One-Pot Synthesis of Copper Iodide-Polypyrrole Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Methods
2.2. One-Pot Composite Preparation and Deposition
2.3. UV–Vis Spectroscopy
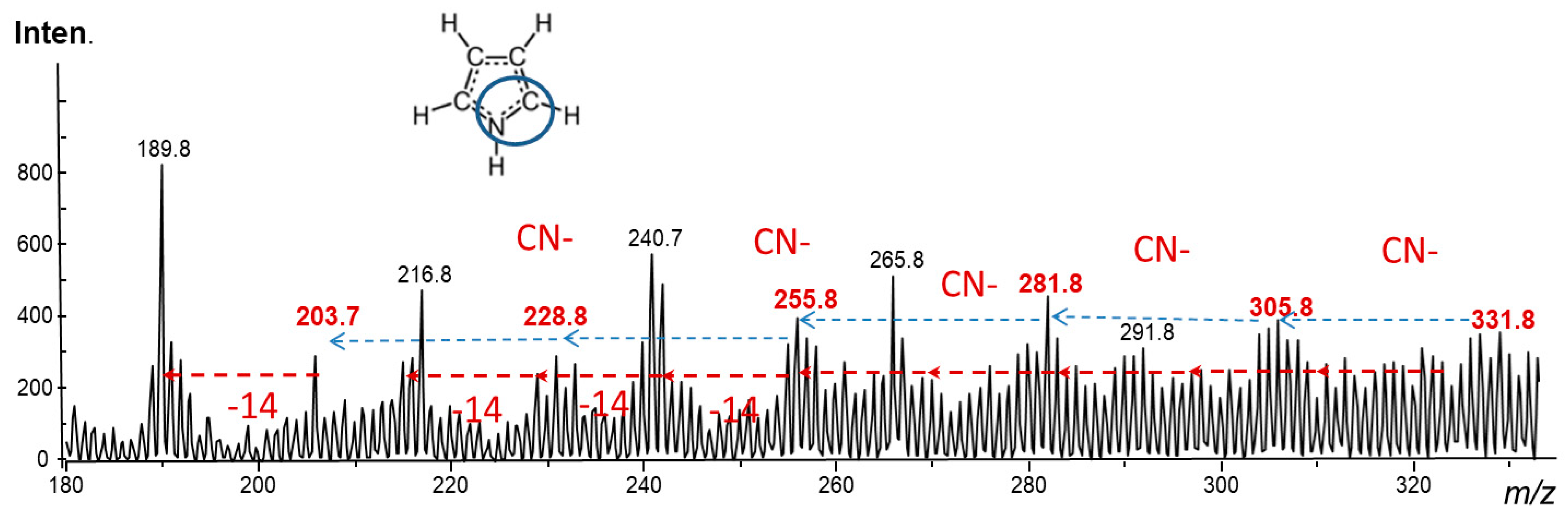
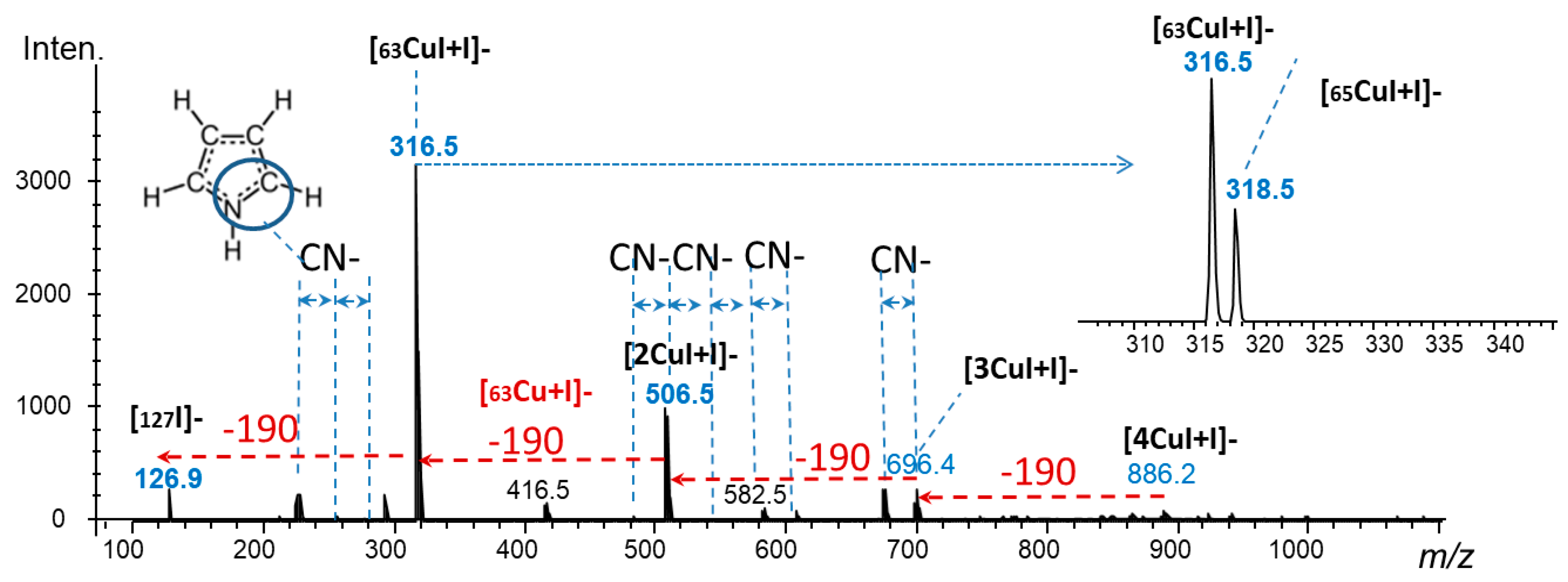
2.4. Laser Desorption Ionization Mass Spectrometry (LDI–MS)
2.5. Scanning Electron Microscopy (SEM) with Coupled X-ray Analysis (EDX)
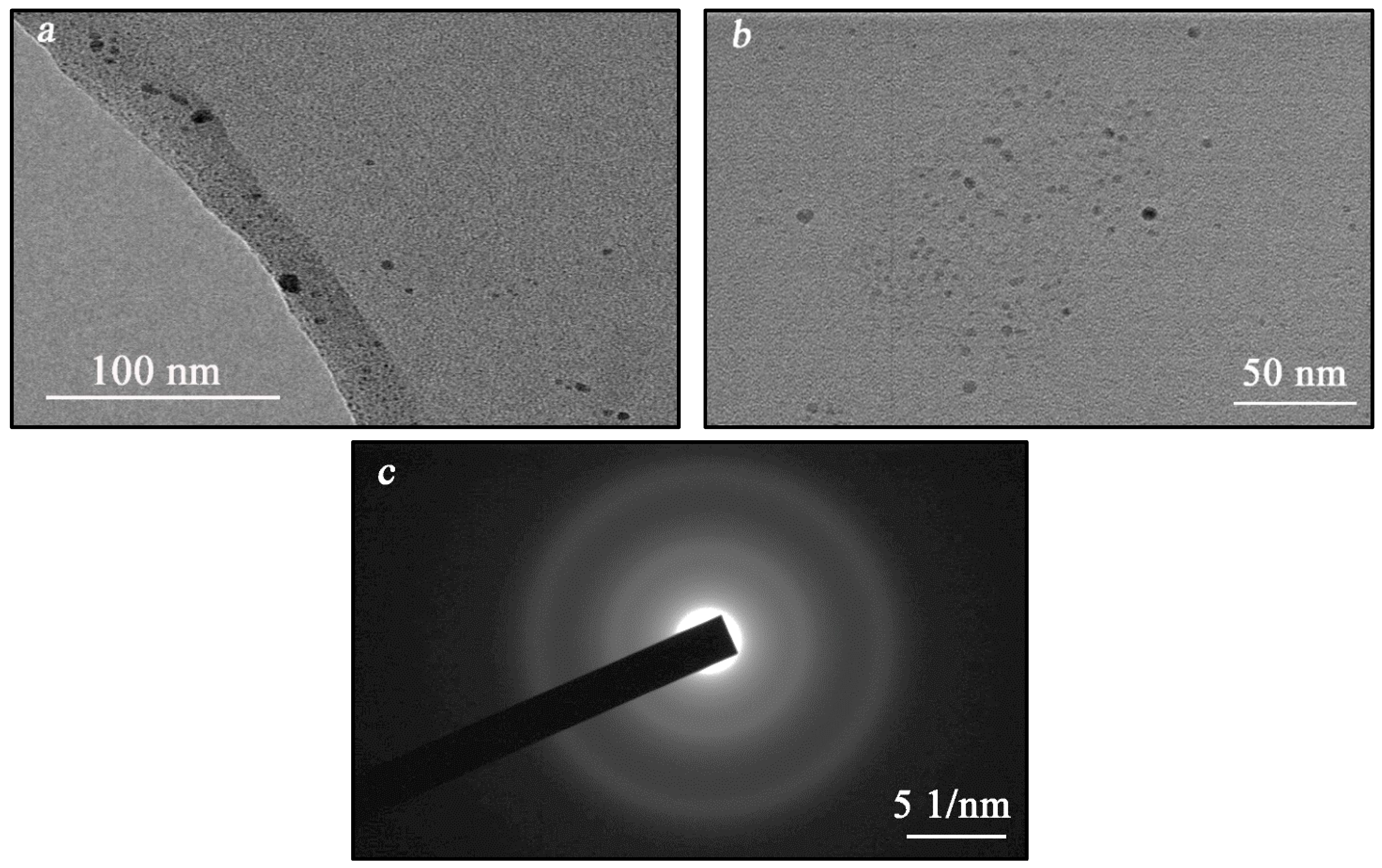
2.6. Transmission Electron Microscopy (TEM)
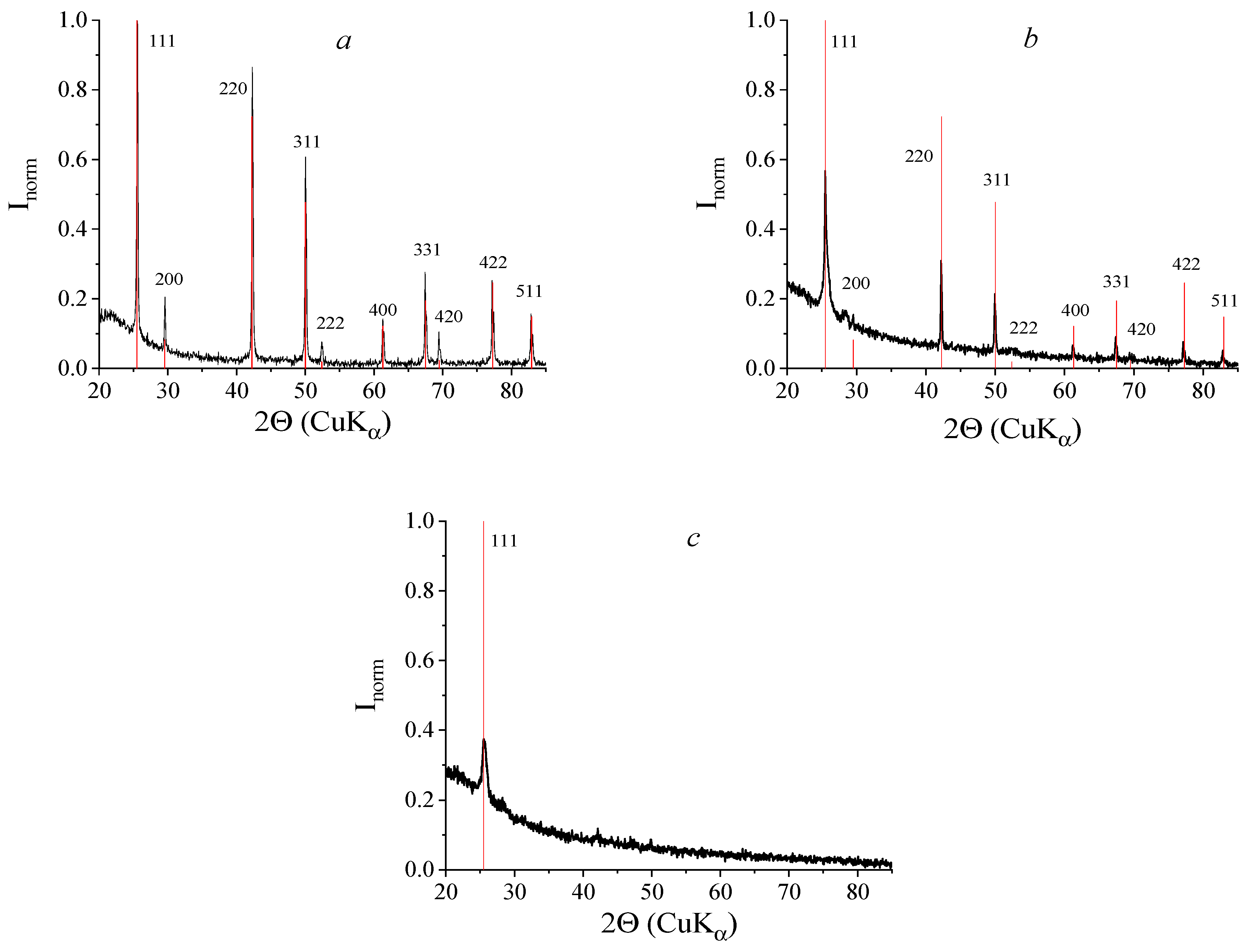
2.7. Elemental and Phase Analysis
2.8. Electrochemical Studies
2.9. pH Measurements
3. Results
3.1. Fundamental Chemical Aspects and Optimization of the Synthesis Conditions
3.2. Characterization of the Hybrid Composites
3.3. Morphological Reproducibility and Storage Stability of CuI-PPy Composite
3.4. Electrochemical Performance of CuI-PPy
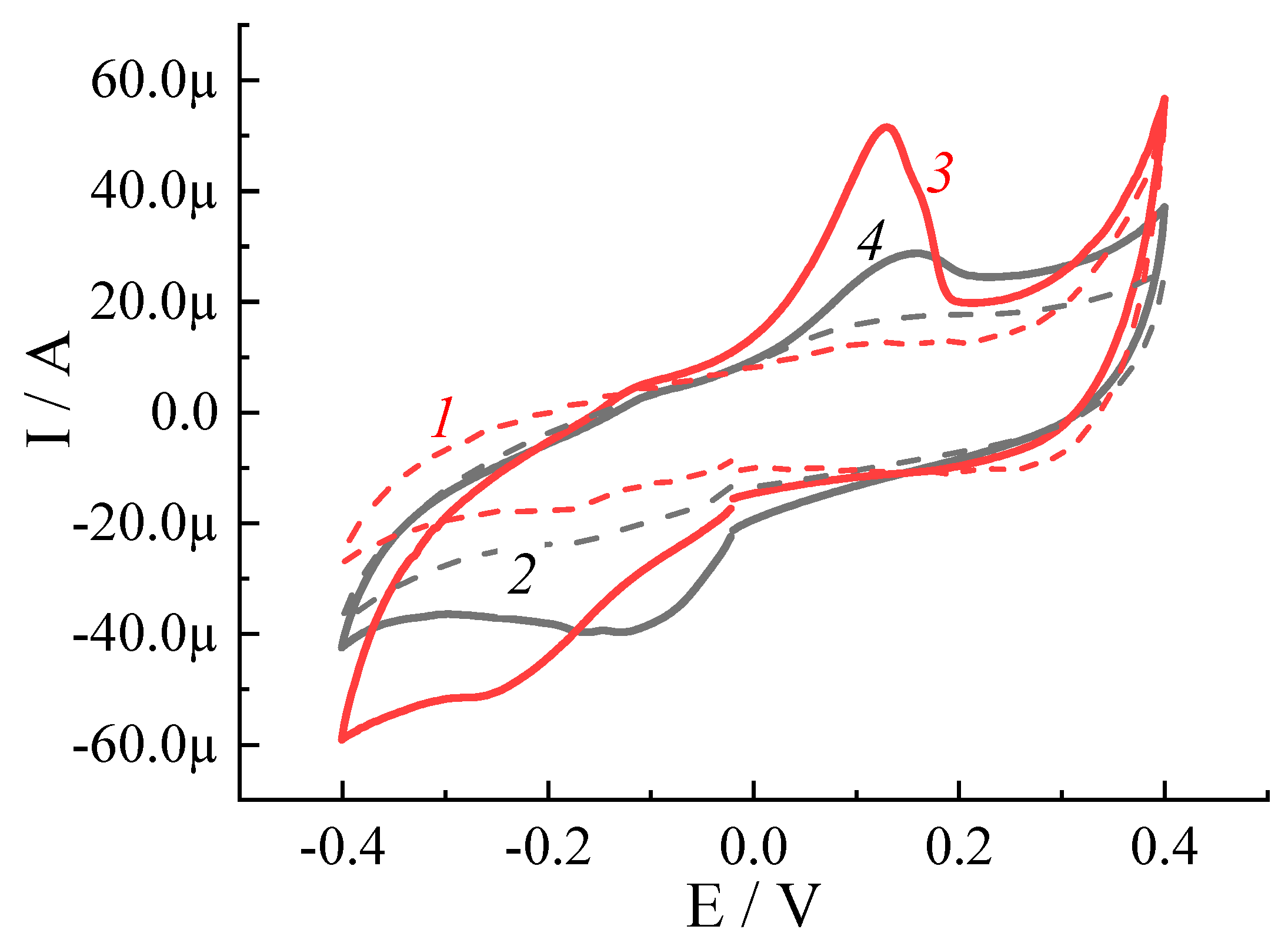
3.4.1. Electrochemical Response Estimated on a Glassy Carbon Electrode under Inert Gas and Oxygen Atmospheres
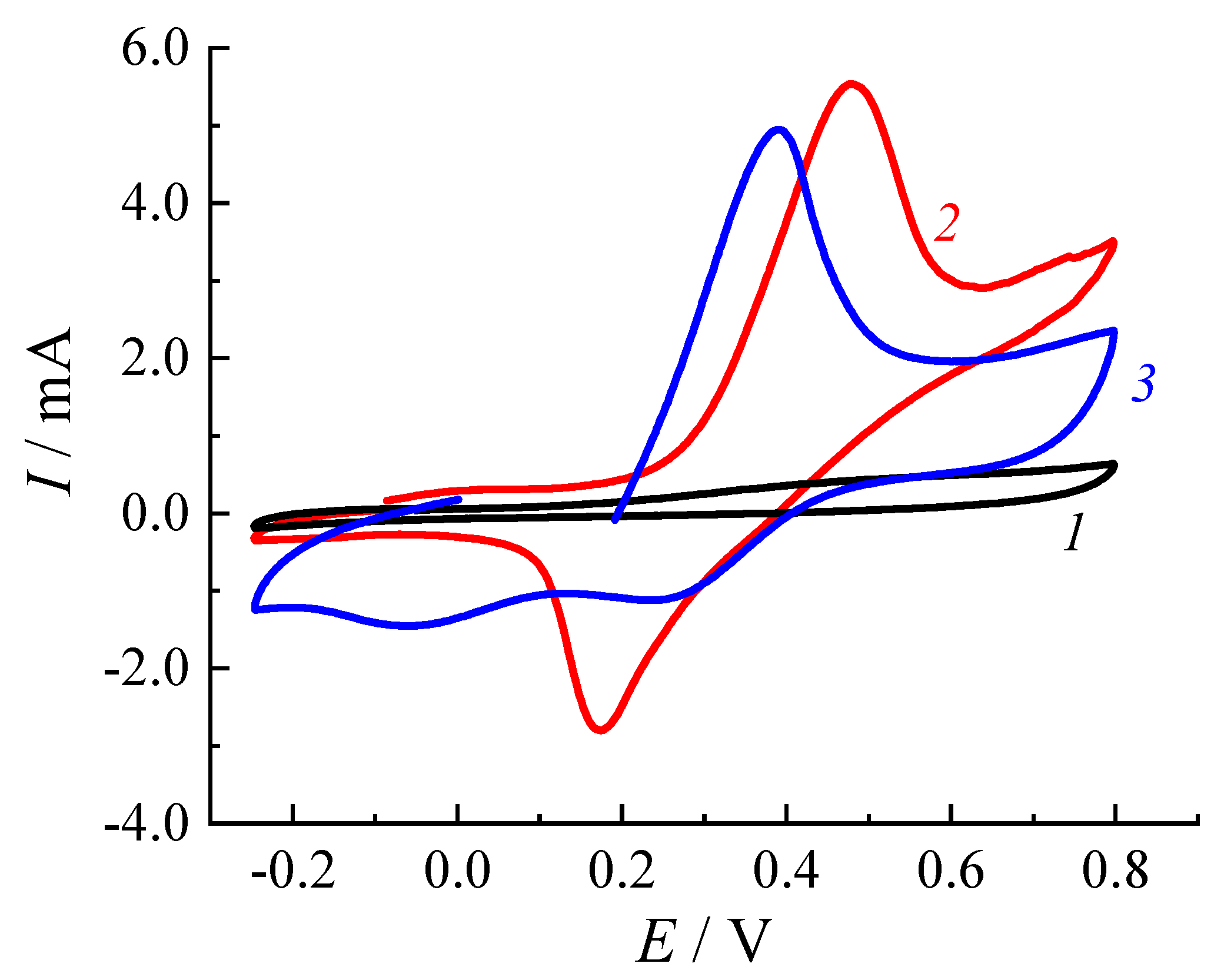
3.4.2. Electrochemical Performance of CuI-PPy Modified SPEs
3.4.3. Application for Chemical Sensing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sekkal, W.; Zaoui, A.; Laref, A.; Certier, M.; Aourag, H. Molecular dynamics simulation of CuI using a three-body potential. J. Phys. Condens. Matter. 2000, 12, 6173–6182. [Google Scholar] [CrossRef]
- Apsana, G.; George, P.P.; Devanna, N.; Yuvasravana, R. One-Step green synthesis, characterization, optical, and photocatalytic properties of metal iodide (MI, M = Ag and Cu) nanoparticles. J. Bionanoscience 2018, 12, 191–199. [Google Scholar] [CrossRef]
- Christians, J.A.; Fung, R.C.M.; Kamat, P.V. An inorganic hole conductor for Organo-lead halide perovskite solar cells. Improved hole conductivity with copper iodide. J. Am. Chem. Soc. 2014, 136, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Hu, J.; Zhai, C.; Zhu, M.; Pan, J. A p-n heterojunction of CuI/TiO2 with enhanced photoelectrocatalytic activity for methanol electro-oxidation. Electrochim. Acta 2017, 245, 863–871. [Google Scholar] [CrossRef]
- Wang, J.J.; Chen, C.; Chen, W.G.; Yao, J.S.; Yang, J.N.; Wang, K.H.; Yin, Y.C.; Yao, M.M.; Feng, L.Z.; Ma, C.; et al. Highly Luminescent Copper Iodide Cluster Based Inks with Photoluminescence Quantum Efficiency Exceeding 98%. J. Am. Chem. Soc. 2020, 142, 3686–3690. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.H.; Jung, D.Y. Air-Stable Transparent Silver Iodide-Copper Iodide Heterojunction Diode. ACS Appl. Mater. Interfaces 2017, 9, 43807–43813. [Google Scholar] [CrossRef]
- Prokopenko, S.L.; Mazurenko, R.V.; Gunja, G.M.; Abramov, N.V.; Makhno, S.M.; Gorbyk, P.P. Electrophysical properties of polymeric nanocomposites based on cobalt and nickel ferrites modified with copper iodide. J. Magn. Magn. Mater. 2020, 494, 165824. [Google Scholar] [CrossRef]
- Crisp, S.; Meddle, D.W.; Nunan, J.M.; Smith, A.F. A copper (I) iodide paper for the detection and determination of the concentration of mercury vapour in the workplace atmosphere. Analyst 1981, 106, 1318–1325. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Q.; Yang, L.; Shi, J.; Zhang, M. CuI/TBAB as a novel efficient catalytic system for Heck reaction in water. RSC Adv. 2013, 3, 21251–21255. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M. Schiff Base/CuI as a Novel Efficient Catalyst System for Cu-Catalyzed Heck Reaction in Water. ChemistrySelect 2019, 4, 9673–9676. [Google Scholar] [CrossRef]
- Lee, V. Application of copper (I) salt and fluoride promoted Stille coupling reactions in the synthesis of bioactive molecules. Org. Biomol. Chem. 2019, 17, 9095–9123. [Google Scholar] [CrossRef]
- Zhang, C.; Zhan, Z.; Lei, M.; Hu, L. Ullmann-type C-N coupling reaction catalyzed by CuI/metformin. Tetrahedron 2014, 70, 8817–8821. [Google Scholar] [CrossRef]
- Xie, J.; Zhu, X.; Huang, M.; Meng, F.; Chen, W.; Wan, Y. Pyrrole-2-carbohydrazides as Ligands for Cu-Catalyzed Amination of Aryl Halides with Amines in Pure Water. Eur. J. Org. Chem. 2010, 2010, 3219–3223. [Google Scholar] [CrossRef]
- Gao, S.; Li, Z.; Jia, X.; Jiang, K.; Zeng, H. Bioinspired synthesis of well faceted CuI nanostructures and evaluation of their catalytic performance for coupling reactions. Green Chem. 2010, 12, 1442–1447. [Google Scholar] [CrossRef]
- Kwong, F.Y.; Klapars, A.; Buchwald, S.L. Copper-catalyzed coupling of alkylamines and aryl iodides: An efficient system even in an air atmosphere. Org. Lett. 2002, 4, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Cai, Q.; Zhang, H. Mild method for ullmann coupling reaction of amines and aryl halides. Org. Lett. 2003, 5, 2453–2455. [Google Scholar] [CrossRef]
- Tao, C.Z.; Liu, W.W.; Sun, J.Y.; Cao, Z.L.; Li, H.; Zhang, Y.F. 3-acetylcoumarin as a practical ligand for copper-catalyzed C-N coupling reactions at room temperature. Synthesis 2010, 8, 1280–1284. [Google Scholar] [CrossRef]
- Yong, F.F.; Teo, Y.C. Efficient ligand-free copper-catalyzed arylation of aliphatic amines. Synlett 2010, 3, 3068–3072. [Google Scholar] [CrossRef]
- Kumar, M.; Bhatt, V.; Nayal, O.S.; Sharma, S.; Kumar, V.; Thakur, M.S.; Kumar, N.; Bal, R.; Singh, B.; Sharma, U. CuI nanoparticles as recyclable heterogeneous catalysts for C-N bond formation reactions. Catal. Sci. Technol. 2017, 7, 2857–2864. [Google Scholar] [CrossRef]
- Li, J.H.; Li, J.L.; Wang, D.P.; Pi, S.F.; Xie, Y.X.; Zhang, M.B.; Hu, X.C. CuI-catalyzed Suzuki-Miyaura and Sonogashira cross-coupling reactions using DABCO as ligand. J. Org. Chem. 2007, 72, 2053–2057. [Google Scholar] [CrossRef]
- Wang, S.; Wang, M.; Wang, L.; Wang, B.; Li, P.; Yang, J. CuI-catalyzed Suzuki coupling reaction of organoboronic acids with alkynyl bromides. Tetrahedron 2011, 67, 4800–4806. [Google Scholar] [CrossRef]
- Zhang, H.-F. Copper-catalyzed Ligand-Free Suzuki-Miyaura Coupling Reaction of Aryl Halides with Arylboronic Acid. J. Med. Chem. Sci. 2019, 2, 47–49. [Google Scholar] [CrossRef]
- Alavinia, S.; Ghorbani-Vaghei, R.; Rakhtshah, J.; Seyf, J.Y.; Arabian, I.A. Copper iodide nanoparticles-decorated porous polysulfonamide gel: As effective catalyst for decarboxylative synthesis of N-Arylsulfonamides. Appl. Organomet. Chem. 2020, 34. [Google Scholar] [CrossRef]
- Solgi, S.; Ghorbani-Vaghei, R.; Alavinia, S. Application of copper iodide nanoparticles immobolized porous polysulfonamide gel as an effective nanocatalyst for synthesis of aminoindolizines. J. Porous Mater. 2020, 28, 289–298. [Google Scholar] [CrossRef]
- Ezzatzadeh, E.; Hossaini, Z.; Moradi, A.V.; Salimifard, M.; Abad, S.A.F. Copper iodide and zno nanoparticles catalyzed multicomponent synthesis of 1,3-cyclopentadiene: Study of antioxidant activity. Can. J. Chem. 2019, 97, 270–276. [Google Scholar] [CrossRef]
- Mallick, S.; Mukhi, P.; Kumari, P.; Mahato, K.R.; Verma, S.K.; Das, D. Synthesis, Characterization and Catalytic Application of Starch Supported Cuprous Iodide Nanoparticles. Catal. Lett. 2019, 149, 3501–3507. [Google Scholar] [CrossRef]
- Dutta, P.K.; Dhar, B.; Sen, S. Aerobic oxidative amidation of alkynes using titanium oxide encapsulated cuprous iodide nanoparticles (CuI@TiO2). New J. Chem. 2018, 42, 12062–12071. [Google Scholar] [CrossRef]
- Karim-Nezhad, G.; Alipour, N. Electrocatalytic oxidation of hydrazine by copper iodide modified sol-gel derived carbon-ceramic composite Electrode. Curr. Chem. Lett. 2014, 3, 133–140. [Google Scholar] [CrossRef]
- Myeni, N.; Ghosh, S.K.; Perla, V.K.; Mallick, K. Copper iodide nanoparticles within the organic matrix: An efficient catalyst for the electro-oxidation of formic acid. Mater. Res. Express 2019, 6, 1050a7. [Google Scholar] [CrossRef]
- Goodgame, D.M.L.; Goodgame, M.; Canham, G.W.R. Reversible Oxidation of Copper(I) Iodide in the Presence of Imidazole. Nature 1969, 222, 866. [Google Scholar] [CrossRef]
- Gor’kov, K.V.; Talagaeva, N.V.; Hierso, J.C.; Bezverkhyy, I.S.; Pisareva, P.A.; Vorotyntsev, M.; Zolotukhina, E.V. Pd-PPy nanocomposite on the surface of carbon nanotubes: Synthesis and catalytic activity. Surf. Innov. 2017, 5, 121–129. [Google Scholar] [CrossRef]
- Talagaeva, N.V.; Zolotukhina, E.V.; Bezverkhyy, I.; Konev, D.V.; Lacroute, Y.; Maksimova, E.Y.; Koryakin, S.L.; Vorotyntsev, M.A. Stability of Prussian Blue-polypyrrole (PB/PPy) composite films synthesized via one-step redox-reaction procedure. J. Solid State Electrochem. 2015, 19, 2701–2709. [Google Scholar] [CrossRef]
- Talagaeva, N.V.; Zolotukhina, E.V.; Pisareva, P.A.; Vorotyntsev, M.A. Nanostructured Prussian Blue-polypyrrole composite coatings with electrochromic properties. Mendeleev Commun. 2016, 26, 119–120. [Google Scholar] [CrossRef]
- Li, X.; Wan, M. Morphology and hydrophobicity of micro/nanoscaled cuprous iodide crystal. Cryst. Growth Des. 2006, 6, 2661–2666. [Google Scholar] [CrossRef]
- Tavakoli, F.; Salavati-Niasari, M.; Mohandes, F. Green synthesis of flower-like CuI microstructures composed of trigonal nanostructures using pomegranate juice. Mater. Lett. 2013, 100, 133–136. [Google Scholar] [CrossRef]
- Li, H.T.; Li, X.G. Preparation and characterization of cuprous iodide nanoparticles. Inorg. Mater. 2007, 43, 85–89. [Google Scholar] [CrossRef]
- Silina, Y.E.; Morgan, B. LDI-MS scanner: Laser desorption ionization mass spectrometry-based biosensor standardization. Talanta 2021, 223, 121688. [Google Scholar] [CrossRef]
- Semenova, D.; Silina, Y.E. The role of nanoanalytics in the development of organic-inorganic nanohybrids—Seeing nanomaterials as they are. Nanomaterials 2019, 9, 1673. [Google Scholar] [CrossRef] [Green Version]
- Almario, Á.A.A.; Vieira, R.L. Study of polypyrrole films modified with copper and silver microparticles by electrochemical cementation process. J. Chil. Chem. Soc. 2006, 51, 971–974. [Google Scholar] [CrossRef]
- Madhu, R.N.; Awasthi, R. Polypyrrole Composites: Electrochemical Synthesis, Characterizations and Applications. Electropolymerization 2011. [Google Scholar] [CrossRef] [Green Version]
- Salado, M.; Lanceros-Mendez, S.; Lizundia, E. Erratum to “Free-standing intrinsically conducting polymer membranes based on cellulose and poly(vinylidene fluoride) for energy storage applications”. Eur. Polym. J. 2021, 144, 110240. [Google Scholar] [CrossRef]
- Talagaeva, N.V.; Zolotukhina, E.V.; Pisareva, P.A.; Vorotyntsev, M.A. Electrochromic properties of Prussian blue-polypyrrole composite films in dependence on parameters of synthetic procedure. J. Solid State Electrochem. 2016, 20, 1235–1240. [Google Scholar] [CrossRef]
- Talagaeva, N.V.; Kleinikova, S.A.; Gor’kov, K.V.; Zolotukhina, E.V. Electrochemical and electrocatalytic stability of Prussian blue/Berlin green redox transformation in Prussian blue-polypyrrole composite films. J. Solid State Electrochem. 2020, 24, 2935–2941. [Google Scholar] [CrossRef]
- Mao, J.; Yang, L.; Yu, P.; Wei, X.; Mao, L. Electrocatalytic four-electron reduction of oxygen with copper (II)-based metal-organic frameworks. Electrochem. Commun. 2012, 19, 29–31. [Google Scholar] [CrossRef]
- Wang, X.; Ke, Y.; Pan, H.; Ma, K.; Xiao, Q.; Yin, D.; Wu, G.; Swihart, M.T. Cu-Deficient Plasmonic Cu2−xS Nanoplate Electrocatalysts for Oxygen Reduction. ACS Catal. 2015, 5, 2534–2540. [Google Scholar] [CrossRef]
- Espejo, A.; Ortiz, J.; Rios, E.; Herrera, F.; Alburquenque, D.; Gautier, J.L. Oxygen reduction on Cu1, Mn16O4 spinel particles composite electrodes effect of particles size. J. Chil. Chem. Soc. 2014, 59. [Google Scholar] [CrossRef] [Green Version]
- Jiao, C.; Jiang, X.; Chu, H.; Jiang, H.; Sun, L. A mixed-valent CuI/CuII metal-organic framework with selective chemical sensing properties. CrystEngComm 2016, 18, 8683–8687. [Google Scholar] [CrossRef]
- Hassan, H.S.; Kashyout, A.B.; Morsi, I.; Nasser, A.A.A.; Abuklill, H. Development of polypyrrole coated copper nanowires for gas sensor application. Sens. Bio Sens. Res. 2015, 5, 50–54. [Google Scholar] [CrossRef] [Green Version]
- Migneault, I.; Catherine, D.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 2004, 37, 790–802. [Google Scholar] [CrossRef]
- Bi, X.; Yang, K.L. Real-time liquid crystal-based glutaraldehyde sensor. Sens. Actuators B Chem. 2008, 134, 432–437. [Google Scholar] [CrossRef]
- Miao, K.; Zhang, H.; Sun, L.; Zhu, Z.; Fan, L.J. Detection of glutaraldehyde in aqueous environments based on fluorescence quenching of a conjugated polymer with pendant protonated primary amino groups. J. Mater. Chem. C 2017, 5, 5010–5017. [Google Scholar] [CrossRef]
- Wang, X.; Wu, P.; Lv, Y.; Hou, X. Ultrasensitive fluorescence detection of glutaraldehyde in water samples with bovine serum albumin-Au nanoclusters. Microchem. J. 2011, 99, 327–331. [Google Scholar] [CrossRef]
- Karim-Nezhad, G.; Hasanzadeh, M.; Saghatforoush, L.; Ershadb, S.; Shadjoua, N. Kinetic study of the electro-catalytic oxidation of acetaldehyde on copper electrode. J. Chin. Chem. Soc. 2009, 56, 554–560. [Google Scholar] [CrossRef]






| Sample | Reaction Mixture | Description of Synthesis | Duration, h | Product Yield *, wt.% | ** CuI, wt.% | CuI Agglomerates Size, μm |
|---|---|---|---|---|---|---|
| Synthesis in accordance with a reaction (1) | ||||||
| 1 | CuSO4—15 mM KI—30 mM Py—100 mM | CuSO4 50 mL solution added after 5 min to the mixture of KI and Py | 1.7 | 89 | 67 ± 2 | 1.2−5.3 |
| 2 | CuSO4—15 mM KI—30 mM Py—100 mM I2—0.5 mM | CuSO4 added after 5 min to the mixture of KI, Py and I2 | 2.5–3 | 91 | 83 ± 3 | 1.4−4.2 |
| 3 | CuSO4—15 mM KI—30 mM Py—100 mM I2—1 mM | CuSO4 added after 5 min to the mixture of KI, Py and I2 | 3–3.5 | 88 | 87 ± 3 | 2.0−3.1 |
| 4 | CuSO4—1.5 mM KI—3 mM Py—10 mM I2—0.5mM | CuSO4 added after 1 h in the mixture of KI, Py and I2 | 7 | 14 | 90 ± 2 | 0.9−2.3 |
| 5 | CuSO4 ‒ 1.5 mM KI—3 mM Py—10 mM I2—0.5 mM | CuSO4 added after 2 h to the mixture of KI, Py and I2 | 5.5 | 32 | 89 ± 2 | 1.2−5.2 |
| Synthesis in accordance with a reaction (2) | ||||||
| 6 | CuSO4—1.5 mM Py—10 mM I2—1 mM | CuSO4 added after 4 h to the mixture of Py and I2 | 168 | 3 | 56 ± 2 | 0.1−0.4 |
| 7 | CuSO4—1.5 mM Py—10 mM I2—0.5 mM | CuSO4 added after 4 h to the mixture of Py and I2 | 168 | 3 | 67 ± 2 | absent |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konakov, A.O.; Dremova, N.N.; Khodos, I.I.; Koch, M.; Zolotukhina, E.V.; Silina, Y.E. One-Pot Synthesis of Copper Iodide-Polypyrrole Nanocomposites. Chemosensors 2021, 9, 56. https://doi.org/10.3390/chemosensors9030056
Konakov AO, Dremova NN, Khodos II, Koch M, Zolotukhina EV, Silina YE. One-Pot Synthesis of Copper Iodide-Polypyrrole Nanocomposites. Chemosensors. 2021; 9(3):56. https://doi.org/10.3390/chemosensors9030056
Chicago/Turabian StyleKonakov, Artem O., Nadejda N. Dremova, Igor I. Khodos, Marcus Koch, Ekaterina V. Zolotukhina, and Yuliya E. Silina. 2021. "One-Pot Synthesis of Copper Iodide-Polypyrrole Nanocomposites" Chemosensors 9, no. 3: 56. https://doi.org/10.3390/chemosensors9030056
APA StyleKonakov, A. O., Dremova, N. N., Khodos, I. I., Koch, M., Zolotukhina, E. V., & Silina, Y. E. (2021). One-Pot Synthesis of Copper Iodide-Polypyrrole Nanocomposites. Chemosensors, 9(3), 56. https://doi.org/10.3390/chemosensors9030056






