Sensitive Materials and Coating Technologies for Surface Acoustic Wave Sensors
Abstract
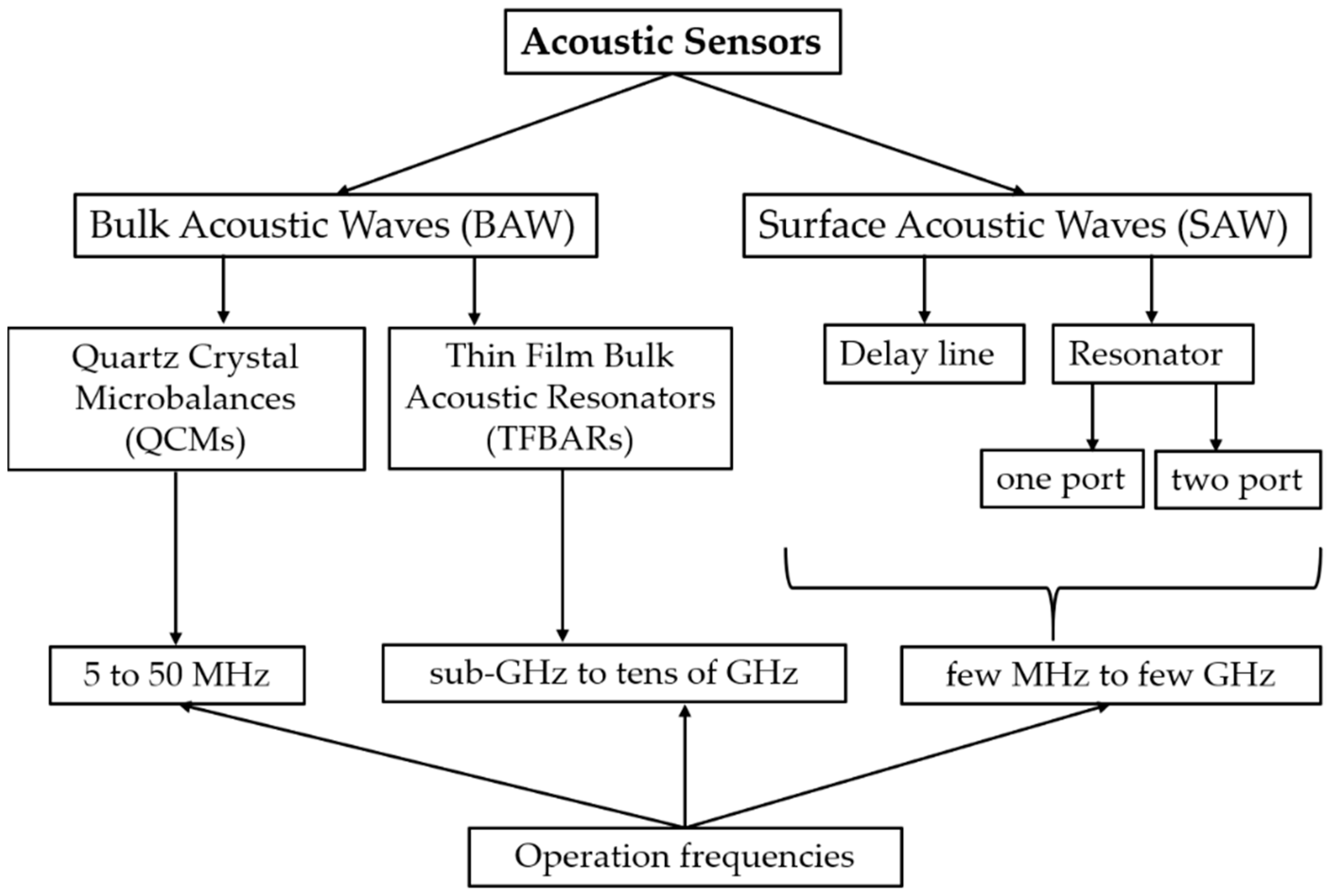
1. Introduction
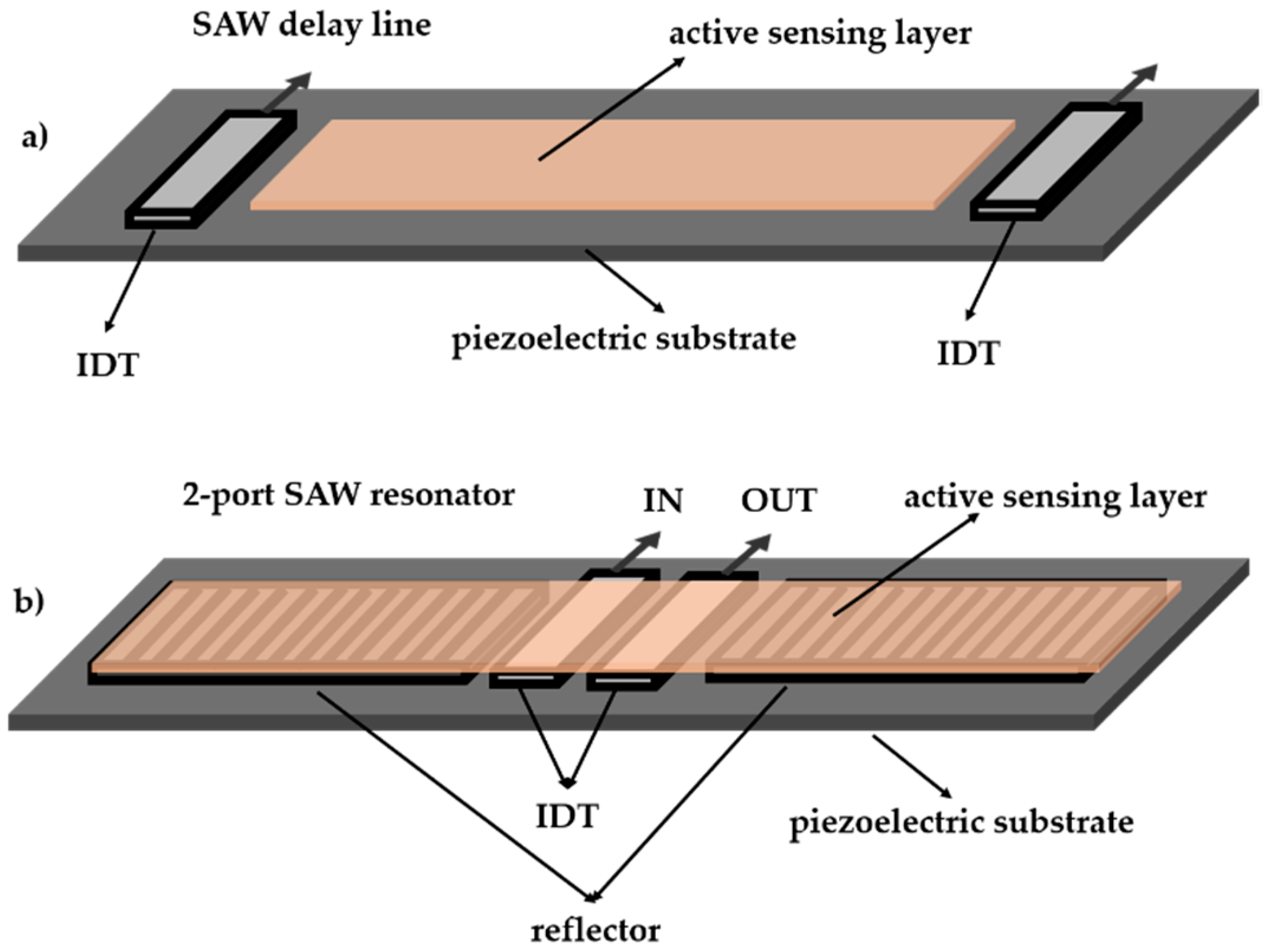
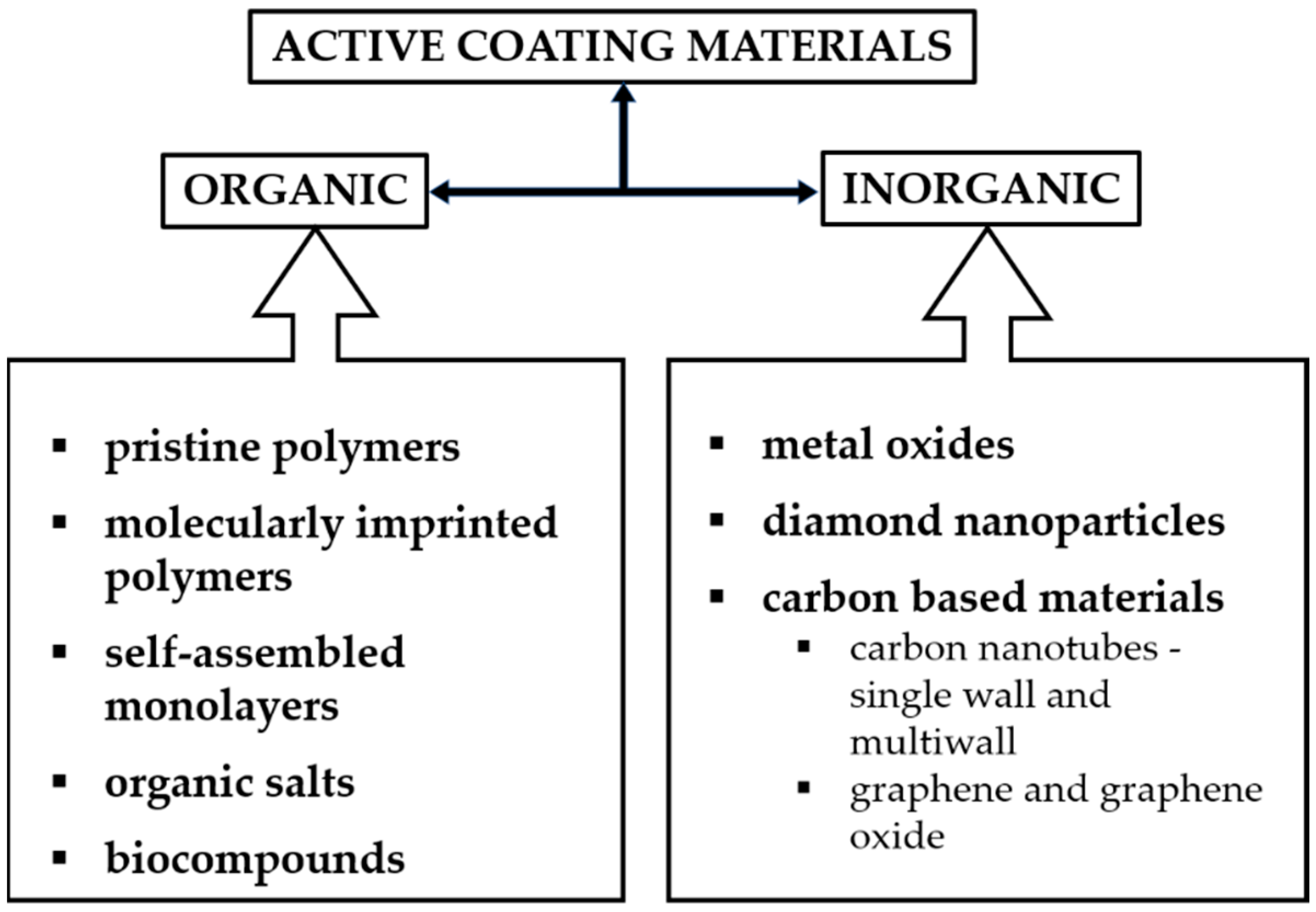
2. Active Materials in SAW Sensors
2.1. Pristine Polymer Sensing Layers in SAW Sensors
2.2. Carbonaceous Structures as Matrices for SAW Sensors
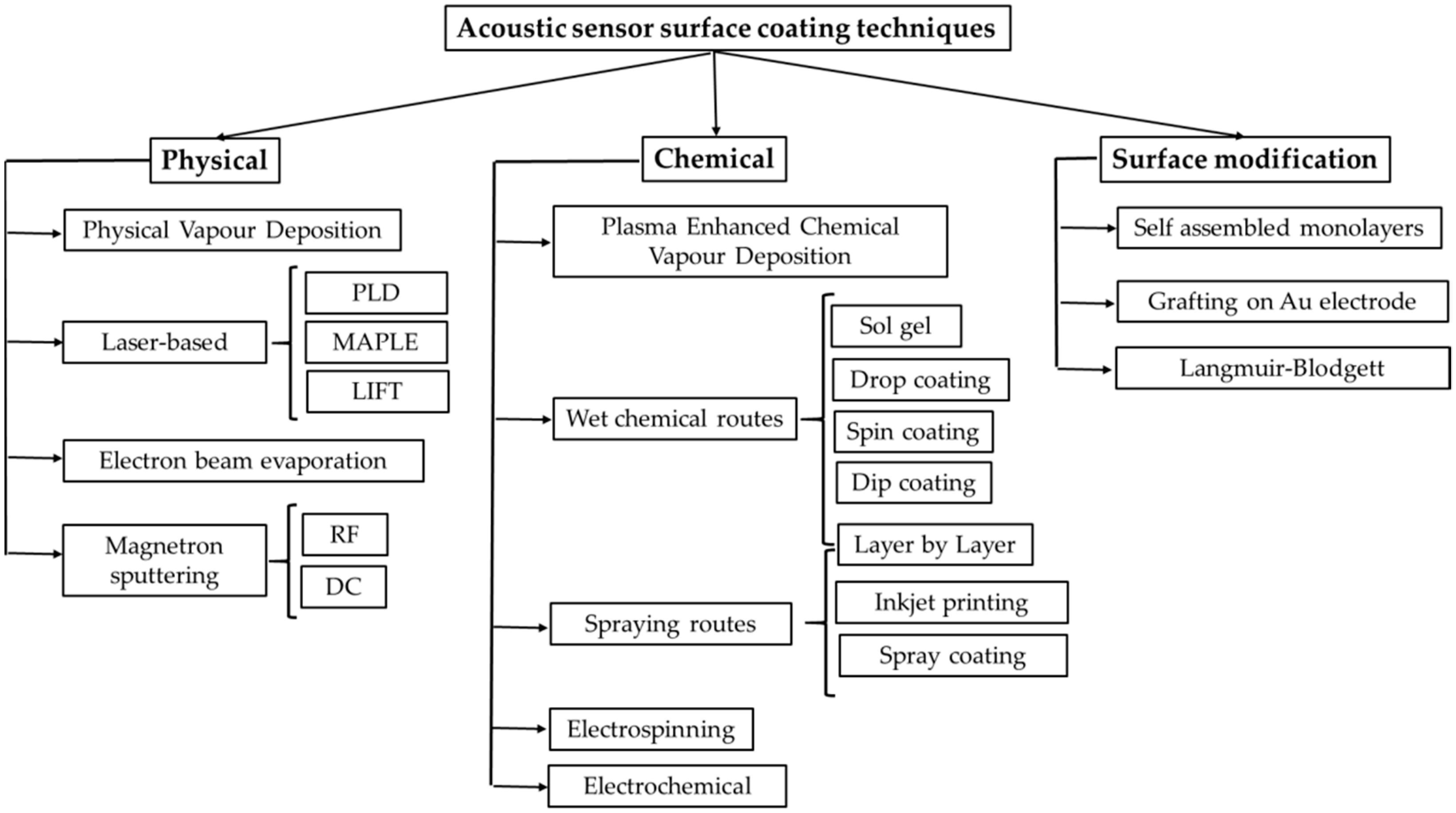
3. Coating Techniques of SAW Sensors
3.1. Spin Coating
3.2. Spray Coating
3.3. Inkjet Printing
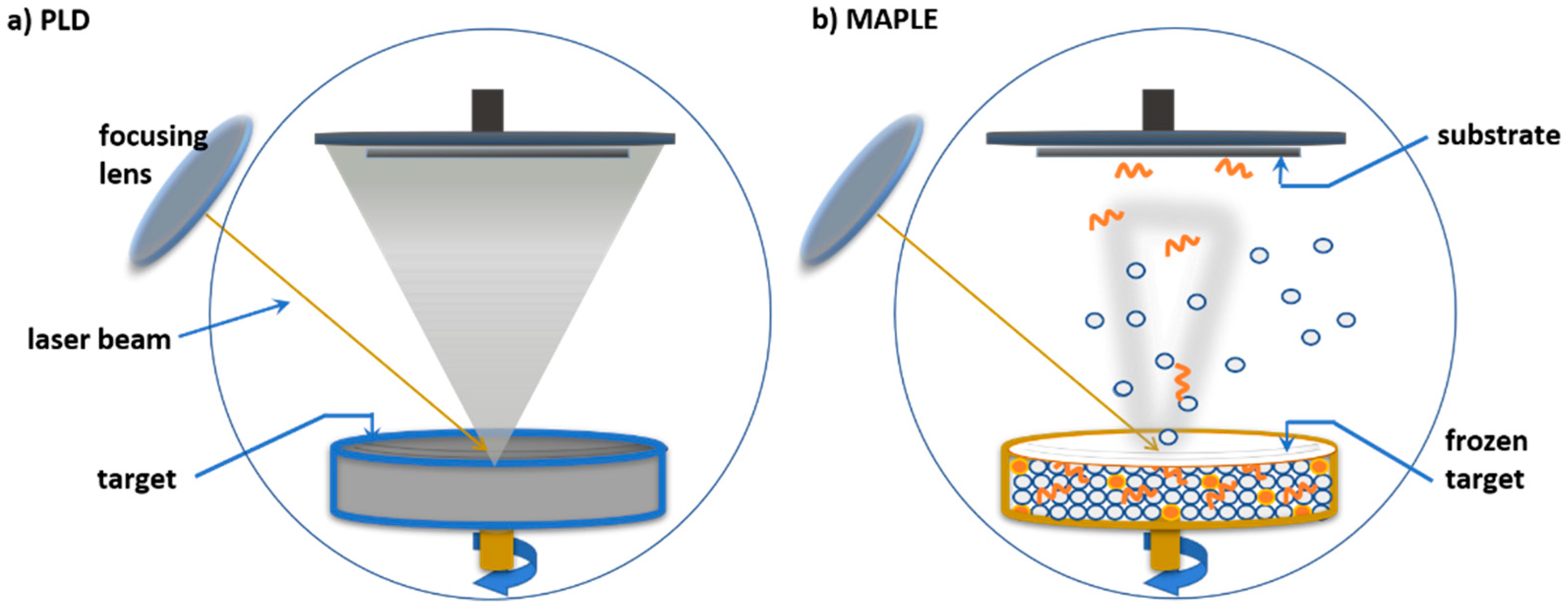
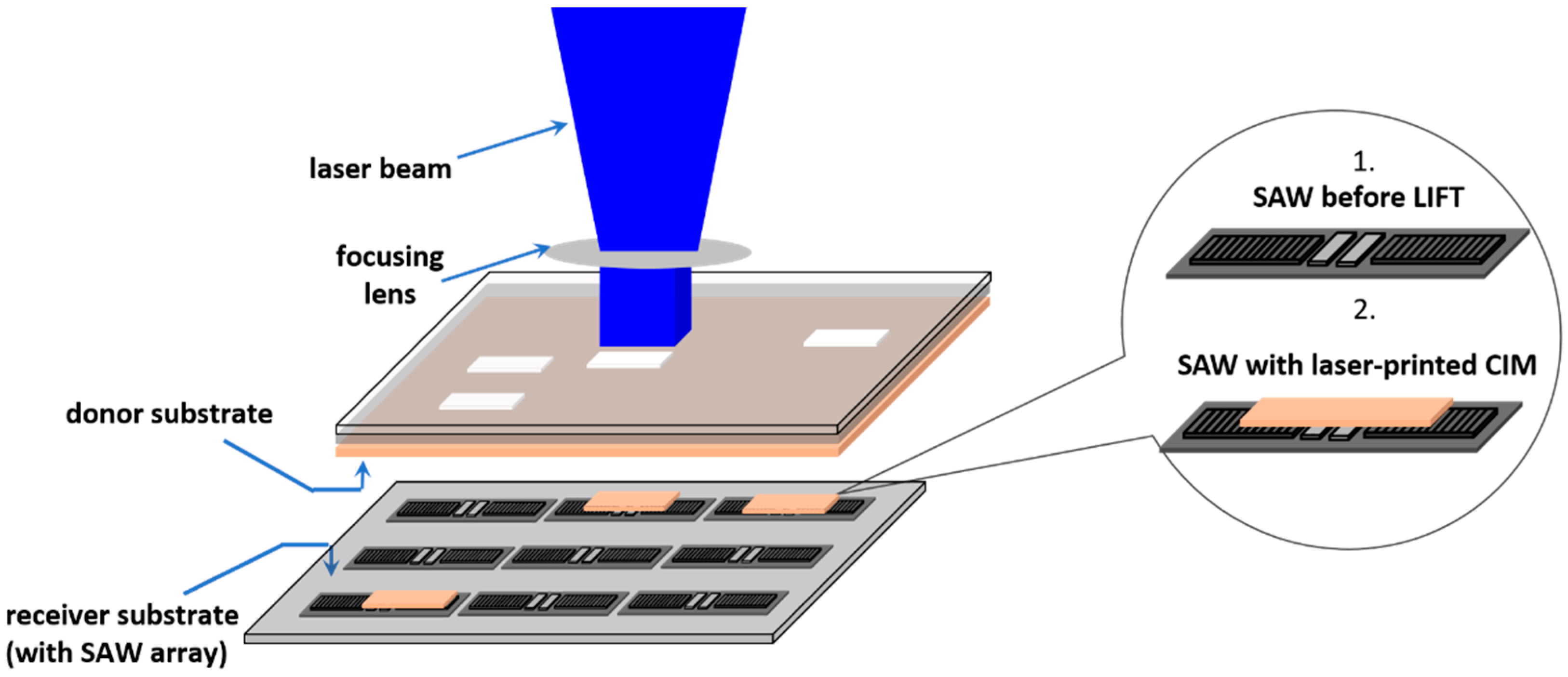
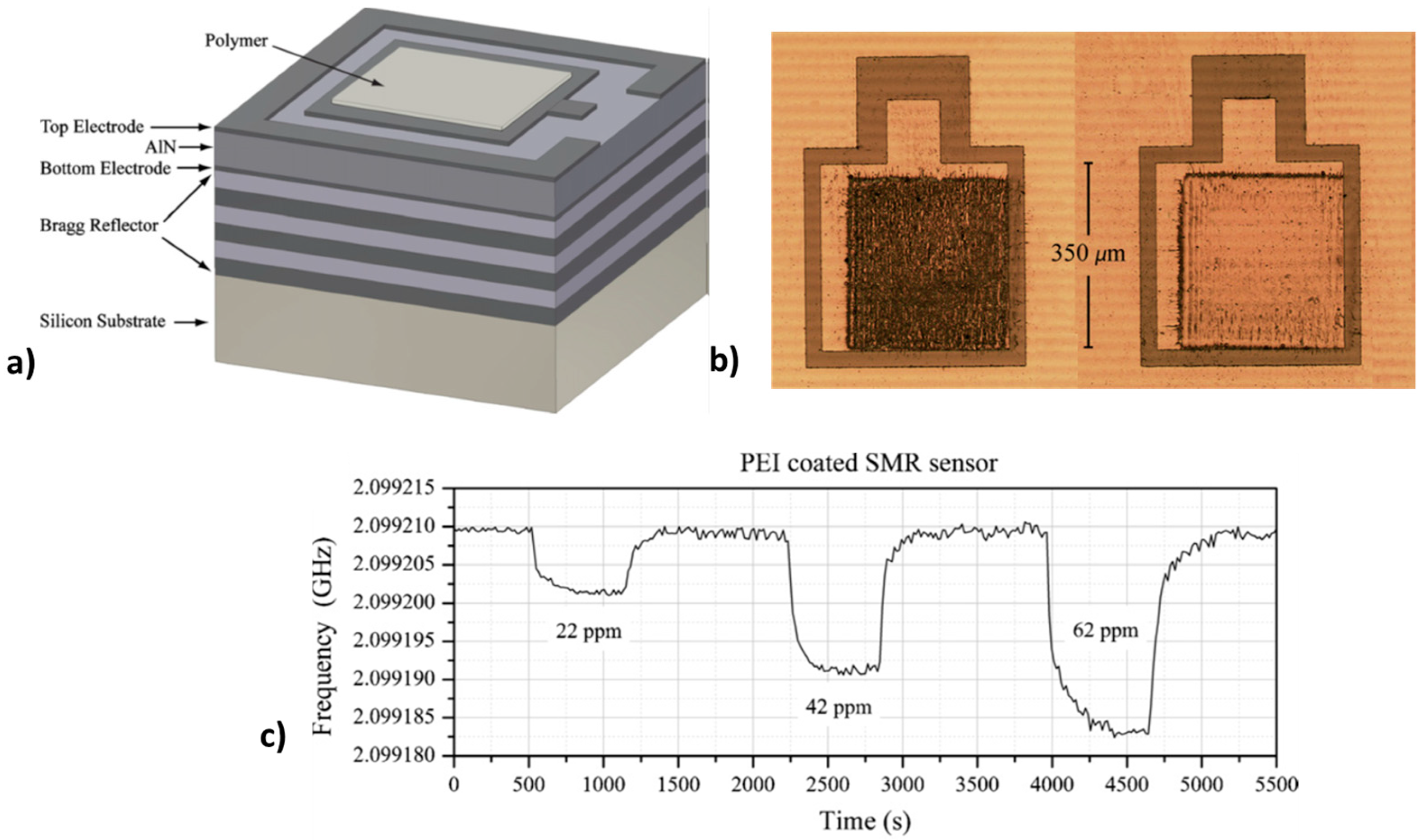
3.4. Laser-Based Methods
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X.; Zhang, J.; Wei, G. Recent advances in the design of colorimetric sensors for environmental monitoring. Environ. Sci. Nano 2020, 7, 2195–2213. [Google Scholar] [CrossRef]
- Ebralidze, I.I.; Laschuk, N.O.; Poisson, J.; Zenkina, O.V. Chapter 1—Colorimetric Sensors and Sensor Arrays. In Nanomaterials Design for Sensing Applications; in Micro and Nano Technologies; Zenkina, O.V., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–39. ISBN 978-0-12-814505-0. [Google Scholar]
- Sun, Y.; Ong, K.Y. Chapter 10: Colorimetric technology. In Detection Technologies for Chemical Warfare Agents and Toxic Vapors; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar] [CrossRef]
- Endo, T.; Yanagida, Y.; Hatsuzawa, T. Colorimetric detection of volatile organic compounds using a colloidal crystal-based chemical sensor for environmental applications. Sens Actuators B Chem. 2007, 125, 589–595. [Google Scholar] [CrossRef]
- Jung, C.; Chung, J.W.; Kim, U.O.; Kim, M.H.; Park, H.G. Real-time colorimetric detection of target DNA using isothermal target and signaling probe amplification and gold nanoparticle cross-linking assay. Biosens. Bioelectron. 2011, 26, 1953–1958. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, R.; Chen, T.-W.; Chen, S.-M.; Baskar, T.; Kannan, R.; Elumalai, P.; Raja, P.; Jeyapragasam, T.; Dinakaran, K.; Gnana kumar, G.P. A review of the advanced developments of electrochemical sensors for the detection of toxic and bioactive molecules. Inorg. Chem. Front. 2019, 6, 3418–3439. [Google Scholar] [CrossRef]
- Cumeras, R.; Figueras, E.; Davis, C.E.; Baumbach, J.I.; Gracia, I. Review on Ion Mobility Spectrometry. Part 1: Current instrumentation. Analyst 2015, 140, 1376–1390. [Google Scholar] [CrossRef]
- Fair, J.D.; Bailey, W.F.; Felty, R.A.; Gifford, A.E.; Shultes, B.; Volles, L.H. Method for rapid on-site identification of VOCs. J. Environ. Sci. 2009, 21, 1005–1008. [Google Scholar] [CrossRef]
- Kovács, T. Developed Physical Detection-Possibilities of Chemical Agents. Acta Polytech. Hung. 2006, 3, 133–141. [Google Scholar]
- Liu, G.; Lin, Y. Biosensor based on self-assembling acetylcholinesterase on carbon nanotubes for flow injection/amperometric detection of organophosphate pesticides and nerve agents. Anal. Chem. 2006, 78, 835–843. [Google Scholar] [CrossRef]
- Saito, M.; Uchida, N.; Furutani, S.; Murahashi, M.; Espulgar, W.; Nagatani, N.; Nagai, H.; Inoue, Y.; Ikeuchi, T.; Kondo, S.; et al. Field-deployable rapid multiple biosensing system for detection of chemical and biological warfare agents. Microsyst. Nanoeng. 2018, 4, 17083. [Google Scholar] [CrossRef]
- Mujahid, A.; Dickert, F.L. Surface Acoustic Wave (SAW) for Chemical Sensing Applications of Recognition Layers. Sensors 2017, 17, 2716. [Google Scholar] [CrossRef] [PubMed]
- Devkota, J.; Ohodnicki, P.R.; Grev, D.W. SAW Sensors for Chemical Vapors and Gases. Sensors 2017, 17, 801. [Google Scholar] [CrossRef] [PubMed]
- Länge, K. Bulk and Surface Acoustic Wave Sensor Arrays for Multi-Analyte Detection: A Review. Sensors 2019, 19, 5382. [Google Scholar] [CrossRef]
- Oprea, A.; Weimar, U. Gas sensors based on mass-sensitive transducers part 1: Transducers and receptors—Basic understanding. Anal. Bioanal. Chem. 2019, 411, 1761–1787. [Google Scholar] [CrossRef]
- King, W.H., Jr. Piezoelectric sorption detector. Anal. Chem. 1964, 36, 1735–1739. [Google Scholar] [CrossRef]
- White, R.M.; Voltmer, F.W. Direct piezoelectric coupling to surface elastic waves. Appl. Phys. Lett. 1965, 12, 314. [Google Scholar] [CrossRef]
- Ippolito, S.J.; Trinchi, A.; Powell, D.A.; Wlodarski, W. Acoustic Wave Gas and Vapor Sensors. In Solid State Gas Sensing; Comini, E., Faglia, G., Sberveglieri, G., Eds.; Springer Science Business Media: Springer, Boston, MA, USA, 2009. [Google Scholar] [CrossRef]
- Wohltjen, H.; Dessy, R. Surface acoustic wave probe for chemical analysis. I. Introduction and instrument description. Anal. Chem. 1979, 51, 1458–1464. [Google Scholar] [CrossRef]
- Wohltjen, H.; Dessy, R. Surface acoustic wave probes for chemical analysis. II. Gas chromatography detector. Anal. Chem. 1979, 51, 1465–1470. [Google Scholar] [CrossRef]
- Wohltjen, H.; Dessy, R. Surface acoustic wave probes for chemical analysis. III. Thermomechanical polymer analyzer. Anal. Chem. 1979, 51, 1470–1475. [Google Scholar] [CrossRef]
- Länge, K.; Rapp, B.E.; Rapp, M. Surface acoustic wave biosensors: A review. Anal. Bioanal. Chem. 2008, 391, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Caliendo, C.; Verardi, P.; Verona, E.; D’Amico, A.; Di Natale, C.; Saggio, G.; Serafini, M.; Paolesse, R.; Huq, S.E. Advances in SAW-based gas sensors. Smart Mater. Struct. 1997, 6, 689–699. [Google Scholar] [CrossRef]
- Grate, J.W.; Abraham, M.H. Solubility interactions and the design of chemically selective sorbent coatings for chemical sensors and arrays. Sens Actuators B Chem. 1991, 3, 85. [Google Scholar] [CrossRef]
- Grate, J.W.; McGill, R.A. Dewetting Effects on Polymer-Coated Surface Acoustic Wave Vapor Sensors. Anal. Chem. 1995, 67, 4015–4019. [Google Scholar] [CrossRef]
- Grate, J.W.; Kaganove, S.N.; Nelson, D.A. Carbosiloxane polymers for sensors. Chem. Innov. 2000, 30, 29–37. [Google Scholar]
- Ballantine, D.S.; Rose, S.L.; Grate, J.W.; Wohltjen, H. Correlation of surface acoustic wave device coating responses with solubility properties and chemical structure using pattern recognition. Anal. Chem. 1986, 58, 3058–3066. [Google Scholar] [CrossRef]
- Haupt, K. Molecularly Imprinted Polymers as Recognition Elements in Sensors. In Ultrathin Electrochemical Chemo- and Biosensors; Springer Series on Chemical Sensors and Biosensors (Methods and Applications); Mirsky, V.M., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; Volume 2. [Google Scholar]
- Fresco-Cala, B.; Batista, A.D.; Cárdenas, S. Molecularly Imprinted Polymer Micro- and Nano-Particles: A Review. Molecules 2020, 25, 4740. [Google Scholar] [CrossRef]
- Tortora, L.; Pomarico, G.; Nardis, S.; Martinelli, E.; Catini, A.; D’Amico, A.; Natale, C.; Paolesse, R. Supramolecular sensing mechanism of corrole thin films. Sens. Actuators B Chem. 2013, 187, 72–77. [Google Scholar] [CrossRef]
- Singh, M.; Kaur, N.; Comini, E. The role of self-assembled monolayers in electronic devices. J. Mater. Chem. C 2020, 8, 3938–3955. [Google Scholar] [CrossRef]
- Chang, Y.; Tang, N.; Qu, H.; Liu, J.; Zhang, D.; Zhang, H.; Pang, W.; Duan, X. Detection of Volatile Organic Compounds by Self-assembled Monolayer Coated Sensor Array with Concentration-independent Fingerprints. Sci. Rep. 2016, 6, 23970. [Google Scholar] [CrossRef]
- Constantinoiu, I.; Viespe, C. ZnO Metal Oxide Semiconductor in Surface Acoustic Wave Sensors: A Review. Sensors 2020, 20, 5118. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, A.; Kim, H.W.; Kim, S.S.; Neri, G. Nanostructured Semiconducting Metal Oxide Gas Sensors for Acetaldehyde Detection. Chemosensors 2019, 7, 56. [Google Scholar] [CrossRef]
- Miu, D.; Birjega, R.; Viespe, C. Surface Acoustic Wave Hydrogen Sensors Based on Nanostructured Pd/WO3 Bilayers. Sensors 2018, 18, 3636. [Google Scholar] [CrossRef]
- Penza, M.; Antolini, F.; Antisari, V.M. Carbon nanotubes as SAW chemical sensors materials. Sens. Actuators B Chem. 2004, 100, 47–59. [Google Scholar] [CrossRef]
- Penza, M.; Rossi, R.; Alvisi, M.; Aversa, P.; Cassano, G.; Suriano, D.; Benneti, M.; Cannata, D.; Di Pietrantonio, F.; Verona, E. SAW Gas. Sensors with Carbon Nanotubes Films; IEEE Ultrasonics Symposium: Beijing, China, 2008; pp. 1850–1853. [Google Scholar]
- Afzal, A.; Iqbal, N.; Mujahida, A.; Schirhagl, R. Advanced vapor recognition materials for selective and fast responsive surface acoustic wave sensors: A review. Anal. Chim. Acta 2013, 787, 36–49. [Google Scholar] [CrossRef]
- Peer, H.K. Reviewed: Molecularly imprinted polymers: The next generation. Anal. Chem. 2003, 75, 376. [Google Scholar]
- Yan, H.; Row, K. Characteristic and synthetic approach of molecularly imprinted polymer. Int. J. Mol. Sci. 2006, 7, 155–178. [Google Scholar] [CrossRef]
- Whitcombe, M.J.; Kirsch, N.; Nicholls, I.A. Molecular imprinting science and technology: A survey of the literature for the years 2004–2011. J. Mol. Recognit. 2014, 27, 297–401. [Google Scholar]
- Oprea, A.; Weimar, U. Gas sensors based on mass-sensitive transducers. Part 2: Improving the sensors towards practical application. Anal. Bioanal. Chem. 2010, 412, 6707–6776. [Google Scholar] [CrossRef]
- Paolesse, R.; Nardis, S.; Monti, D.; Stefanelli, M.; Di Natale, C. Porphyrinoids for chemical sensor applications. Chem. Rev. 2017, 117, 2517–2583. [Google Scholar] [CrossRef] [PubMed]
- Bohrer, F.I.; Colesniuc, C.N.; Park, J.; Ruidiaz, M.E.; Schuller, I.K.; Kummel, A.C.; Trogler, W.C. Comparative gas sensing in cobalt, nickel, copper, zinc, and metal-free phthalocyanine chemiresistors. J. Am. Chem. Soc. 2009, 131, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-F.; Han, Y. Triptycene-derived macrocyclic arenes: From calixarenes to helicarenes. Acc. Chem. Res. 2018, 51, 2093–2106. [Google Scholar] [CrossRef] [PubMed]
- Regmi, B.P.; Galpothdeniya, W.I.S.; Siraj, N.; Webb, M.H.; Speller, N.C.; Warner, I.M. Phthalocyanine- and porphyrin-based GUMBOS for rapid and sensitive detection of organic vapors. Sens. Actuators B Chem. 2014, 209, 172–179. [Google Scholar] [CrossRef]
- Hur, Y.; Han, J.; Seon, J.; Pak, Y.E.; Roh, Y. Development of an SH-SAW sensor for the detection of DNA hybridization. Sens. Actuators A 2005, 120, 462–467. [Google Scholar] [CrossRef]
- Sakong, J.; Roh, H.; Roh, Y. Surface acoustic wave DNA sensor with micro-fluidic channels. Jpn. J. Appl. Phys. 2007, 46, 4729–4733. [Google Scholar] [CrossRef]
- Gronewold, T.M.A.; Baumgartner, A.; Quandt, E.; Famulok, M. Discrimination of Single Mutations in Cancer-Related Gene Fragments with a Surface Acoustic Wave Sensor. Anal. Chem. 2006, 78, 4865–4871. [Google Scholar] [CrossRef] [PubMed]
- Porter, T.L.; Eastman, M.P.; Pace, D.L.; Bradley, M. Sensor based on piezoresistive microcantilever technology. Sens Actuators A Phys. 2001, 88, 45–51. [Google Scholar] [CrossRef]
- Shaw, G. Quenching by oxygen diffusion of phosphorescence emission of aromatic molecules in polymethyl methacrylate. Trans. Faraday Soc. 1967, 63, 2181–2189. [Google Scholar] [CrossRef]
- Bergman, I. Rapid-response Atmospheric Oxygen Monitor based on Fluorescence Quenching. Nature 1968, 218, 396. [Google Scholar] [CrossRef]
- Hormats, E.I.; Unterleitner, F.C. Rates of decay phosphorescence from triphenylene in acrylic polymers. J. Phys. Chem. 1965, 69, 3677–3681. [Google Scholar] [CrossRef]
- Potyrailo, R.A. Polymeric Sensor materials: Toward an alliance of combinatorial and rational design tools. Angew. Chem. Int. Ed. 2006, 45, 702–723. [Google Scholar] [CrossRef]
- Zellers, E.T.; Han, M. Effects of Temperature and Humidity on the Performance of Polymer-Coated Surface Acoustic Wave Vapor Sensor Arrays. Anal. Chem. 1996, 68, 2409–2418. [Google Scholar] [CrossRef] [PubMed]
- Grate, J.W.; Snow, A.; Ballantine, D.S.; Wohltjen, H.; Abraham, M.H.; McGill, R.A.; Sasson, P. Determination of partition coefficients from surface acoustic wave vapor sensor responses and correlation with gas-liquid chromatographic partition coefficients. Anal. Chem. 1988, 60, 869–875. [Google Scholar] [CrossRef]
- Grate, J.W. Hydrogen-Bond Acidic Polymers for Chemical Vapor Sensing. Chem. Rev. 2008, 108, 726–745. [Google Scholar] [CrossRef]
- Zellers, E.T.; Batterman, S.A.; Han, M.; Patrash, S.J. Optimal Coating Selection for the Analysis of Organic Vapor Mixtures with Polymer-Coated Surface Acoustic Wave Sensor Arrays. Anal. Chem. 1995, 67, 1092–1106. [Google Scholar] [CrossRef]
- Park, J.; Groves, W.A.; Zeller, E.T. Vapor Recognition with Small Arrays of Polymer-Coated Microsensors. A Comprehensive Analysis. Anal. Chem. 1999, 71, 3877–3886. [Google Scholar] [CrossRef] [PubMed]
- Patrash, S.J.; Zellers, E.T. Characterization of polymeric surface acoustic wave sensor coatings and semiempirical models of sensor responses to organic vapors. Anal. Chem. 1993, 65, 2055–2066. [Google Scholar] [CrossRef]
- Wang, W.; Xie, X.; He, S. Optimal Design of a Polyaniline-Coated Surface Acoustic Wave Based Humidity Sensor. Sensors 2013, 13, 16816–16828. [Google Scholar] [CrossRef]
- Ansari, R. Polypyrrole Conducting Electroactive Polymers: Synthesis and Stability Studies. E-J. Chem. 2006, 3, 186–201. [Google Scholar] [CrossRef]
- Penza, M.; Milella, E.; Anisimkin, V.I. Monitoring of NH3 gas by LB polypyrrole-based SAW sensor. Sens Actuators B Chem. 1998, 47, 218–224. [Google Scholar] [CrossRef]
- Mazouz, Z.; Rahali, S.; Fourat, N.; Zerrouki, C.; Aloui, N.; Seydou, M.; Yaakoubi, N.; Chehimi, M.M.; Othmane, A.; Kalfat, R. Highly Selective Polypyrrole MIP-Based Gravimetric and Electrochemical Sensors for Picomolar Detection of Glyphosate. Sensors 2017, 17, 2586. [Google Scholar] [CrossRef] [PubMed]
- Eslami, M.R.; Alizadeh, N. Ultrasensitive and selective QCM sensor for detection of trace amounts of nitroexplosive vapors in ambient air based on polypyrrole—Bromophenol blue nanostructure. Sens. Actuators B Chem. 2019, 278, 55–63. [Google Scholar] [CrossRef]
- Toal, S.J.; Trogler, W.C. Polymer sensors for nitroaromatic explosives detection. J. Mater. Chem. 2006, 16, 2871–2883. [Google Scholar] [CrossRef]
- McGill, R.A.; Mlsna, T.E.; Chung, R.; Nguyen, V.K.; Stepnowski, J. The design of functionalized silicone polymers for chemical sensor detection of nitroaromatic compounds. Sens. Actuators B 2000, 65, 5–9. [Google Scholar] [CrossRef]
- Bekkar, F.; Belbachir, M. Chemical modification of poly(epichlorohydrin) using montmorillonite clay. Chin. J. Chem. 2009, 27, 1174–1178. [Google Scholar] [CrossRef]
- Joo, B.S.; Huh, J.S.; Lee, D.D. Fabrication of polymer SAW sensor array to classify chemical warfare agents. Sens Actuators B Chem. 2007, 121, 47–53. [Google Scholar] [CrossRef]
- Beck, K.; Kunzelmann, T.; Schickfus, M.; Hunklinger, S. Contactless surface acoustic wave gas sensor. Sens. Actuators 1999, 76, 103–106. [Google Scholar] [CrossRef]
- Alizadeh, T.; Zeynali, S. Electronic nose based on the polymer coated SAW sensors array for the warfare agent simulants classification. Sens. Actuators B Chem. 2008, 129, 412–423. [Google Scholar] [CrossRef]
- Di Pietrantonio, F.; Benetti, M.; Cannatà, C.; Verona, E.; Palla-Papavlu, A.; Dinca, V.; Dinescu, M.; Mattle, T.; Lippert, T. Volatile toxic compound detection by surface acoustic wave sensor array coated with chemoselective polymers deposited by laser induced forward transfer: Application to sarin. Sens. Actuators B 2012, 174, 158–167. [Google Scholar] [CrossRef]
- Benetti, M.; Cannatà, C.; Verona, E.; Palla Papavlu, A.; Dinca, V.C.; Lippert, T.; Dinescu, M.; Di Pietrantonio, F. Highly selective surface acoustic wave e-nose implemented by laser direct writing. Sens. Actuators B. Chem. 2019, 283, 154–162. [Google Scholar] [CrossRef]
- Dinca, V.; Fardel, R.; Di Pietrantonio, F.; Cannatà, D.; Benetti, M.; Verona, E.; Palla-Papavlu, A.; Dinescu, M.; Lippert, T. Laser induced forward transfer: An approach to single-step polymer microsensor fabrication. Sens. Lett. 2010, 8, 436–440. [Google Scholar] [CrossRef]
- Louis, M.H.; Dutoit, S.; Denoux, Y.; Erbacher, P.; Deslandes, E.; Behr, J.P.; Gauduchon, P.; Poulain, L. Intraperitoneal linear polyethylenimine (L-PEI)-mediated gene delivery to ovarian carcinoma nodes in miceIntraperitoneal PEI-mediated gene delivery. Cancer Gene Ther. 2006, 13, 367–374. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dinca, V.; Palla-Papavlu, A.; Matei, A.; Luculescu, C.; Dinescu, M.; Lippert, T.; Di Pietrantonio, F.; Cannata, D.; Benetti, M.; Verona, E. A comparative study of DRL-lift and lift on integrated polyisobutylene polymer matrices. Appl. Phys. A 2010, 101, 429–434. [Google Scholar] [CrossRef]
- Matatagui, D.; Martí, J.; Fernández, M.J.; Fontecha, J.L.; Gutiérrez, J.; Gràcia, I.; Cane, C.; Horrillo, M.C. Chemical warfare agents simulants detection with an optimized SAW sensor array. Sens Actuators B Chem. 2011, 154, 199–205. [Google Scholar] [CrossRef]
- Palla-Papavlu, A.; Dinca, V.; Dinescu, M.; Di Pietrantonio, F.; Cannata, D.; Benetti, M.; Verona, E. Matrix-assisted pulsed laser evaporation of chemoselective polymers. Appl. Phys. A 2011, 105, 651–659. [Google Scholar] [CrossRef]
- McGill, R.A.; Nguyen, V.K.; Chung, R.; Shaffer, R.E.; DiLella, D.; Stepnowski, J.L.; Mlsna, T.E.; Venezky, D.L.; Dominguez, D. The “NRL-SAWRHINO”: A nose for toxic gases. Sens. Actuators B Chem. 2000, 65, 10–13. [Google Scholar] [CrossRef]
- Khot, L.R.; Panigrahi, S.; Lin, D. Development and evaluation of piezoelectric-polymer thin film sensors for low concentration detection of volatile organic compounds related to food safety applications. Sens. Actuators B Chem. 2011, 153, 1. [Google Scholar] [CrossRef]
- Stahl, U.; Voigt, A.; Dirschka, M.; Barié, N.; Richter, C.; Waldbaur, A.; Gruhl, F.J.; Rapp, B.E.; Rapp, M.; Länge, K. Long-term capability of polymer-coated surface transverse wave sensors for distinguishing vapors of similar hydrocarbons. Sens. Actuators B Chem. 2018, 274, 560–564. [Google Scholar] [CrossRef]
- Oprea, M.; Voicu, S.I. Recent advances in composites based on cellulose derivatives for biomedical applications. Carbohydr. Polym. 2020, 247, 116683. [Google Scholar] [CrossRef]
- Oprea, M.; Voicu, S.I. Recent advances in applications of cellulose derivatives-based composite membranes with hydroxyapatite. Materials 2020, 13, 2481. [Google Scholar] [CrossRef]
- Pandele, A.M.; Serbanescu, O.S.; Voicu, S.I. Polysulfone Composite Membranes with Carbonaceous Structure. Synthesis and Applications. Coatings 2020, 10, 609. [Google Scholar] [CrossRef]
- Raicopol, M.D.; Andronescu, C.; Voicu, S.I.; Vasile, E.; Pandele, A.M. Cellulose acetate/layered double hydroxide adsorptive membranes for efficient removal of pharmaceutical environmental contaminants. Carbohydr. Polym. 2019, 214, 204–212. [Google Scholar] [CrossRef]
- Pandele, A.M.; Constantinescu, A.; Radu, I.C.; Miculescu, F.; Voicu, S.I.; Ciocan, L.T. Synthesis and characterization of PLA-microstructured hydroxyapatite composite films. Materials 2020, 13, 274. [Google Scholar] [CrossRef]
- Snow, E.S.; Perkins, F.K.; Robinson, J.A. Chemical vapor detection using single walled carbon nanotubes. Chem. Soc. Rev. 2006, 35, 790–798. [Google Scholar] [CrossRef]
- Tabtimsai, C.; Keawwangchai, S.; Wanno, B.; Ruangpornvisuti, V. Gas adsorption on the Zn-, Pd- and Os-doped armchair (5,5) single-walled carbon nanotubes. J. Mol. Model. 2012, 18, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Cho, K. Ab initio study of doped carbon nanotube sensors. Nano Lett. 2003, 3, 513–517. [Google Scholar] [CrossRef]
- Yoosefian, M.; Barzgari, Z.; Yoosefian, J. Ab initio study of Pd-decorated single-walled carbon nanotube with C-vacancy as CO sensor. Struct. Chem. 2014, 25, 9–19. [Google Scholar] [CrossRef]
- Zhou, X.; Tian, W.Q.; Wang, X.L. Adsorption sensitivity of Pd-doped SWCNTs to small gas molecules. Sens. Actuators B 2010, 151, 56–64. [Google Scholar] [CrossRef]
- Yoosefian, M.; Raissi, H.; Mola, A. The hybrid of Pd and SWCNT (Pd loaded on SWCNT) as an efficient sensor for the formaldehyde molecule detection: A DFT study. Sens. Actuators B 2015, 212, 55–62. [Google Scholar] [CrossRef]
- Yoosefian, M. Powerful greenhouse gas nitrous oxide adsorption onto intrinsic and Pd doped Single walled carbon nanotube. Appl. Surf. Sci. 2017, 392, 225–230. [Google Scholar] [CrossRef]
- Buasaeng, P.; Rakrai, W.; Wanno, B.; Tabtimsai, C. DFT investigation of NH3, PH3, and AsH3 adsorptions on Sc-, Ti-, V-, and Cr-doped single-walled carbon nanotubes. Appl. Surf. Sci. 2007, 400, 506–514. [Google Scholar] [CrossRef]
- Li, D.; Luo, H.; Cai, J.; Cheng, Y.; Shao, X.; Dong, C. First-principles study of H, O, and N adsorption on metal embedded carbon nanotubes. Appl. Surf. Sci. 2017, 403, 645–651. [Google Scholar] [CrossRef]
- Penza, M.; Antolini, F.; Vittori-Antisari, M. Carbon nanotubes-based surface acoustic waves oscillating sensor for vapour detection. Thin Solid Film. 2005, 472, 246–252. [Google Scholar] [CrossRef]
- Serban, B.C.; Voicu, S.I.; Costea, S.D.; Cobianu, C. Matrix Nanocomposite Sensing Film for SAW/BAW Based Hydrogen Sulphide Sensor and Method for Making Same. U.S. Patent 7,695,993, 13 April 2010. [Google Scholar]
- Serban, B.C.; Dumitru, V.G.; Cobianu, C.; Costea, S.D.; Varachiu, N.; Voicu, S.I. Methods for Use of a Sensitive Layer for Hydrogen Sulphide Detection with SAW/BAW Devices. U.S. Patent 7,867,552, 11 January 2011. [Google Scholar]
- Serban, B.C.; Cobianu, C.; Bercu, M.; Varachiu, N.; Mihaila, M.; Bostan, C.; Voicu, S.I. Matrix Nanocomposite Containing Aminocarbon Nanotubes for Carbon Dioxide Sensor Detection. U.S. Patent 7,913,541, 29 March 2011. [Google Scholar]
- Arsat, R.; He, X.; Spizzirri, P.; Shafiei, M.; Arsat, M.; Wlodarski, W. Hydrogen Gas Sensor Based on Highly Ordered Polyaniline/Multiwall Carbon Nanotubes Composite. Sens. Lett. 2011, 9, 940–943. [Google Scholar] [CrossRef]
- Wang, Y.; Chyu, M.K.; Wang, Q.-M. Passive wireless surface acoustic wave CO2 sensor with carbon nanotube nanocomposite as an interface layer. Sens. Actuators A 2014, 220, 34–44. [Google Scholar] [CrossRef]
- Wang, Y.; Chyu, M.K.; Wang, Q.-M. Passive Wireless Surface Acoustic Wave CO2 Sensor for Geological Sequestration Sites Monitoring. In Proceedings of the 2013 Joint European Frequency and Time Forum & International Frequency Control Symposium (EFTF/IFC), Prague, Czech Republic, 21–25 July 2013; pp. 470–473. [Google Scholar]
- Sivaramakrishnan, S.; Rajamani, R.; Smith, C.S.; McGee, K.A.; Mann, K.R.; Yamashita, N. Carbon nanotube-coated surface acoustic wave sensor for carbon dioxide sensing. Sens. Actuators B 2008, 132, 296–304. [Google Scholar] [CrossRef]
- Vevericik, M.; Bury, P.; Kopcansky, P.; Timko, M.; Mitroova, Z. Effect of carbon nanotubes on liquid crystal behavior in electric and magnetic fields studied by SAW. Procedia Eng. 2017, 192, 935–940. [Google Scholar] [CrossRef]
- Sayago, I.; Fernández, M.J.; Fontecha, J.L.; Horrillo, M.C.; Vera, C.; Obieta, I.; Bustero, I. New sensitive layers for surface acoustic wave gas sensors based on polymer and carbon nanotube composites. Sens. Actuators B 2012, 175, 67–72. [Google Scholar] [CrossRef]
- Sayago, I.; Fernández, M.J.; Fontecha, J.L.; Horrillo, M.C.; Vera, C.; Obieta, I.; Bustero, I. Surface acoustic wave gas sensors based on polyisobutylene and carbon nanotube composites. Sens. Actuators B 2011, 156, 1–5. [Google Scholar] [CrossRef]
- Penza, M.; Aversa, P.; Cassano, G.; Wlodarski, W.; Kalantar-Zadeh, K. Layered SAW gas sensor with single-walled carbon nanotube-based nanocomposite coating. Sens. Actuators B 2007, 127, 168–178. [Google Scholar] [CrossRef]
- Muhulet, A.; Tuncel, C.; Miculescu, F.; Pandele, A.M.; Bobirica, C.; Orbeci, C.; Bobirica, L.; Palla Papavlu, A.; Voicu, S.I. Synthesis and characterization of polysulfone-TiO2 doped MWCNT composite membranes by sonochemical method. Appl. Phys. A 2020, 126, 233. [Google Scholar] [CrossRef]
- David, M.; Arab, M.; Martino, C.; Delmas, L.; Guinneton, F.; Gavarri, J.-R. Carbon nanotubes/ceria composite layers deposited on surface acoustic wave devices for gas detection at room temperature. Thin Solid Film. 2012, 520, 4786–4791. [Google Scholar] [CrossRef]
- Abraham, N.; Krishnakumar, R.R.; Unni, C.; Philip, D. Simulation studies on the responses of ZnO-CuO/CNT nanocomposite based SAW sensor to various volatile organic chemicals. J. Sci. Adv. Mat. Dev. 2019, 4, 125–131. [Google Scholar] [CrossRef]
- Asad, M.; Sheikhi, M.H. Surface acoustic wave based H2S gas sensors incorporating sensitive layers of single wall carbon nanotubes decorated with Cu nanoparticles. Sens. Actuators B 2014, 198, 134–141. [Google Scholar] [CrossRef]
- Kus, F.; Altinkok, C.; Zayim, E.; Erdemir, S.; Tasaltin, C.; Gurol, I. Surface acoustic wave (SAW) sensor for volatile organic compounds (VOCs) detection with calix[4]arene functionalized Gold nanorods (AuNRs) and silver nanocubes (AgNCs). Sens. Actuators B 2021, 330, 129402. [Google Scholar] [CrossRef]
- García-Gancedoa, L.; Zhua, Z.; Iborra, E.; Clement, M.; Olivares, J.; Flewitt, A.J.; Milnea, W.I.; Ashleyc, G.M.; Luoc, J.K.; Zhao, X.B.; et al. AlN-based BAW resonators with CNT electrodes for gravimetric biosensing. Sens. Actuators B 2011, 160, 1386–1393. [Google Scholar] [CrossRef]
- Oprea, M.; Voicu, S.I. Cellulose Composites with Graphene for Tissue Engineering Applications. Materials 2020, 13, 5347. [Google Scholar] [CrossRef] [PubMed]
- Voicu, S.I.; Pandele, M.A.; Vasile, E.; Rughinis, R.; Crica, L.; Pilan, L.; Ionita, M. The impact of sonication time through polysulfone graphene oxide composite films properties. Dig. J. Nanomater. Biostruct. 2013, 8, 1389–1394. [Google Scholar]
- Ionita, M.; Crica, L.E.; Voicu, S.I.; Pandele, A.M.; Iovu, H. Fabrication of Cellulose Triacetate/Graphene Oxide Porous Membrane. Polym. Adv. Technol. 2016, 27, 350–357. [Google Scholar] [CrossRef]
- Satulu, V.; Mitu, B.; Pandele, A.M.; Voicu, S.I.; Kravets, L.; Dinescu, G. Composite polyethylene terephthalate track membranes with thin teflon-like layers: Preparation and surface properties. Appl. Surf. Sci. 2019, 476, 452–459. [Google Scholar] [CrossRef]
- Pandele, A.M.; Iovu, H.; Orbeci, C.; Tuncel, C.; Miculescu, F.; Nicolescu, A.; Deleanu, C.; Voicu, S.I. Surface Modified Cellulose Acetate Membranes for the Reactive Retention of Tetracycline. Sep. Purif. Technol. 2020, 249, 117145. [Google Scholar] [CrossRef]
- Sebanescu, O.S.; Pandele, A.M.; Miculescu, F.; Voicu, S.I. Synthesis and characterization of cellulose acetate membranes with self-indicating properties by changing the membrane surface color for separation of Gd (III). Coatings 2020, 10, 468. [Google Scholar] [CrossRef]
- Yu, S.-Y.; Sun, X.-C.; Ni, X.; Wang, Q.; Yan, X.-J.; He, C.; Liu, X.-P.; Feng, L.; Lu, M.-H.; Chen, Y.-F. Surface phononic graphene. Nat. Mater. 2016, 15, 1243–1247. [Google Scholar] [CrossRef]
- Bandhu, L.; Nash, G.R. Controlling the properties of surface acoustic waves using graphene. Nano Res. 2016, 9, 685–691. [Google Scholar] [CrossRef]
- Roshchupkin, D.; Ortega, L.; Zizak, I.; Plotitcyna, O.; Matveev, V.; Kononenko, O.; Emelin, E.; Erko, A.; Tynyshtykbayev, K.; Irzhak, D.; et al. Surface acoustic wave propagation in graphene film. J. Appl. Phys. 2015, 118, 104901. [Google Scholar] [CrossRef]
- Okuda, S.; Ono, T.; Kanai, Y.; Ikuta, T.; Shimatani, M.; Ogawa, S.; Maehashi, K.; Inoue, K.; Matsumoto, K. Graphene Surface Acoustic Wave Sensor for Simultaneous Detection of Charge and Mass. ACS Sens. 2018, 3, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Le, X.; Wang, X.; Pang, J.; Liu, Y.; Fang, B.; Xu, Z.; Gao, C.; Xu, Y.; Xie, J. A high performance humidity sensor based on surface acoustic wave and graphene oxide on AlN/Si layered structure. Sens. Actuators B 2018, 255, 2454–2461. [Google Scholar] [CrossRef]
- Guo, Y.J.; Zhang, J.; Zhao, C.; Hu, P.A.; Zu, X.T.; Fu, Y.Q. Graphene/LiNbO3 surface acoustic wave device based relative humidity sensor. Optik 2014, 125, 5800–5802. [Google Scholar] [CrossRef]
- Xuan, W.; He, M.; Meng, N.; He, X.; Wang, W.; Chen, J.; Shi, T.; Hasan, T.; Xu, Z.; Xu, Y.; et al. Fast Response and High Sensitivity ZnO/ glass Surface Acoustic Wave Humidity Sensors Using Graphene Oxide Sensing Layer. Sci. Rep. 2014, 4, 7206. [Google Scholar] [CrossRef] [PubMed]
- Balashov, S.M.; Balachova, O.V.; Braga, A.V.U.; Pavani Filho, A.; Moshkalev, S. Influence of the deposition parameters of graphene oxide nanofilms on the kinetic characteristics of the SAW humidity sensor. Sens Actuators B Chem. 2015, 217, 88–91. [Google Scholar] [CrossRef]
- Li, D.; Le, X.; Pang, J.; Peng, J.; Xu, Z.; Gao, C.; Xie, J. A SAW hydrogen sensor based on decoration of graphene oxide by palladium nanoparticles on AIN/Si layered structure. J. Micromech. Microeng. 2019, 29, 045007. [Google Scholar] [CrossRef]
- Ha, N.H.; Nam, N.H.; Dung, D.D.; Phuong, N.H.; Thach, P.D.; Hong, H.S. Hydrogen Gas Sensing Using Palladium-Graphene Nanocomposite Material Based on Surface Acoustic Wave. J. Nanomater. 2017, 2017, 9057250. [Google Scholar] [CrossRef]
- Constantinoiu, I.; Miu, D.; Viespe, C. Surface Acoustic Wave Sensors for Ammonia Detection at Room Temperature Based on SnO2/Co3O4 Bilayers. J. Sens. 2019, 2019, 8203810. [Google Scholar] [CrossRef]
- Hung, T.-T.; Chung, M.-H.; Chiu, J.-J.; Yang, M.-W.; Tien, T.-N.; Shen, C.-Y. Poly(4-styrenesulfonic acid) doped polypyrrole/tungsten oxide/reduced graphene oxide nanocomposite films based surface acoustic wave sensors for NO sensing behavior. Org. Electron. 2021, 88, 106006. [Google Scholar] [CrossRef]
- Thomas, S.; Cole, M.; De Luca, A.; Torrisi, F.; Ferrari, A.C.; Udrea, F.; Gardner, J.W. Graphene-coated Rayleigh SAW resonators for NO2 detection. Procedia Eng. 2014, 87, 999–1002. [Google Scholar] [CrossRef]
- Xu, S.; Li, C.; Li, H.; Li, M.; Qu, M.; Yang, B. Carbon dioxide sensors based on a surface acoustic wave device with a graphene–nickel–l-alanine multilayer film. J. Mater. Chem. C 2015, 3, 3882–3890. [Google Scholar] [CrossRef]
- Sayago, I.; Matatagui, D.; Jesús Fernández, M.; Fontecha, J.L.; Jurewicz, I.; Garriga, R.; Muñoz, E. Graphene oxide as sensitive layer in Love-wave surface acoustic wave sensors for the detection of chemical warfare agent simulants. Talanta 2016, 148, 393–400. [Google Scholar] [CrossRef]
- Shen, C.; Lu, S.; Tian, Z.; Yang, S.; Cardenas, J.A.; Li, J.; Peng, X.; Huang, T.J.; Franklin, A.D.; Cummer, S.A. Electrically Tunable Surface Acoustic Wave Propagation at MHz Frequencies Based on Carbon Nanotube Thin-Film Transistors. Adv. Funct. Mater. 2021, 31, 2010744. [Google Scholar] [CrossRef]
- Haaland, P.; McKibben, J.; Paradi, M. Fundamental constraints on thin film coatings for flat-panel display manufacturing. In Proceedings of the Display Manufacturing Technology Conference; SID: San Jose, CA, USA, 1995; pp. 79–81. [Google Scholar]
- Garcıa, M.; Fernandez, M.J.; Fontecha, J.L.; Lozano, J.; Santos, J.P.; Aleixandre, M.; Sayago, I.; Gutierrez, J.; Horrillo, M.C. Differentiation of red wines using an electronic nose based on surface acoustic wave devices. Talanta 2006, 68, 1162–1165. [Google Scholar] [CrossRef]
- Bruening, M.; Dotzauer, D. Polymer films: Just spray it. Nat. Mater. 2009, 8, 449–450. [Google Scholar] [CrossRef] [PubMed]
- Manoosingh, L.L. Design of a Chemical Agent Detector Based on Polymer Coated Surface Acoustic Wave (SAW) Resonator Technology. Ph.D. Thesis, University of South Florida, Tampa, FL, USA, 18 June 2004. [Google Scholar]
- Tepper, G.; Levit, N. Polymer Deposition from Supercritical Solutions for Sensing Applications. Am. Chem. Soc. 2000, 39, 4445–4449. [Google Scholar] [CrossRef]
- Kuznetsova, I.; Smirnov, A.; Anisimkin, V.; Gubin, S.; Signore, M.A.; Francioso, L.; Kondoh, J.; Kolesov, V. Inkjet Printing of Plate Acoustic Wave Devices. Sensors 2020, 20, 3349. [Google Scholar] [CrossRef] [PubMed]
- Kirbus, B.; Brachmann, E.; Hengst, C.; Menzel, S. Additive manufacturing of 96MHz surface acoustic wave devices by means of superfine inkjet printing. Smart Mater. Struct. 2018, 27, 075042. [Google Scholar] [CrossRef]
- de Gans, B.J.; Duineveld, P.C.; Schubert, U.S. Inkjet printing of polymers: State of the art and future developments. Adv. Mater. 2004, 16, 203–213. [Google Scholar] [CrossRef]
- Chang, J.B.; Liu, V.; Subramanian, V.; Sivula, K.; Luscombe, C.; Murphy, A.; Liu, J.; Fréchet, J.M.J. Printable polythiophene gas sensor array for low-cost electronic noses. J. Appl. Phys. 2006, 100, 014506. [Google Scholar] [CrossRef]
- Nikolaou, I.; Hallil, H.; Conedera, V.; Deligeorgis, G.; Dejous, C.; Rebiere, D. Inkjet-printed graphene oxide thin layers on love wave devices for humidity and vapor detection. IEEE Sens. J. 2016, 16, 7620–7627. [Google Scholar] [CrossRef]
- Chrisey, D.B.; Piqué, A.; McGill, R.A.; Horwitz, J.S.; Ringeisen, B.R.; Bubb, D.M.; Wu, P.K. Laser Deposition of Polymer and Biomaterial Films. Chem. Rev. 2003, 103, 553–576. [Google Scholar] [CrossRef]
- Meinschien, J.; Behme, G.; Falk, F.; Stafast, H. Smooth and oriented AlN thin films deposited by laser ablation and their application for SAW devices. Appl. Phys. A 1999, 69, 683–686. [Google Scholar] [CrossRef]
- Shibata, Y.; Kaya, K.; Akashi, K.; Kanai, M.; Kawai, T.; Kawai, S. Epitaxial growth and surface-acoustic-wave properties of LiTaO3 films grown by pulsed laser deposition. Appl. Phys. Lett. 1993, 62, 3046–3048. [Google Scholar] [CrossRef]
- Shibata, Y.; Kuze, N.; Matsui, M.; Kanno, Y.; Kaya, K.; Ozaki, M.; Kanai, M.; Kawai, T. Surface Acoustic Wave Properties of Lithium Tantalate Films Grown by Pulsed Laser Deposition. Jpn. J. Appl. Phys. 1995, 34, 249. [Google Scholar] [CrossRef]
- Benetti, M.; Cannatá, D.; Di Pietrantonio, F.; Verona, E.; Verardi, P.; Scarisoreanu, N.; Matei, D.; Dinescu, G.; Moldovan, A.; Dinescu, M. Structural and piezoelectric properties of pulsed laser deposited ZnO thin films. Superlattices Microstruct. 2006, 39, 366–375. [Google Scholar] [CrossRef]
- Viespe, C.; Miu, D. Surface Acoustic Wave Sensor with Pd/ZnO Bilayer Structure for Room Temperature Hydrogen Detection. Sensors 2017, 17, 1529. [Google Scholar] [CrossRef]
- Constantinoiu, I.; Viespe, C. Development of Pd/TiO2 Porous Layers by Pulsed Laser Deposition for Surface Acoustic Wave H2 Gas Sensor. Nanomaterials 2020, 10, 760. [Google Scholar] [CrossRef] [PubMed]
- Marcu, A.; Viespe, C. Surface Acoustic Wave Sensors for Hydrogen and Deuterium Detection. Sensors 2017, 17, 1417. [Google Scholar] [CrossRef]
- Marcu, A.; Viespe, C. Laser-grown ZnO nanowires for room-temperature SAW-sensor applications. Sens Actuators B Chem. 2015, 208, 1–6. [Google Scholar] [CrossRef]
- Schou, J. Physical aspects of the pulsed laser deposition technique: The stoichiometric transfer of material from target to film. Appl. Surf. Sci. 2009, 255, 5191. [Google Scholar] [CrossRef]
- Pique, A.; Auyeung, R.C.Y.; Stepnowski, J.L.; Weir, D.W.; Arnold, C.B.; McGill, R.A.; Chrisey, D.B. Laser processing of polymer thin films for chemical sensor applications. Surf. Coat. Technol. 2003, 163–164, 293–299. [Google Scholar] [CrossRef]
- Dinca, V.; Viespe, C.; Brajnicov, S.; Constantinoiu, I.; Moldovan, A.; Bonciu, A.; Toader, C.N.; Ginghina, R.E.; Grigoriu, N.; Dinescu, M.; et al. MAPLE Assembled Acetylcholinesterase–Polyethylenimine Hybrid and Multilayered Interfaces for Toxic Gases Detection. Sensors 2018, 18, 4265. [Google Scholar] [CrossRef]
- Bubb, D.M.; Horwitz, J.S.; McGill, R.A.; Chrisey, D.B.; Papantonakis, M.R.; Haglund, R.F., Jr.; Toftmann, B. Resonant infrared pulsed-laser deposition of a sorbent chemoselective polymer. Appl. Phys. Lett. 2001, 79, 2847. [Google Scholar] [CrossRef]
- Houser, E.J.; Chrisey, D.B.; Bercu, M.; Scarisoreanu, N.D.; Purice, A.; Colceag, D.; Constantinescu, C.; Moldovan, A.; Dinescu, M. Functionalized polysiloxane thin films deposited by matrix-assisted pulsed laser evaporation for advanced chemical sensor applications. Appl. Surf. Sci. 2006, 252, 4871–4876. [Google Scholar] [CrossRef]
- Pique, A.; Serra, P. (Eds.) Laser Printing of Functional Materials: 3D Microfabrication, Electronics and Biomedicine; Wiley-VCH: Weinheim, Germany, 2018; ISBN 978-3-527-34212-9. [Google Scholar]
- Braudy, R.S. Laser Writing. Proc. IEEE 1969, 57, 1771–1772. [Google Scholar] [CrossRef]
- Levene, M.L.; Scott, R.D.; Siryj, B.W. Material Transfer Recording. Appl. Optics 1970, 9, 2260. [Google Scholar] [CrossRef]
- Brisbane, A.D. Pattern Deposit by Laser. U.S. Patent 3,560,258, 2 February 1971. [Google Scholar]
- Bohandy, J.; Kim, B.F.; Adrian, F.J.; Jette, A.N. Metal deposition at 532 nm using a laser transfer technique. J. Appl. Phys. 1988, 63, 1538. [Google Scholar] [CrossRef]
- Cannatà, D.; Benetti, M.; Di Pietrantonio, F.; Verona, E.; Palla-Papavlu, A.; Dinca, V.; Dinescu, M.; Lippert, T. Nerve agent simulant detection by solidly mounted resonators (SMRs) polymer coated using laser induced forward transfer (LIFT) technique. Sens. Actuators B Chem. 2012, 173, 32–39. [Google Scholar] [CrossRef]
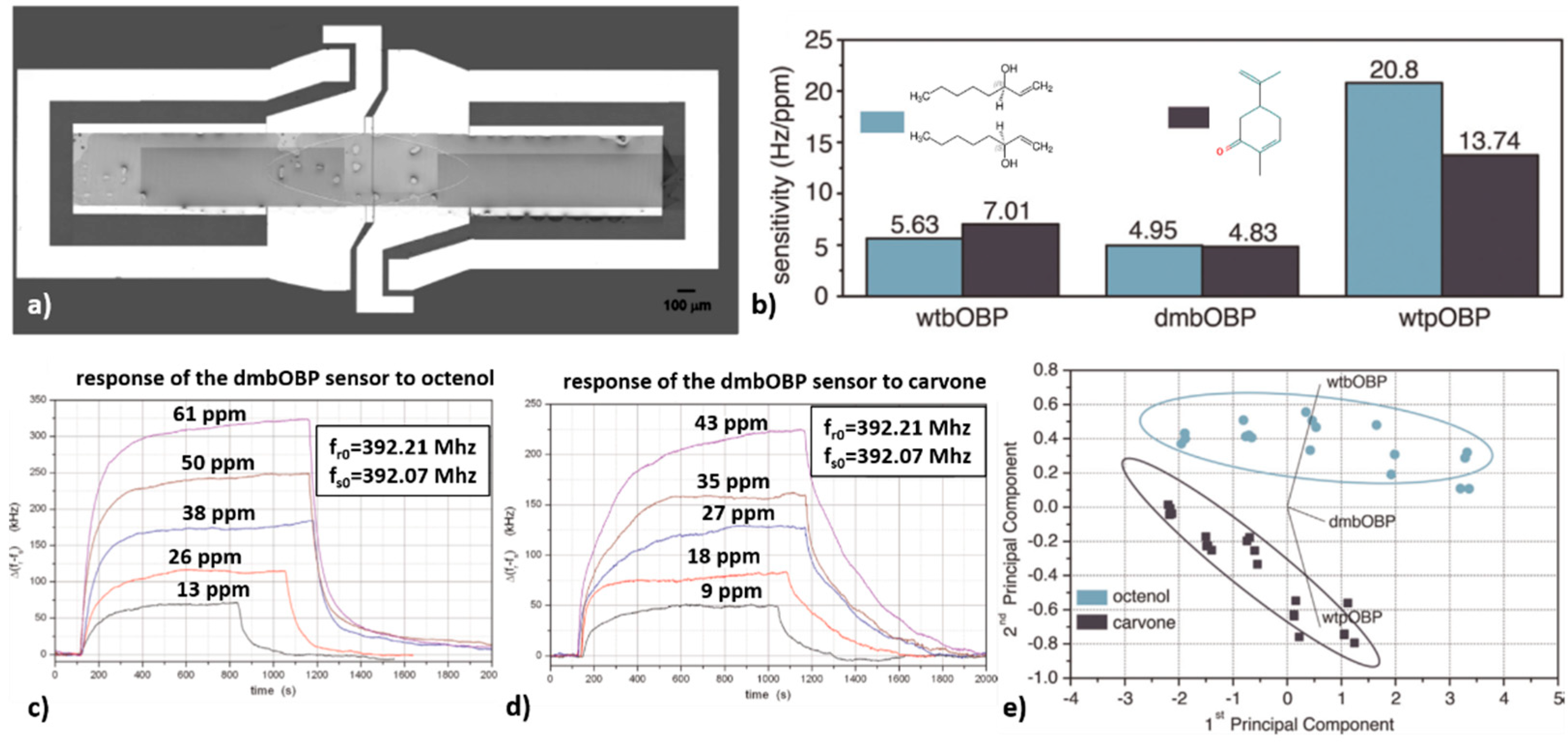
- Di Pietrantonio, F.; Benetti, M.; Cannatà, D.; Varriale, A.; D’Auria, S.; Palla-Papavlu, A.; Serra, P.; Verona, E. Surface acoustic wave biosensor based on odorant binding proteins deposited by laser induced forward transfer. In Proceedings of the 2013 IEEE International Ultrasonics Symposium (IUS), Prague, Czech Republic, 21–25 July 2013; pp. 2144–2147. [Google Scholar] [CrossRef]
- Palla-Papavlu, A.; Patrascioiu, A.; Di Pietrantonio, F.; Fernández-Pradas, J.-M.; Cannatà, D.; Benetti, M.; D’Auria, S.; Verona, E.; Serra, P. Preparation of surface acoustic wave odor sensors by laser-induced forward transfer. Sens. Actuators B 2014, 192, 369–377. [Google Scholar] [CrossRef]
- Di Pietrantonio, F.; Benetti, M.; Cannatà, D.; Verona, E.; Palla-Papavlu, A.; Fernández-Pradas, J.-M.; Serra, P.; Staiano, M.; Varriale, A.; D’Auria, S. A surface acoustic wave bio-electronic nose for detection of volatile odorant molecules. Biosens. Bioelectron. 2015, 67, 516–523. [Google Scholar] [CrossRef] [PubMed]
| Sensitive Layer | Sensor Type | Target Analytes | Sensitivity | Detection Limit | Remarks | Ref. |
|---|---|---|---|---|---|---|
| polypyrrole | SAW | NH3 | - | - | Interferents: (CO, CH4, H2, O2 Good response time to NH3 | [63] |
| polypyrrole | SAW | glyphosate | (6.9 ± 2.9) × 10−20/nM | 1 pM | - | [64] |
| polyprole-bromophenol blue | Q-TSMR | Nitroaromatic compounds (TNT, PETN, RDX, HMX) | - | 500 ppt for TNT; 800 ppt for PETN; 1 ppb for RDX; 2 ppb for HMX | Selectivity toward TNT | [65] |
| HFIP functionalized siloxane polymers | SAW/250 MHz | Nitroaromatic compounds | - | 235 ppt | - | [67] |
| poly(siloxanes), PECH | Q-SAW (ST) | Warfare agents simulants | - | - | classification | [71] |
| PIB, PECH, PEI | Q-SAW/392 MHz | sarin DMMP | 649 Hz/ppm | 15.41 × 10−3 ppm | Best values for PECH, followed by PEI, and PIB | [72] |
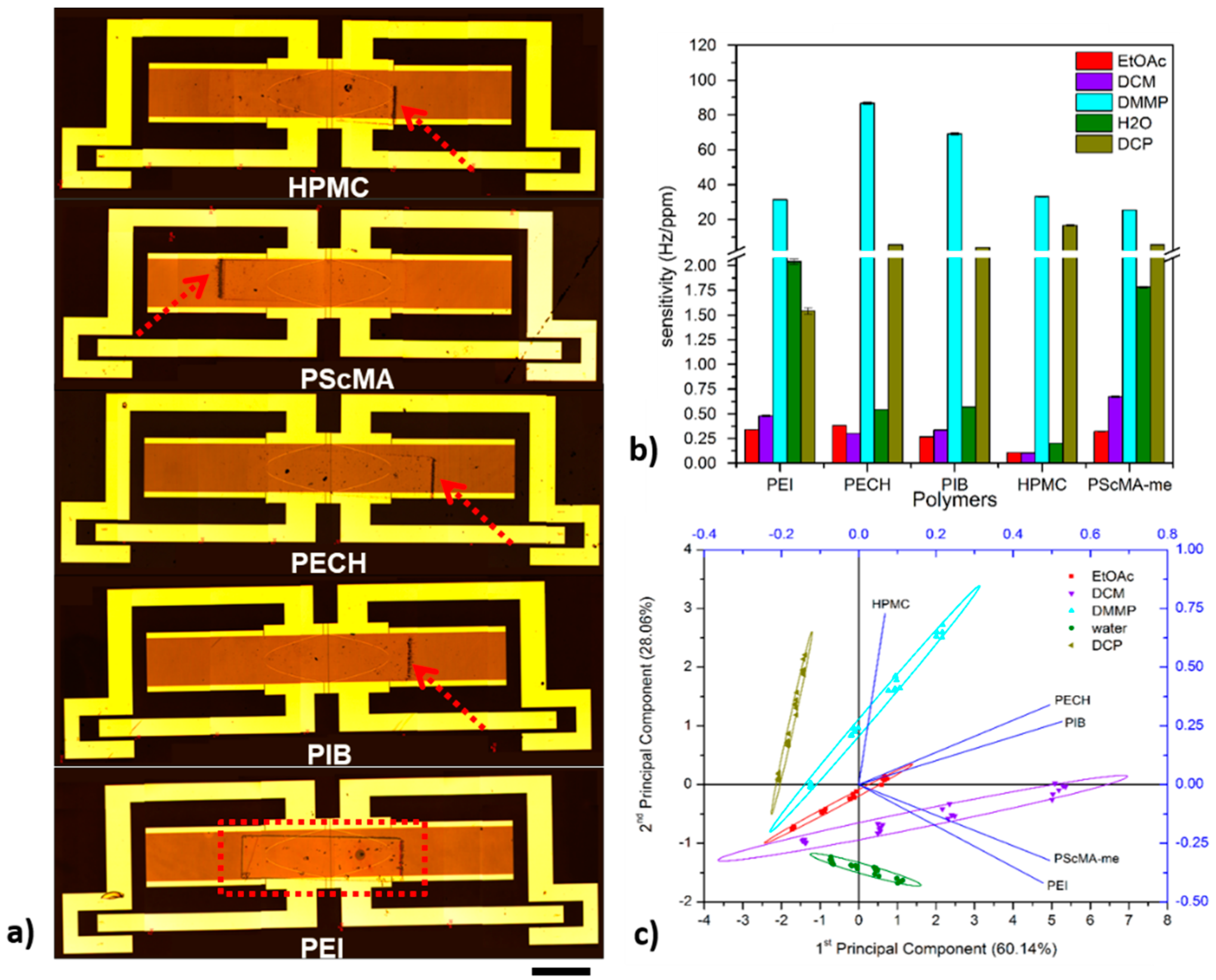
| PIB, PECH, PEI, PScMA-me, HPMC | Q-SAW/392 MHz | EtOAc, DCM, DMMP, H2O, DCP | 86 Hz/ppm | 0.3 ppm | Best values for PECH; Good discrimination; | [73] |
| chemoselective polymers | Q-SAW/250 kHz | CWA | - | GB = 4 ppb/mustard = 80 ppb | - | [79] |
| chemoselective polymers (PIB, PECH, PDMS, PIP, PBD) | Q-SAW/264 MHz | CWA/DMMP, acetonitrile, DCM, DCP | - | - | Good discrimination | [69] |
| poly (3-hexyl thiophene) | QCM | VOCs | - | 1.5 | LDL = 3% permissible exposure limit 180/120 sec response and recovery times | [80] |
| poly(siloxanes), PEI, PECH, Carbowax | Q-LOWE SAW | DMMP and CWA | 40,200 Hz/ppmv | 40 ppbv | Principal component 2 = 1% variance Good discrimination | [77] |
| polymer array (PBMA, PIB, PDMS, PCFV, PECH, silar, L grease, polyurethane alkyd resin with trace isocyanates) | STW | VOC | - | - | Polar plots | [81] |
| No | Sensitive Layer | Detected Species | Sensitivity | Reference |
|---|---|---|---|---|
| 1. | MWNTs/polyethylene imine | CO2 | 0.5–100% in air | [101] |
| 2. | Poly(diallyldimethylammonium chloride)/SWCNT | CO2 | 0–10% in air | [103] |
| 3. | Polyepichlorohydrin/polyetherurethane/MWNT | Octane | 0.59–1.01 Hz/ppm | [105] |
| 4. | Polyepichlorohydrin/ polyetherurethane/MWNT | Toluene | 0.61–4.38 Hz/ppm | [105] |
| 5. | Polyisobutylene/MWNT | Octane | 4.596–7.034 Hz/ppm | [106] |
| 6. | Polyisobutylene/MWNT | Toluene | 2.016–3.002 Hz/ppm | [106] |
| 7. | SWCNT Langmuir–Blodgett film | H2 | 0.030–1% | [107] |
| 8. | SWCNT Langmuir–Blodgett film | NH3 | 30–1000 ppm | [107] |
| 9. | SWCNT Langmuir–Blodgett film | NO2 | 1–10 ppm | [107] |
| 10. | ZnO–CuO/SWCNT | 2-propanol | 200.26 kHz/100 ppm | [110] |
| 11. | ZnO–CuO/SWCNT | Trichloromethane | 59.8 kHz/100 ppm | [110] |
| 12. | ZnO–CuO/SWCNT | Dichloromethane | 33.42 kHz/100 ppm | [110] |
| 13. | ZnO–CuO/SWCNT | n-Hexane | 30.76 kHz/100 ppm | [110] |
| 14. | ZnO–CuO/SWCNT | n-Pentane | 10.89 kHz/100 ppm | [110] |
| 15. | ZnO–CuO/SWCNT | Diethylether | 89.46 kHz/100 ppm | [110] |
| 16. | ZnO–CuO/SWCNT | Acetone | 107.23 kHz/100 ppm | [110] |
| 17. | ZnO–CuO/SWCNT | Acetonitrile | 10.64 kHz/100 ppm | [110] |
| 18. | ZnO–CuO/SWCNT | Ethanol | 100.69 kHz/100 ppm | [110] |
| 19. | ZnO–CuO/SWCNT | Methanol | 20.99 kHz/100 ppm | [110] |
| 20. | Cu nanoparticles/SWCNT | H2S | 5–200 ppm | [111] |
| Coating Technique | Coating Materials | Velocity of Transfer Material | Cost | Problems |
|---|---|---|---|---|
| Spray coating | Wide range of materials (metal, alloy, plastic, polymer, etc.) | 450–1000 m s−1, depending on the spray device, spray material, and operating conditions | Inexpensive | Finish quality |
| Inkjet printing | Specially designed inks | 1–5 m s−1 | Inexpensive | Properties of the liquid limited ink types |
| LIFT | Can work for all types of material phases | From 2000 m s−1 (gold particles) to 100 m s−1 (flyer material) | Moderately expensive; requires laser, works also in air | UV and thermal load; Preparation of multilayer donor |
| MAPLE direct write | Polymers material is embedded in a matrix | 200 m s−1 | Expensive; requires vacuum | UV and thermal load; Preparation of the matrix |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palla-Papavlu, A.; Voicu, S.I.; Dinescu, M. Sensitive Materials and Coating Technologies for Surface Acoustic Wave Sensors. Chemosensors 2021, 9, 105. https://doi.org/10.3390/chemosensors9050105
Palla-Papavlu A, Voicu SI, Dinescu M. Sensitive Materials and Coating Technologies for Surface Acoustic Wave Sensors. Chemosensors. 2021; 9(5):105. https://doi.org/10.3390/chemosensors9050105
Chicago/Turabian StylePalla-Papavlu, Alexandra, Stefan Ioan Voicu, and Maria Dinescu. 2021. "Sensitive Materials and Coating Technologies for Surface Acoustic Wave Sensors" Chemosensors 9, no. 5: 105. https://doi.org/10.3390/chemosensors9050105
APA StylePalla-Papavlu, A., Voicu, S. I., & Dinescu, M. (2021). Sensitive Materials and Coating Technologies for Surface Acoustic Wave Sensors. Chemosensors, 9(5), 105. https://doi.org/10.3390/chemosensors9050105







