Metal Nanoparticle and Quantum Dot Tags for Signal Amplification in Electrochemical Immunosensors for Biomarker Detection
Abstract
1. Introduction
2. Biomarkers
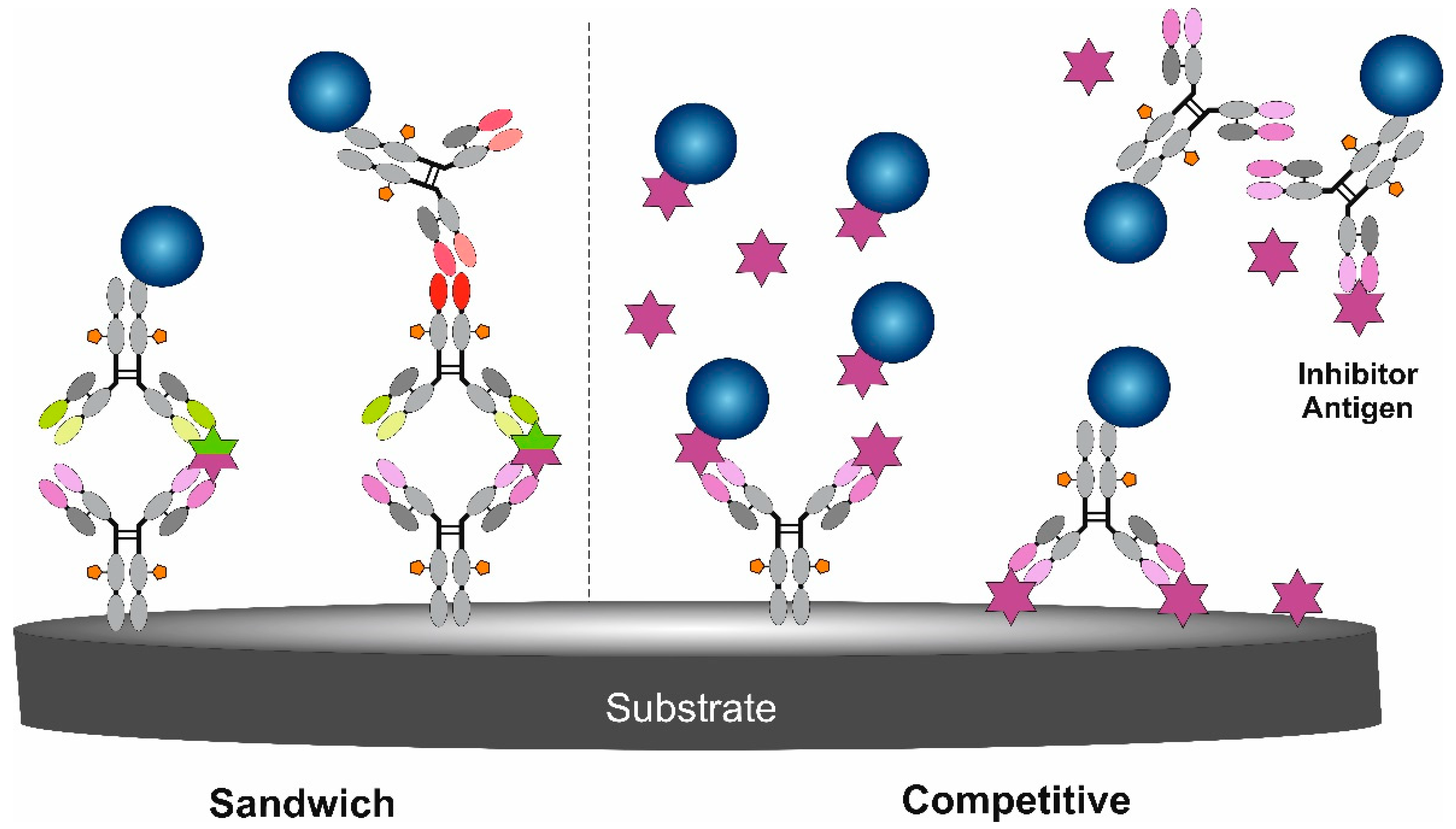
3. Immunoassay Formats Using Nanoparticles as Signal Amplifying Tags
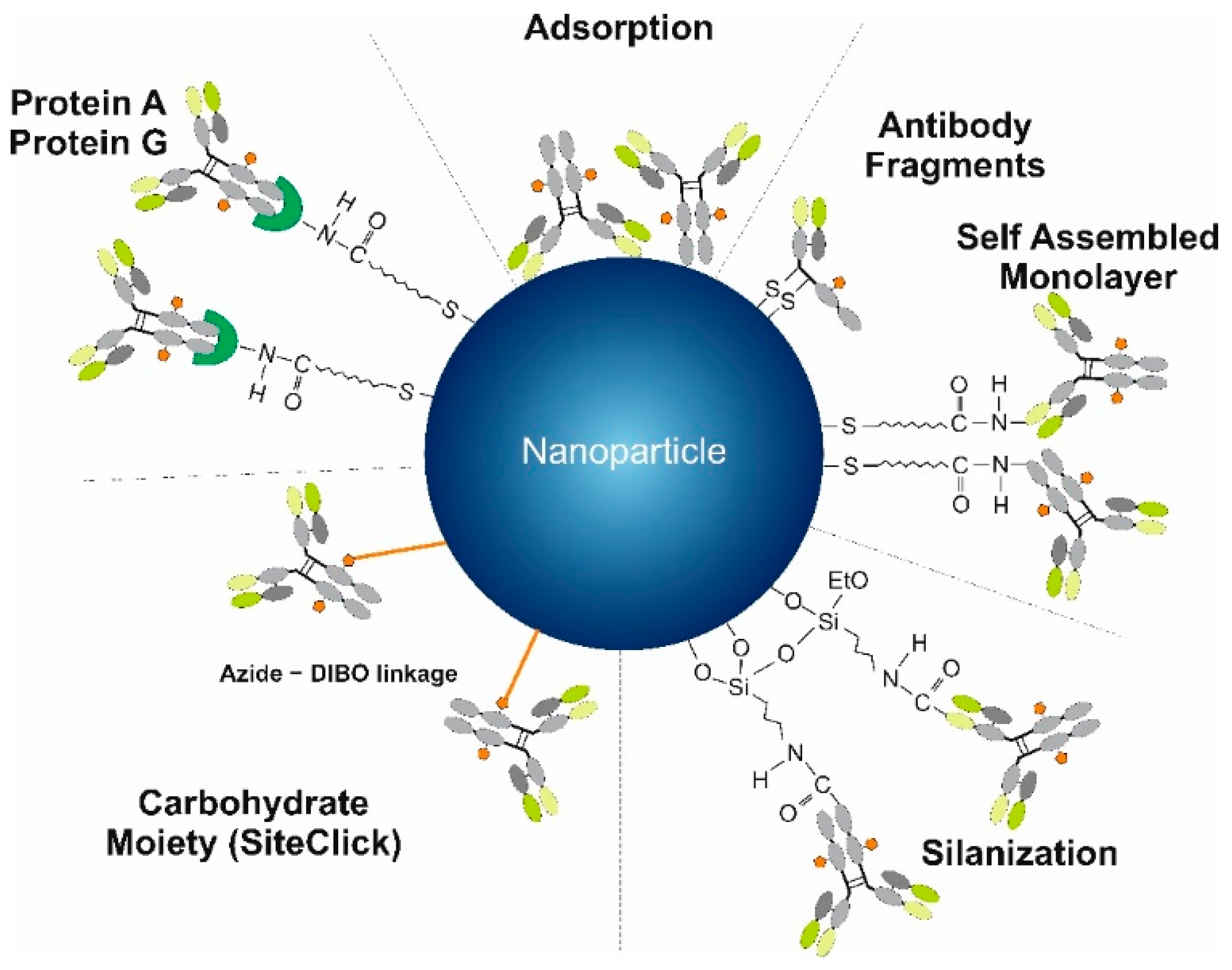
4. Methods Used for the Modification of MNPs and Qdots by Antibodies
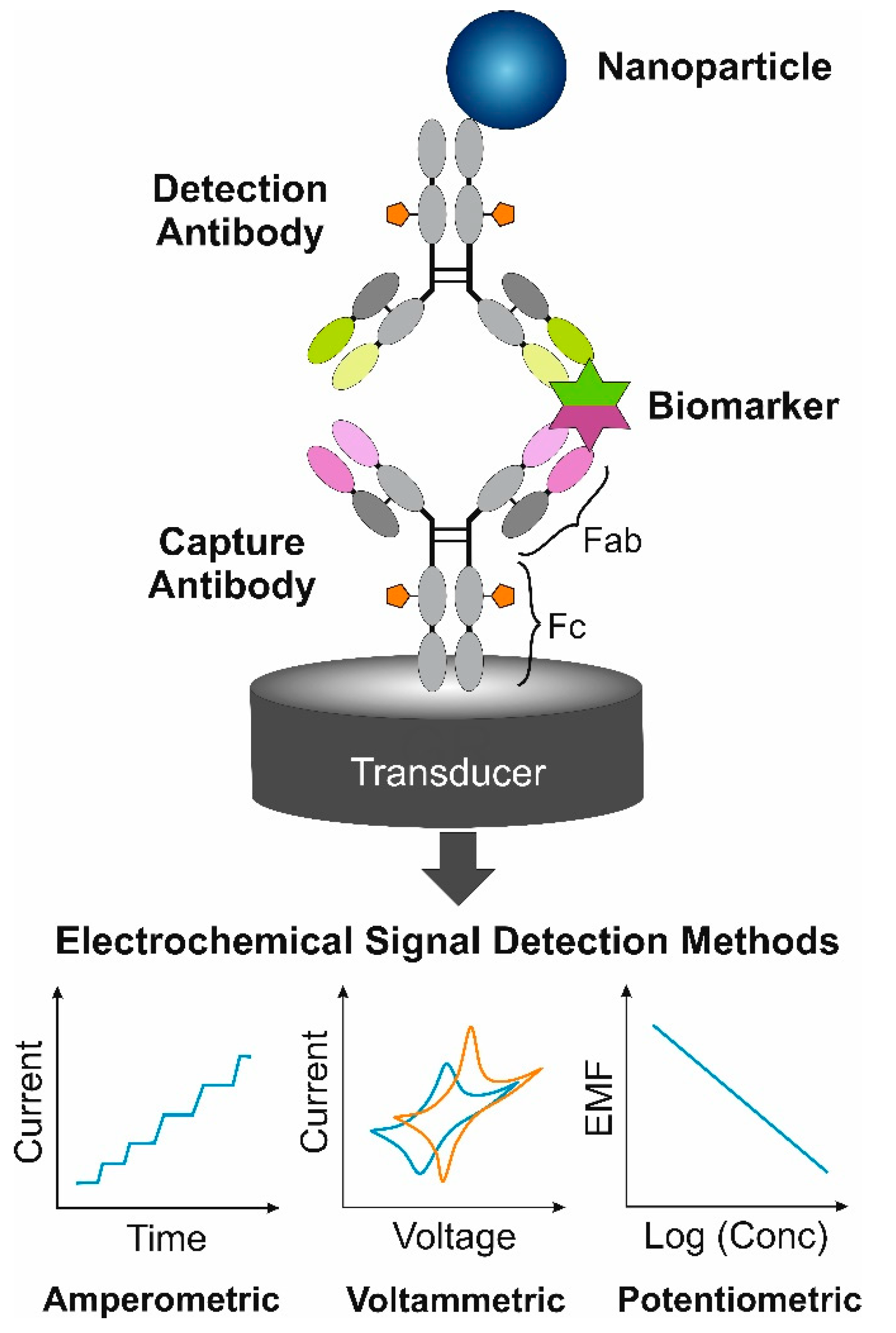
5. Electrochemical Immunosensors
5.1. Amperometric Immunosensors
5.2. Voltammetric Immunosensors
5.3. Potentiometric Immunosensors
5.4. Photoelectrochemical Immunosensors
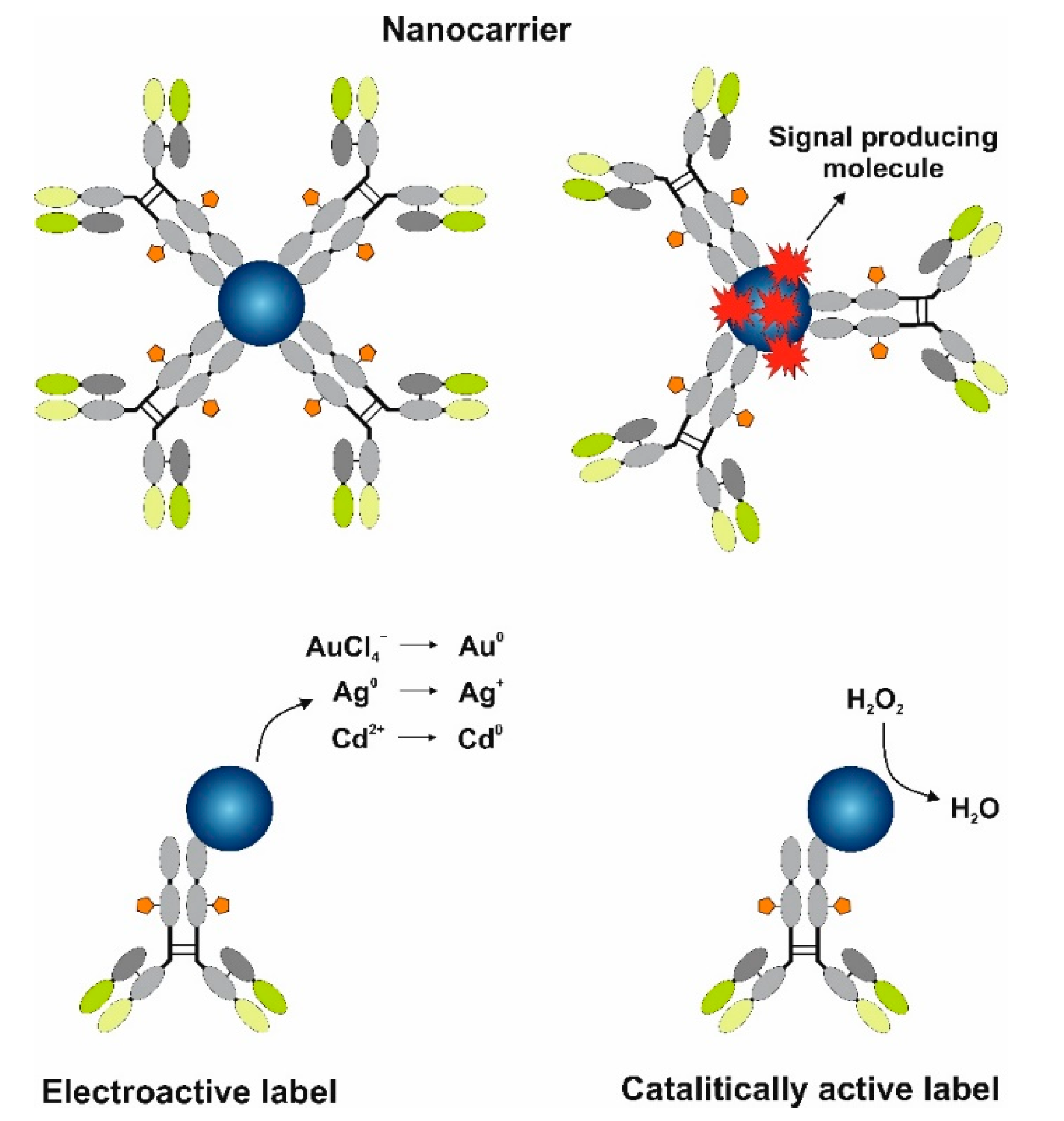
6. MNP and Qdot Tags for Electrochemical Signal Amplification
- Nanocarriers—transport numerous molecules close to the electrode.
- Electroactive labels for biomarker detection.
- Catalytically active labels.
6.1. MNPs and Qdots as Nanocarriers
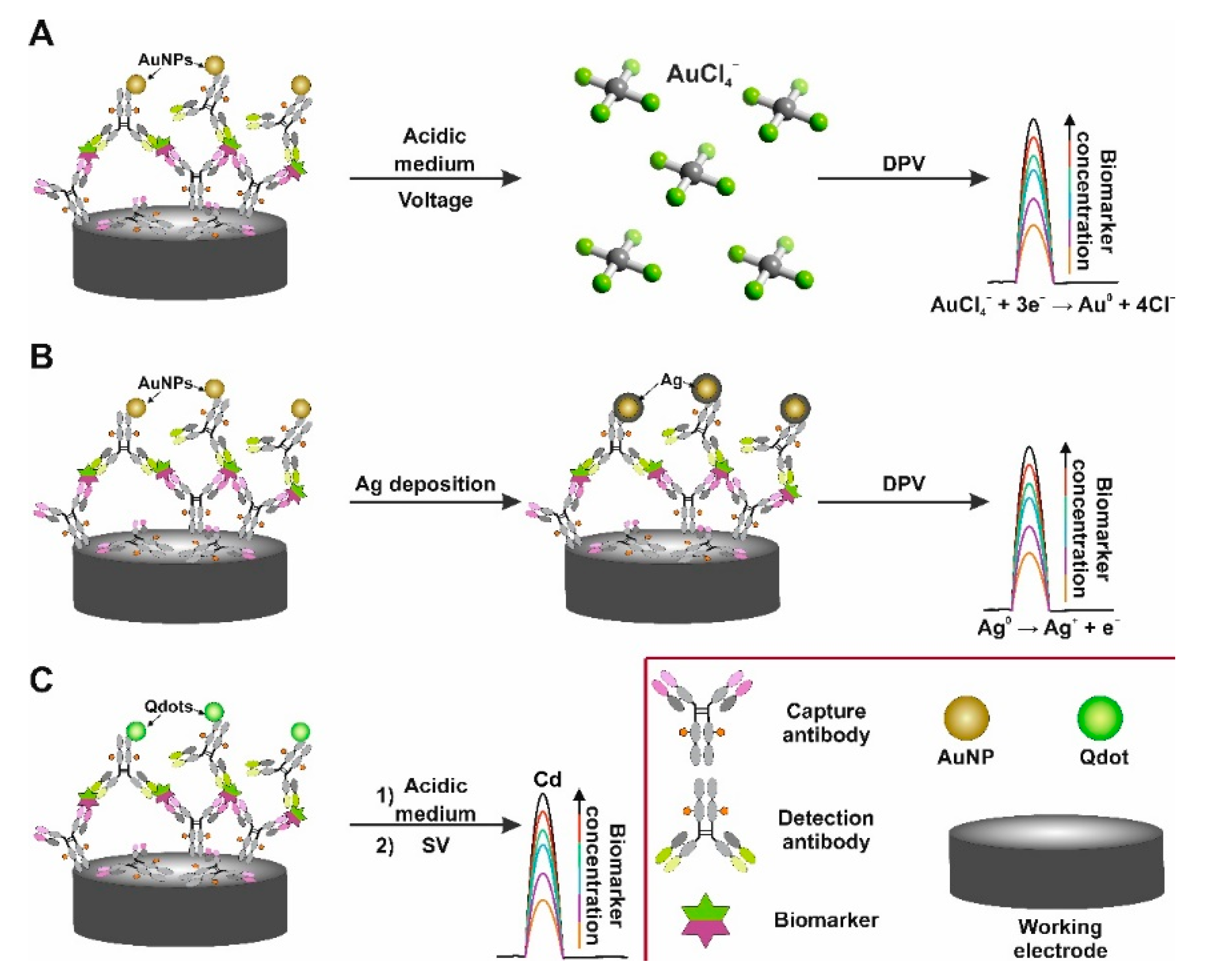
6.2. MNPs and Qdots as Electroactive Labels in Electrochemical Immunosensors
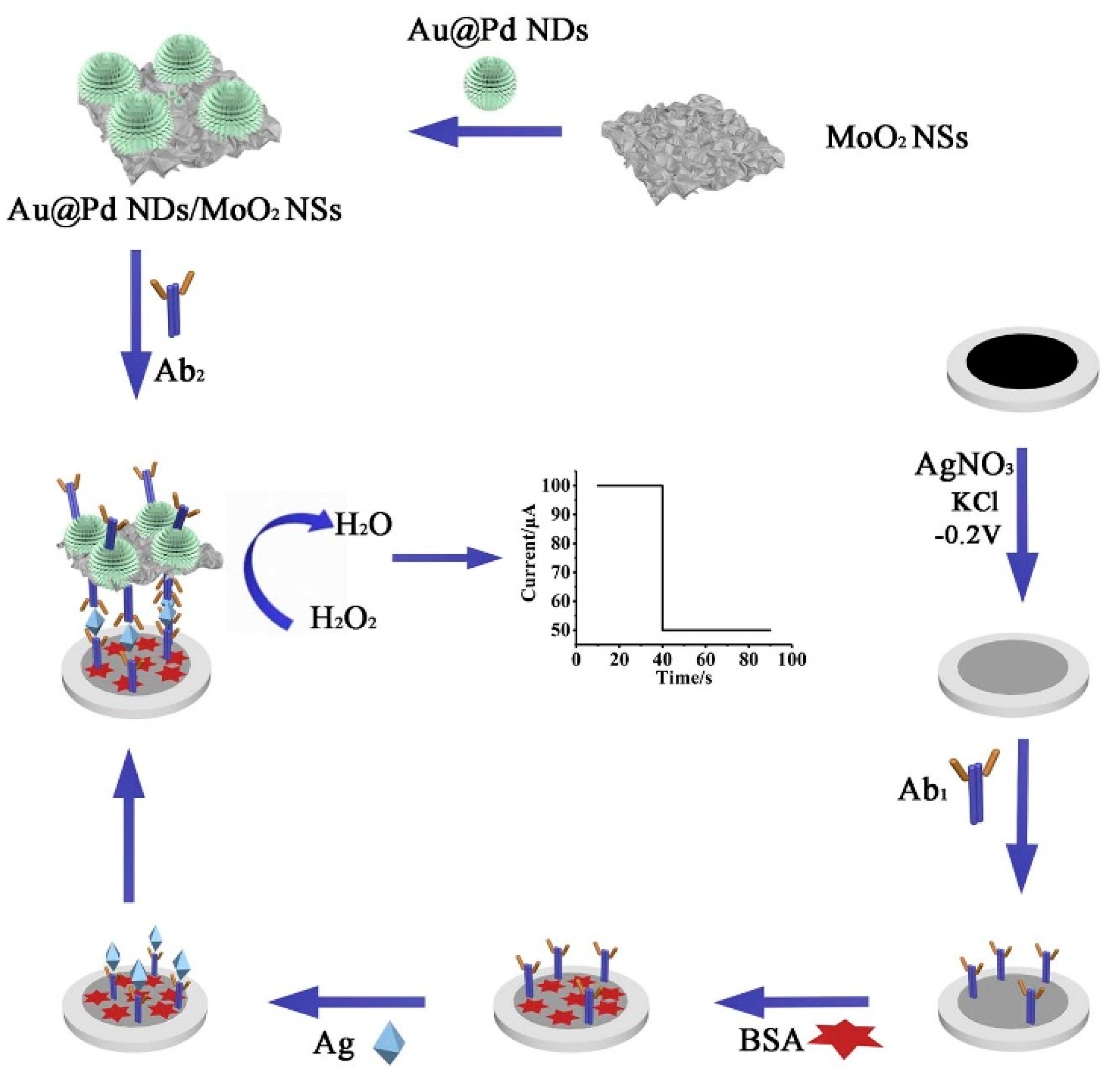
6.3. MNPs as Catalytically Active Labels
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yalow, R.S.; Berson, S.A. Immunoassay of endogenous plasma insulin in man. J. Clin. Investig. 1960, 39, 1157–1175. [Google Scholar] [CrossRef] [PubMed]
- Nakane, P.K.; Pierce, G.B. Enzyme-labeled antibodies: Preparation and application for the localization of antigens. J. Histochem. Cytochem. 1966, 14, 929–931. [Google Scholar] [CrossRef] [PubMed]
- Evtugyn, G.; Hianik, T. Electrochemical immuno- and aptasensors for mycotoxin determination. Chemosensors 2019, 7, 10. [Google Scholar] [CrossRef]
- Engvall, E.; Perlmann, P. Enzyme-linked immunosorbent assay (ELISA) quantitative assay of immunoglobulin G. Immunochemistry 1971, 8, 871–874. [Google Scholar] [CrossRef]
- Van Weemen, B.K.; Schuurs, A.H.W.M. Immunoassay using antigen-enzyme conjugates. FEBS Lett. 1971, 15, 232–236. [Google Scholar] [CrossRef]
- Mollarasouli, F.; Kurbanoglu, S.; Ozkan, S.A. The Role of Electrochemical Immunosensors in Clinical Analysis. Biosensors 2019, 9, 86. [Google Scholar] [CrossRef]
- Ramanavicius, A.; Oztekin, Y.; Ramanaviciene, A. Electrochemical formation of polypyrrole-based layer for immunosensor design. Sens. Actuators B Chem. 2014, 197. [Google Scholar] [CrossRef]
- Duffy, G.F.; Moore, E.J. Electrochemical Immunosensors for Food Analysis: A Review of Recent Developments. Anal. Lett. 2017, 50, 1–32. [Google Scholar] [CrossRef]
- Pollap, A.; Kochana, J. Electrochemical Immunosensors for Antibiotic Detection. Biosensors 2019, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.-H.; Lee, J.; Kim, J.; Kang, M.-S.; Paik, J.K.; Ku, S.; Cho, H.-M.; Irudayaraj, J.; Kim, D.-H. Current Technologies of Electrochemical Immunosensors: Perspective on Signal Amplification. Sensors 2018, 18, 207. [Google Scholar] [CrossRef]
- Makaraviciute, A.; Ramanaviciene, A. Site-directed antibody immobilization techniques for immunosensors. Biosens. Bioelectron. 2013, 50, 460–471. [Google Scholar] [CrossRef]
- Baniukevic, J.; Kirlyte, J.; Ramanavicius, A.; Ramanaviciene, A. Application of oriented and random antibody immobilization methods in immunosensor design. Sens. Actuators B Chem. 2013, 189. [Google Scholar] [CrossRef]
- Huang, H.; Zhu, J.-J. The electrochemical applications of quantum dots. Analyst 2013, 138, 5855–5865. [Google Scholar] [CrossRef]
- Varghese, R.J.; Oluwafemi, O.S. The photoluminescence and biocompatibility of cuins2-based ternary quantum dots and their biological applications. Chemosensors 2020, 8, 101. [Google Scholar] [CrossRef]
- WHO. Biomarkers in Risk Assessment: Validity and Validation; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Website for the National Cancer Institute (NCI), the U.S. Government’s Principal Agency for Cancer Research. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/biomarker (accessed on 13 March 2021).
- Atkinson, A.J.; Colburn, W.A.; DeGruttola, V.G.; DeMets, D.L.; Downing, G.J.; Hoth, D.F.; Oates, J.A.; Peck, C.C.; Schooley, R.T.; Spilker, B.A.; et al. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar]
- Sherwani, S.I.; Khan, H.A.; Ekhzaimy, A.; Masood, A.; Sakharkar, M.K. Significance of HbA1c Test in Diagnosis and Prognosis of Diabetic Patients. Biomark. Insights 2016, 11, 95–104. [Google Scholar] [CrossRef]
- Basu, N.N.; Ingham, S.; Hodson, J.; Lalloo, F.; Bulman, M.; Howell, A.; Evans, D.G. Risk of contralateral breast cancer in BRCA1 and BRCA2 mutation carriers: A 30-year semi-prospective analysis. Fam. Cancer 2015, 14, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Gundogdu, F.; Soylu, F.; Erkan, L.; Tatli, O.; Mavi, S.; Yavuzcan, A. The role of serum CA-125 levels and CA-125 tissue expression positivity in the prediction of the recurrence of stage III and IV epithelial ovarian tumors (CA-125 levels and tissue CA-125 in ovarian tumors). Arch. Gynecol. Obstet. 2011, 283, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Mitterhauser, M.; Wadsak, W. Imaging biomarkers or biomarker imaging? Pharmaceuticals 2014, 7, 765–778. [Google Scholar] [CrossRef]
- Huss, R. Biomarkers. In Translational Regenerative Medicine; Atala, A., Allickson, J.G.B.T.-T.R.M., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 235–241. ISBN 9780124104570. [Google Scholar]
- Laterza, O.F.; Hendrickson, R.C.; Wagner, J.A. Molecular Biomarkers. Drug Inf. J. 2007, 41, 573–585. [Google Scholar] [CrossRef]
- Darwish, I.A. Immunoassay Methods and their Applications in Pharmaceutical Analysis: Basic Methodology and Recent Advances. Int. J. Biomed. Sci. 2006, 2, 217–235. [Google Scholar]
- Website for Bio-Rad Company. Available online: https://www.bio-rad-antibodies.com/elisa-types-direct-indirect-sandwich-competition-elisa-formats.htm (accessed on 14 March 2021).
- Kausaite-Minkstimiene, A.; Ramanaviciene, A.; Kirlyte, J.; Ramanavicius, A. Comparative study of random and oriented antibody immobilization techniques on the binding capacity of immunosensor. Anal. Chem. 2010, 82. [Google Scholar] [CrossRef]
- Byzova, N.A.; Safenkova, I.V.; Slutskaya, E.S.; Zherdev, A.V.; Dzantiev, B.B. Less is More: A Comparison of Antibody–Gold Nanoparticle Conjugates of Different Ratios. Bioconjug. Chem. 2017, 28, 2737–2746. [Google Scholar] [CrossRef]
- Mühlpfordt, H. The preparation of colloidal gold particles using tannic acid as an additional reducing agent. Experientia 1982, 38, 1127–1128. [Google Scholar] [CrossRef]
- Correard, F.; Maximova, K.; Estève, M.-A.; Villard, C.; Roy, M.; Al-Kattan, A.; Sentis, M.; Gingras, M.; Kabashin, A.V.; Braguer, D. Gold nanoparticles prepared by laser ablation in aqueous biocompatible solutions: Assessment of safety and biological identity for nanomedicine applications. Int. J. Nanomed. 2014, 9, 5415–5430. [Google Scholar] [CrossRef]
- Ruiz, G.; Tripathi, K.; Okyem, S.; Driskell, J.D. pH Impacts the Orientation of Antibody Adsorbed onto Gold Nanoparticles. Bioconjug. Chem. 2019, 30, 1182–1191. [Google Scholar] [CrossRef]
- Makaraviciute, A.; Ruzgas, T.; Ramanavicius, A.; Ramanaviciene, A. Antibody fragment immobilization on planar gold and gold nanoparticle modified quartz crystal microbalance with dissipation sensor surfaces for immunosensor applications. Anal. Methods 2014, 6. [Google Scholar] [CrossRef]
- Baniukevic, J.; Hakki Boyaci, I.; Goktug Bozkurt, A.; Tamer, U.; Ramanavicius, A.; Ramanaviciene, A. Magnetic gold nanoparticles in SERS-based sandwich immunoassay for antigen detection by well oriented antibodies. Biosens. Bioelectron. 2013, 43. [Google Scholar] [CrossRef]
- Kaminiaris, M.D.; Mavrikou, S.; Georgiadou, M.; Paivana, G.; Tsitsigiannis, D.I.; Kintzios, S. An Impedance Based Electrochemical Immunosensor for Aflatoxin B1 Monitoring in Pistachio Matrices. Chemosensors 2020, 8, 121. [Google Scholar] [CrossRef]
- Dvorakova, V.; Cadkova, M.; Datinska, V.; Kleparnik, K.; Foret, F.; Bilkova, Z.; Korecka, L. An advanced conjugation strategy for the preparation of quantum dot-antibody immunoprobes. Anal. Methods 2017, 9, 1991–1997. [Google Scholar] [CrossRef]
- Sahoo, S.L.; Liu, C.-H.; Kumari, M.; Wu, W.-C.; Wang, C.-C. Biocompatible quantum dot-antibody conjugate for cell imaging, targeting and fluorometric immunoassay: Crosslinking, characterization and applications. RSC Adv. 2019, 9, 32791–32803. [Google Scholar] [CrossRef]
- Thomas, J.A.; Schnell, F.; Kaveh-Baghbaderani, Y.; Berensmeier, S.; Schwaminger, S.P. Immunomagnetic Separation of Microorganisms with Iron Oxide Nanoparticles. Chemosensors 2020, 8, 17. [Google Scholar] [CrossRef]
- Jampasa, S.; Siangproh, W.; Laocharoensuk, R.; Vilaivan, T.; Chailapakul, O. Electrochemical detection of c-reactive protein based on anthraquinone-labeled antibody using a screen-printed graphene electrode. Talanta 2018, 183, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Makaraviciute, A.; Ramanavicius, A.; Ramanaviciene, A. Development of a reusable protein G based SPR immunosensor for direct human growth hormone detection in real samples. Anal. Methods 2015, 7. [Google Scholar] [CrossRef]
- Bodanszky, A.; Bodanszky, M. Sepharose-avidin column for the binding of biotin or biotin-containing peptides. Experientia 1970, 26, 327. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Kitova, E.N.; Klassen, J.S. Dissociation Kinetics of the Streptavidin–Biotin Interaction Measured Using Direct Electrospray Ionization Mass Spectrometry Analysis. J. Am. Soc. Mass Spectrom. 2013, 24, 49–56. [Google Scholar] [CrossRef]
- Melnyk, O.; Duburcq, X.; Olivier, C.; Urbès, F.; Auriault, C.; Gras-Masse, H. Peptide Arrays for Highly Sensitive and Specific Antibody-Binding Fluorescence Assays. Bioconjug. Chem. 2002, 13, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Quash, G.; Roch, A.-M.; Niveleau, A.; Grange, J.; Keolouangkhot, T.; Huppert, J. The preparation of latex particles with covalently bound polyamines, IgG and measles agglutinins and their use in visual agglutination tests. J. Immunol. Methods 1978, 22, 165–174. [Google Scholar] [CrossRef]
- Website for Thermo Fisher Scientific Company. Available online: https://www.thermofisher.com/order/catalog/product/S10469#/S10469 (accessed on 14 March 2021).
- Kondzior, M.; Grabowska, I. Antibody-Electroactive Probe Conjugates Based Electrochemical Immunosensors. Sensors 2020, 20, 2014. [Google Scholar] [CrossRef] [PubMed]
- Warsinke, A.; Benkert, A.; Scheller, F.W. Electrochemical immunoassays. Fresenius. J. Anal. Chem. 2000, 366, 622–634. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Li, F.; Li, M.; Chen, L.; Dong, Y.; Wei, Q. Sandwich-type amperometric immunosensor using functionalized magnetic graphene loaded gold and silver core-shell nanocomposites for the detection of Carcinoembryonic antigen. J. Electroanal. Chem. 2017, 795, 1–9. [Google Scholar] [CrossRef]
- Yan, Q.; Cao, L.; Dong, H.; Tan, Z.; Liu, Q.; Zhang, W.; Zhao, P.; Li, Y.; Liu, Y.; Dong, Y. Sensitive amperometric immunosensor with improved electrocatalytic Au@Pd urchin-shaped nanostructures for human epididymis specific protein 4 antigen detection. Anal. Chim. Acta 2019, 1069, 117–125. [Google Scholar] [CrossRef]
- Thunkhamrak, C.; Reanpang, P.; Ounnunkad, K.; Jakmunee, J. Sequential injection system with amperometric immunosensor for sensitive determination of human immunoglobulin G. Talanta 2017, 171, 53–60. [Google Scholar] [CrossRef]
- Shi, W.; Ma, Z. A novel label-free amperometric immunosensor for carcinoembryonic antigen based on redox membrane. Biosens. Bioelectron. 2011, 26, 3068–3071. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Q.; Liu, Y.; Cui, J.; Liu, H.; Wang, P.; Li, Y.; Chen, L.; Zhao, Z.; Dong, Y. A novel label-free electrochemical immunosensor based on functionalized nitrogen-doped graphene quantum dots for carcinoembryonic antigen detection. Biosens. Bioelectron. 2017, 90, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, G.; Sánchez-Tirado, E.; González-Cortés, A.; Yáñez-Sedeño, P.; Pingarrón, J.M. Amperometric immunoassay for the obesity biomarker amylin using a screen printed carbon electrode functionalized with an electropolymerized carboxylated polypyrrole. Microchim. Acta 2018, 185, 323. [Google Scholar] [CrossRef]
- Yáñez-Sedeño, P.; Campuzano, S.; Pingarrón, J.M. Multiplexed Electrochemical Immunosensors for Clinical Biomarkers. Sensors 2017, 17, 965. [Google Scholar] [CrossRef] [PubMed]
- Mansuriya, B.D.; Altintas, Z. Graphene Quantum Dot-Based Electrochemical Immunosensors for Biomedical Applications. Materials 2019, 13, 96. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Zhou, Z.; Zhou, H.S. Review—Measurement and Analysis of Cancer Biomarkers Based on Electrochemical Biosensors. J. Electrochem. Soc. 2020, 167, 37525. [Google Scholar] [CrossRef]
- Trindade, E.K.G.; Silva, B.V.M.; Dutra, R.F. A probeless and label-free electrochemical immunosensor for cystatin C detection based on ferrocene functionalized-graphene platform. Biosens. Bioelectron. 2019, 138, 111311. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Bao, J.; Zhao, Y.; Huo, D.; Chen, M.; Qi, Y.; Yang, M.; Fa, H.; Hou, C. A sandwich-type electrochemical immunoassay for ultrasensitive detection of non-small cell lung cancer biomarker CYFRA21-1. Bioelectrochemistry 2018, 120, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Hui, N.; Luo, X. Reagentless and label-free voltammetric immunosensor for carcinoembryonic antigen based on polyaniline nanowires grown on porous conducting polymer composite. Microchim. Acta 2017, 184, 889–896. [Google Scholar] [CrossRef]
- Feng, C.; Xu, Y.; Song, L. Study on highly sensitive potentiometric IgG immunosensor. Sens. Actuators B Chem. 2000, 66, 190–192. [Google Scholar] [CrossRef]
- Tang, D.P.; Yuan, R.; Chai, Y.Q.; Zhong, X.; Liu, Y.; Dai, J.Y.; Zhang, L.Y. Novel potentiometric immunosensor for hepatitis B surface antigen using a gold nanoparticle-based biomolecular immobilization method. Anal. Biochem. 2004, 333, 345–350. [Google Scholar] [CrossRef]
- Ibupoto, Z.H.; Jamal, N.; Khun, K.; Willander, M. Development of a disposable potentiometric antibody immobilized ZnO nanotubes based sensor for the detection of C-reactive protein. Sens. Actuators B Chem. 2012, 166–167, 809–814. [Google Scholar] [CrossRef]
- Fowler, J.M.; Wong, D.K.Y.; Brian Halsall, H.; Heineman, W.R. Recent developments in electrochemical immunoassays and immunosensors. In Electrochemical Sensors, Biosensors and their Biomedical Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2008; pp. 115–143. ISBN 9780123737380. [Google Scholar]
- Cao, Y.; Zheng, M.; Cai, W.; Wang, Z. Enzyme-loaded liposome with biocatalytic precipitation for potentiometric immunoassay of thyroid-stimulating hormone in thyroid carcinoma. Chinese Chem. Lett. 2020, 31, 463–467. [Google Scholar] [CrossRef]
- Lv, S.; Lin, Z.; Zhang, K.; Lu, M.; Tang, D. Polyion oligonucleotide-decorated gold nanoparticles with tunable surface charge density for amplified signal output of potentiometric immunosensor. Anal. Chim. Acta 2017, 964, 67–73. [Google Scholar] [CrossRef]
- Thürer, R.; Vigassy, T.; Hirayama, M.; Wang, J.; Bakker, E.; Pretsch, E. Potentiometric immunoassay with quantum dot labels. Anal. Chem. 2007, 79, 5107–5110. [Google Scholar] [CrossRef]
- Shu, J.; Tang, D. Current Advances in Quantum-Dots-Based Photoelectrochemical Immunoassays. Chem. Asian J. 2017, 12, 2780–2789. [Google Scholar] [CrossRef]
- Li, R.; Gao, J.; Gao, P.; Zhang, S.; Liu, Y.; Du, B.; Wei, Q. A sensitive photoelectrochemical immunoassay based on mesoporous carbon/core–shell quantum dots as donor–acceptor light-harvesting architectures. New J. Chem. 2015, 39, 731–738. [Google Scholar] [CrossRef]
- Liang, M.; Liu, S.; Wei, M.; Guo, L.-H. Photoelectrochemical Oxidation of DNA by Ruthenium Tris(bipyridine) on a Tin Oxide Nanoparticle Electrode. Anal. Chem. 2006, 78, 621–623. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.-W.; Xu, J.-J.; Chen, H.-Y. Photoelectrochemical bioanalysis: The state of the art. Chem. Soc. Rev. 2015, 44, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, G.-C. A novel signal-on photoelectrochemical immunosensor for detection of alpha-fetoprotein by in situ releasing electron donor. Biosens. Bioelectron. 2017, 98, 155–160. [Google Scholar] [CrossRef]
- Tabrizi, M.A.; Ferré-Borrull, J.; Kapruwan, P.; Marsal, L.F. A photoelectrochemical sandwich immunoassay for protein S100β, a biomarker for Alzheimer’s disease, using an ITO electrode modified with a reduced graphene oxide-gold conjugate and CdS-labeled secondary antibody. Microchim. Acta 2019, 186, 117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, Y.; Zhao, G.; Wang, C.; Li, Y.; Zhang, Y.; Wang, H.; Wei, Q. A photoelectrochemical immunosensor based on CdS/CdTe-cosensitized SnO2 as a platform for the ultrasensitive detection of amyloid β-protein. Analyst 2020, 145, 619–625. [Google Scholar] [CrossRef]
- Sun, X.; Li, C.; Zhu, Q.; Chen, J.; Li, J.; Ding, H.; Sang, F.; Kong, L.; Chen, Z.; Wei, Q. A novel ultrasensitive sandwich-type photoelectrochemical immunoassay for PSA detection based on dual inhibition effect of Au/MWCNTs nanohybrids on N-GQDs/CdS QDs dual sensitized urchin-like TiO2. Electrochim. Acta 2020, 333, 135480. [Google Scholar] [CrossRef]
- Fan, D.; Li, N.; Ma, H.; Li, Y.; Hu, L.; Du, B.; Wei, Q. Electrochemical immunosensor for detection of prostate specific antigen based on an acid cleavable linker into MSN-based controlled release system. Biosens. Bioelectron. 2016, 85, 580–586. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, T.; Yan, T.; Kuang, X.; Wang, H.; Wu, D.; Wei, Q. Novel electrochemical immunosensor for sensitive monitoring of cardiac troponin I using antigen–response cargo released from mesoporous Fe3O4. Biosens. Bioelectron. 2019, 143, 111608. [Google Scholar] [CrossRef]
- Song, D.; Zheng, J.; Myung, N.V.; Xu, J.; Zhang, M. Sandwich-type electrochemical immunosensor for CEA detection using magnetic hollow Ni/C@SiO2 nanomatrix and boronic acid functionalized CPS@PANI@Au probe. Talanta 2021, 225, 122006. [Google Scholar] [CrossRef]
- Kumar, N.; Sharma, S.; Nara, S. Dual gold nanostructure-based electrochemical immunosensor for CA125 detection. Appl. Nanosci. 2018, 8, 1843–1853. [Google Scholar] [CrossRef]
- Liu, C.; Dong, J.; Waterhouse, G.I.N.; Cheng, Z.; Ai, S. Electrochemical immunosensor with nanocellulose-Au composite assisted multiple signal amplification for detection of avian leukosis virus subgroup J. Biosens. Bioelectron. 2018, 101, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, X.; Mao, K.; Li, Y.; Du, B.; Zhang, Y.; Wei, Q. Electrochemical immunosensor for α-fetoprotein detection using ferroferric oxide and horseradish peroxidase as signal amplification labels. Anal. Biochem. 2014, 465, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, H.; Chen, Z.; Wei, X.; Yang, C.F. An electrochemical immunosensor for carcinoembryonic antigen enhanced by self-assembled nanogold coatings on magnetic particles. Anal. Chim. Acta 2010, 665, 98–104. [Google Scholar] [CrossRef]
- Tufa, L.T.; Oh, S.; Tran, V.T.; Kim, J.; Jeong, K.J.; Park, T.J.; Kim, H.J.; Lee, J. Electrochemical immunosensor using nanotriplex of graphene quantum dots, Fe3O4 and Ag nanoparticles for tuberculosis. Electrochim. Acta 2018, 290, 369–377. [Google Scholar] [CrossRef]
- López-Marzo, A.M.; Hoyos-De-La-Torre, R.; Baldrich, E. NaNO3/NaCl Oxidant and Polyethylene Glycol (PEG) Capped Gold Nanoparticles (AuNPs) as a Novel Green Route for AuNPs Detection in Electrochemical Biosensors. Anal. Chem. 2018, 90, 4010–4018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ma, H.; Yan, L.; Cao, W.; Yan, T.; Wei, Q.; Du, B. Copper-doped titanium dioxide nanoparticles as dual-functional labels for fabrication of electrochemical immunosensors. Biosens. Bioelectron. 2014, 59, 335–341. [Google Scholar] [CrossRef]
- Feng, L.N.; Bian, Z.P.; Peng, J.; Jiang, F.; Yang, G.H.; Zhu, Y.D.; Yang, D.; Jiang, L.P.; Zhu, J.J. Ultrasensitive multianalyte electrochemical immunoassay based on metal ion functionalized titanium phosphate nanospheres. Anal. Chem. 2012, 84, 7810–7815. [Google Scholar] [CrossRef]
- Lah, Z.M.A.N.H.; Ahmad, S.A.A.; Zaini, M.S.; Kamarudin, M.A. An Electrochemical Sandwich Immunosensor for the Detection of HER2 using Antibody-Conjugated PbS Quantum Dot as a label. J. Pharm. Biomed. Anal. 2019, 174, 608–617. [Google Scholar] [CrossRef]
- Ehzari, H.; Amiri, M.; Safari, M. Enzyme-free sandwich-type electrochemical immunosensor for highly sensitive prostate specific antigen based on conjugation of quantum dots and antibody on surface of modified glassy carbon electrode with core–shell magnetic metal-organic frameworks. Talanta 2020, 210, 120641. [Google Scholar] [CrossRef]
- Martín-Yerga, D.; González-García, M.B.; Costa-García, A. Electrochemical immunosensor for anti-tissue transglutaminase antibodies based on the in situ detection of quantum dots. Talanta 2014, 130, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Jiang, Y.; Xia, L.; Zhang, T.; Xu, L.; Zhang, S.; Liu, D.; Song, H. A sensitive photoelectrochemical biosensor for AFP detection based on ZnO inverse opal electrodes with signal amplification of CdS-QDs. Biosens. Bioelectron. 2015, 74, 411–417. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, C.; Jia, Y.; Zhang, X.; Dong, Y.; Li, X.; Liu, Q.; Li, Y.; Zhao, Z. Sandwich-type electrochemical immunosensor based on Au@Pt DNRs/NH 2 -MoSe 2 NSs nanocomposite as signal amplifiers for the sensitive detection of alpha-fetoprotein. Bioelectrochemistry 2019, 128, 140–147. [Google Scholar] [CrossRef]
- Lou, Y.; He, T.; Jiang, F.; Shi, J.J.; Zhu, J.J. A competitive electrochemical immunosensor for the detection of human interleukin-6 based on the electrically heated carbon electrode and silver nanoparticles functionalized labels. Talanta 2014, 122, 135–139. [Google Scholar] [CrossRef]
- Song, W.; Li, H.; Liu, H.; Wu, Z.; Qiang, W.; Xu, D. Fabrication of streptavidin functionalized silver nanoparticle decorated graphene and its application in disposable electrochemical sensor for immunoglobulin e. Electrochem. Commun. 2013, 31, 16–19. [Google Scholar] [CrossRef]
- Chen, P.; Wang, T.; Zheng, X.; Tian, D.; Xia, F.; Zhou, C. An ultrasensitive electrochemical immunosensor based on C60-modified polyamidoamine dendrimers and Au NPs for co-catalytic silver deposition. New J. Chem. 2018, 42, 4653–4660. [Google Scholar] [CrossRef]
- Chen, S.; Yang, Y.; Li, W.; Song, Y.; Shi, L.; Hong, C. A sandwich-type electrochemical immunosensor using Ag@CeO2-Au as a lable for sensitive detection of carcinoembryonic antigen. Microchem. J. 2020, 159, 105415. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, Y.; Chen, Y.; Xiao, H.; Sui, Y.; Xie, Q.; Liu, R.; Yang, X. Dual-signal sandwich-type electrochemical immunoassay of galectin-3 using methylene blue and gold nanoparticles biolabels. J. Electroanal. Chem. 2020, 861, 113952. [Google Scholar] [CrossRef]
- Sui, Y.; Xu, A.; Jin, X.; Zheng, J.; He, X.; Cheng, Y.; Xie, Q.; Liu, R. In situ enzymatic generation of gold for ultrasensitive amperometric sandwich immunoassay of procalcitonin. Biosens. Bioelectron. 2018, 117, 422–428. [Google Scholar] [CrossRef]
- Qin, X.; Xu, A.; Liu, L.; Deng, W.; Chen, C.; Tan, Y.; Fu, Y.; Xie, Q.; Yao, S. Ultrasensitive electrochemical immunoassay of proteins based on in situ duple amplification of gold nanoparticle biolabel signals. Chem. Commun. 2015, 51, 8540–8543. [Google Scholar] [CrossRef]
- Shamsipur, M.; Emami, M.; Farzin, L.; Saber, R. A sandwich-type electrochemical immunosensor based on in situ silver deposition for determination of serum level of HER2 in breast cancer patients. Biosens. Bioelectron. 2018, 103, 54–61. [Google Scholar] [CrossRef]
- Duangkaew, P.; Wutikhun, T.; Laocharoensuk, R. Triple signal amplification strategy based on size and shape transformation of ultrasmall sub-10 nm gold nanoparticles tag towards sensitivity improvement of electrochemical immunosensors. Sens. Actuators B Chem. 2017, 239, 430–437. [Google Scholar] [CrossRef]
- Wang, C.; Ding, L.; Qu, F. Sensitive electrochemical immunosensor for platelet-derived growth factor in serum with electron transfer mediated by gold nanoparticles initiated silver enhancement. Measurement 2013, 46, 279–283. [Google Scholar] [CrossRef]
- Yang, G.; Lai, Y.; Xiao, Z.; Tang, C.; Deng, Y. Ultrasensitive electrochemical immunosensor of carcinoembryonic antigen based on gold-label silver-stain signal amplification. Chinese Chem. Lett. 2018, 29, 1857–1860. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Zhang, N.; Fan, D.; Liu, L.; Yan, T.; Yang, X.; Ding, C.; Wei, Q.; Ju, H. Magnetic electrode-based electrochemical immunosensor using amorphous bimetallic sulfides of CoSnSx as signal amplifier for the NTpro BNP detection. Biosens. Bioelectron. 2019, 131, 250–256. [Google Scholar] [CrossRef]
- Tan, X.; Zhang, L.; Deng, X.; Miao, L.; Li, H.; Zheng, G. Redox active molybdophosphate produced by Cu3(PO4)2 nanospheres for enhancing enzyme-free electrochemical immunoassay of C-reactive protein. New J. Chem. 2017, 41, 11867–11871. [Google Scholar] [CrossRef]
- Wu, D.; Guo, Z.; Liu, Y.; Guo, A.; Lou, W.; Fan, D.; Wei, Q. Sandwich-type electrochemical immunosensor using dumbbell-like nanoparticles for the determination of gastric cancer biomarker CA72-4. Talanta 2015, 134, 305–309. [Google Scholar] [CrossRef]
- Cui, Z.; Wu, D.; Zhang, Y.; Ma, H.; Li, H.; Du, B.; Wei, Q.; Ju, H. Ultrasensitive electrochemical immunosensors for multiplexed determination using mesoporous platinum nanoparticles as nonenzymatic labels. Anal. Chim. Acta 2014, 807, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Ma, H.; Cao, W.; Wu, D.; Yan, T.; Du, B.; Wei, Q. Highly sensitive electrochemical immunosensor for the detection of alpha fetoprotein based on PdNi nanoparticles and N-doped graphene nanoribbons. Biosens. Bioelectron. 2015, 74, 786–791. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, P.; Ma, E.; Yu, H.; Zhou, K.; Tang, C.; Ren, J.; Li, Y.; Liu, Q.; Dong, Y. A sandwich-type electrochemical immunosensor based on Au@Pd nanodendrite functionalized MoO2 nanosheet for highly sensitive detection of HBsAg. Bioelectrochemistry 2021, 138, 107713. [Google Scholar] [CrossRef]
- Li, Y.; Tian, L.; Liu, L.; Khan, M.S.; Zhao, G.; Fan, D.; Cao, W.; Wei, Q. Dual-responsive electrochemical immunosensor for detection of insulin based on dual-functional zinc silicate spheres-palladium nanoparticles. Talanta 2018, 179, 420–425. [Google Scholar] [CrossRef]
- Kang, J.; Li, Z.; Wang, G. A novel signal amplification strategy electrochemical immunosensor for ultra-sensitive determination of p53 protein. Bioelectrochemistry 2021, 137, 107647. [Google Scholar] [CrossRef]
- Brasiunas, B.; Popov, A.; Ramanavicius, A.; Ramanaviciene, A. Gold nanoparticle based colorimetric sensing strategy for the determination of reducing sugars. Food Chem. 2021, 351, 129238. [Google Scholar] [CrossRef]
- Serafín, V.; Valverde, A.; Martínez-García, G.; Martínez-Periñán, E.; Comba, F.; Garranzo-Asensio, M.; Barderas, R.; Yáñez-Sedeño, P.; Campuzano, S.; Pingarrón, J.M. Graphene quantum dots-functionalized multi-walled carbon nanotubes as nanocarriers in electrochemical immunosensing. Determination of IL-13 receptor A2 in colorectal cells and tumor tissues with different metastatic potential. Sens. Actuators B Chem. 2019, 284, 711–722. [Google Scholar] [CrossRef]
- Serafín, V.; Valverde, A.; Garranzo-Asensio, M.; Barderas, R.; Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Simultaneous amperometric immunosensing of the metastasis-related biomarkers IL-13Rα2 and CDH-17 by using grafted screen-printed electrodes and a composite prepared from quantum dots and carbon nanotubes for signal amplification. Microchim. Acta 2019. [Google Scholar] [CrossRef]
- Soelberg, S.D.; Stevens, R.C.; Limaye, A.P.; Furlong, C.E. Surface Plasmon Resonance Detection Using Antibody-Linked Magnetic Nanoparticles for Analyte Capture, Purification, Concentration, and Signal Amplification. Anal. Chem. 2009, 81, 2357–2363. [Google Scholar] [CrossRef]
- Lara, S.; Perez-Potti, A. Applications of Nanomaterials for Immunosensing. Biosensors 2018, 8, 104. [Google Scholar] [CrossRef]
- Ozsoz, M.; Erdem, A.; Kerman, K.; Ozkan, D.; Tugrul, B.; Topcuoglu, N.; Ekren, H.; Taylan, M. Electrochemical Genosensor Based on Colloidal Gold Nanoparticles for the Detection of Factor V Leiden Mutation Using Disposable Pencil Graphite Electrodes. Anal. Chem. 2003, 75, 2181–2187. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, P.A.; Sandhyarani, N. Electrochemical DNA sensors based on the use of gold nanoparticles: A review on recent developments. Microchim. Acta 2017, 184, 981–1000. [Google Scholar] [CrossRef]
- Kokkinos, C.; Economou, A.; Prodromidis, M.I. Electrochemical immunosensors: Critical survey of different architectures and transduction strategies. TrAC Trends Anal. Chem. 2016, 79, 88–105. [Google Scholar] [CrossRef]
- Lim, S.A.; Ahmed, M.U. Electrochemical immunosensors and their recent nanomaterial-based signal amplification strategies: A review. RSC Adv. 2016, 6, 24995–25014. [Google Scholar] [CrossRef]
- Szymanski, M.; Porter, R.; Dep, G.V.; Wang, Y.; Haggett, B.G.D. Silver nanoparticles and magnetic beads with electrochemical measurement as a platform for immunosensing devices. Phys. Chem. Chem. Phys. 2011, 13, 5383–5387. [Google Scholar] [CrossRef]
- Cunningham, J.C.; Scida, K.; Kogan, M.R.; Wang, B.; Ellington, A.D.; Crooks, R.M. Paper diagnostic device for quantitative electrochemical detection of ricin at picomolar levels. Lab Chip 2015, 15, 3707–3715. [Google Scholar] [CrossRef]
- Ting, B.P.; Zhang, J.; Khan, M.; Yang, Y.Y.; Ying, J.Y. The solid-state Ag/AgCl process as a highly sensitive detection mechanism for an electrochemical immunosensor. Chem. Commun. 2009, 0, 6231–6233. [Google Scholar] [CrossRef]
- Iglesias-Mayor, A.; Amor-Gutiérrez, O.; Costa-García, A.; de la Escosura-Muñiz, A. Nanoparticles as emerging labels in electrochemical immunosensors. Sensors 2019, 19, 5137. [Google Scholar] [CrossRef]
- Ramanaviciene, A.; Voronovic, J.; Popov, A.; Drevinskas, R.; Kausaite-Minkstimiene, A.; Ramanavicius, A. Investigation of biocatalytic enlargement of gold nanoparticles using dynamic light scattering and atomic force microscopy. Colloids Surf. A Physicochem. Eng. Asp. 2016, 510, 183–189. [Google Scholar] [CrossRef]
- Jiang, P.; Wang, Y.; Zhao, L.; Ji, C.; Chen, D.; Nie, L. Applications of gold nanoparticles in non-optical biosensors. Nanomaterials 2018, 8, 977. [Google Scholar] [CrossRef]
- Prodromidis, M.I.; Economou, A. New Trends in Antibody-Based Electrochemical Biosensors. Compr. Anal. Chem. 2017, 77, 55–100. [Google Scholar] [CrossRef]
- Cadkova, M.; Kovarova, A.; Dvorakova, V.; Metelka, R.; Bilkova, Z.; Korecka, L. Electrochemical quantum dots-based magneto-immunoassay for detection of HE4 protein on metal film-modified screen-printed carbon electrodes. Talanta 2018, 182, 111–115. [Google Scholar] [CrossRef]
- Zhong, M.; Yang, L.; Yang, H.; Cheng, C.; Deng, W.; Tan, Y.; Xie, Q.; Yao, S. An electrochemical immunobiosensor for ultrasensitive detection of Escherichia coli O157:H7 using CdS quantum dots-encapsulated metal-organic frameworks as signal-amplifying tags. Biosens. Bioelectron. 2019, 126, 493–500. [Google Scholar] [CrossRef]
- Zupančič, U.; Rainbow, J.; Flynn, C.; Aidoo-Brown, J.; Estrela, P.; Moschou, D. Strategies for Multiplexed Electrochemical Sensor Development; Springer: Singapore, 2021; pp. 63–93. [Google Scholar]
- Tang, D.; Hou, L.; Niessner, R.; Xu, M.; Gao, Z.; Knopp, D. Multiplexed electrochemical immunoassay of biomarkers using metal sulfide quantum dot nanolabels and trifunctionalized magnetic beads. Biosens. Bioelectron. 2013, 46, 37–43. [Google Scholar] [CrossRef]
- Fan, G.C.; Zhu, H.; Du, D.; Zhang, J.R.; Zhu, J.J.; Lin, Y. Enhanced Photoelectrochemical Immunosensing Platform Based on CdSeTe@CdS:Mn Core-Shell Quantum Dots-Sensitized TiO2 Amplified by CuS Nanocrystals Conjugated Signal Antibodies. Anal. Chem. 2016, 88, 3392–3399. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.W.; Wang, J.; Xu, J.J.; Chen, H.Y. Energy transfer between CdS quantum dots and Au nanoparticles in photoelectrochemical detection. Chem. Commun. 2011, 47, 10990–10992. [Google Scholar] [CrossRef]
- Dong, Y.X.; Cao, J.T.; Liu, Y.M.; Ma, S.H. A novel immunosensing platform for highly sensitive prostate specific antigen detection based on dual-quenching of photocurrent from CdSe sensitized TiO2 electrode by gold nanoparticles decorated polydopamine nanospheres. Biosens. Bioelectron. 2017, 91, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.; Wu, D.; Ma, H.; Zhang, Y.; Li, H.; Du, B.; Wei, Q. An ultrasensitive enzyme-free electrochemical immunosensor for CA125 using Au@Pd core–shell nanoparticles as labels and platforms for signal amplification. J. Mater. Chem. B 2013, 1, 4052–4058. [Google Scholar] [CrossRef]
- Zhao, L.; Li, S.; He, J.; Tian, G.; Wei, Q.; Li, H. Enzyme-free electrochemical immunosensor configured with Au–Pd nanocrystals and N-doped graphene sheets for sensitive detection of AFP. Biosens. Bioelectron. 2013, 49, 222–225. [Google Scholar] [CrossRef]
- Dong, S.; Wang, S.; Gyimah, E.; Zhu, N.; Wang, K.; Wu, X.; Zhang, Z. A novel electrochemical immunosensor based on catalase functionalized AuNPs-loaded self-assembled polymer nanospheres for ultrasensitive detection of tetrabromobisphenol A bis(2-hydroxyethyl) ether. Anal. Chim. Acta 2019, 1048, 50–57. [Google Scholar] [CrossRef]
- Wu, L.; Chen, J.; Du, D.; Ju, H. Electrochemical immunoassay for CA125 based on cellulose acetate stabilized antigen/colloidal gold nanoparticles membrane. Electrochim. Acta 2006, 51, 1208–1214. [Google Scholar] [CrossRef]
- Wu, L.; Yan, F.; Ju, H. An amperometric immunosensor for separation-free immunoassay of CA125 based on its covalent immobilization coupled with thionine on carbon nanofiber. J. Immunol. Methods 2007, 322, 12–19. [Google Scholar] [CrossRef]
- Gu, L.; Luo, N.; Miley, G.H. Cathode electrocatalyst selection and deposition for a direct borohydride/hydrogen peroxide fuel cell. J. Power Sources 2007, 173, 77–85. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, S.; Xue, Y.; Liang, J.; Cui, L.; Li, Q.; Zhou, S.; Huang, Y.; Li, G.; Zhao, Y. A Fe3O4@Au-basedpseudo-homogeneous electrochemical immunosensor for AFP measurement using AFP antibody-GNPs-HRP as detection probe. Anal. Biochem. 2017, 534, 56–63. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, R.; Chai, Y.; Zhuo, Y.; Su, H.; Zhang, Y. Horseradish peroxidase-loaded nanospheres attached to hollow gold nanoparticles as signal enhancers in an ultrasensitive immunoassay for alpha-fetoprotein. Microchim. Acta 2014, 181, 679–685. [Google Scholar] [CrossRef]

| Size (nm) | Technique(s) | Biomarker | Linear Range (ng·mL−1) | LOD (pg·mL−1) | Real Sample | Reference | |
|---|---|---|---|---|---|---|---|
| Nanocarriers | |||||||
| AuNPs (MSNP-Thi-Au) | 80 (MSN) 5 (AuNPs) | DPV | PSA | 10−3–5 | 0.31 | Serum | [73] |
| Mesoporous Fe3O4 | 25 | CA | cTnI | 10−3–100 | 0.39 | Serum | [74] |
| Ni/C@SiO2 | 300 | DPV | CEA | 6 × 10−3–12 | 1.56 | Serum | [75] |
| AuNPs | 18 | DPV | CA-125 | 20–100 U | 3.4 U | Serum | [76] |
| AuNPs/Thi/MWCNT | 15 (AuNPs) | DPV | CYFRA21-1 | 0.1–150 | 43 | Serum | [56] |
| AuNPs | 30 | PT | PSA | 0.05–20 | 13.6 | Serum | [63] |
| NC-AuNPs | 30 (NC-AuNPs) 7.5 (AuNPs) | DPV | ALV-J | 120–104 TCID50 | 95 TCID50 | – | [77] |
| MSNP-Fe3O4 | 100 (MSN) 8 (Fe3O4) | CV | AFP | 0.01–25 | 4 | Serum | [78] |
| Fe3O4/AuNPs | 29 (Fe3O4/AuNPs) 12 (AuNPs) | DPV | CEA | 5 × 10−3–50 | 1 | Serum | [79] |
| Electroactive labels | |||||||
| AuNPs | 13 | DPV | Mtb | 5 × 103–5 × 105 | 330 | Urine | [80] |
| AuNPs | 20 | DPV | hMMP9 | 0.18–23 | 60 | Plasma | [81] |
| Cu@TiO2 | 250 | SWV CA | IgG | 10−4–100 10−5–100 | 0.052 4.3 × 10−3 | Serum | [82] |
| TiNPs-Zn TiNPs-Cd | 50 (TiNPs) | SWV | cTnI FABP | 5 × 10−5–50 | 10−3 3 × 10−3 | Serum | [83] |
| PbS Qdots | – | SP | HER2 | 1–100 | 280 | Serum | [84] |
| CdTe:Ni Qdots | – | DPV | PSA | 10−3–100 | 0.45 | Serum | [85] |
| CdS Qdots | – | DPV | anti-tTG IgA | 40–100 U | 2.2 U | Serum | [86] |
| CdS QDots | – | CA | AFP | 0.1–500 | 10 | Serum | [87] |
| CdSe | – | PT | Mouse IgG | 0.15–4.0 pM | 10 fM | - | [64] |
| CdS | 4 | PEC | S100ß | 0.25–10 | 0.15 | Serum | [70] |
| Au@Pt-MoSe2 | 45 × 16 (Au) 80 × 58 (Au@Pt) | CA | AFP | 10−5–200 | 3.3 × 10−3 | Serum | [88] |
| PS@PDA−AgNPs | 200 | LSV | IL-6 | 10−4–100 | 0.059 | Serum | [89] |
| Graphene/AgNPs | - | SW ASV | IgE | 10–1000 | 3.6 × 103 | – | [90] |
| Au@PAMAM-C60 | 100 | LSV | AFP | 10−4–10 | 0.03 | Serum | [91] |
| Ag@CeO2-Au | 50–100 (Ag@CeO2) | CV | CEA | 10−4–5 | 3.2 × 10−3 | Serum | [92] |
| AuNPs/MB/MSNP | 80 (MSN) | ASV | Gal-3 | 5 × 10−7–500 | 1.7 × 10−4 | Serum | [93] |
| AuNPs-PDC-GOx | – | ASV | PCT | 5 × 10−7–500 | 4 × 10−5 | Serum | [94] |
| AuNPs | 13 | ASV | IgG PSA | 4 × 10−7–400 1.8 × 10−7–450 | 3 × 10−4 10−4 | Serum | [95] |
| AuNPs-Fe3O4 | 30 (Fe3O4) 25 (AuNPs) | DPV | HER2 | 5 × 10−4–50 | 0.02 | Serum | [96] |
| AuNPs/Au/spiky Au/Ag | 250 | LSV | PSA | 1.9 × 10−3–0.125 0.125–10 | 1.2 | – | [97] |
| AuNPs | 13 | SWE | PDGF | 5 × 10−3–10 | 2 | Serum | [98] |
| Ag@Au | – | LSV | CEA | 0.1–120 | 55 | – | [99] |
| Catalytically active labels | |||||||
| CoSnSx–Pd | 200 - 600 | CA | NT-pro BNP | 10−4–50 | 0.0315 | Serum | [100] |
| Cu3(PO4)2 | 200 | SWV | CRP | 5 × 10−4–1 | 0.13 | Serum | [101] |
| PtPd-Fe3O4 | 10 | CA | CA72-4 | 10−3–10 U | 0.3 mU | Serum | [102] |
| Mesoporous Pt NPs | 30 | DPV | CEA CA-125 CA-153 | 0.05–20 U 8 × 10−3–24 U 0.02–20 | 2 mU 1 mU 7 | Serum | [103] |
| PdNi NPs/ graphene nanoribbon | 10 (PdNi NPs) | CA | AFP | 10−4–16 | 0.03 | Serum | [104] |
| Au@Pd NDs/NH2–MoO2 NSs | 20 (Au@Pd NDs) | CA | HBsAg | 10−5–100 | 3.3 × 10−3 | Serum | [105] |
| Zn2SiO4-PdNPs | 100–200 (Zn2SiO4) | SWV | Insulin | 10−4–50 | 2.5 × 10−4 | Serum | [106] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popov, A.; Brasiunas, B.; Kausaite-Minkstimiene, A.; Ramanaviciene, A. Metal Nanoparticle and Quantum Dot Tags for Signal Amplification in Electrochemical Immunosensors for Biomarker Detection. Chemosensors 2021, 9, 85. https://doi.org/10.3390/chemosensors9040085
Popov A, Brasiunas B, Kausaite-Minkstimiene A, Ramanaviciene A. Metal Nanoparticle and Quantum Dot Tags for Signal Amplification in Electrochemical Immunosensors for Biomarker Detection. Chemosensors. 2021; 9(4):85. https://doi.org/10.3390/chemosensors9040085
Chicago/Turabian StylePopov, Anton, Benediktas Brasiunas, Asta Kausaite-Minkstimiene, and Almira Ramanaviciene. 2021. "Metal Nanoparticle and Quantum Dot Tags for Signal Amplification in Electrochemical Immunosensors for Biomarker Detection" Chemosensors 9, no. 4: 85. https://doi.org/10.3390/chemosensors9040085
APA StylePopov, A., Brasiunas, B., Kausaite-Minkstimiene, A., & Ramanaviciene, A. (2021). Metal Nanoparticle and Quantum Dot Tags for Signal Amplification in Electrochemical Immunosensors for Biomarker Detection. Chemosensors, 9(4), 85. https://doi.org/10.3390/chemosensors9040085









