Construction of a Highly Selective Membrane Sensor for the Determination of Cobalt (II) Ions
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Products, Materials and Reagents
2.2. Preparation of the Cited Reagent
2.3. Preparation of Stock Solutions
2.4. Sample Preparation for the Estimation of Cobalt Ions
2.5. Construction of the Membrane Sensor
2.6. Active Component of Liquid-Membrane Layer
2.7. The Potential Layer Preparation
2.8. EM F Measurements
3. Results
3.1. Calibration Curves
3.2. Selectivity Coefficient Sensor’s Measurements
3.3. Response Time of the Proposed Cobalt Electrode
3.4. Dependence of The Electrode Potential on the pH
3.5. Lifetime of the Cobalt Sensor
3.6. Determination of Cobalt in Food Products and Pharmaceutical Formulations Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ebert, B.; Jelkman, W. Intolerability of cobalt salt as erythropoietic agent. Drug Test. Anal. 2014, 6, 185–193. [Google Scholar] [CrossRef]
- Yari, A.; Darvishi, L.; Shamsipur, M. Cobalt metabolism and toxicology. Anal. Chim. Acta 2006, 555, 329–334. [Google Scholar] [CrossRef]
- Kalayci, S.; Somer, G.; Ekmekci, G. Cobalt use and regulation in horseracing. Talanta 2005, 65, 87–93. [Google Scholar]
- Karimian, F.; Rounaghi, G.H.; Arbab-Zavar, M.H. Horse welfare is the issue with use of cobalt. Aust Chin. Chem. Lett. 2014, 25, 809–815. [Google Scholar] [CrossRef]
- Ahmed, F.A. Spectrophotometric determination of cobalt in biological and environmental samples using 2, 6-pyridinedicarboxaldehyde thiosemicarbazone. Int. J. Anal. Pharm. Bio. Sci. 2013, 2, 6–12. [Google Scholar]
- Basha, V.S.; Chowdary, P.G.; Renuka, M. Non-extractive spectrophotometric determination of cobalt (II) using 2- acetylthiophene isonico tinoylhydrazone in environmental and pharmaceutical samples. J. Pharma. Drug Res. 2019, 2, 102–109. [Google Scholar]
- Sulaiman, S.T.; Hamoudi, T.A. Spectrophotometric determination of cobalt(II) with mordant blue 9-application to vitamin B12 (injections and powder). Raf. J. Sci. 2018, 27, 93–100. [Google Scholar]
- Kuliyev, K.A.; Verdizadeh, N.A.; Suleymanova, G.S. Spectrophotometric determination of cobalt (II) with 2, 6-dithiolphenol and its derivatives in the presence of hydrophobic amines. Am. J. Chem. 2016, 6, 95–101. [Google Scholar]
- Rinda, A.S.; Uaisin, K.; Sabarudin, A.; Nacapricha, D.; Wilairat, P. Spectrophotometric determination of cobalt in horse urine using 2-(5- bromo-2-pyridylazo)-5-[N-n-propyl-N-(3-sulfopropyl)amino]aniline as chromogenic reagent. Mater. Sci. Eng. 2018, 299, 12–18. [Google Scholar] [CrossRef]
- Wardak, C.; Grabarczyk, M. Single-piece all-solid-state Co(II) ion-selective electrode for cobalt monitoring in real samples. Int. Agrophys. 2020, 34, 17–23. [Google Scholar] [CrossRef]
- Dragan, F.; Hincu, L.; Bratu, I. Determination of cobalt in human biological liquids from electrothermal atomic absorption spectrometry. J. Physi. Conf. Ser. 2009, 182, 6–13. [Google Scholar] [CrossRef]
- Morfobos, M.; Economous, A.; Voulgaropoulos, A. Simultaneous determination of nickel(II) and cobalt(II) by square wave adsorptive stripping voltammetry on arotating-disc bismuth-film electrode. Anal. Chim. Acta 2004, 519, 57–64. [Google Scholar] [CrossRef]
- Orolczuk, M.K.; Tyszczuk, K.; Grabarczyk, M. Adsorptive stripping voltammetry of nickel and cobalt at in situ plated lead film electrode. Electrochem. Commun. 2005, 7, 1185. [Google Scholar] [CrossRef]
- Zhao, L.; Zhoug, S.; Fang, K.; Qima, Z.; Chen, J. Determination of cadmium(II), cobalt(II), nickel(II), lead(II), zinc(II), and copper(II) in water samples using dual-cloud point. J. Hazard Mater. 2012, 15, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Khuhawar, M.Y.; Lanjuani, S.N. High performance liquid chromatographic separation and UV determination of cobalt, copper iron and platinum in pharmaceutical preparations using bis(isovalerylacetone)ethylenediimine as complexing reagent. Microchim. Acta 1998, 129, 65–70. [Google Scholar] [CrossRef]
- Gupta, V.K.; Chandra, S.; Agarwal, S.; Lang, A. A PVC based electrochemical sensor for cobalt (II) determination. Proc. Indian Natn. Sci. Acad. 2004, 70, 399–406. [Google Scholar]
- Singh, A.K.; Mehtab, S.; Saxena, P. Cobalt(II)-selective electrode based on a newly synthesized macrocyclic compound. Sens. Actuators B Chem. 2006, 114, 578–583. [Google Scholar] [CrossRef]
- Gangali, M.R.; Rezapour, M.; Pourjavid, M.R.; Niasari, M.S. Cobalt(II)-selective membrane electrode based on a recently synthesized benzo-substituted macrocyclic diamide. Anal. Sci. 2003, 19, 1127–1131. [Google Scholar]
- Gupta, V.K.; Singh, A.K.; Mehtab, S.; Gupta, B. Electrochemical sensor for cobalt (II) determination. Anal. Chimica Acta 2006, 566, 5–10. [Google Scholar] [CrossRef]
- Vlascici, D.; Cosma, E.F.; Spiridon, O.B. A New Composition for Co(II)-porphyrin-based membranes used in thiocyanate-selective electrodes. Sensors 2006, 6, 892–900. [Google Scholar] [CrossRef]
- Ghiaci, M.; Rezaei, B.; Ghomi, J.S. Determination of cobalt(II) by square wave adsorptive stripping Voltammetry. J. Chem. Eng. Data 2010, 13, 287–292. [Google Scholar]
- Rofouei, M.K.; Mohammadi, M.; Khodadian, M.; Jalalvand, A.R. Beiza, Cobalt (II)-selective membrane electrode based on N, N′-di (thiazol-2-yl) formimidamide. Int. J. Environ. Anal. Chem. 2012, 92, 665–675. [Google Scholar] [CrossRef]
- Tyagi, D.S.; Singh, A. Highly selective and sensitive cobalt(II) membrane sensors based on 6-methyl-4-(1-phenylmethylidene) amino-3-thioxo-1,2,4-triazin-5-one as a new neutral ionophore. J. Chinese Adv. Maters. Soc. 2013, 1, 177–184. [Google Scholar] [CrossRef]
- Eren, H.; Uzun, H.; Andac, M.; Bilir, S. Potentiometric monitoring of cobalt in beer sample by solid contact ion selective electrode. J. Food Drug Anal. 2014, 22, 413–417. [Google Scholar] [CrossRef]
- Gupta, V.K.; Singh, L.P. Encyclopedia of Sensors; Grimes, C.A., Dickey, B., Eds.; American Scientific Publishers: Los Angeles, CA, USA, 2006; p. 133. [Google Scholar]
- Khalil, S.; Al Harthi, S.S. Ion-selective membrane sensor for magnesium determination in pharmaceutical formulations. Int. J. Electrochem. Sci. 2020, 15, 9223–9231. [Google Scholar] [CrossRef]
- Panggabean, A.S. Preparation and characterization ion selective electrode Cd(II) based on Chitosan in PVC membrane. Indo. J. Chem. 2011, 11, 285–289. [Google Scholar] [CrossRef]
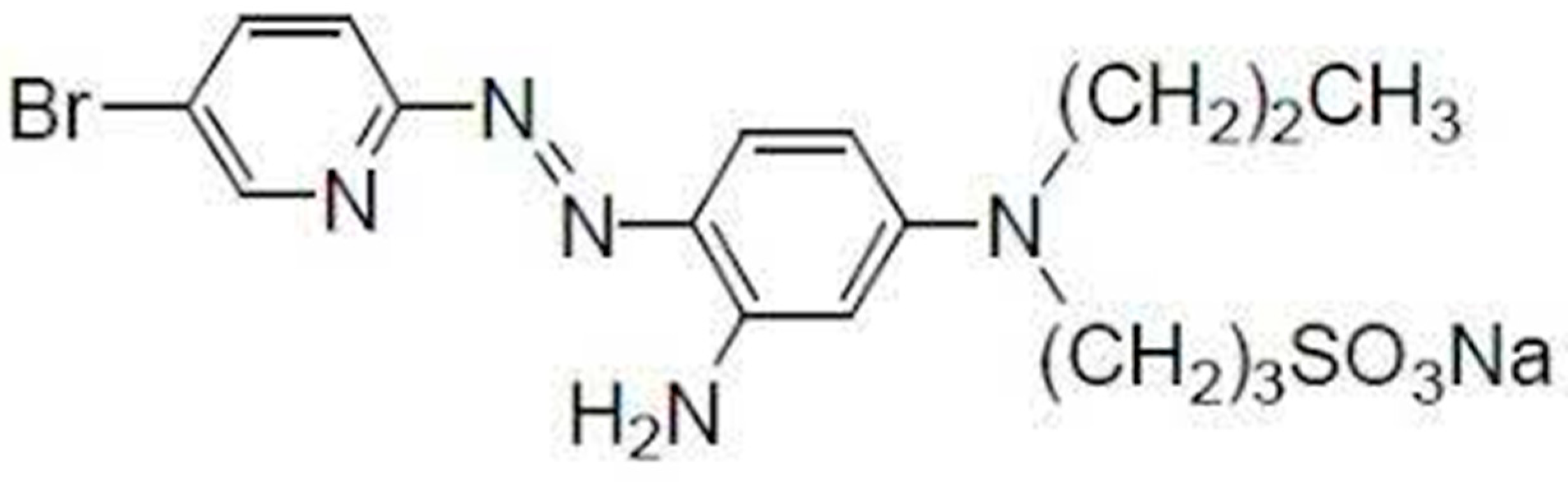
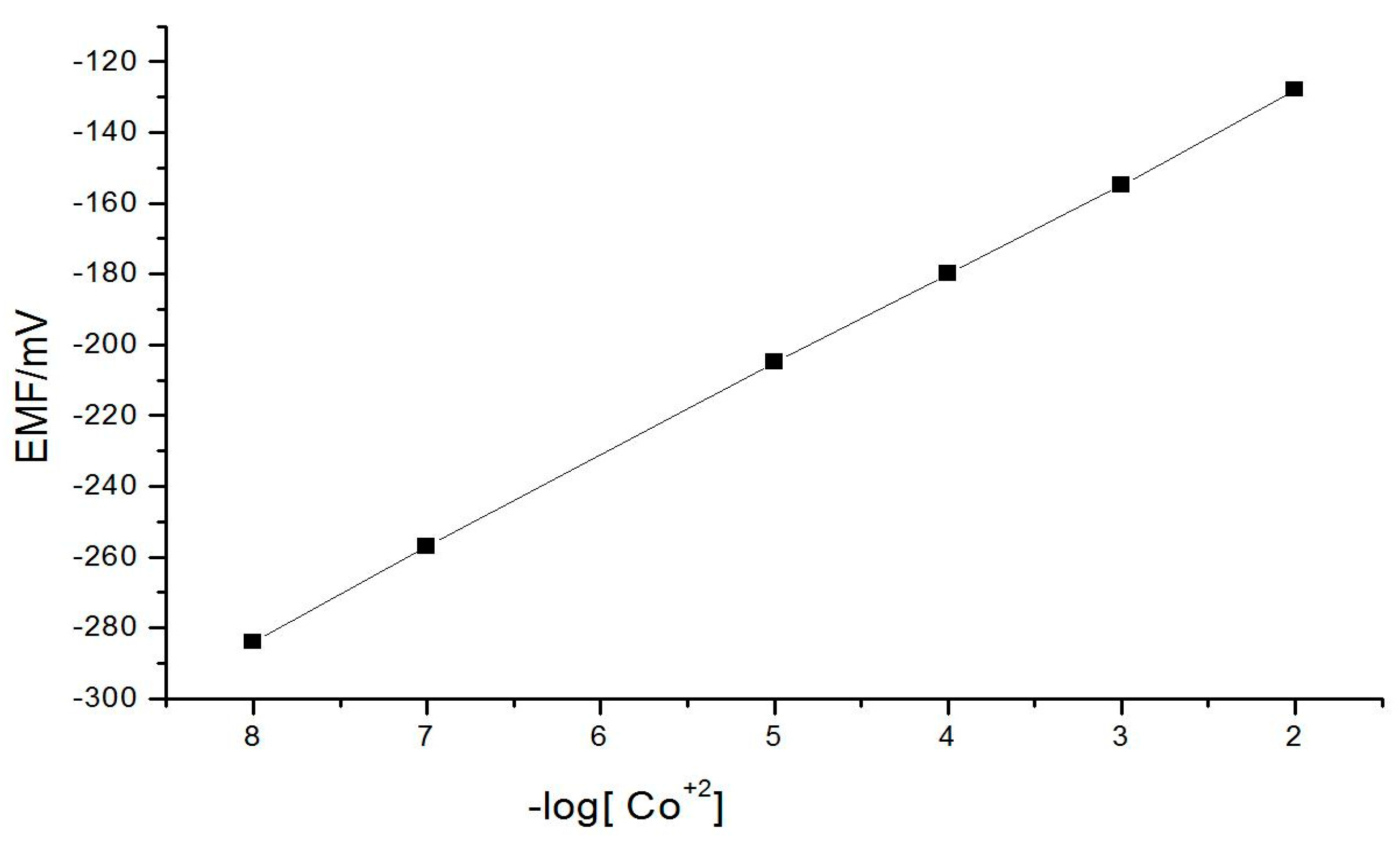
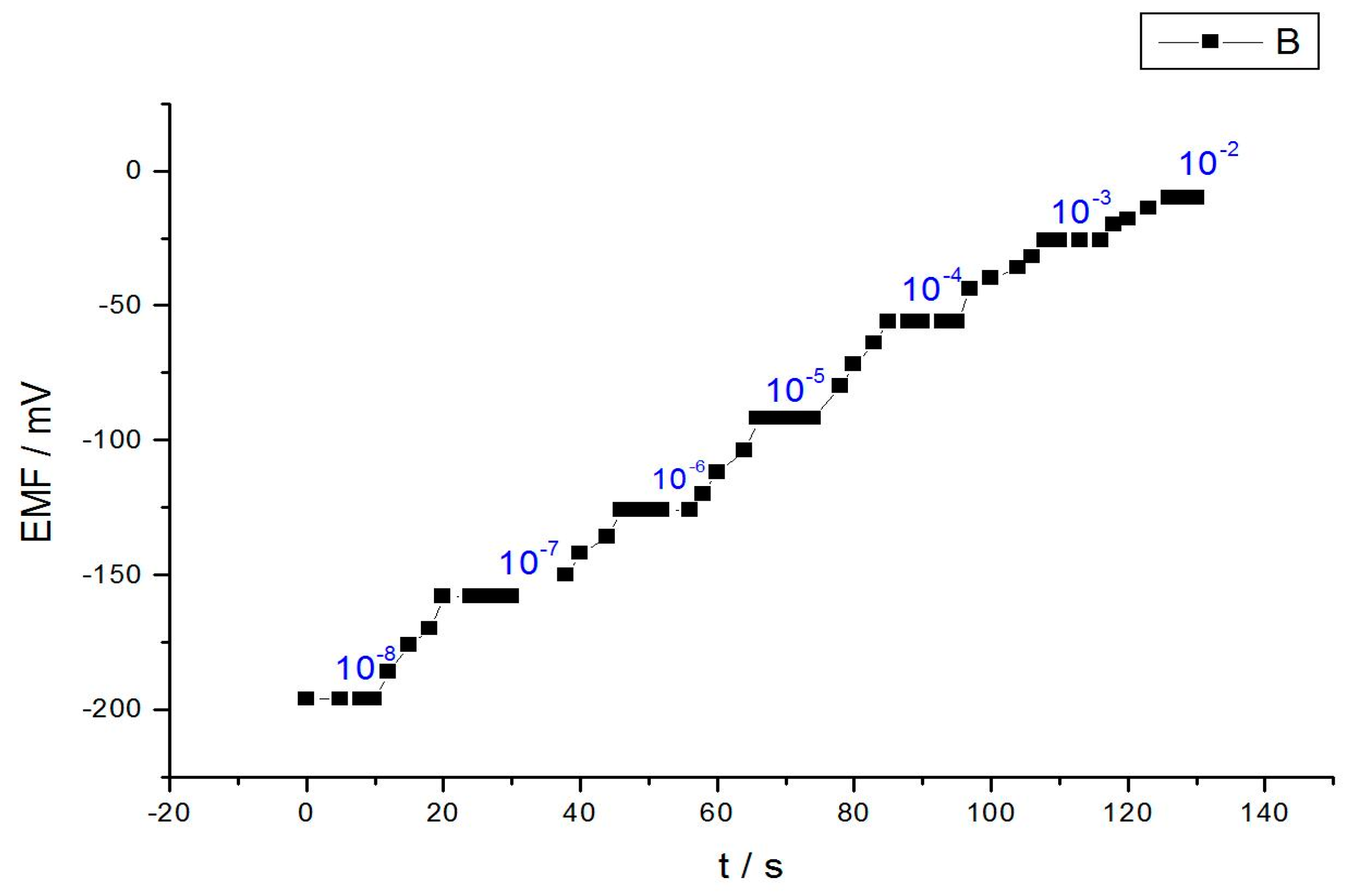

| Slope of the characteristics/mV | 56.66 |
| Intercept/mV | −48.5 + 0.3 |
| Limit of detection/mol dm−3 | 2.7 × 10−8 |
| Measuring range/mol dm−3 | 3.4 × 10−8–2.4 × 10−2 |
| Response time/s | 10 |
| Lifetime/d | 210 |
| pH range | 3.5–8.5 |
| Separate Solution Method (SSM) | Matched Potential Method | ||
|---|---|---|---|
| K | Ei = Ej | ai = aj | MPM |
| CoCl2 | 0.326 + 0.0210 | 0.366 + 0.012 | 0.345 + 0.0180 |
| CdCl2 | 0.240 + 0.0048 | 0.312 + 0.032 | 0.287 + 0.0110 |
| NiCl2 | 0.066+ 0.0018 | 0.151 + 0.003 | 0.256 + 0.0230 |
| AlCl3 | 0.046 + 0.0130 | 0.054 + 0.002 | 0.004 + 0.0012 |
| CaCl2 | 0.245 + 0.0050 | 0.321 + 0.040 | 0.308 + 0.0060 |
| CuCl2 | 0.014 + 0.0003 | 0.066 + 0.002 | 0.016 + 0.0003 |
| Sample (Active Principal) | Calibration Curve Method | Standard Addition in the Sample with Dilution | ||||||
|---|---|---|---|---|---|---|---|---|
| Sample Data mg/Kg | Co+2 Found mg/Kg | Relative Error % | V % | Sample Data mg/Kg | Co+2 Found mg/Kg | Relative Error % | V % | |
| Garlic Root | 430 | 430.65 | 0.14 | 0.08 | 430 | 432.75 | 0.60 | 0.15 |
| Flour | 170 | 171.45 | 0.97 | 0.05 | 170 | 172.86 | 1.91 | 0.12 |
| Powder Milk | 650 | 651.55 | 0.21 | 0.23 | 650 | 652.89 | 0.39 | 0.25 |
| Spinach Leaves | 450 | 451.86 | 0.34 | 0.12 | 450 | 452.95 | 0.54 | 0.16 |
| Red Onion Root | 165 | 166.76 | 1.00 | 0.26 | 165 | 167.54 | 1.45 | 0.16 |
| Cimamon | 600 | 601.85 | 0.46 | 0.35 | 600 | 602.75 | 0.69 | 0.28 |
| Neurobion forte (a) | 500 | 501.65 | 0.33 | 0.11 | 500 | 502.15 | 0.43 | 0.51 |
| Basiton forte (b) | 550 | 551.75 | 0.35 | 0.13 | 550 | 502.65 | 0.53 | 0.26 |
| Ref. | Slope (mV) | Linear Range (M) | Lifetime | Detection Limit (M) | pH Range |
|---|---|---|---|---|---|
| this work data | 56.66 | 3.4 × 10−8–2.4 × 10−2 | 7 months | 2.7 × 10−8 | 3.5–8.5 |
| 16 | 30.5 | 1.9 × 10−5–1.0 × 10−1 | 4 months | ----------- | 1.9–5.8 |
| 17 | 29.5 ± 0.2 | 6.3 × 10−7–1.0 × 10−1 | ----------- | 3.9 × 10−7 | 2.5–6.5 |
| 18 | --------- | 1.0 × 10−6–1.0 × 10−1 | 2 months | 5.0 × 10−7 | 4.0–9.5 |
| 19 | 30 ± 0.2 | 7.9 × 10−8–1.0 × 10−1 | 5 months | 5.0 × 10−8 | 2.0–9.0 |
| 20 | 65.8 ± 1.0 | 1.0 × 10−5–1.0 × 10−1 | ----------- | 6.0 × 10−6 | 2.0–5.5 |
| 21 | ---------- | 6.58 × 10−7–1.0 × 10−1 | ----------- | 6.82 × 10−8 | 2.5–8.5 |
| 22 | 29.5 ± 0.4 | 2.5 × 10−7–1.0 × 10−1 | 2 months | 6.1 × 10−8 | 2.0–7.0 |
| 23 | 30.2 ± 1.0 | 1.1 × 10−7–1.0 × 10−1 | --------- | --------- | 2.0–8.0 |
| 24 | 25.3 ± 1.0 | 1.0 × 10−6–1.0 × 10−1 | 4 months | 3.5 × 10−7 | 3.5–6.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalil, S.; El-Sharnouby, M. Construction of a Highly Selective Membrane Sensor for the Determination of Cobalt (II) Ions. Chemosensors 2021, 9, 86. https://doi.org/10.3390/chemosensors9050086
Khalil S, El-Sharnouby M. Construction of a Highly Selective Membrane Sensor for the Determination of Cobalt (II) Ions. Chemosensors. 2021; 9(5):86. https://doi.org/10.3390/chemosensors9050086
Chicago/Turabian StyleKhalil, Sabry, and Mohamed El-Sharnouby. 2021. "Construction of a Highly Selective Membrane Sensor for the Determination of Cobalt (II) Ions" Chemosensors 9, no. 5: 86. https://doi.org/10.3390/chemosensors9050086
APA StyleKhalil, S., & El-Sharnouby, M. (2021). Construction of a Highly Selective Membrane Sensor for the Determination of Cobalt (II) Ions. Chemosensors, 9(5), 86. https://doi.org/10.3390/chemosensors9050086





