Biochar from Spent Malt Rootlets and Its Application to an Energy Conversion and Storage Device
Abstract
1. Introduction
2. Materials and Methods
2.1. Biochar Preparation
2.2. Biochar Characterization
2.3. Construction of the Electrodes
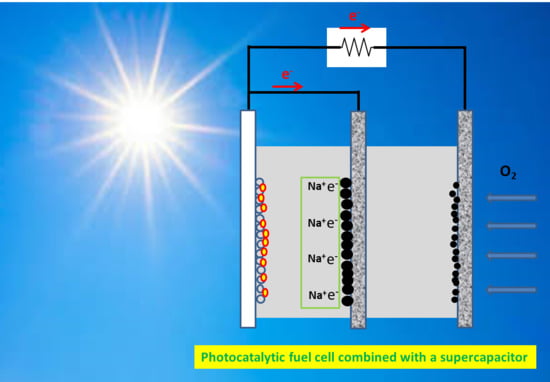
2.4. Apparatus for Photoelectrochemical Measurements
3. Results and Discussion
3.1. Physicochemical Characterization of Na–BC
3.1.1. Specific Surface Area
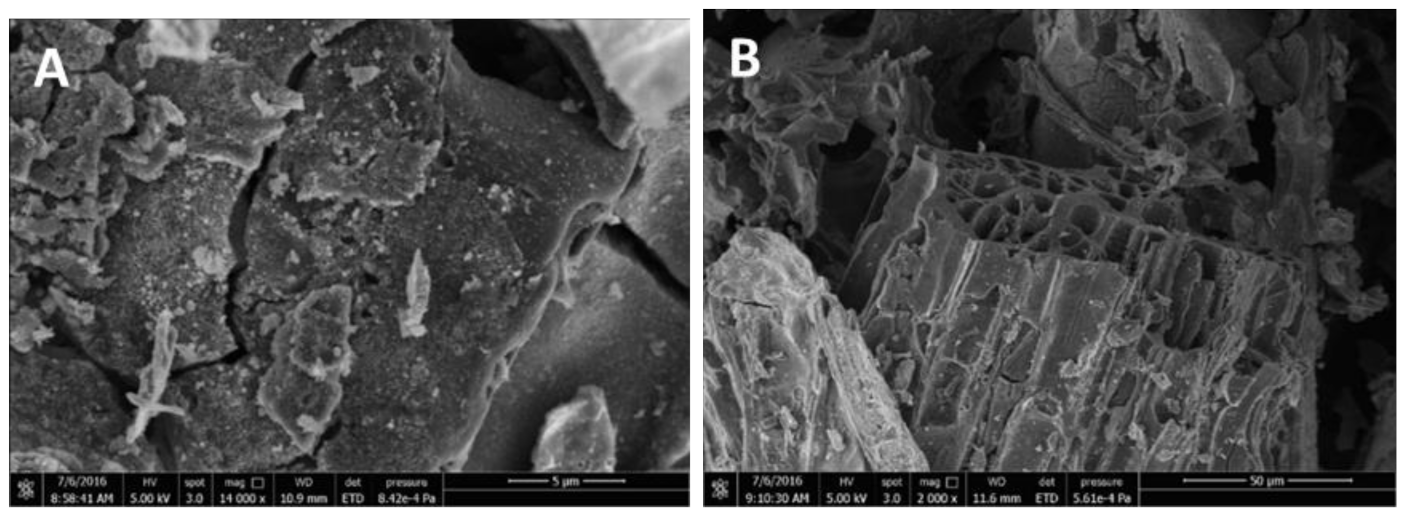
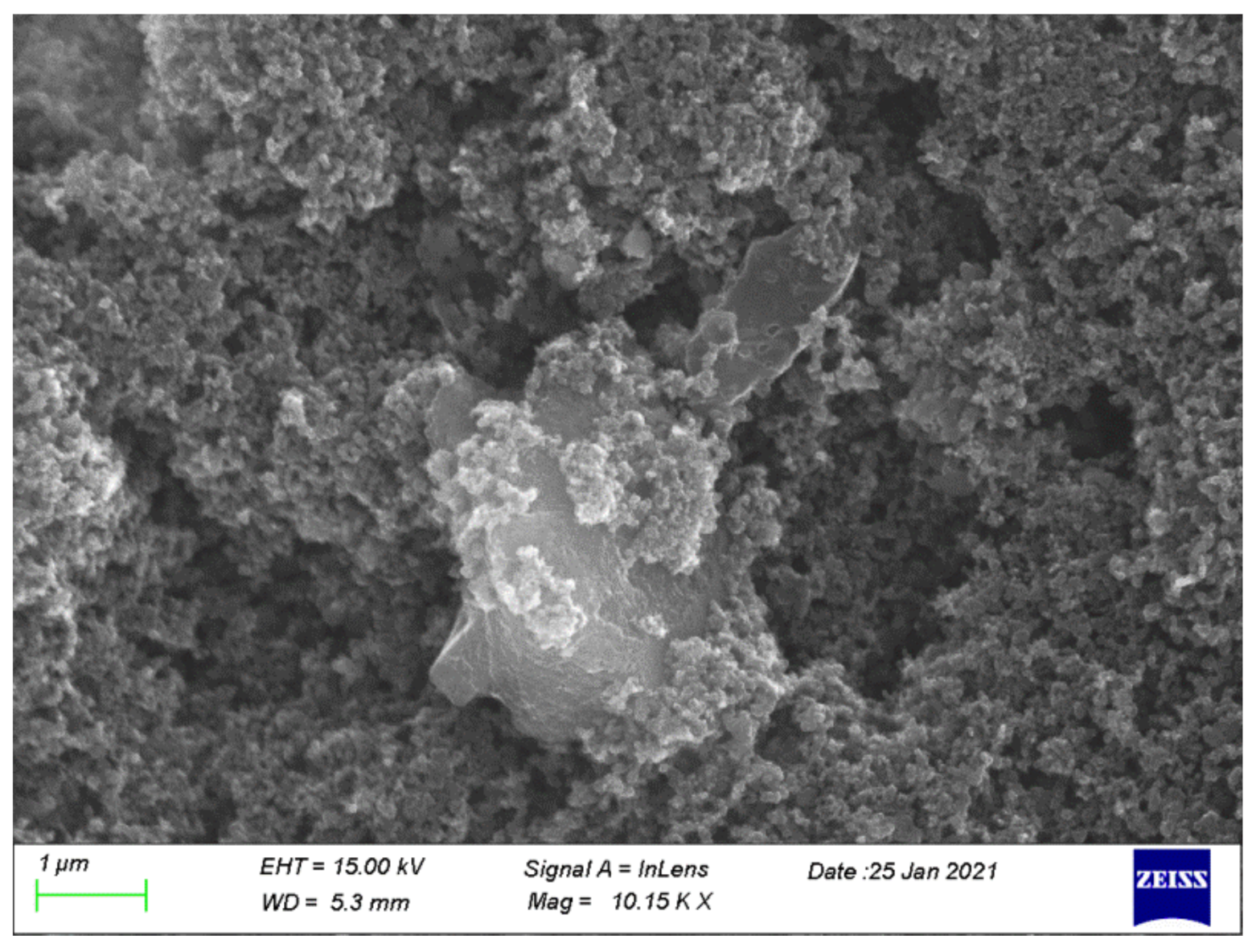
3.1.2. SEM and EDX Analysis
3.1.3. TGA Analysis
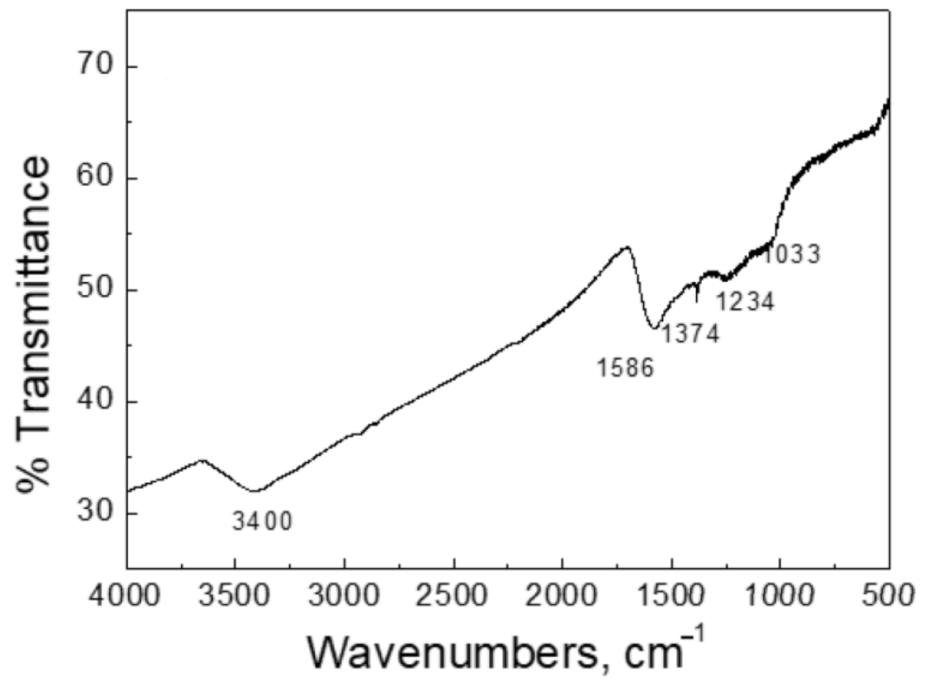
3.1.4. FTIR Spectroscopy
3.1.5. XRD Analysis
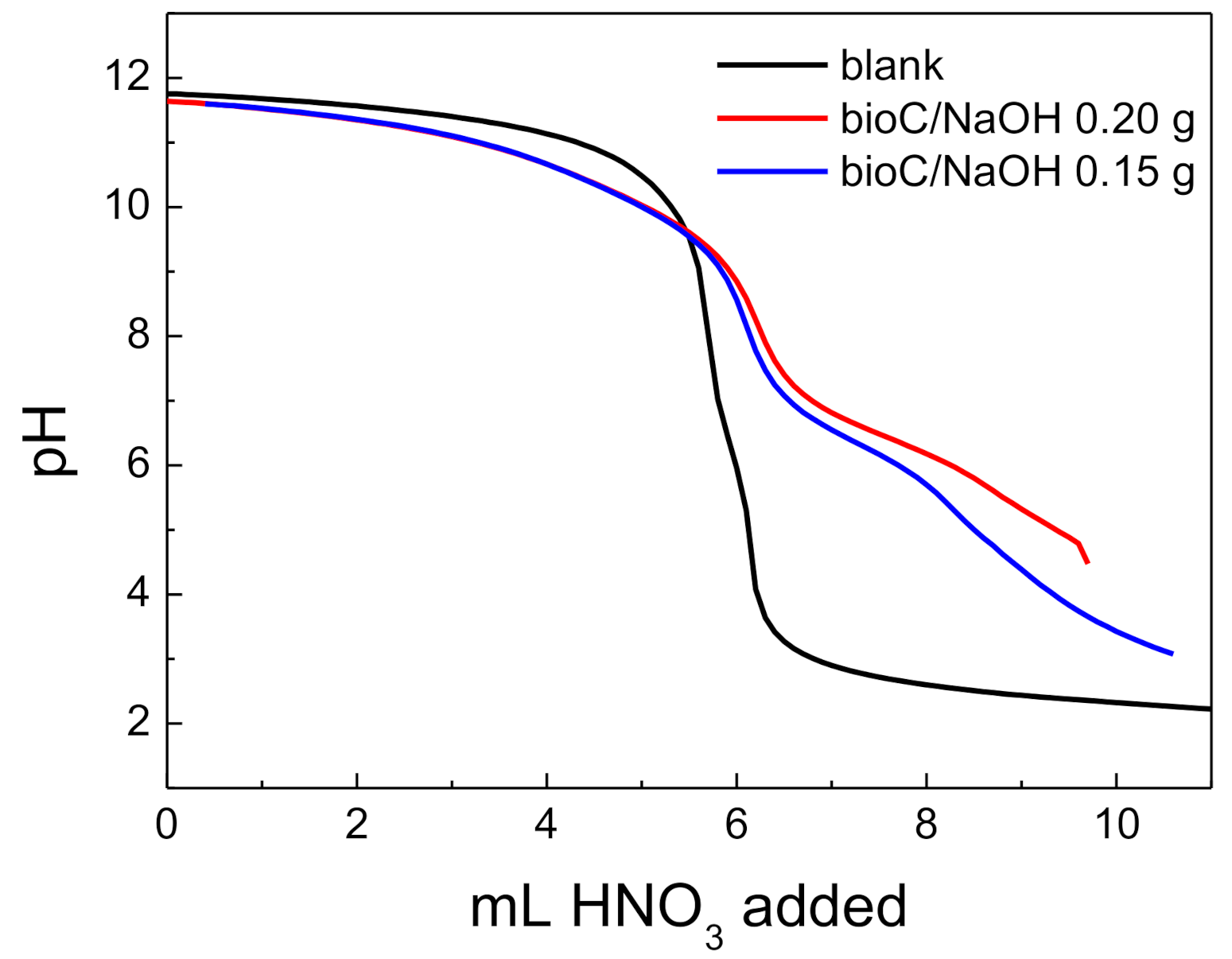
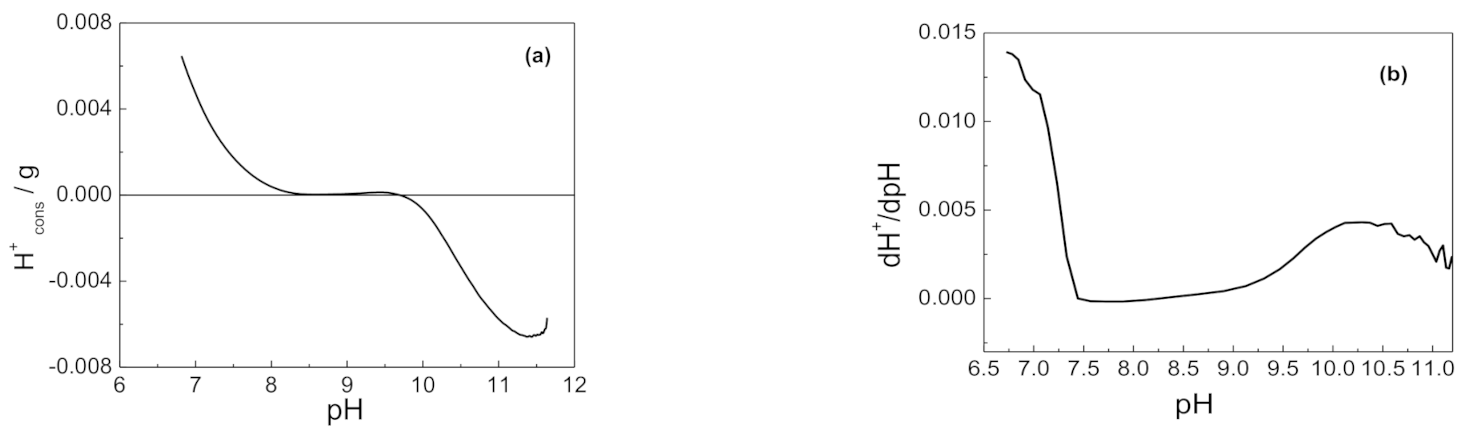
3.1.6. Acid-Base Behavior
3.2. Characterization of the Supercapacitor Electrode
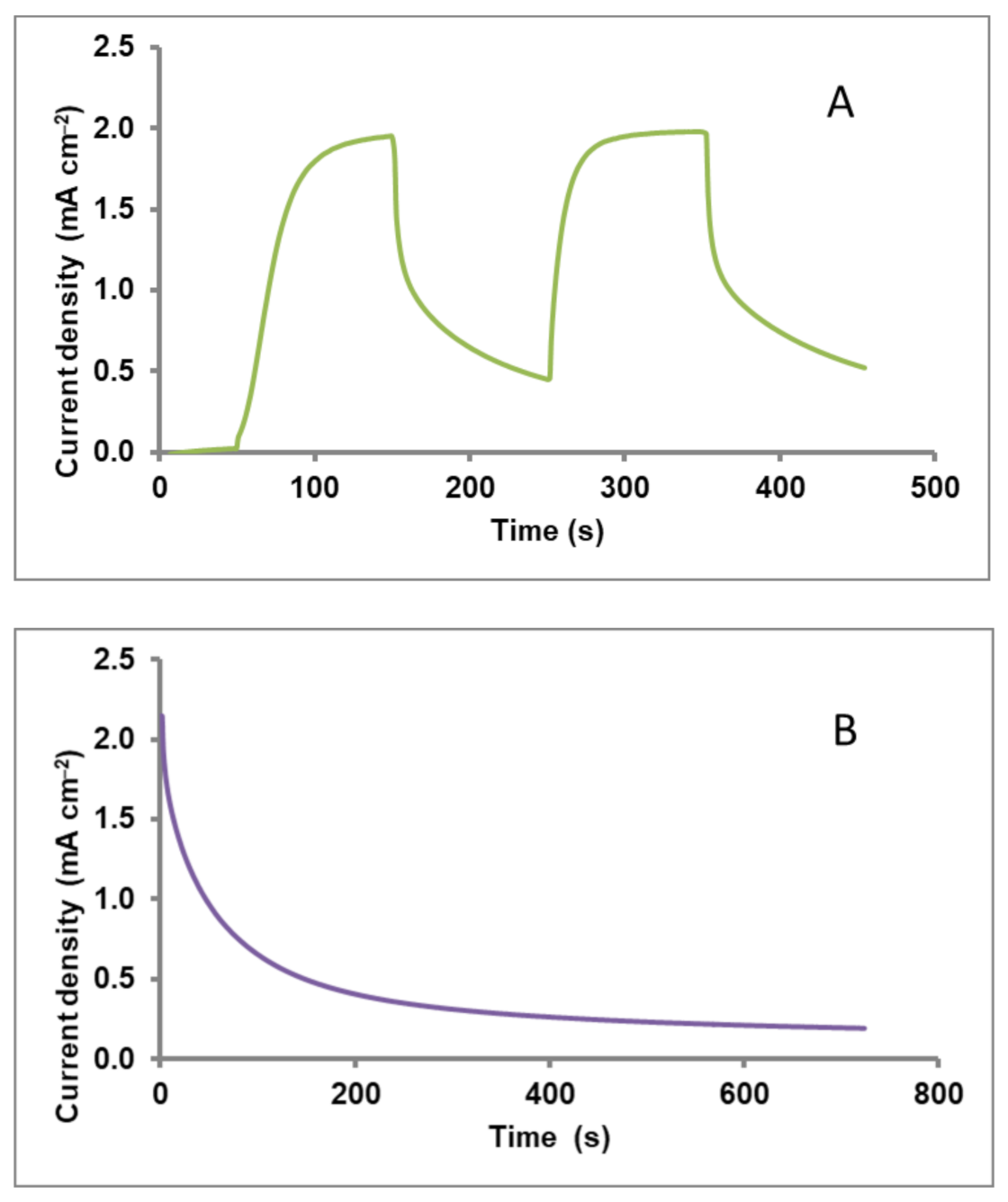
3.3. Current-Voltage Characteristics of the Photocatalytic Fuel Cell with or without a Supercapacitor Electrode
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Qian, L.; Guo, F.; Jia, X.; Zhan, Y.; Zhou, H.; Jiang, X.; Tao, C. Recent development in the synthesis of agricultural and forestry biomass-derived porous carbons for supercapacitor applications: A review. Ionics 2020, 26, 3705–3723. [Google Scholar] [CrossRef]
- Liu, W.-J.; Jiang, H.; Yu, H.-Q. Development of biochar-based functional materials: Toward a sustainable platform carbon material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef]
- Yuan, J.-H.; Xu, R.-K. The amelioration effects of low temperature biochar generated from nine crop residues on an acidic Ultisol. Soil Use Manag. 2011, 27, 110–115. [Google Scholar] [CrossRef]
- Cha, J.S.; Park, S.H.; Jung, S.C.; Ryu, C.; Jeon, J.K.; Shin, M.C.; Park, Y.K. Production and utilization of biochar: A review. Ind. Eng. Chem. 2016, 40, 1–15. [Google Scholar] [CrossRef]
- Inyang, M.; Dickenson, E. The potential role of biochar in the removal of organic and microbial contaminants from potable and reuse water: A review. Chemosphere 2015, 134, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Vakros, J. Biochars and their use as transesterification catalysts for biodiesel production: A short review. Catalysts 2018, 8, 562. [Google Scholar] [CrossRef]
- Shen, B.; Chen, J.; Yue, S.; Li, G. A comparative study of modified cotton biochar and activated carbon based catalysts in low temperature SCR. Fuel 2015, 156, 47. [Google Scholar] [CrossRef]
- Hasa, B.; Martino, E.; Vakros, J.; Trakakis, G.; Galiotis, C.; Katsaounis, A. Effect of carbon support on the electrocatalytic properties of Pt-Ru catalysts. Chem. Electron. Chem. 2019, 6, 4970–4979. [Google Scholar]
- Mian, M.; Liu, G. Recent progress in biochar-supported photocatalysts: Synthesis, role of biochar, and applications. RSC Adv. 2018, 8, 14237. [Google Scholar] [CrossRef]
- Tan, X.F.; Liu, Y.G.; Gu, Y.L.; Xu, Y.; Zeng, G.M.; Hu, X.J.; Liu, S.B.; Wang, X.; Liu, S.M.; Li, J. Biochar-based nano-composites for the decontamination of wastewater: A review. Bioresour. Technol. 2016, 212, 318–333. [Google Scholar] [CrossRef]
- Spanu, D.; Binda, G.; Dossi, C.; Monticelli, D. Biochar as an alternative sustainable platform for sensing applications: A review. Microchem. J. 2020, 159, 105506. [Google Scholar] [CrossRef]
- Muhammad, H.; Wei, T.; Cao, G.; Yu, S.H.; Ren, X.H.; Jia, H.L.; Saleem, A.; Hua, L.; Guo, J.K.; Li, Y. Study of soil microorganisms modified wheat straw and biochar for reducing cadmium leaching potential and bioavailability. Chemosphere 2021, 273, 129644. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ouyang, D.; Zhou, Z.; Page, S.J.; Liu, D.; Zhao, X. Lignocellulosic biomass as sustainable feedstock and materials for power generation and energy storage. J. Energy Chem. 2021, 57, 247–280. [Google Scholar] [CrossRef]
- Anae, J.; Ahmad, N.; Kumar, V.; Thakur, V.K.; Gutierrez, T.; Yang, X.J.; Cai, C.; Yang, Z.; Coulon, F. Recent advances in biochar engineering for soil contaminated with complex chemical mixtures: Remediation strategies and future perspectives. Sci. Total Environ. 2021, 767, 144351. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, S.; Kour, N.; Manhas, S.; Zahid, S.; Wani, O.A.; Sharma, V.; Wijaya, L.; Alyemeni, M.N.; Alsahli, A.A.; El-Serehy, H.A.; et al. Biochar as a tool for effective management of drought and heavy metal toxicity. Chemosphere 2021, 271, 129458. [Google Scholar] [CrossRef]
- Leng, L.; Xiong, Q.; Yang, L.; Li, H.; Zhou, Y.; Zhang, W.; Jiang, S.; Li, H.; Huang, H. An overview on engineering the surface area and porosity of biochar. Sci. Total Environ. 2021, 763, 144204. [Google Scholar] [CrossRef] [PubMed]
- Kamran, U.; Park, S.J. Chemically modified carbonaceous adsorbents for enhanced CO2 capture: A review. J. Clean. Prod. 2021, 290, 125776. [Google Scholar] [CrossRef]
- Chi, L.N.T.; Anto, S.; Ahamed, S.T.; Kumar, S.S.; Shanmugam, S.; Samuel, M.S.; Mathimani, T.; Brindhadevi, K.; Pugazhendhi, A. A review on biochar production techniques and biochar based catalyst for biofuel production from algae. Fuel 2021, 287, 119411. [Google Scholar] [CrossRef]
- Yang, C.S.; Jang, Y.S.; Jeong, H.K. Bamboo-based activated carbon for supercapacitor applications. Curr. Appl. Phys. 2014, 14, 1616–1620. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, J.; Yang, W. Green supercapacitor assisted photocatalytic fuel cell system for sustainable hydrogen production. Chem. Eng. J. 2021, 403, 126368–126377. [Google Scholar] [CrossRef]
- Wang, H.; Wen, J. Biomass porous carbon-based composite for high performance supercapacitor. Mater. Res. Express 2020, 7, 115601–115612. [Google Scholar] [CrossRef]
- Chen, H.; Hu, H.; Han, F.; Liu, J.; Zhang, Y.; Zheng, Y. CoMoO4/bamboo charcoal hybrid material for high-energy-density and high cycling stability supercapacitors. Dalt. Trans. 2020, 49, 10799–10807. [Google Scholar] [CrossRef]
- Cheng, J.; Hu, S.-C.; Sun, G.-T.; Kang, K.; Zhu, M.-Q.; Geng, Z.-C. Comparison of activated carbons prepared by one-step and two-step chemical activation process based on cotton stalk for supercapacitors application. Energy 2020, 215, 119144–119158. [Google Scholar] [CrossRef]
- Mensah-Darkwa, K.; Zequine, C.; Kahol, P.K.; Gupta, R.K. Supercapacitor energy storage device using biowastes: A sustainable approach to green energy. Sustainability 2019, 11, 414. [Google Scholar] [CrossRef]
- Andrade, S.T.; Vakros, J.; Mantzavinos, D.; Lianos, P. Biochar obtained by carbonization of spent coffee grounds and its application in the construction of an energy storage device. Chem. Eng. J. Adv. 2020, 4, 100061. [Google Scholar] [CrossRef]
- Jin, H.; Wang, X.; Shen, Y.; Gu, Z. A high-performance carbon derived from corn stover via microwave and slow pyrolysis for supercapacitors. J. Anal. Appl. Pyrolysis 2014, 110, 18–23. [Google Scholar] [CrossRef]
- Genovese, M.; Jiang, J.; Lian, K.; Holm, N. High capacitive performance of exfoliated biochar nanosheets from biomass waste corn cob. J. Mater. Chem. A 2015, 3, 2903–2913. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, T.; Dong, D.; Zhang, Y. Using biochar and coal as the electrode material for supercapacitor applications. Front. Energy Res. 2020, 7, 159. [Google Scholar] [CrossRef]
- Zhong-Yu, W.; Lei, F.; You-Rong, T.; Wei, W.; Xing-Cai, W.; Jian-Wei, Z. Pomelo peel derived hierarchical porous carbon as electrode materials for high-performance supercapacitor. Chin. J. Inorg. Chem. 2018, 34, 1249–1260. [Google Scholar]
- Subramanian, V.; Luo, C.; Stephan, A.M.; Nahm, K.S.; Thomas, S.; Wei, B. Supercapacitors from activated carbon derived from banana fibers. J. Phys. Chem. C 2007, 111, 7527–7531. [Google Scholar] [CrossRef]
- Neylon, E.; Arendt, E.K.; Lynch, K.M.; Zannini, E.; Bazzoli, P.; Monin, T.; Sahin, A.W. Rootlets, a malting by-product with great potential. Fermentation 2020, 6, 117. [Google Scholar] [CrossRef]
- Ntaflou, M.; Vakros, J. Transesterification activity of modified biochars from spent malt rootlets using triacetin. J. Clean. Prod. 2020, 259, 120931. [Google Scholar] [CrossRef]
- Bourikas, K.; Vakros, J.; Kordulis, C.; Lycourghiotis, A. Potentiometric mass titrations: Experimental and theoretical establishment of a new technique for determining the point of zero charge (PZC) of metal (Hydr)oxides. J. Phys. Chem. B 2003, 107, 9441–9451. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Martínez, P.M.; Bakker, R.; Harmsen, P.; Gruppen, H.; Kabela, M. Importance of acid or alkali concentration on the removal of xylan and lignin for enzymatic cellulose hydrolysis. Ind. Crop Prod. 2015, 64, 88–96. [Google Scholar] [CrossRef]
- Tala, W.; Chantara, S. Use of spent coffee ground biochar as ambient PAHs sorbent and novel extraction method for GC-MS analysis. Environ. Sci. Poll. Res. 2019, 26, 13025–13040. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, functional, and structural properties of spent coffee grounds and coffee silverskin. Food Bioprocess. Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- Magioglou, E.; Frontistis, Z.; Vakros, J.; Manariotis, I.D.; Mantzavinos, D. Activation of persulfate by biochars from valorized olive stones for the degradation of sulfamethoxazole. Catalysts 2019, 9, 419. [Google Scholar] [CrossRef]
- Bao, N.; Miao, X.; Hu, X.; Zhang, Q.; Jie, X.; Zheng, X. Novel synthesis of plasmonic Ag/AgCl@TiO2 continues fibers with enhanced broadband photocatalytic performance. Catalysts 2017, 7, 117. [Google Scholar] [CrossRef]
- Grilla, E.; Vakros, J.; Konstantinou, I.; Manariotis, I.D.; Mantzavinos, D. Activation of persulfate by biochar from spent malt rootlets for the degradation of trimethoprim in the presence of inorganic ions. J. Chem. Technol. Biotechnol. 2020, 95, 2348–2358. [Google Scholar] [CrossRef]


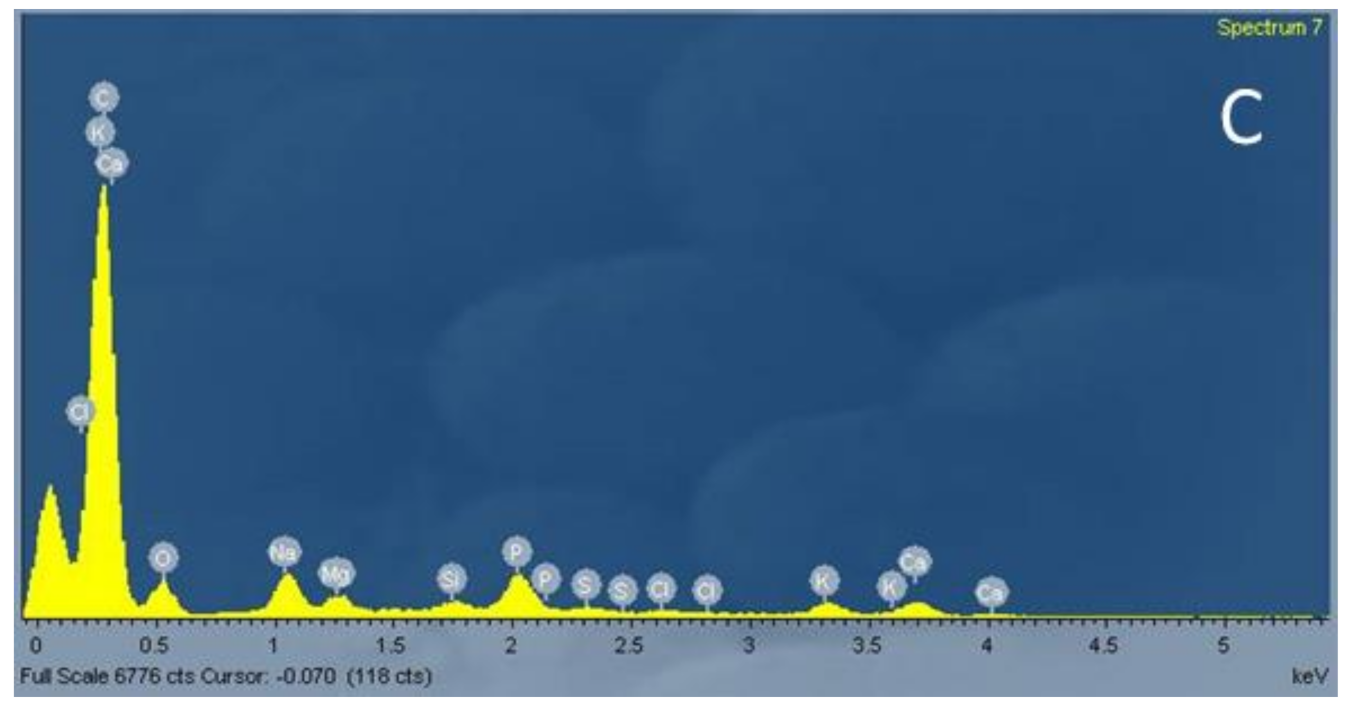




| Element | % Atomic Concentration |
|---|---|
| C | 83.24 |
| O | 11.14 |
| Na | 1.70 |
| Mg | 0.57 |
| Si | 0.32 |
| P | 1.41 |
| S | 0.19 |
| Cl | 0.13 |
| K | 0.57 |
| Ca | 0.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vakros, J.; Manariotis, I.D.; Dracopoulos, V.; Mantzavinos, D.; Lianos, P. Biochar from Spent Malt Rootlets and Its Application to an Energy Conversion and Storage Device. Chemosensors 2021, 9, 57. https://doi.org/10.3390/chemosensors9030057
Vakros J, Manariotis ID, Dracopoulos V, Mantzavinos D, Lianos P. Biochar from Spent Malt Rootlets and Its Application to an Energy Conversion and Storage Device. Chemosensors. 2021; 9(3):57. https://doi.org/10.3390/chemosensors9030057
Chicago/Turabian StyleVakros, John, Ioannis D. Manariotis, Vassilios Dracopoulos, Dionissios Mantzavinos, and Panagiotis Lianos. 2021. "Biochar from Spent Malt Rootlets and Its Application to an Energy Conversion and Storage Device" Chemosensors 9, no. 3: 57. https://doi.org/10.3390/chemosensors9030057
APA StyleVakros, J., Manariotis, I. D., Dracopoulos, V., Mantzavinos, D., & Lianos, P. (2021). Biochar from Spent Malt Rootlets and Its Application to an Energy Conversion and Storage Device. Chemosensors, 9(3), 57. https://doi.org/10.3390/chemosensors9030057