidity. Langmuir 2015, 31, 7410–7417. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.K.; Trinh, T.P.; Nguyen, V.C.; Kim, J. Facile synthesis of graphite oxide/MIL-101(Cr) hybrid composites for enhanced adsorption performance towards industrial toxic dyes. J. Ind. Eng. Chem. 2021, 95, 224–234. [Google Scholar] [CrossRef]
- Esfandiari, K.; Mandavi, A.R.; Ghoreyshri, A.A.; Jahanshahi, M. Optimizing parameters affecting synthetize of CuBTC using response surface methodology and development of AC@CuBTC composite for enhanced hydrogen uptake. Int. J. Hydrog. Energy 2018, 43, 6654–6665. [Google Scholar] [CrossRef]
- Dastbaz, A.; Karimi-Sabet, J.; Moosavian, M.A. Sonochemical synthesis of novel decorated graphene nanosheets with amine functional Cu-terephthalate MOF for hydrogen adsorption: Effect of ultrasound and graphene content. Int. J. Hydrog. Energy 2019, 44, 26444–26458. [Google Scholar] [CrossRef]
- Zheng, Y.; Zheng, S.; Xue, H.; Pang, H. Metal-Organic Frameworks/Graphene-Based Materials: Preparations and Applications. Adv. Funct. Mater. 2018, 28, 1804950. [Google Scholar] [CrossRef]
- Wen, P.; Gong, P.; Sun, J.; Wang, J.; Yang, S. Design and synthesis of Ni-MOF/CNT composites and rGO/carbon nitride composites for an asymmetric supercapacitor with high energy and power density. J. Mater. Chem. A 2015, 3, 13874–13883. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, L.; Tan, X.; Sang, X.; Zhang, J.; Liu, C.; Zhang, B.; Han, B.; Yang, G. Pickering emulsions stabilized by a metal-organic framework (MOF) and graphene oxide (GO) for producing MOF/GO composites. Soft Matter 2017, 13, 7365–7370. [Google Scholar] [CrossRef] [PubMed]
- Pera-Titus, M.; Leclercq, L.; Clacens, J.-M.; De Campo, F.; Nardello-Rataj, V. Pickering Interfacial Catalysis for Biphasic Systems: From Emulsion Design to Green Reactions. Angew. Chem. Int. Ed. 2015, 54, 2006–2021. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, Q.; Zhu, S. Assembly of a Metal-Organic Framework into 3D Hierarchical Porous Monoliths Using a Pickering High Internal Phase Emulsion Template. Chem. A Eur. J. 2016, 22, 8751–8755. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, J.; Liu, C.; Peng, L.; Sang, X.; Han, B.; Ma, X.; Luo, T.; Tan, X.; Yang, G. High-internal-phase emulsions stabilized by metal-organic frameworks and derivation of ultralight metal-organic aerogels. Sci. Rep. 2016, 6, 21401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, J.; Sang, X.; Liu, C.; Luo, T.; Peng, L.; Han, B.; Tan, X.; Ma, X.; Wang, D.; et al. Cellular graphene aerogel combines ultralow weight and high mechanical strength: A highly efficient reactor for catalytic hydrogenation. Sci. Rep. 2016, 6, 25830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, J.-J.; Lv, W.; Yang, Q.-H. Self-Assembly of Graphene Oxide at Interfaces. Adv. Mater. 2014, 26, 5586–5612. [Google Scholar] [CrossRef]
- Levasseur, B.; Petit, C.; Bandosz, T.J. Reactive Adsorption of NO2 on Copper-Based Metal-Organic Framework and Graphite Oxide/Metal-Organic Framework Composites. ACS Appl. Mater. Interfaces 2010, 2, 3606–3613. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wu, F.; Sun, X.; Li, R.; Guo, Y.; Li, C.; Zhang, L.; Xing, F.; Wang, W.; Gao, J. Factors that Affect Pickering Emulsions Stabilized by Graphene Oxide. ACS Appl. Mater. Interfaces 2013, 5, 4843–4855. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sun, L.; Xu, F.; Zhang, J.; Jiao, C.; Li, F.; Li, Z.; Wang, S.; Wang, Z.; Jiang, X.; et al. Nanosized Cu-MOFs induced by graphene oxide and enhanced gas storage capacity. Energy Environ. Sci. 2013, 6, 818–823. [Google Scholar] [CrossRef]
- Bashkova, S.; Bandosz, T.J. Insight into the role of the oxidized graphite precursor on the properties of copper-based MOF/graphite oxide composites. Microporous Mesoporous Mater. 2013, 179, 205–211. [Google Scholar] [CrossRef]
- Ahsan, M.A.; Jabbari, V.; Islam, M.T.; Turley, R.S.; Dominguez, N.; Kim, H.; Castro, E.; Hernandez-Viezcas, J.A.; Curry, M.L.; Lopez, J.; et al. Sustainable synthesis and remarkable adsorption capacity of MOF/graphene oxide and MOF/CNT based hybrid nanocomposites for the removal of Bisphenol A from water. Sci. Total Environ. 2019, 673, 306–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chappanda, K.N.; Shekhah, O.; Yassine, O.; Patole, S.P.; Eddaoudi, M.; Salama, K.N. The quest for highly sensitive QCM humidity sensors: The coating of CNT/MOF composite sensing films as case study. Sens. Actuators B Chem. 2018, 257, 609–619. [Google Scholar] [CrossRef] [Green Version]
- Tran Thanh, T.; Manh Trung, T.; Feller, J.-F.; Castro, M.; Truc Van, N.; Hassan, K.; Nine, M.J.; Losic, D. Graphene and metal organic frameworks (MOFs) hybridization for tunable chemoresistive sensors for detection of volatile organic compounds (VOCs) biomarkers. Carbon 2020, 159, 333–344. [Google Scholar] [CrossRef]
- Ellis, J.E.; Zeng, Z.; Hwang, S.I.; Li, S.; Luo, T.-Y.; Burkert, S.C.; White, D.L.; Rosi, N.L.; Gassensmith, J.J.; Star, A. Growth of ZIF-8 on molecularly ordered 2-methylimidazole/single-walled carbon nanotubes to form highly porous, electrically conductive composites. Chem. Sci. 2019, 10, 737–742. [Google Scholar] [CrossRef] [Green Version]
- Jian, M.; Liu, B.; Liu, R.; Qu, J.; Wang, H.; Zhang, X. Water-based synthesis of zeolitic imidazolate framework-8 with high morphology level at room temperature. RSC Adv. 2015, 5, 48433–48441. [Google Scholar] [CrossRef]
- Lo, Y.; Lam, C.H.; Chang, C.-W.; Yang, A.-C.; Kang, D.-Y. Polymorphism/pseudopolymorphism of metalorganic frameworks composed of Zinc(II) and 2methylimidazole: Synthesis, stability, and application in gas storage. RSC Adv. 2016, 6, 89148–89156. [Google Scholar] [CrossRef]
- Chen, C.; Li, B.; Zhou, L.; Xia, Z.; Feng, N.; Ding, J.; Wang, L.; Wan, H.; Guan, G. Synthesis of Hierarchically Structured Hybrid Materials by Controlled Self-Assembly of Metal Organic Framework with Mesoporous Silica for CO2 Adsorption. ACS Appl. Mater. Interfaces 2017, 9, 23060–23071. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-F.; Zhang, H.; Liu, Q.; Sun, L.; Stanciu, L.; Xie, J. Fabrication of High-Surface-Area Graphene/Polyaniline Nanocomposites and Their Application in Supercapacitors. ACS Appl. Mater. Interfaces 2013, 5, 2685–2691. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.; Chen, Y.; Kim, J. Cellulose-titanium dioxide-multiwalled carbon nanotube hybrid nanocomposite and its ammonia gas sensing properties at room temperature. Sens. Actuators B Chem. 2012, 171, 1186–1191. [Google Scholar] [CrossRef]
- Liu, J.; Wei, Y.; Li, P.; Zhao, Y.; Zou, R. Selective H2S/CO2 Separation by Metal-Organic Frameworks Based on Chemical-Physical Adsorption. J. Phys. Chem. C 2017, 121, 13249–13255. [Google Scholar] [CrossRef]
- Wang, H.; Qu, Z.G.; Zhang, W.; Yu, Q.N.; He, Y.L. Experimental and numerical study of CO2 adsorption on copper benzene-1,3,5-tricarboxylate (Cu-BTC) metal organic framework. Int. J. Heat Mass Transf. 2016, 92, 859–863. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Z.; Lin, Y.S. Adsorption and Diffusion of Carbon Dioxide on Metal-Organic Framework (MOF-5). Ind. Eng. Chem. Res. 2009, 48, 10015–10020. [Google Scholar] [CrossRef]
- Gautam, S.; Cole, D. CO2 Adsorption in Metal-Organic Framework Mg-MOF-74: Effects of Inter-Crystalline Space. Nanomaterials 2020, 10, 2274. [Google Scholar] [CrossRef]
- Barea, E.; Montoro, C.; Navarro, J.A.R. Toxic gas removal—metal-organic frameworks for the capture and degradation of toxic gases and vapours. Chem. Soc. Rev. 2014, 43, 5419–5430. [Google Scholar] [CrossRef] [Green Version]
- Petit, C.; Levasseur, B.; Mendoza, B.; Bandosz, T.J. Reactive adsorption of acidic gases on MOF/graphite oxide composites. Microporous Mesoporous Mater. 2012, 154, 107–112. [Google Scholar] [CrossRef]
- Zhao, Y.; Seredych, M.; Zhong, Q.; Bandosz, T.J. Aminated graphite oxides and their composites with copper-based metal-organic framework: In search for efficient media for CO2 sequestration. RSC Adv. 2013, 3, 9932–9941. [Google Scholar] [CrossRef]
- Ning, H.; Yang, Z.; Yin, Z.; Wang, D.; Meng, Z.; Wang, C.; Zhang, Y.; Chen, Z. A Novel Strategy to Enhance the Performance of CO2 Adsorption Separation: Grafting Hyper-cross-linked Polyimide onto Composites of UiO-66-NH2 and GO. ACS Appl. Mater. Interfaces 2021, 13, 17781–17790. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Seredych, M.; Zhong, Q.; Bandosz, T.J. Superior Performance of Copper Based MOF and Aminated Graphite Oxide Composites as CO2 Adsorbents at Room Temperature. ACS Appl. Mater. Interfaces 2013, 5, 4951–4959. [Google Scholar] [CrossRef] [PubMed]
- Policicchio, A.; Zhao, Y.; Zhong, Q.; Agostino, R.G.; Bandosz, T.J. Cu-BTC/Aminated Graphite Oxide Composites As High-Efficiency CO2 Capture Media. ACS Appl. Mater. Interfaces 2014, 6, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Seredych, M.; Jagiello, J.; Zhong, Q.; Bandosz, T.J. Insight into the mechanism of CO2 adsorption on Cu-BTC and its composites with graphite oxide or aminated graphite oxide. Chem. Eng. J. 2014, 239, 399–407. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef] [PubMed]
- Daraee, M.; Ghasemy, E.; Rashidi, A. Synthesis of novel and engineered UiO-66/graphene oxide nanocomposite with enhanced H2S adsorption capacity. J. Environ. Chem. Eng. 2020, 8, 104351. [Google Scholar] [CrossRef]
- McCullough, R.D. The chemistry of conducting polythiophenes. Adv. Mater. 1998, 10, 93–116. [Google Scholar] [CrossRef]
- Wong, Y.C.; Ang, B.C.; Haseeb, A.S.M.A.; Baharuddin, A.A.; Wong, Y.H. Review-Conducting Polymers as Chemiresistive Gas Sensing Materials: A Review. J. Electrochem. Soc. 2019, 167, 037503. [Google Scholar] [CrossRef]
- Waghuley, S.A.; Yenorkar, S.M.; Yawale, S.S.; Yawale, S.P. Application of chemically synthesized conducting polymer-polypyrrole as a carbon dioxide gas sensor. Sens. Actuators B Chem. 2008, 128, 366–373. [Google Scholar] [CrossRef]
- Joshi, A.; Gangal, S.A.; Gupta, S.K. Ammonia sensing properties of polypyrrole thin films at room temperature. Sens. Actuators B Chem. 2011, 156, 938–942. [Google Scholar] [CrossRef]
- Hassanzadeh, N.; Omidvar, H.; Tabaian, S.H. Chemical synthesis of high density and long polypyrrole nanowire arrays using alumina membrane and their hydrogen sensing properties. Superlattices Microstruct. 2012, 51, 314–323. [Google Scholar] [CrossRef]
- Su, P.-G.; Lee, C.-T.; Chou, C.-Y. Flexible NH3 sensors fabricated by in situ self-assembly of polypyrrole. Talanta 2009, 80, 763–769. [Google Scholar] [CrossRef]
- Malkeshi, H.; Moghaddam, H.M. Ammonia gas-sensing based on polythiophene film prepared through electrophoretic deposition method. J. Polym. Res. 2016, 23, 108. [Google Scholar] [CrossRef]
- Bai, S.; Guo, J.; Sun, J.; Tang, P.; Chen, A.; Luo, R.; Li, D. Enhancement of NO2-Sensing Performance at Room Temperature by Graphene-Modified Polythiophene. Ind. Eng. Chem. Res. 2016, 55, 5788–5794. [Google Scholar] [CrossRef]
- Dunst, K.; Karczewski, J.; Jasinski, P. Nitrogen dioxide sensing properties of PEDOT polymer films. Sens. Actuators B Chem. 2017, 247, 108–113. [Google Scholar] [CrossRef]
- Yoon, H.; Chang, M.; Jang, J. Formation of 1D poly(3,4-ethylenedioxythiophene) nanomaterials in reverse microemulsions and their application to chemical sensors. Adv. Funct. Mater. 2007, 17, 431–436. [Google Scholar] [CrossRef]
- Shaik, M.; Rao, V.K.; Sinha, A.K.; Murthy, K.S.R.C.; Jain, R. Sensitive detection of nitrogen dioxide gas at room temperature using poly(3,4-ethylenedioxythiophene) nanotubes. J. Environ. Chem. Eng. 2015, 3, 1947–1952. [Google Scholar] [CrossRef]
- Harale, N.S.; Nagare, A.B.; Mali, S.S.; Suryawanshi, M.P.; Sharma, K.K.K.; Rao, V.K.; Hong, C.K.; Kim, J.H.; Patil, P.S. Facile Synthesis of Nanofibrous Polyaniline Thin Films for Ammonia Gas Detection. J. Electron. Mater. 2020, 49, 1338–1347. [Google Scholar] [CrossRef]
- Liu, J.; Cui, N.; Xu, Q.; Wang, Z.; Gu, L.; Dou, W. High-Performance PANI-Based Ammonia Gas Sensor Promoted by Surface Nanostructuralization. ECS J. Solid State Sci. Technol. 2021, 10, 027007. [Google Scholar] [CrossRef]
- Abe, S.; Kijima, M.; Shirakawa, H. Effect of mesogenic cores and length of spacers on liquid crystallinity of N-substituted polypyrrole derivatives. Synth. Met. 2001, 119, 421–422. [Google Scholar] [CrossRef]
- Goren, M.; Lennox, R.B. Nanoscale polypyrrole patterns using block copolymer surface micelles as templates. Nano Lett. 2001, 1, 735–738. [Google Scholar] [CrossRef]
- Somani, P.R.; Marimuthu, R.; Mulik, U.P.; Sainkar, S.R.; Amalnerkar, D.P. High piezoresistivity and its origin in conducting polyaniline/TiO2 composites. Synth. Met. 1999, 106, 45–52. [Google Scholar] [CrossRef]
- Wang, S.X.; Tan, Z.C.; Li, Y.S.; Sun, L.X.; Zhang, T. Synthesis, characterization and thermal analysis of polyaniline/ZrO2 composites. Thermochim. Acta 2006, 441, 191–194. [Google Scholar] [CrossRef]
- Yuvaraja, S.; Surya, S.G.; Chernikova, V.; Vijjapu, M.T.; Shekhah, O.; Bhatt, P.M.; Chandra, S.; Eddaoudi, M.; Salama, K.N. Realization of an Ultrasensitive and Highly Selective OFET NO2 Sensor: The Synergistic Combination of PDVT-10 Polymer and Porphyrin-MOF. ACS Appl. Mater. Interfaces 2020, 12, 18748–18760. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, S.; Soccol, D.; Gravesteijn, D.J.; Kapteijn, F.; Sudholter, E.J.R.; Gascon, J.; de Smett, L.C.P.M. Polymer-Metal Organic Framework Composite Films as Affinity Layer for Capacitive Sensor Devices. ACS Sens. 2016, 1, 1188–1192. [Google Scholar] [CrossRef]
- Yang, S.; Karve, V.V.; Justin, A.; Kochetygov, I.; Espin, J.; Asgari, M.; Trukhina, O.; Sun, D.T.; Peng, L.; Queen, W.L. Enhancing MOF performance through the introduction of polymer guests. Coord. Chem. Rev. 2021, 427, 213525. [Google Scholar] [CrossRef]
- Ding, N.; Li, H.; Feng, X.; Wang, Q.; Wang, S.; Ma, L.; Zhou, J.; Wang, B. Partitioning MOF-5 into Confined and Hydrophobic Compartments for Carbon Capture under Humid Conditions. J. Am. Chem. Soc. 2016, 138, 10100–10103. [Google Scholar] [CrossRef]
- Gamage, N.-D.H.; McDonald, K.A.; Matzger, A.J. MOF-5-Polystyrene: Direct Production from Monomer, Improved Hydrolytic Stability, and Unique Guest Adsorption. Angew. Chem. Int. Ed. 2016, 55, 12099–12103. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Moreton, J.C.; Tavares, S.R.; Semino, R.; Maurin, G.; Cohen, S.M.; Schmidt-Rohr, K. Polymer Infiltration into Metal-Organic Frameworks in Mixed-Matrix Membranes Detected in Situ by NMR. J. Am. Chem. Soc. 2019, 141, 7589–7595. [Google Scholar] [CrossRef] [PubMed]
- Gul-E-Noor, F.; Jee, B.; Poeppl, A.; Hartmann, M.; Himsl, D.; Bertmer, M. Effects of varying water adsorption on a Cu-3(BTC)(2) metal-organic framework (MOF) as studied by H-1 and C-13 solid-state NMR spectroscopy. Phys. Chem. Chem. Phys. 2011, 13, 7783–7788. [Google Scholar] [CrossRef]
- Peng, Y.; Krungleviciute, V.; Eryazici, I.; Hupp, J.T.; Farha, O.K.; Yildirim, T. Methane Storage in Metal-Organic Frameworks: Current Records, Surprise Findings, and Challenges. J. Am. Chem. Soc. 2013, 135, 11887–11894. [Google Scholar] [CrossRef] [Green Version]
- Carne-Sanchez, A.; Stylianou, K.C.; Carbonell, C.; Naderi, M.; Imaz, I.; Maspoch, D. Protecting Metal-Organic Framework Crystals from Hydrolytic Degradation by Spray-Dry Encapsulating Them into Polystyrene Microspheres. Adv. Mater. 2015, 27, 869–873. [Google Scholar] [CrossRef] [Green Version]
- Iizuka, T.; Honjo, K.; Uemura, T. Enhanced mechanical properties of a metal-organic framework by polymer insertion. Chem. Commun. 2019, 55, 691–694. [Google Scholar] [CrossRef]
- Yoo, D.K.; Khan, N.A.; Jhung, S.H. Polyaniline-loaded metal-organic framework MIL-101(Cr): Promising adsorbent for CO2 capture with increased capacity and selectivity by polyaniline introduction. J. CO2 Util. 2018, 28, 319–325. [Google Scholar] [CrossRef]
- Xian, S.; Wu, Y.; Wu, J.; Wang, X.; Xiao, J. Enhanced Dynamic CO2 Adsorption Capacity and CO2/CH4 Selectivity on Polyethylenimine-Impregnated UiO-66. Ind. Eng. Chem. Res. 2015, 54, 11151–11158. [Google Scholar] [CrossRef]
- Xian, S.; Xu, F.; Ma, C.; Wu, Y.; Xia, Q.; Wang, H.; Li, Z. Vapor-enhanced CO2 adsorption mechanism of composite PEI@ZIF-8 modified by polyethyleneimine for CO2/N-2 separation. Chem. Eng. J. 2015, 280, 363–369. [Google Scholar] [CrossRef]
- Yan, Q.; Lin, Y.; Kong, C.; Chen, L. Remarkable CO2/CH4 selectivity and CO2 adsorption capacity exhibited by polyamine-decorated metal-organic framework adsorbents. Chem. Commun. 2013, 49, 6873–6875. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Li, Z.; Wang, L.; Ye, Y.; Liu, Q.; Ma, X.; Chen, Q.; Zhang, Z.; Xiang, S. Cobalt-citrate framework armored with graphene oxide exhibiting improved thermal stability and selectivity for biogas decarburization. J. Mater. Chem. A 2015, 3, 593–599. [Google Scholar] [CrossRef]
- Jadhav, A.; Gupta, K.; Ninawe, P.; Ballav, N. Imparting Multifunctionality by Utilizing Biporosity in a Zirconium-Based Metal-Organic Framework. Angew. Chem. Int. Ed. 2020, 59, 2215–2219. [Google Scholar] [CrossRef] [PubMed]
- Uemura, T.; Kadowaki, Y.; Yanai, N.; Kitagawa, S. Template Synthesis of Porous Polypyrrole in 3D Coordination Nanochannels. Chem. Mater. 2009, 21, 4096–4098. [Google Scholar] [CrossRef]
- Mashao, G.; Modibane, K.D.; Mdluli, S.B.; Iwuoha, E.I.; Hato, M.J.; Makgopa, K.; Molapo, K.M. Polyaniline-Cobalt Benzimidazolate Zeolitic Metal-Organic Framework Composite Material for Electrochemical Hydrogen Gas Sensing. Electrocatalysis 2019, 10, 406–419. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, X.; Li, H.; Chen, Y.; Zhao, J.; Wang, S.; Wang, L.; Wang, B. Photoinduced Postsynthetic Polymerization of a Metal-Organic Framework toward a Flexible Stand-Alone Membrane. Angew. Chem. Int. Ed. 2015, 54, 4259–4263. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.T.; Peng, L.; Reeder, W.S.; Moosavi, S.M.; Tiana, D.; Britt, D.K.; Oveisi, E.; Queen, W.L. Rapid, Selective Heavy Metal Removal from Water by a Metal-Organic Framework/Polydopamine Composite. ACS Cent. Sci. 2018, 4, 349–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, L.; Yang, S.; Sun, D.T.; Asgari, M.; Queen, W.L. MOF/polymer composite synthesized using a double solvent method offers enhanced water and CO2 adsorption properties. Chem. Commun. 2018, 54, 10602–10605. [Google Scholar] [CrossRef] [PubMed]
- Erucar, I.; Yilmaz, G.; Keskin, S. Recent Advances in Metal-Organic Framework-Based Mixed Matrix Membranes. Chem. Asian J. 2013, 8, 1692–1704. [Google Scholar] [CrossRef] [PubMed]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 2017, 356, eaab0530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robeson, L.M. Correlation of Separation Factor Versus Permeability for Polymeric Membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Adams, R.; Carson, C.; Ward, J.; Tannenbaum, R.; Koros, W. Metal organic framework mixed matrix membranes for gas separations. Microporous Mesoporous Mater. 2010, 131, 13–20. [Google Scholar] [CrossRef]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.-H.; Long, J.R. Carbon Dioxide Capture in Metal-Organic Frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef]
- Jeazet, H.B.T.; Staudt, C.; Janiak, C. A method for increasing permeability in O-2/N-2 separation with mixed-matrix membranes made of water-stable MIL-101 and polysulfone. Chem. Commun. 2012, 48, 2140–2142. [Google Scholar] [CrossRef]
- Lin, R.; Hernandez, B.V.; Ge, L.; Zhu, Z. Metal organic framework based mixed matrix membranes: An overview on filler/polymer interfaces. J. Mater. Chem. A 2018, 6, 293–312. [Google Scholar] [CrossRef]
- Azizi, A.; Feijani, E.A.; Ghorbani, Z.; Tavasoli, A. Fabrication and characterization of highly efficient three component CuBTC/graphene oxide/PSF membrane for gas separation application. Int. J. Hydrogen Energy 2021, 46, 2244–2254. [Google Scholar] [CrossRef]
- Seoane, B.; Coronas, J.; Gascon, I.; Etxeberria Benavides, M.; Karvan, O.; Caro, J.; Kapteijn, F.; Gascon, J. Metal-organic framework based mixed matrix membranes: A solution for highly efficient CO2 capture? Chem. Soc. Rev. 2015, 44, 2421–2454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Huang, H.; Ban, Y.; Yang, Q.; Xiao, Y.; Li, Y.; Yang, W.; Zhong, C. Mixed matrix membranes incorporated with amine-functionalized titanium-based metal-organic framework for CO2/CH4 separation. J. Membr. Sci. 2015, 478, 130–139. [Google Scholar] [CrossRef]
- Nik, O.G.; Chen, X.Y.; Kaliaguine, S. Functionalized metal organic framework-polyimide mixed matrix membranes for CO2/CH4 separation. J. Membr. Sci. 2012, 413, 48–61. [Google Scholar] [CrossRef]
- Basu, S.; Cano-Odena, A.; Vankelecom, I.F.J. Asymmetric Matrimid (R)/ Cu-3(BTC)(2) mixed-matrix membranes for gas separations. J. Membr. Sci. 2010, 362, 478–487. [Google Scholar] [CrossRef]
- Sachdeva, S.; Koper, S.J.H.; Sabetghadam, A.; Soccol, D.; Gravesteijn, D.J.; Kapteijn, F.; Sudholter, E.J.R.; Gascon, J.; de Smet, L.C.P.M. Gas Phase Sensing of Alcohols by Metal Organic Framework-Polymer Composite Materials. ACS Appl. Mater. Interfaces 2017, 9, 24926–24935. [Google Scholar] [CrossRef] [PubMed]
- Rodenas, T.; van Dalen, M.; Serra-Crespo, P.; Kapteijn, F.; Gascon, J. Mixed matrix membranes based on NH2-functionalized MIL-type MOFs: Influence of structural and operational parameters on the CO2/CH4 separation performance. Microporous Mesoporous Mater. 2014, 192, 35–42. [Google Scholar] [CrossRef]
- Serra-Crespo, P.; Gobechiya, E.; Ramos-Fernandez, E.V.; Juan-Alcaniz, J.; Martinez-Joaristi, A.; Stavitski, E.; Kirschhock, C.E.A.; Martens, J.A.; Kapteijn, F.; Gascon, J. Interplay of Metal Node and Amine Functionality in NH2-MIL-53: Modulating Breathing Behavior through Intra-framework Interactions. Langmuir 2012, 28, 12916–12922. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.T.; Koros, W.J. Non-ideal effects in organic-inorganic materials for gas separation membranes. J. Mol. Struct. 2005, 739, 87–98. [Google Scholar] [CrossRef]
- Burmann, P.; Zornoza, B.; Tellez, C.; Coronas, J. Mixed matrix membranes comprising MOFs and porous silicate fillers prepared via spin coating for gas separation. Chem. Eng. Sci. 2014, 107, 66–75. [Google Scholar] [CrossRef]
- Cao, R.; Ding, H.; Kim, K.-J.; Peng, Z.; Wu, J.; Culp, J.T.; Ohodnicki, P.R.; Beckman, E.; Chen, K.P. Metal-organic framework functionalized polymer coating for fiber optical methane sensors. Sens. Actuators B Chem. 2020, 324. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, A.L.; Deep, A. Sensitive impedimetric detection of E. coli with metal-organic framework (MIL-53) / polymer (PEDOT) composite modified screen-printed electrodes. J. Environ. Chem. Eng. 2021, 9, 104925. [Google Scholar] [CrossRef]
- Li, C.; Zhang, L.; Chen, J.; Li, X.; Sun, J.; Zhu, J.; Wang, X.; Fu, Y. Recent development and applications of electrical conductive MOFs. Nanoscale 2021, 13, 485–509. [Google Scholar] [CrossRef] [PubMed]
- Zahadiya, H.; Wijesundera, R.P.; Hettiarachchi, C.V.; Perera, I.R. Effect of Benzene Derivatives as Guest Molecules on Semiconductor Properties of MOF-199. Chemistryselect 2021, 6, 425–429. [Google Scholar] [CrossRef]
- Vo Minh Huy, T.; Aguey-Zinsou, K.-F. Encapsulation of silicotungstic acid into chromium (III) terephthalate metal-organic framework for high proton conductivity membranes. Res. Chem. Intermed. 2021, 47, 61–76. [Google Scholar] [CrossRef]
- Yao, M.-S.; Li, W.-H.; Xu, G. Metal-organic frameworks and their derivatives for electrically-transduced gas sensors. Coord. Chem. Rev. 2021, 426. [Google Scholar] [CrossRef]
- Hansen, J.; Kharecha, P.; Sato, M.; Masson-Delmotte, V.; Ackerman, F.; Beerling, D.J.; Hearty, P.J.; Hoegh-Guldberg, O.; Hsu, S.-L.; Parmesan, C.; et al. Assessing “Dangerous Climate Change”: Required Reduction of Carbon Emissions to Protect Young People, Future Generations and Nature. PLoS ONE 2013, 8, e81648. [Google Scholar] [CrossRef] [Green Version]
- Mikkelsen, M.; Jorgensen, M.; Krebs, F.C. The teraton challenge. A review of fixation and transformation of carbon dioxide. Energy Environ. Sci. 2010, 3, 43–81. [Google Scholar] [CrossRef]
- Romero-Hermida, M.I.; Romero-Enrique, J.M.; Morales-Florez, V.; Esquivias, L. Flue gas adsorption by single-wall carbon nanotubes: A Monte Carlo study. J. Chem. Phys. 2016, 145, 074701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lackner, K.S. A guide to CO2 sequestration. Science 2003, 300, 1677–1678. [Google Scholar] [CrossRef] [PubMed]
- Haszeldine, R.S. Carbon Capture and Storage: How Green Can Black Be? Science 2009, 325, 1647–1652. [Google Scholar] [CrossRef] [PubMed]
- Carbon Sequestration. Science 2009, 325, 1644–1645. [CrossRef]
- Firoozabadi, A.; Myint, P.C. Prospects for Subsurface CO2 Sequestration. AIChE J. 2010, 56, 1398–1405. [Google Scholar] [CrossRef]
- Gao, W.H.; Butler, D.; Tomasko, D.L. High-pressure adsorption of CO2 on NaY zeolite and model prediction of adsorption isotherms. Langmuir 2004, 20, 8083–8089. [Google Scholar] [CrossRef]
- Siriwardane, R.V.; Shen, M.S.; Fisher, E.P. Adsorption of CO2 on zeolites at moderate temperatures. Energy Fuels 2005, 19, 1153–1159. [Google Scholar] [CrossRef]
- Siriwardane, R.V.; Shen, M.S.; Fisher, E.P.; Poston, J.A. Adsorption of CO2 on molecular sieves and activated carbon. Energy Fuels 2001, 15, 279–284. [Google Scholar] [CrossRef]
- Przepiorski, J.; Skrodzewicz, M.; Morawski, A.W. High temperature ammonia treatment of activated carbon for enhancement of CO2 adsorption. Appl. Surf. Sci. 2004, 225, 235–242. [Google Scholar] [CrossRef]
- Li, J.-R.; Ma, Y.; McCarthy, M.C.; Sculley, J.; Yu, J.; Jeong, H.-K.; Balbuena, P.B.; Zhou, H.-C. Carbon dioxide capture-related gas adsorption and separation in metal-organic frameworks. Coord. Chem. Rev. 2011, 255, 1791–1823. [Google Scholar] [CrossRef]
- Shekhah, O.; Belmabkhout, Y.; Chen, Z.; Guillerm, V.; Cairns, A.; Adil, K.; Eddaoudi, M. Made-to-order metal-organic frameworks for trace carbon dioxide removal and air capture. Nat. Commun. 2014, 5, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Farha, O.K.; Bae, Y.-S.; Hauser, B.G.; Spokoyny, A.M.; Snurr, R.Q.; Mirkin, C.A.; Hupp, J.T. Chemical reduction of a diimide based porous polymer for selective uptake of carbon dioxide versus methane. Chem. Commun. 2010, 46, 1056–1058. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.-S.; Hauser, B.G.; Farha, O.K.; Hupp, J.T.; Snurr, R.Q. Enhancement of CO2/CH4 selectivity in metal-organic frameworks containing lithium cations. Microporous Mesoporous Mater. 2011, 141, 231–235. [Google Scholar] [CrossRef]
- Babarao, R.; Jiang, J. Unprecedentedly High Selective Adsorption of Gas Mixtures in rho Zeolite-like Metal-Organic Framework: A Molecular Simulation Study. J. Am. Chem. Soc. 2009, 131, 11417–11425. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, Z.; Li, Y.; Yao, K.; Zhu, Y.; Deng, Z.; Yang, F.; Zhou, X.; Li, G.; Wu, H.; et al. Enhanced Binding Affinity, Remarkable Selectivity, and High Capacity of CO2 by Dual Functionalization of a rht-Type Metal-Organic Framework. Angew. Chem. Int. Ed. 2012, 51, 1412–1415. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.-S.; Farha, O.K.; Spokoyny, A.M.; Mirkin, C.A.; Hupp, J.T.; Snurr, R.Q. Carborane-based metal-organic frameworks as highly selective sorbents for CO2 over methane. Chem. Commun. 2008, 4135–4137. [Google Scholar] [CrossRef] [PubMed]
- Dietzel, P.D.C.; Besikiotis, V.; Blom, R. Application of metal-organic frameworks with coordinatively unsaturated metal sites in storage and separation of methane and carbon dioxide. J. Mater. Chem. 2009, 19, 7362–7370. [Google Scholar] [CrossRef]
- Caskey, S.R.; Wong-Foy, A.G.; Matzger, A.J. Dramatic tuning of carbon dioxide uptake via metal substitution in a coordination polymer with cylindrical pores. J. Am. Chem. Soc. 2008, 130, 10870–10871. [Google Scholar] [CrossRef] [PubMed]
- Yazaydin, A.O.; Snurr, R.Q.; Park, T.-H.; Koh, K.; Liu, J.; LeVan, M.D.; Benin, A.I.; Jakubczak, P.; Lanuza, M.; Galloway, D.B.; et al. Screening of Metal-Organic Frameworks for Carbon Dioxide Capture from Flue Gas Using a Combined Experimental and Modeling Approach. J. Am. Chem. Soc. 2009, 131, 18198–18199. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, Z.-Z.; Xiang, S.; Chen, B. Perspective of microporous metal-organic frameworks for CO2 capture and separation. Energy Environ. Sci. 2014, 7, 2868–2899. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, H.; Emge, T.J.; Gong, Q.; Nijem, N.; Chabal, Y.J.; Kong, L.; Langreth, D.C.; Liu, H.; Zeng, H.; et al. Enhancing Gas Adsorption and Separation Capacity through Ligand Functionalization of Microporous Metal-Organic Framework Structures. Chem. A Eur. J. 2011, 17, 5101–5109. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhao, Y.; Zhang, Z.; Nijem, N.; Chabal, Y.J.; Zeng, H.; Li, J. The Effect of Methyl Functionalization on Microporous Metal-Organic Frameworks’ Capacity and Binding Energy for Carbon Dioxide Adsorption. Adv. Funct. Mater. 2011, 21, 4754–4762. [Google Scholar] [CrossRef]
- Samanta, A.; Zhao, A.; Shimizu, G.K.H.; Sarkar, P.; Gupta, R. Post-Combustion CO2 Capture Using Solid Sorbents: A Review. Ind. Eng. Chem. Res. 2012, 51, 1438–1463. [Google Scholar] [CrossRef]
- Lin, Y.; Kong, C.; Chen, L. Direct synthesis of amine-functionalized MIL-101(Cr) nanoparticles and application for CO2 capture. RSC Adv. 2012, 2, 6417–6419. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, S.; Xian, S.; Xi, H.; Li, Z. Adsorption Equilibrium and Kinetics of CO2 on Chromium Terephthalate MIL-101. Energy Fuels 2011, 25, 835–842. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, W.; Miao, J.; Xia, Q.; Zhang, Z.; Wang, H.; Li, Z. Enhanced separation performance of a novel composite material GrO@MIL-101 for CO2/CH4 binary mixture. Chem. Eng. J. 2015, 266, 339–344. [Google Scholar] [CrossRef]
- Xian, S.; Peng, J.; Zhang, Z.; Xia, Q.; Wang, H.; Li, Z. Highly enhanced and weakened adsorption properties of two MOFs by water vapor for separation of CO2/CH4 and CO2/N-2 binary mixtures. Chem. Eng. J. 2015, 270, 385–392. [Google Scholar] [CrossRef]
- Seo, Y.-K.; Yoon, J.W.; Lee, J.S.; Hwang, Y.K.; Jun, C.-H.; Chang, J.-S.; Wuttke, S.; Bazin, P.; Vimont, A.; Daturi, M.; et al. Energy-Efficient Dehumidification over Hierachically Porous Metal-Organic Frameworks as Advanced Water Adsorbents. Adv. Mater. 2012, 24, 806–807. [Google Scholar] [CrossRef]
- Akiyama, G.; Matsuda, R.; Sato, H.; Hori, A.; Takata, M.; Kitagawa, S. Effect of functional groups in MIL-101 on water sorption behavior. Microporous Mesoporous Mater. 2012, 157, 89–93. [Google Scholar] [CrossRef]
- Yan, J.; Yu, Y.; Ma, C.; Xiao, J.; Xia, Q.; Li, Y.; Li, Z. Adsorption isotherms and kinetics of water vapor on novel adsorbents MIL-101(Cr)@GO with super-high capacity. Appl. Therm. Eng. 2015, 84, 118–125. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, S.; Yang, Y.; Li, X.; Li, J.; Li, Z. Competitive adsorption and selectivity of benzene and water vapor on the microporous metal organic frameworks (HKUST-1). Chem. Eng. J. 2015, 259, 79–89. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Kang, D.-Y.; Copeland, J.R.; Brunelli, N.A.; Didas, S.A.; Bollini, P.; Sievers, C.; Kamegawa, T.; Yamashita, H.; Jones, C.W. Dramatic Enhancement of CO2 Uptake by Poly(ethyleneimine) Using Zirconosilicate Supports. J. Am. Chem. Soc. 2012, 134, 10757–10760. [Google Scholar] [CrossRef]
- Chaikittisilp, W.; Khunsupat, R.; Chen, T.T.; Jones, C.W. Poly(allylamine)-Mesoporous Silica Composite Materials for CO2 Capture from Simulated Flue Gas or Ambient Air. Ind. Eng. Chem. Res. 2011, 50, 14203–14210. [Google Scholar] [CrossRef]
- Espinal, L.; Green, M.L.; Fischer, D.A.; DeLongchamp, D.M.; Jaye, C.; Horn, J.C.; Sakwa-Novak, M.A.; Chaikittisilp, W.; Brunelli, N.A.; Jones, C.W. Interrogating the Carbon and Oxygen K-Edge NEXAFS of a CO2-Dosed Hyperbranched Aminosilica. J. Phys. Chem. Lett. 2015, 6, 148–152. [Google Scholar] [CrossRef]
- Sayari, A.; Belmabkhout, Y. Stabilization of Amine-Containing CO2 Adsorbents: Dramatic Effect of Water Vapor. J. Am. Chem. Soc. 2010, 132, 6312–6314. [Google Scholar] [CrossRef] [PubMed]
- Hiyoshi, N.; Yogo, K.; Yashima, T. Reversible adsorption of carbon dioxide on amine-modified SBA-15 from flue gas containing water vapor. In Carbon Dioxide Utilization for Global Sustainability; Studies in Surface Science and Catalysis; Park, S.E., Chang, J.S., Lee, K.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; Volume 153, pp. 417–422. [Google Scholar] [CrossRef]
- Huang, H.Y.; Yang, R.T.; Chinn, D.; Munson, C.L. Amine-grafted MCM-48 and silica xerogel as superior sorbents for acidic gas removal from natural gas. Ind. Eng. Chem. Res. 2003, 42, 2427–2433. [Google Scholar] [CrossRef]
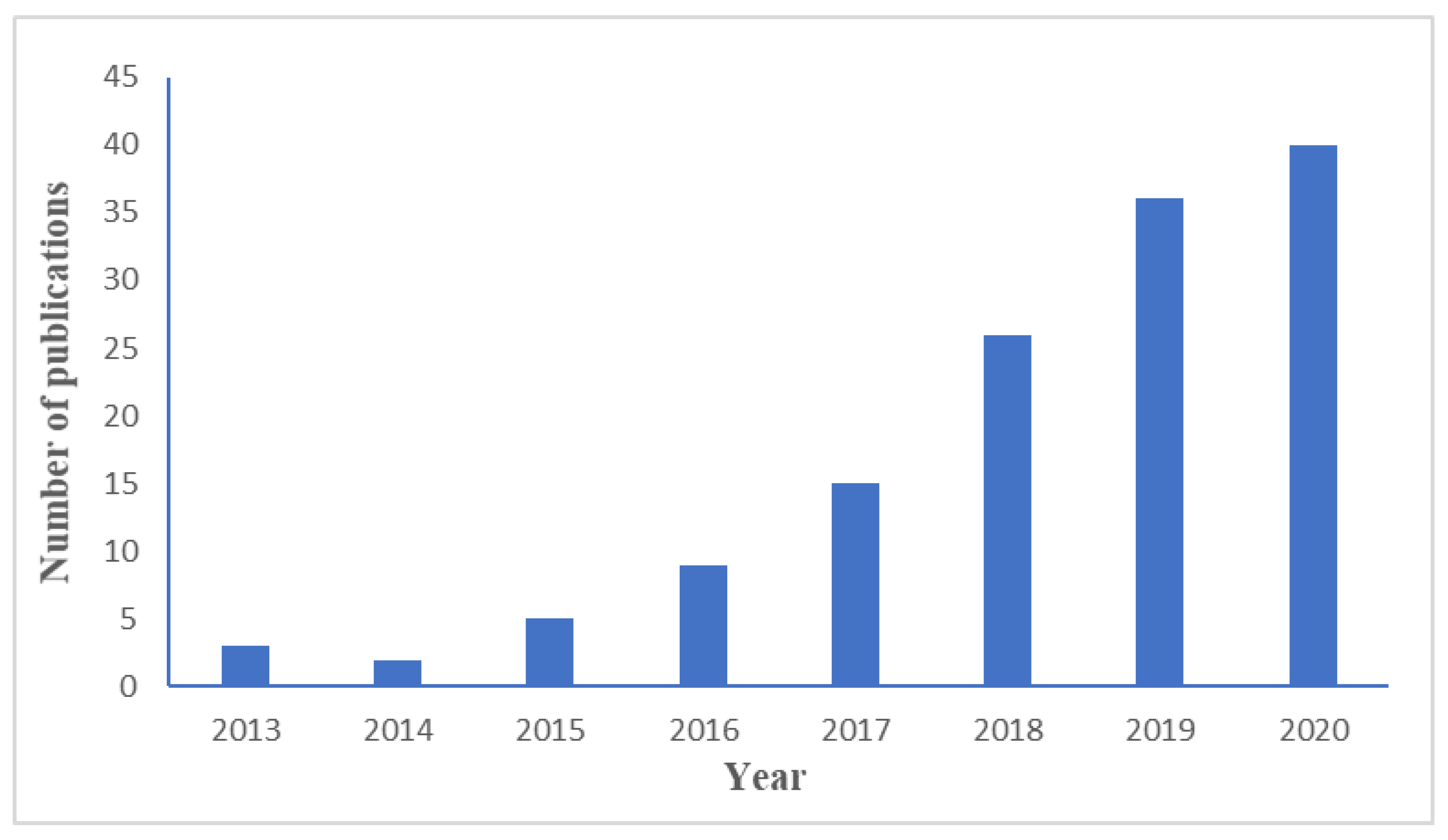

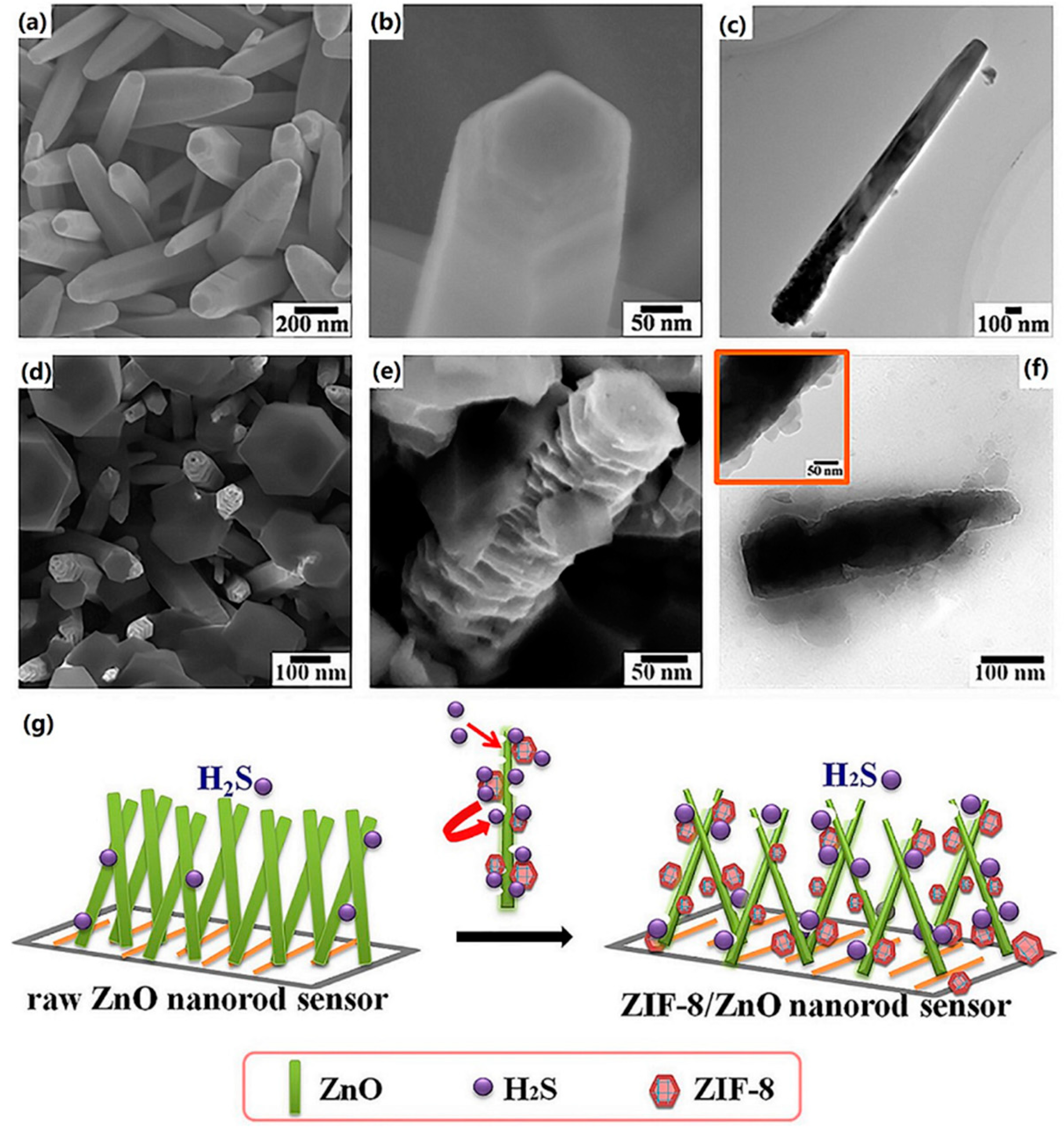
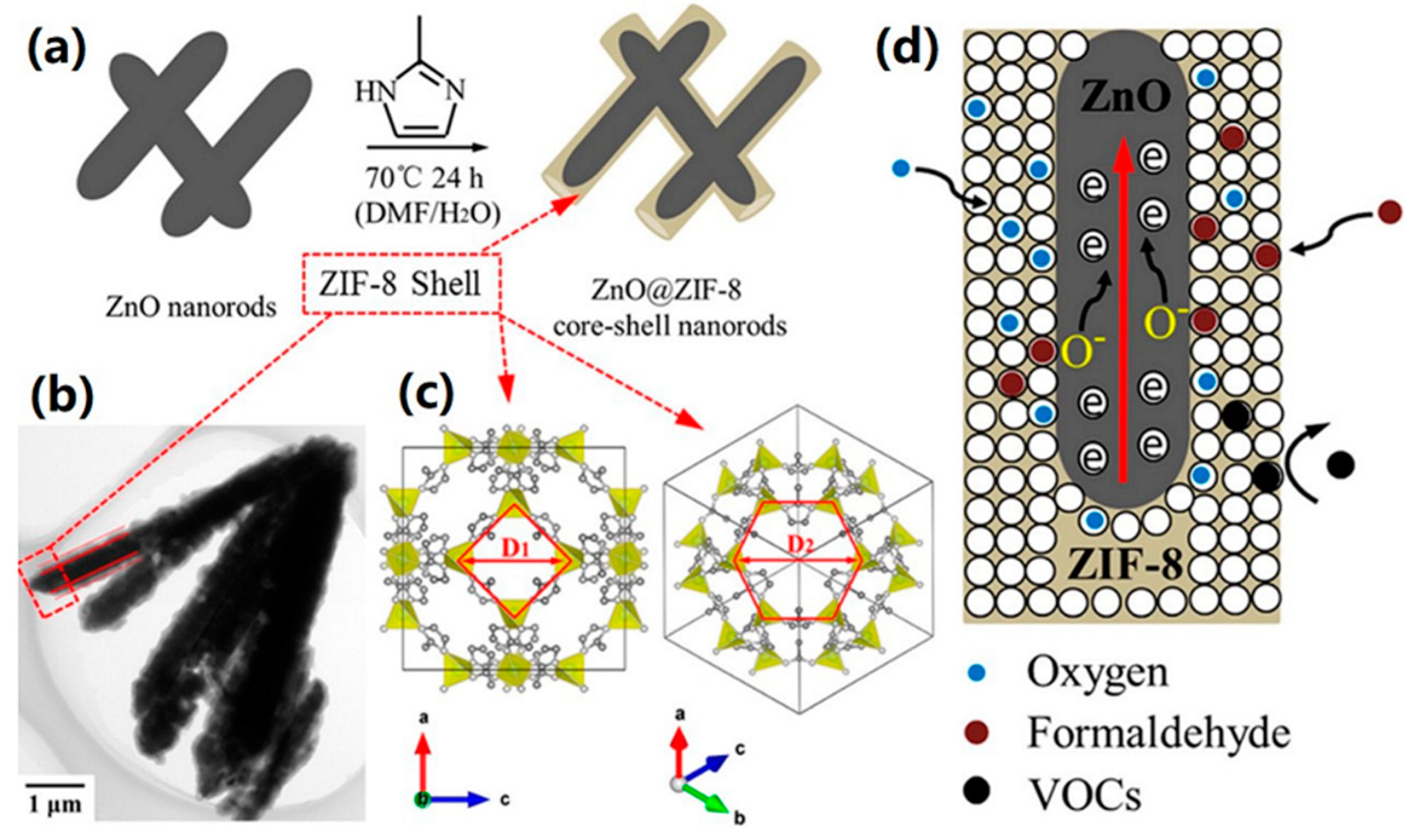
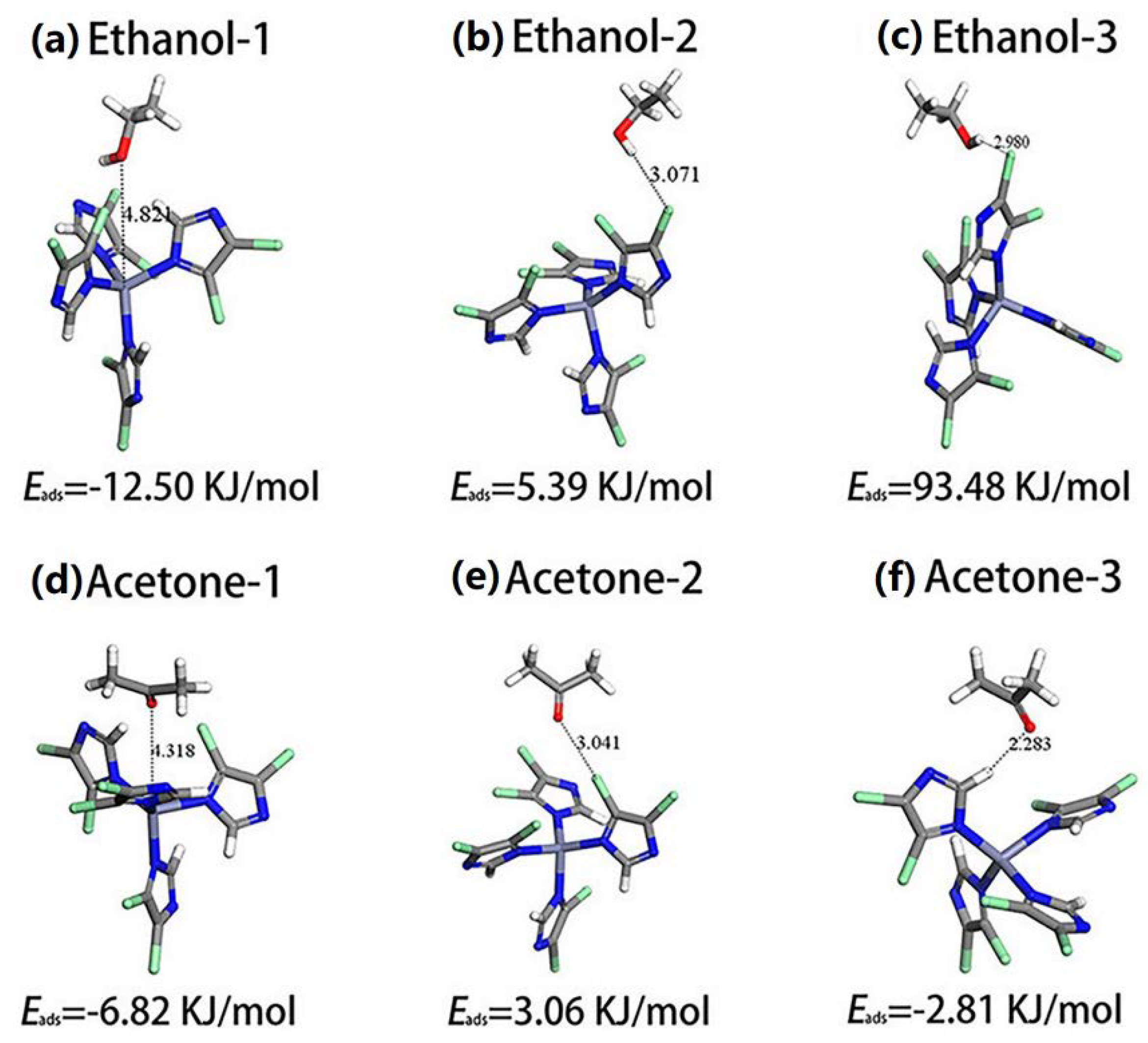
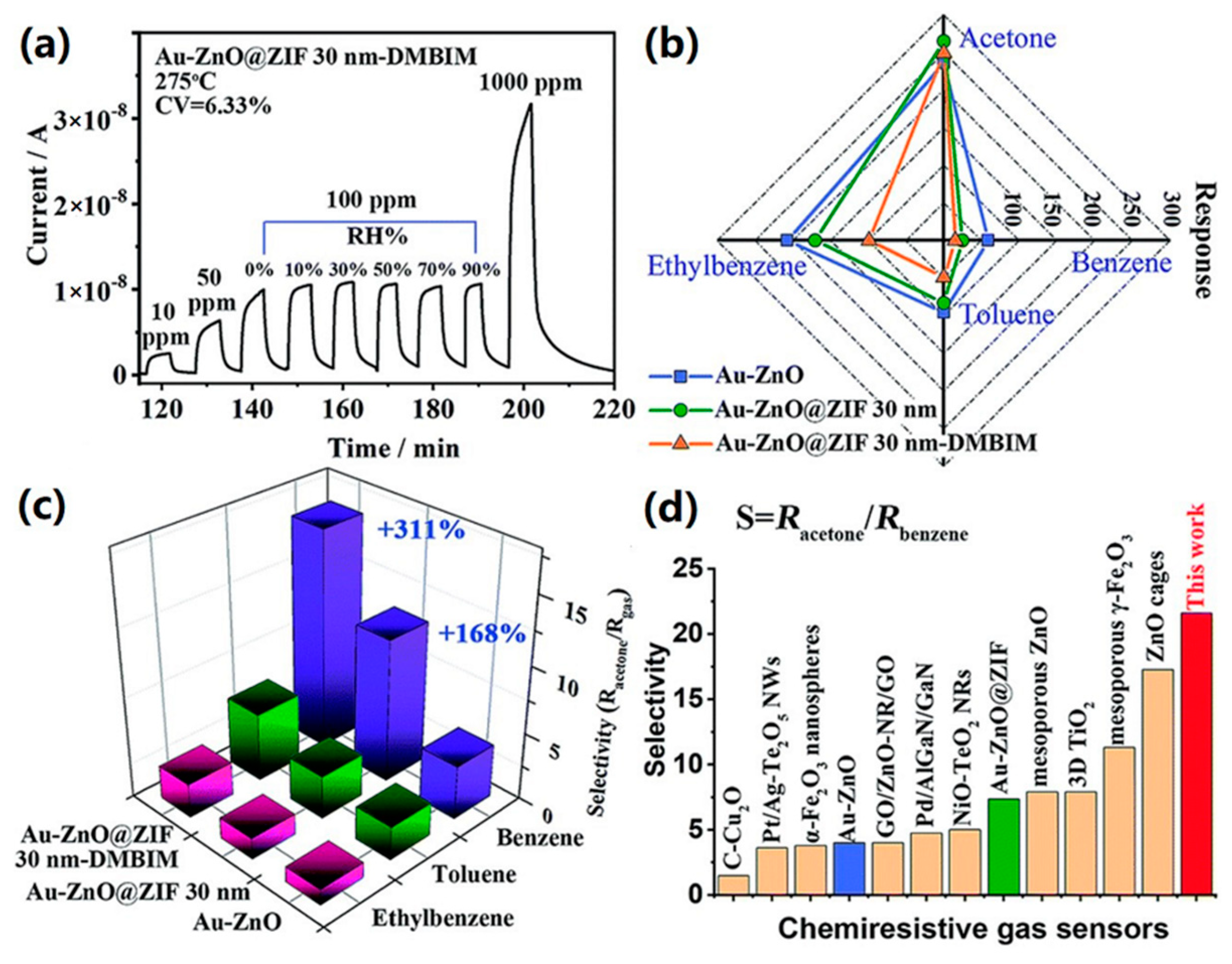
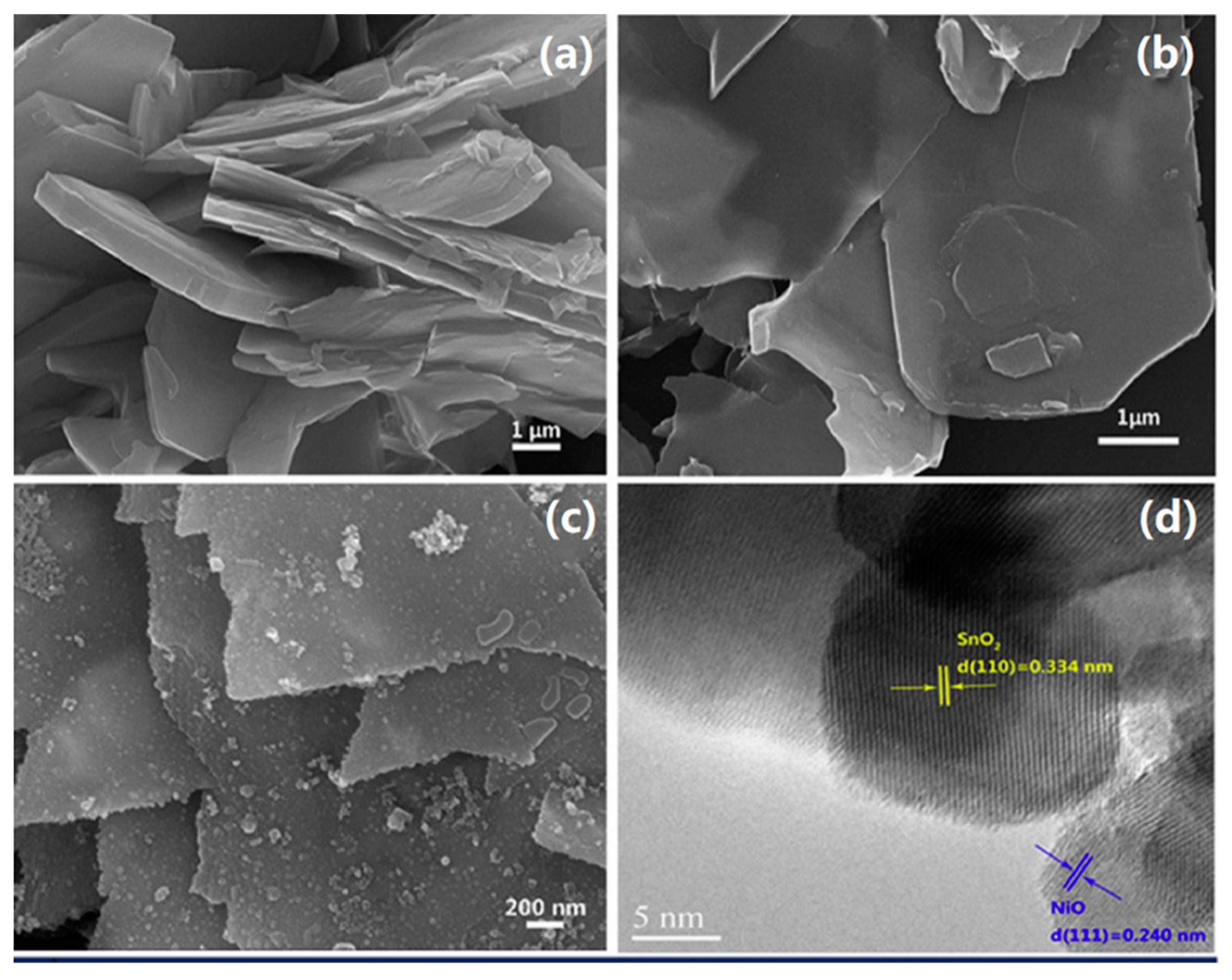
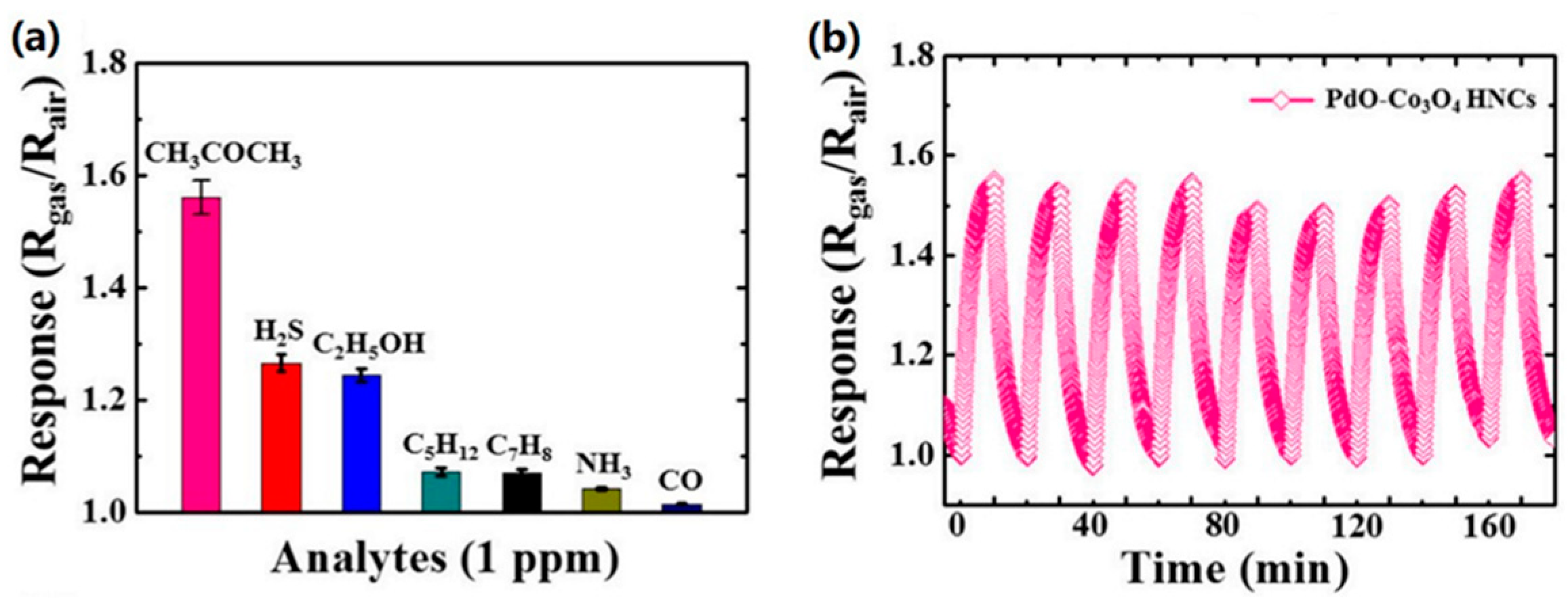

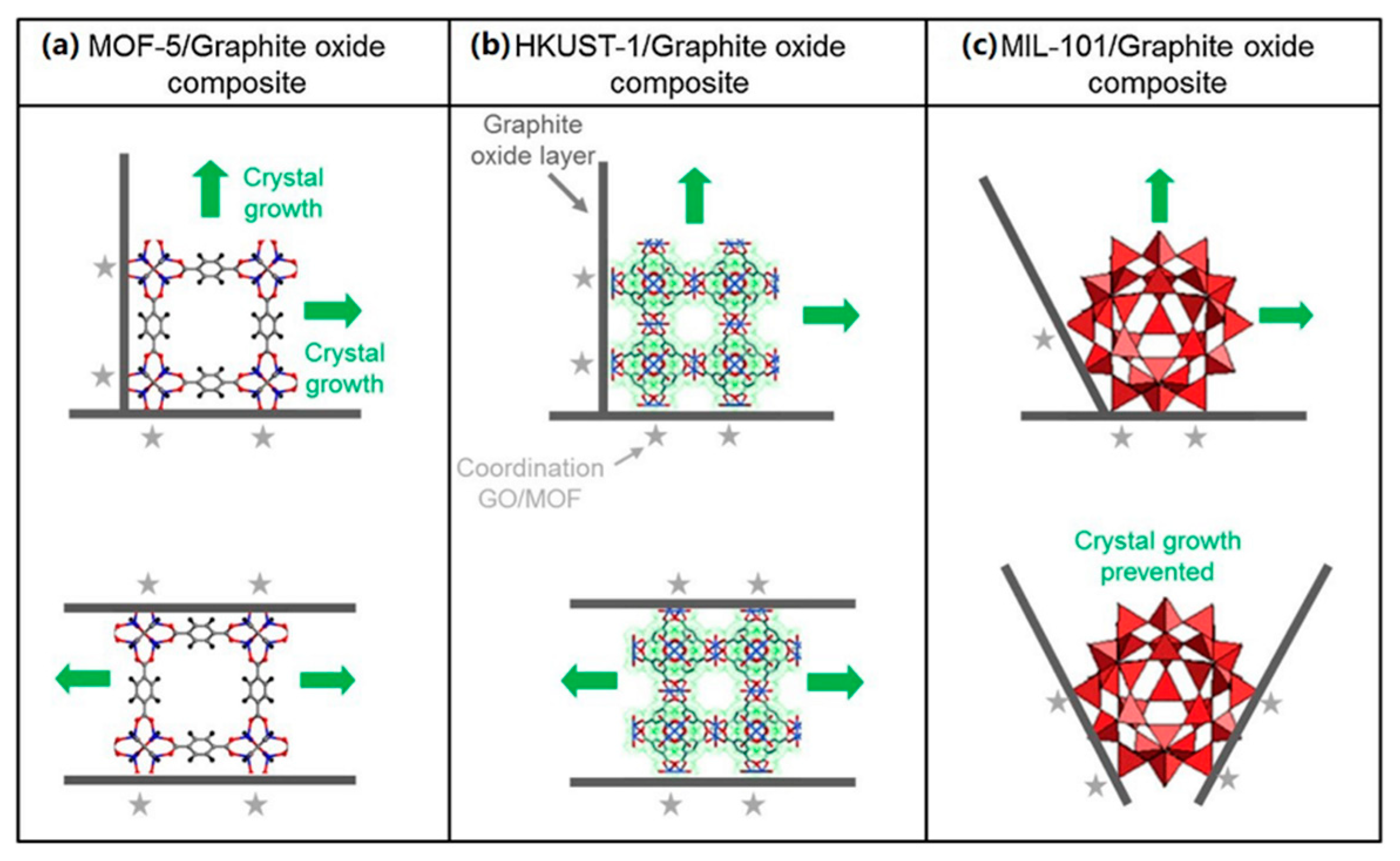

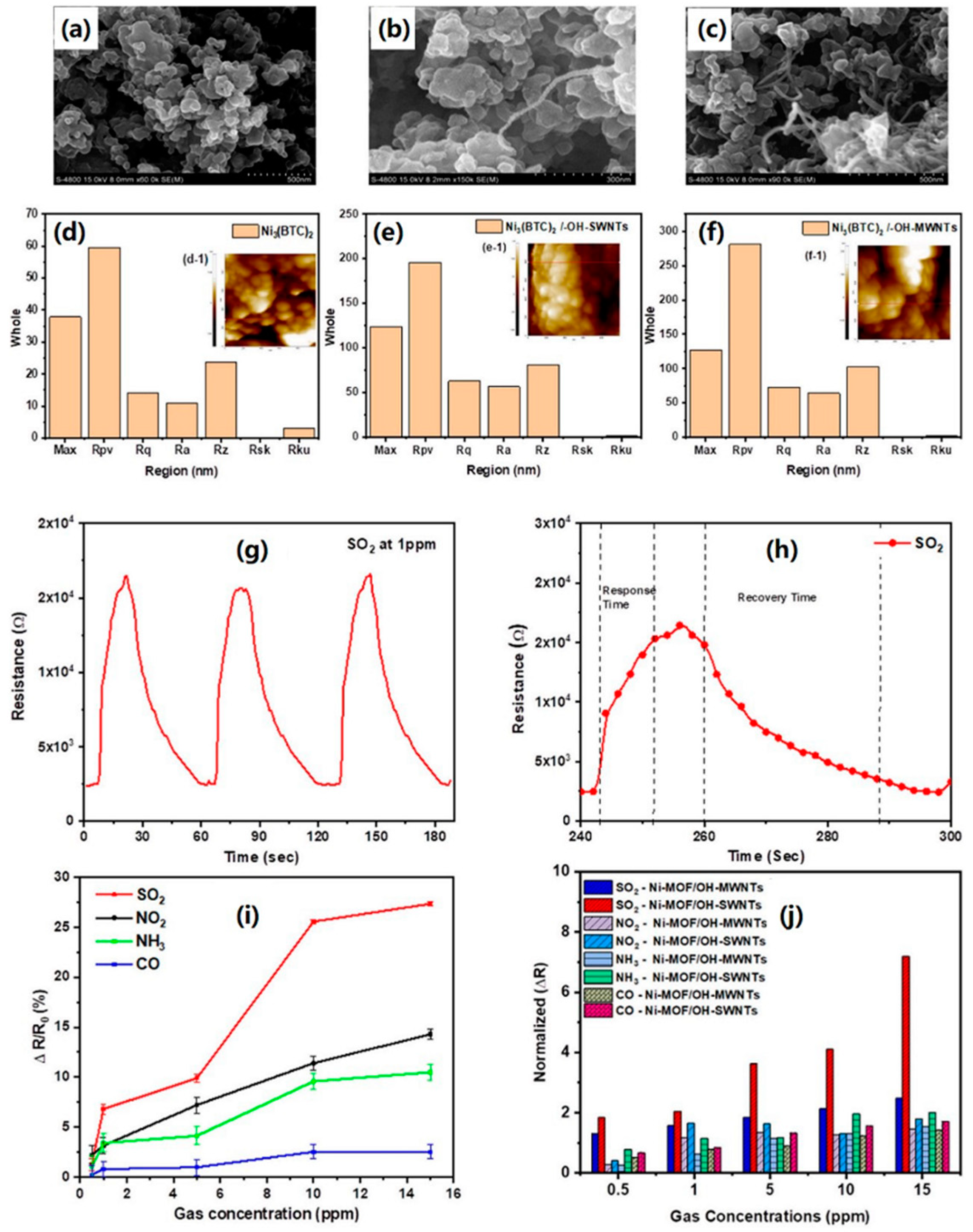


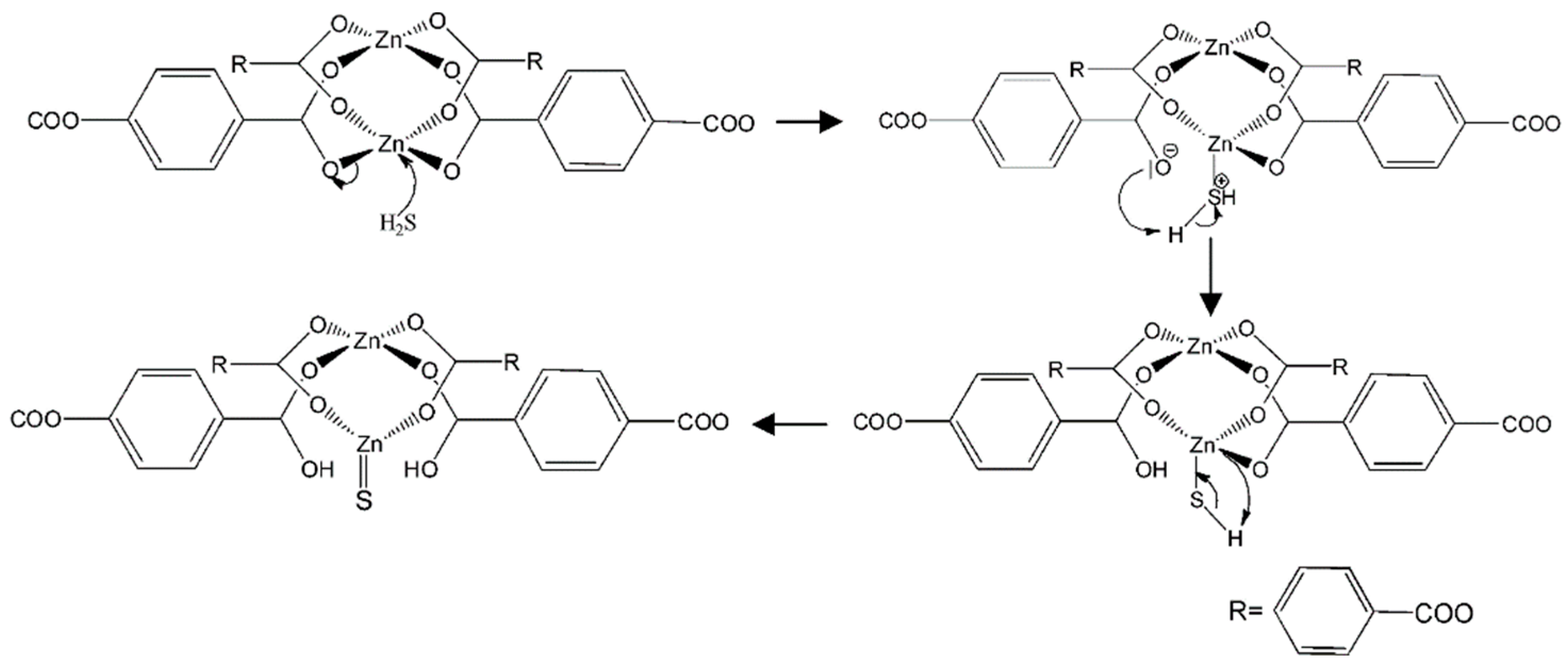

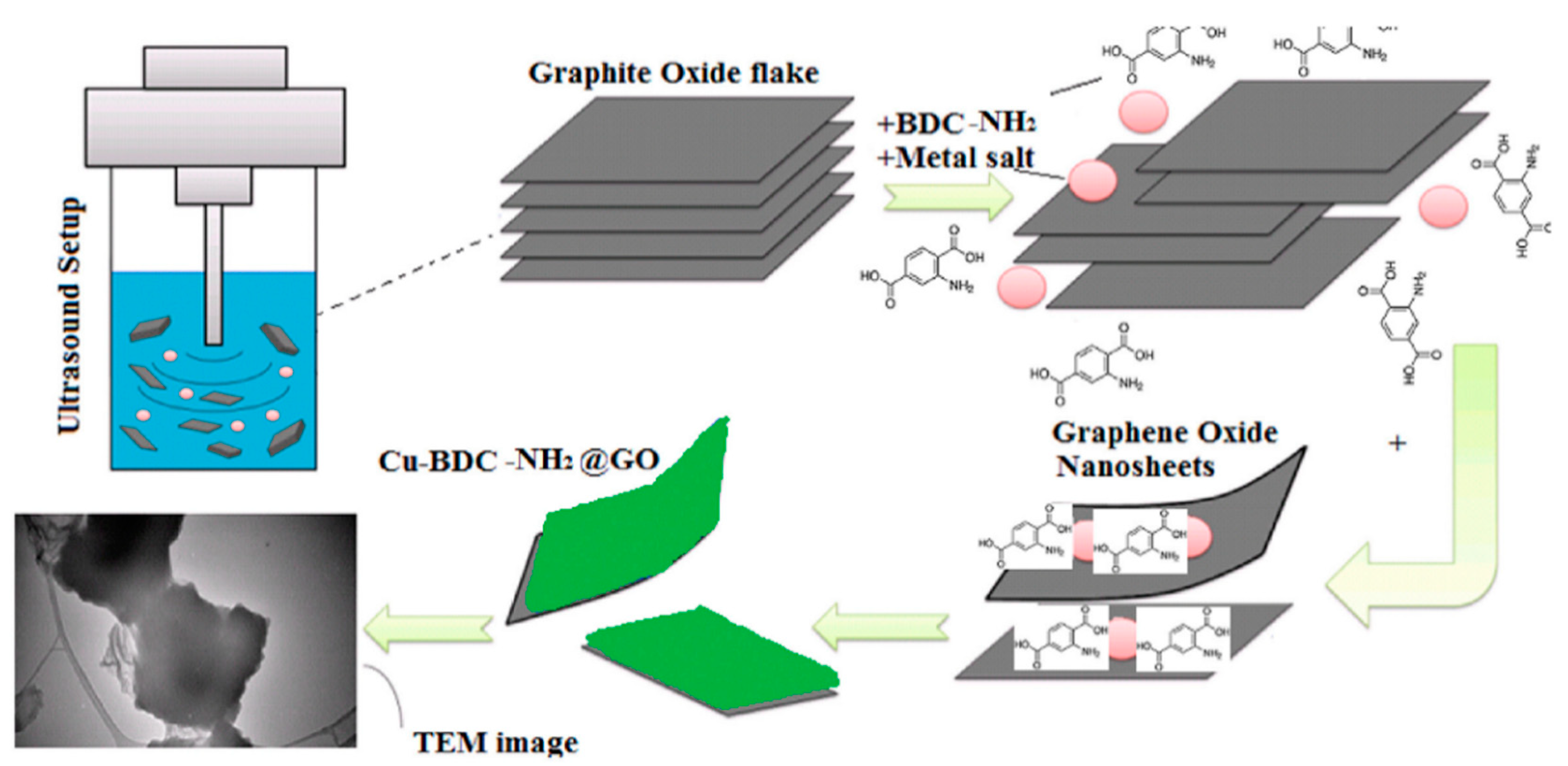
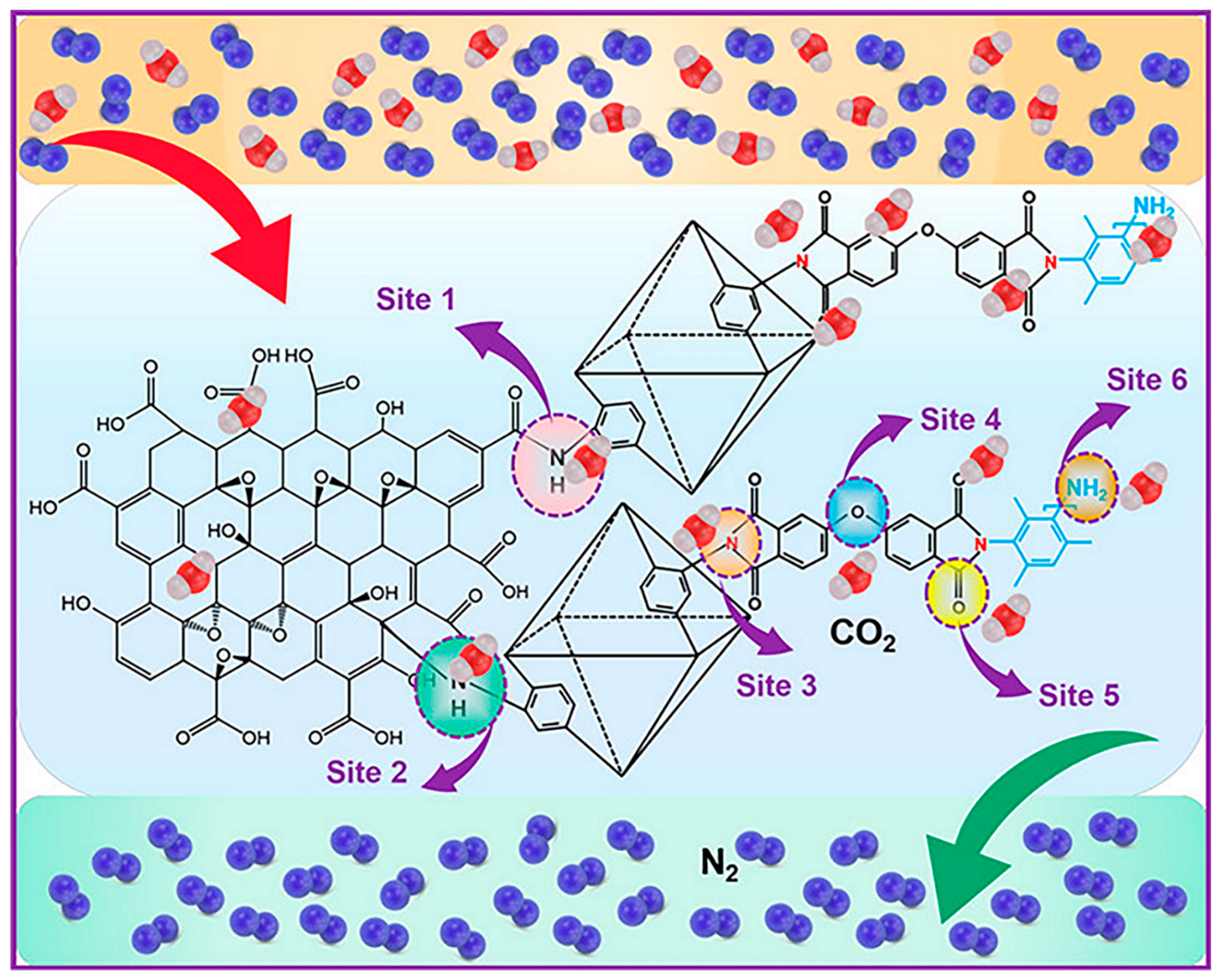

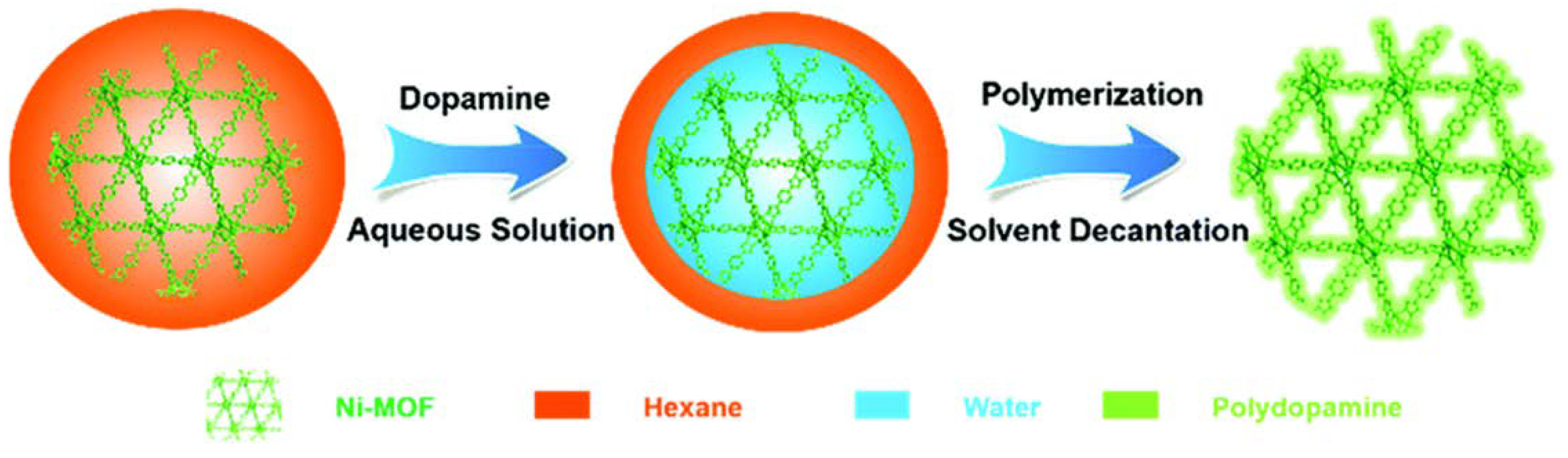
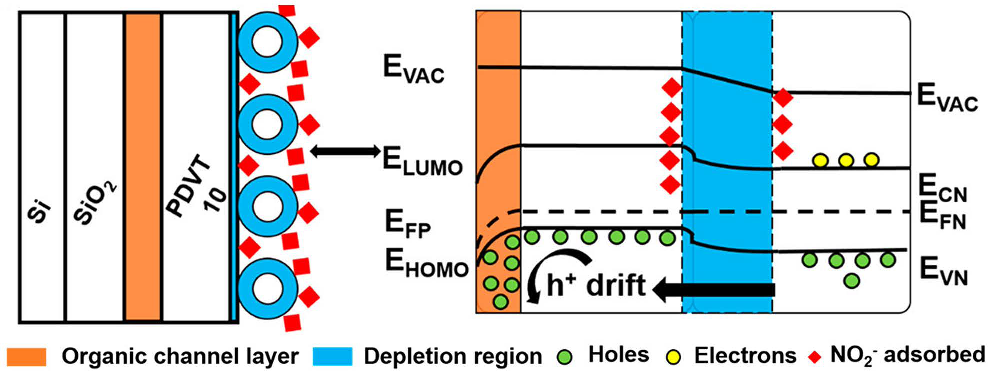
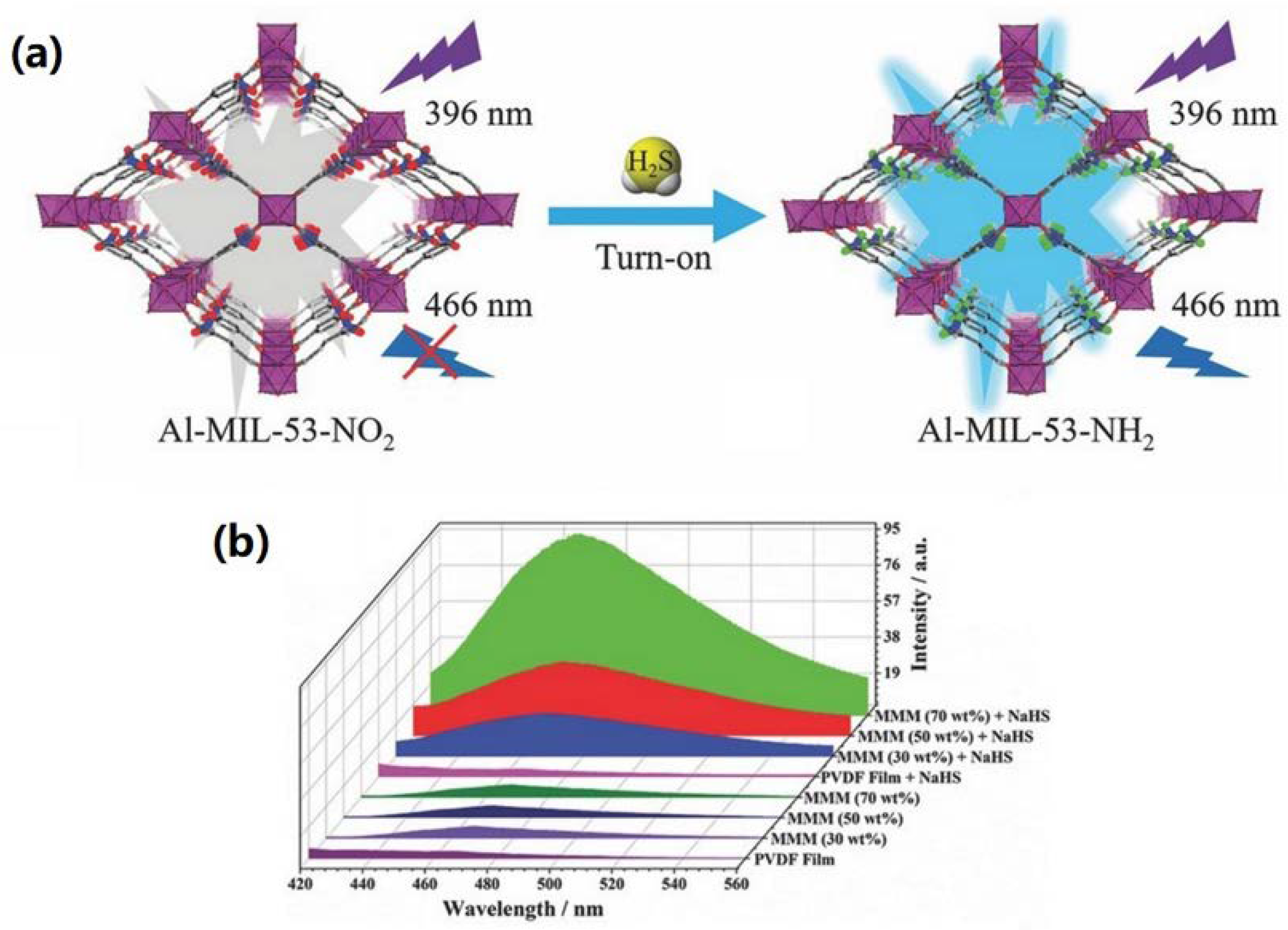
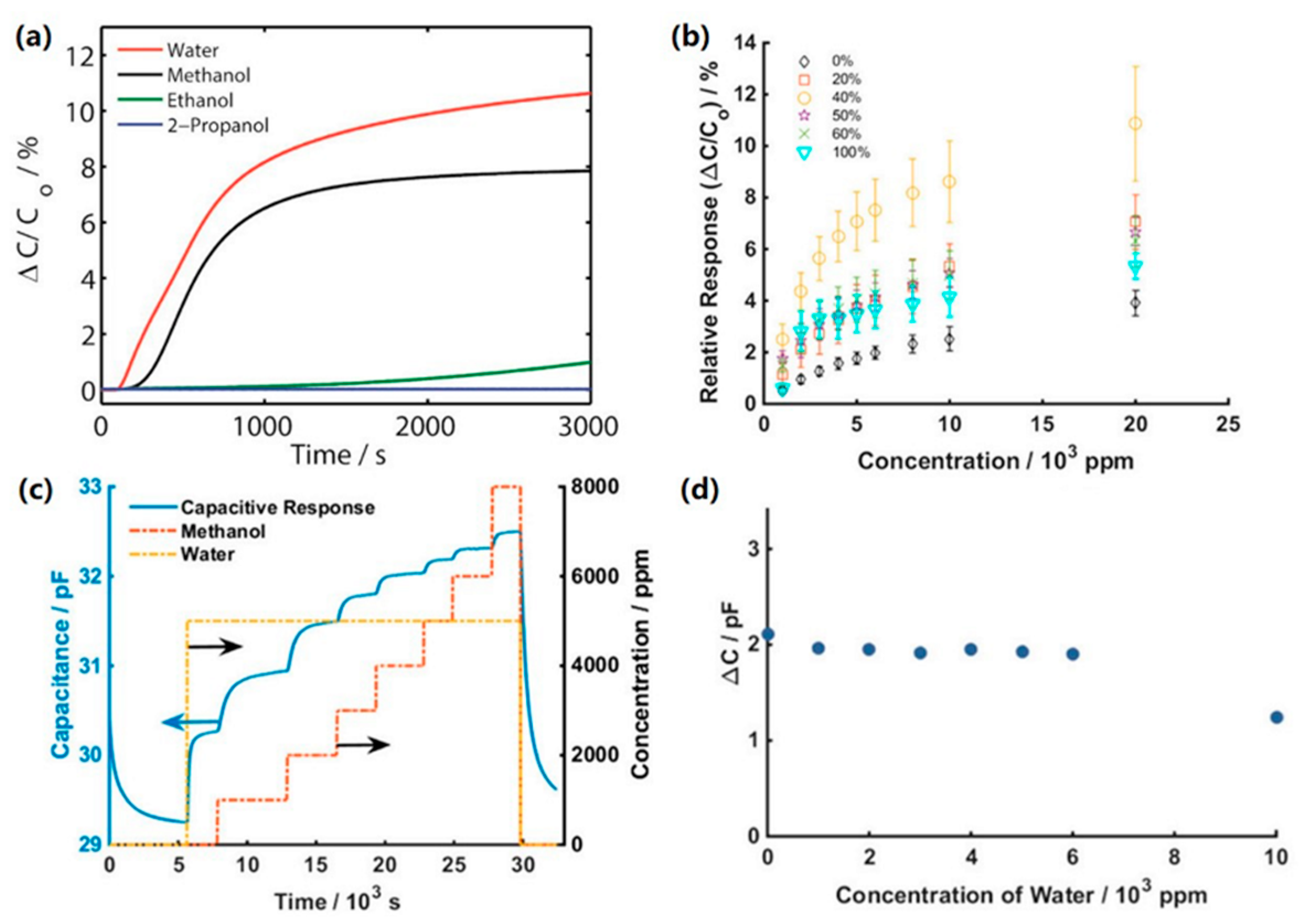
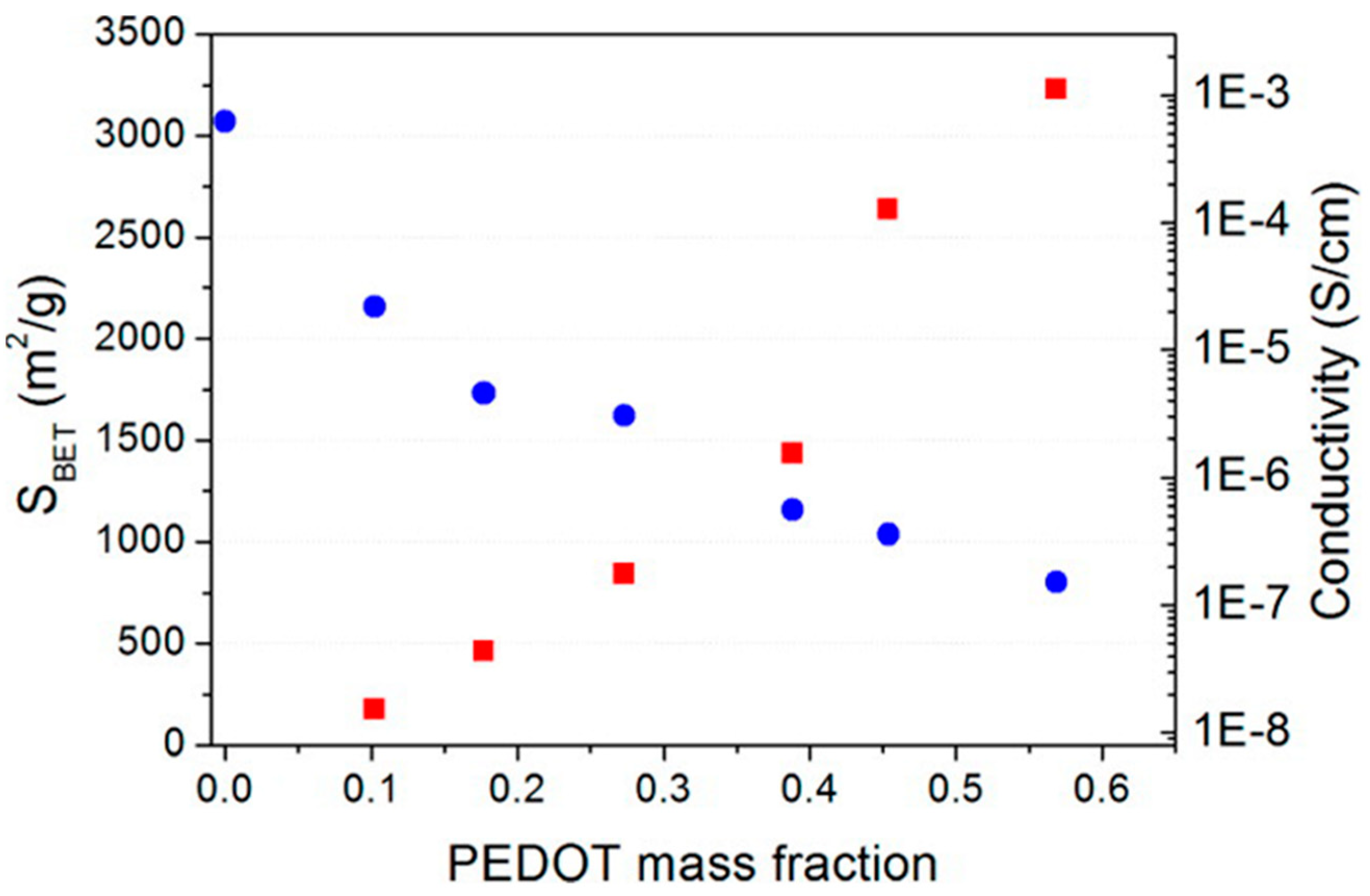
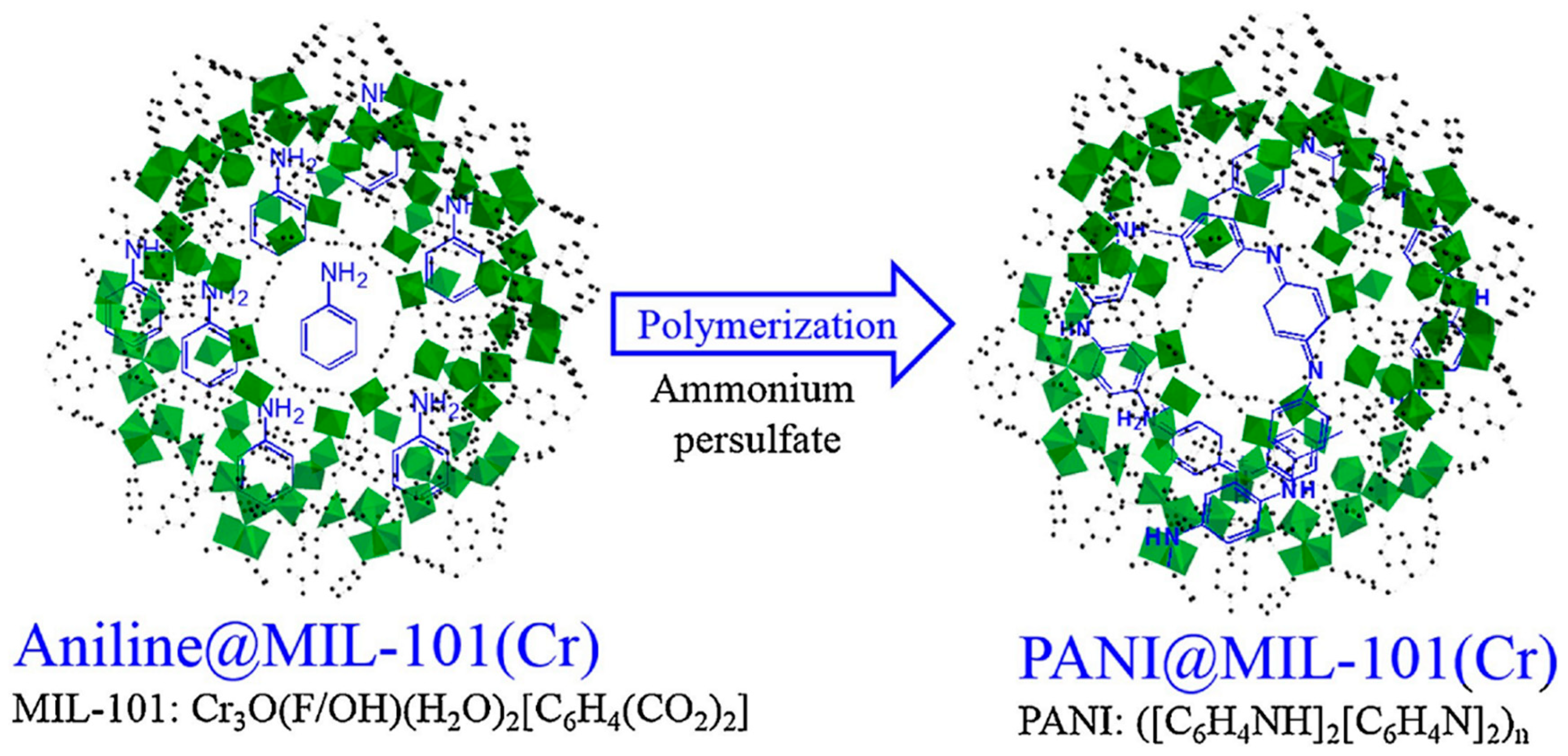
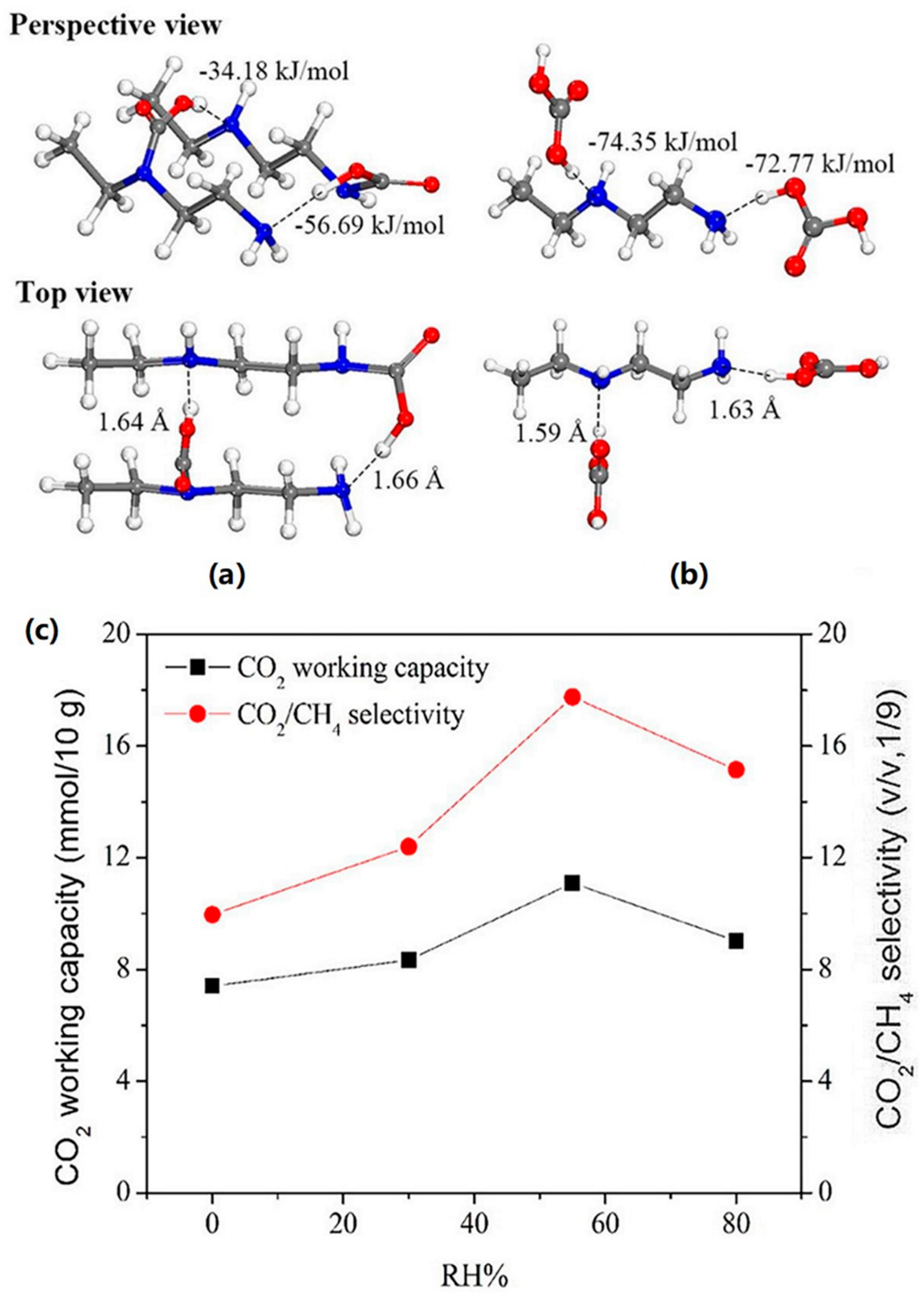
| Material | Target Gas, Concentration (ppm) | Topt (°C) | Response | Response/Recovery Time (s) | BET (m2/g) | Detection Limit | Ref. |
|---|---|---|---|---|---|---|---|
| ZnO@ZIF-8 | H2 50 ppm | 300 | 1.44 | - | 1760 ± 260 | - | [89] |
| ZnO@ZIF-8 | formaldehyde 100 ppm | 300 | ~13 | 16/9 | 307.4 | 5.6 ppm | [80] |
| ZnO@5nm ZIF-CoZn | acetone 10 ppm | 260 | 27 | 43.2/61.2 | - | 0.0019 ppm | [12] |
| ZnO@ZIF-8 | H2 50 ppm | 250 | 3.28 | - | 4813 | - | [90] |
| ZnO@ZIF-8 | H2 10 ppm | 125 | ~5.2 | 100/20 | - | ~1.9 ppm | [91] |
| ZnO@ZIF-8 ZnO@ZIF-71 | ammonia, hydrogen, ethanol, acetone and benzene 50 ppm | 250 | ~20 ~84 ~40 ~25 ~5 | - | ~295 ~348 | - | [92] |
| ~25 ~85 ~325 ~240 ~10 | |||||||
| SnO2@ZIF-67 | CO2 5000 ppm | 205 | 16.5 ± 2.1% | 18.4 ± 3.5/25.5 ± 4.5 | 501 | - | [93] |
| In2O3/ZIF-8 | NO2 1 ppm | 140 | 16.4 | 80/133 | 528.2 | 10 ppb | [53] |
| ZIF-8/ZnO | H2S 1 ppm | 25 | 18.70% | 420/642 | 145.5 | 50 ppb | [94] |
| ZnO@ZIF-71 | ethanol 10 ppm | 150 | 13.40% | 194.37/442.17 | ~375 | 21 ppb | [95] |
| acetone 5 ppm | 150 | 38.90% | 195.9/535.5 | 3 ppb | |||
| Au-ZnO@ZIF 5 nm-DMBIM | acetone 100 ppm | 275 | 231 | 180/60 | - | 0.0034 ppm | [96] |
| ZnO@ZIF-8 | H2 | 275 | ~47.5% | 50/130 | - | - | [87] |
| WO3@ZIF-71 | H2S 20 ppm | 250 | 19.12 | 118/431 | - | 0.697 ppm | [88] |
| ZnO@ZIF-71(Co) | acetone 50 ppm | 250 | 513 | 71/53 | - | 50 ppb | [97] |
| Material | Target Gas, Concentration (ppm) | Topt (°C) | Response | Response/Recovery Time (s) | BET (m2/g) | Detection Limit | Ref. |
|---|---|---|---|---|---|---|---|
| Cu-BTC/GO (25) | NH3 500 ppm | - | 7% | - | 916 | - | [131] |
| Cu-BTC/PPy-rGO | NH3 50 ppm | 25 | 12.4% | 13/22 | 1861 | 2 ppm | [132] |
| SiO2CuOF-graphene-PAni | NH3 40 ppm | - | - | 30/180 | 756 | 0.6 ppm | [133] |
| ZIF-8/MWCNTs/AgNPs | methanol, ethanol, acetone, acetonitrile, n-hexane 1% | RT | 8.0% 12.16% 2.28% 2.02% −0.81% | - | 1176.24 | 1.847% 0.399% 4.996% 4.431% 5.203% | [134] |
| Co-Zn-Ni MOF@CNT | H2S 100 ppm | 325 | ~166 | 126/23 | 363 | - | [135] |
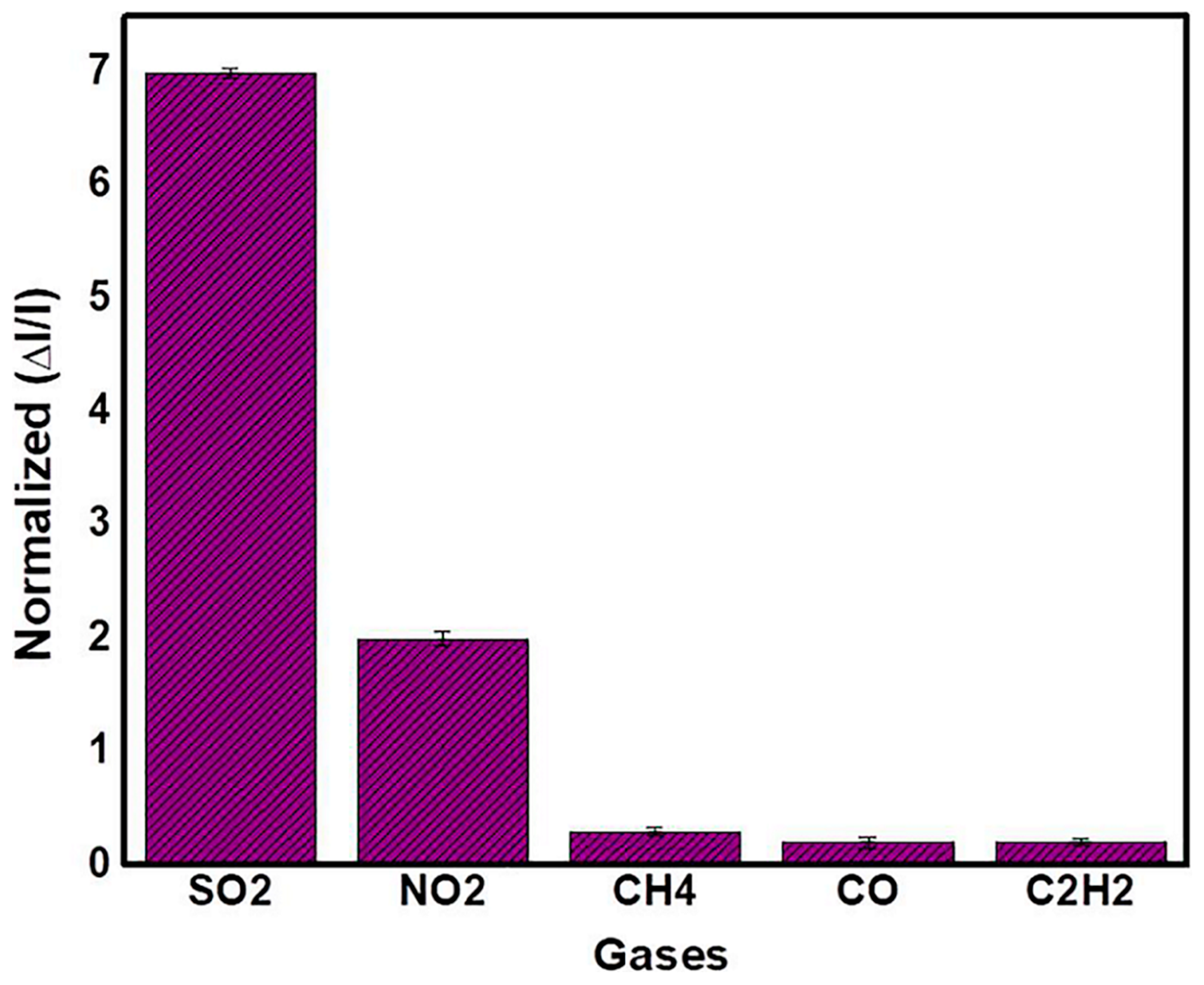
| Ni3BTC2/OH-SWNTs | SO2 15 ppm | 25 | - | 4.59/11.04 | - | 4 ppm | [136] |
| TiO2-SnO2/MWCNTs@Cu-BTC | NH3 - | RT | ~0.58 (10 ppm) ~0.64 (20 ppm) ~0.70 (30 ppm) ~0.83 (40ppm) | 80/15 | - | 0.77 ppm | [137] |
| Ni-MOF/-OH-SWNTs | SO2 1 ppm | RT | - | 10/30 | - | 0.5 ppm | [128] |
| Material | Target Gas, Concentration (ppm) | Topt (°C) | Response | Response/Recovery Time (s) | BET (m2/g) | Detection Limit | Ref. |
|---|---|---|---|---|---|---|---|
| MIL-101(Cr)⊃PEDOT(45) | SO2 200 ppb | RT | 0.9% | <30/- | 1038 | 60 ppb | [61] |
| Cu-BTC/PPy-rGO | NH3 50 ppm | 25 | 12.4% | 13/22 | 1861 | 2 ppm | [132] |
| SiO2CuOF-graphene-PAni | NH3 40 ppm | - | - | 30/180 | 756 | 0.6 ppm | [133] |
| [Ni(TPyP)(TiF6)]n MOF-A/PDVT-10 | NO2 25 ppb | 20 | ~18% | 43/438 | - | 8.25 ppb | [224] |
| Matrimid-NH2-MIL-53(Al) (20%) | methanol 20,000 ppm | 28 | ~8% | - | - | - | [225] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, B.; Li, Y.; Zeng, W. Application of Metal-Organic Framework-Based Composites for Gas Sensing and Effects of Synthesis Strategies on Gas-Sensitive Performance. Chemosensors 2021, 9, 226. https://doi.org/10.3390/chemosensors9080226
Huang B, Li Y, Zeng W. Application of Metal-Organic Framework-Based Composites for Gas Sensing and Effects of Synthesis Strategies on Gas-Sensitive Performance. Chemosensors. 2021; 9(8):226. https://doi.org/10.3390/chemosensors9080226
Chicago/Turabian StyleHuang, Bo, Yanqiong Li, and Wen Zeng. 2021. "Application of Metal-Organic Framework-Based Composites for Gas Sensing and Effects of Synthesis Strategies on Gas-Sensitive Performance" Chemosensors 9, no. 8: 226. https://doi.org/10.3390/chemosensors9080226
APA StyleHuang, B., Li, Y., & Zeng, W. (2021). Application of Metal-Organic Framework-Based Composites for Gas Sensing and Effects of Synthesis Strategies on Gas-Sensitive Performance. Chemosensors, 9(8), 226. https://doi.org/10.3390/chemosensors9080226






