Colorimetric Chemosensor Array for Determination of Halides
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
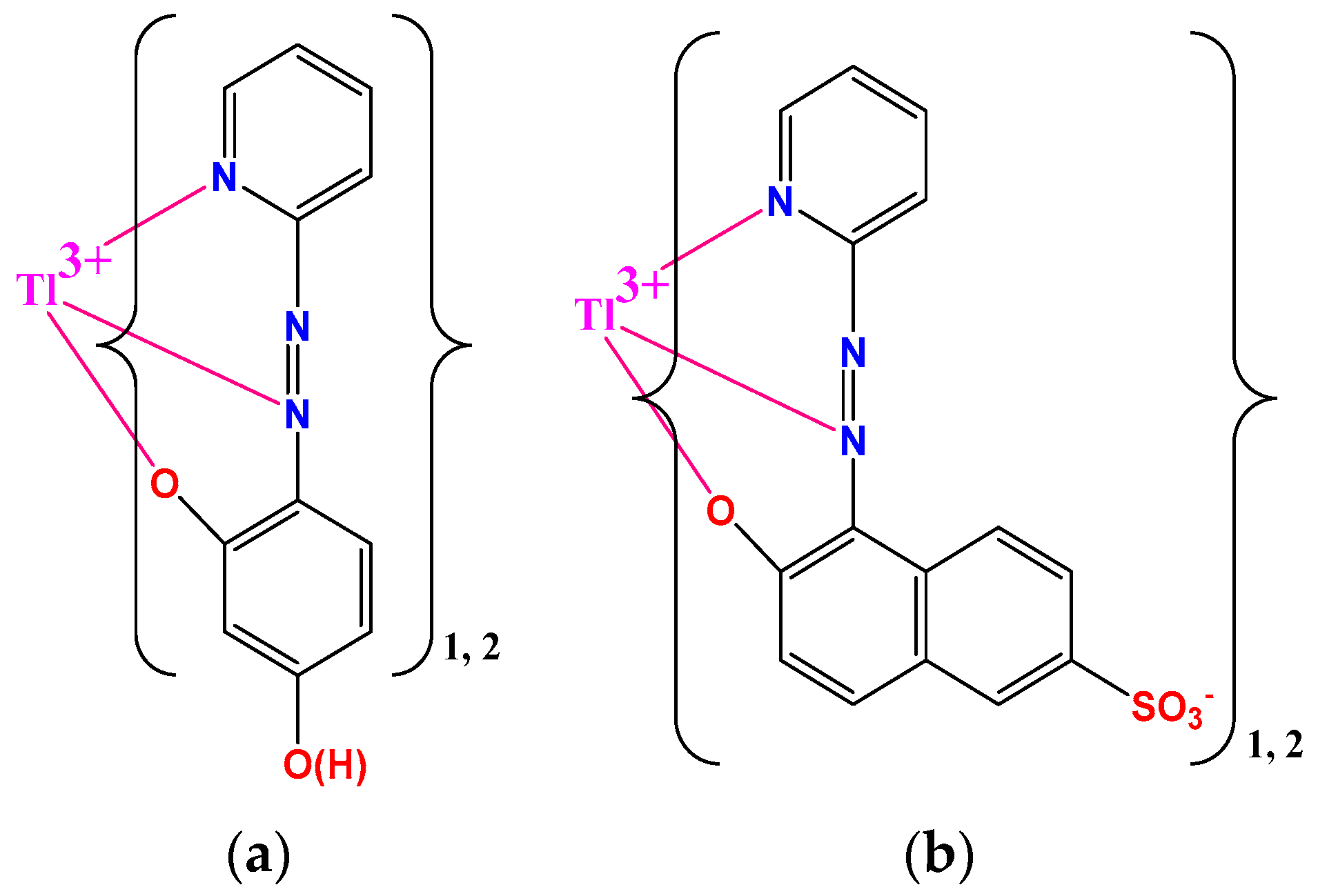
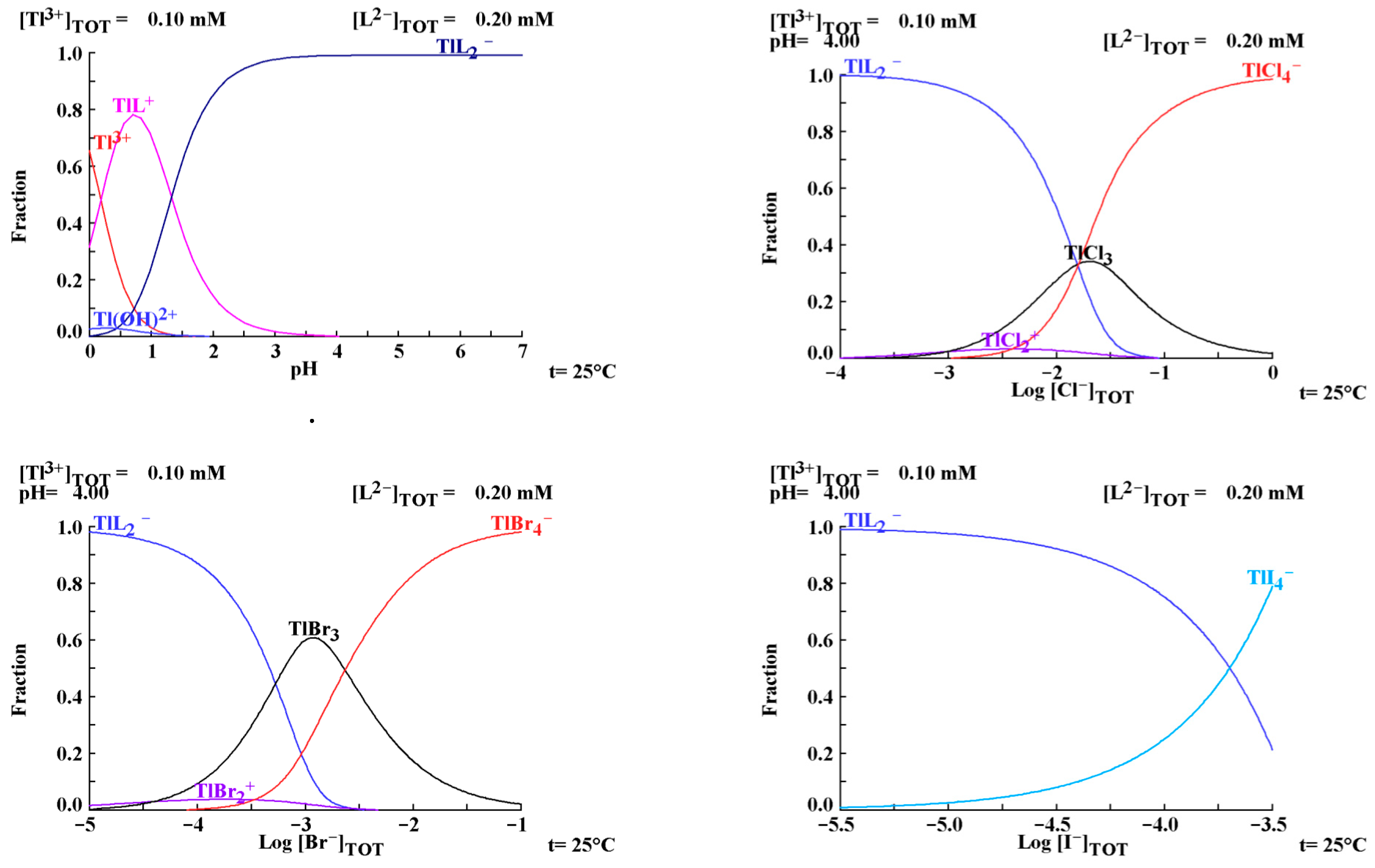
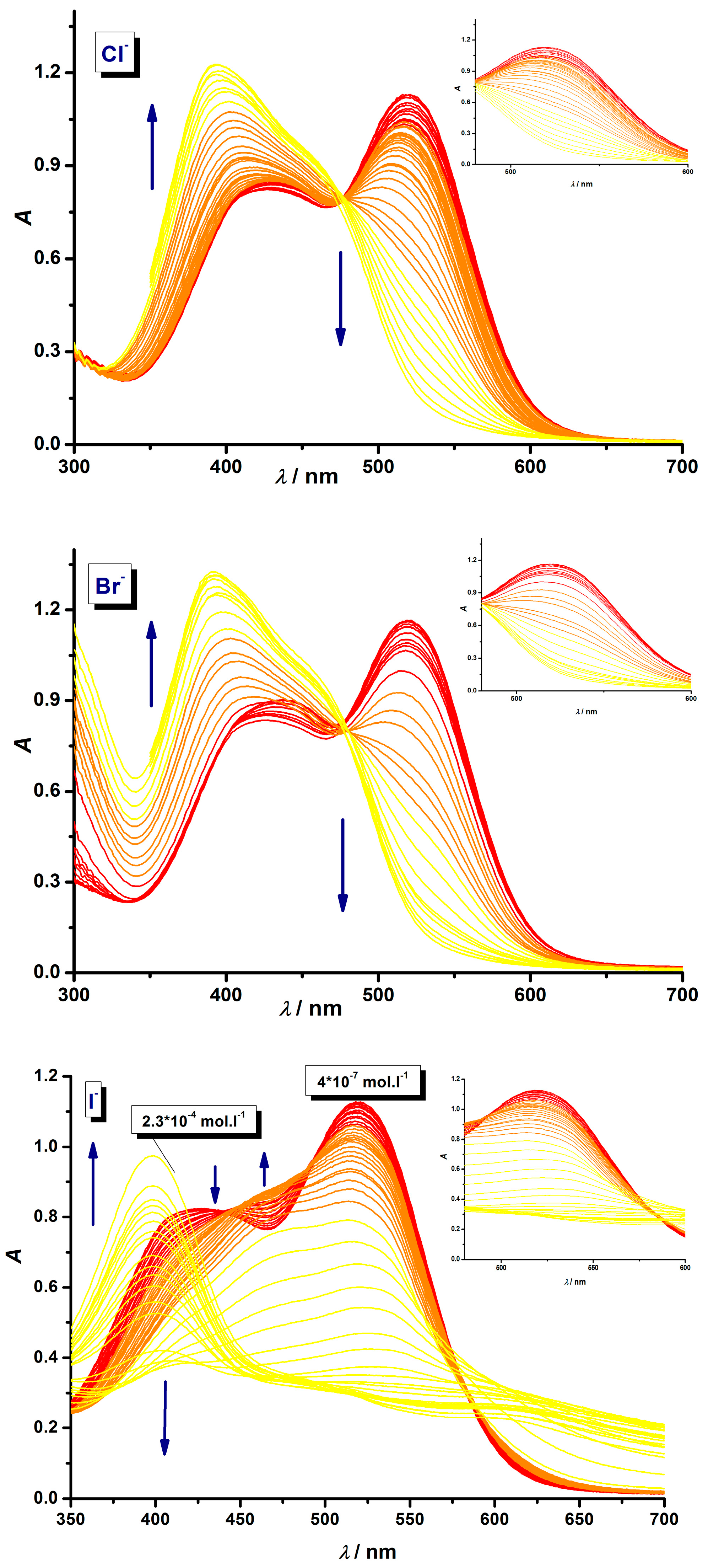
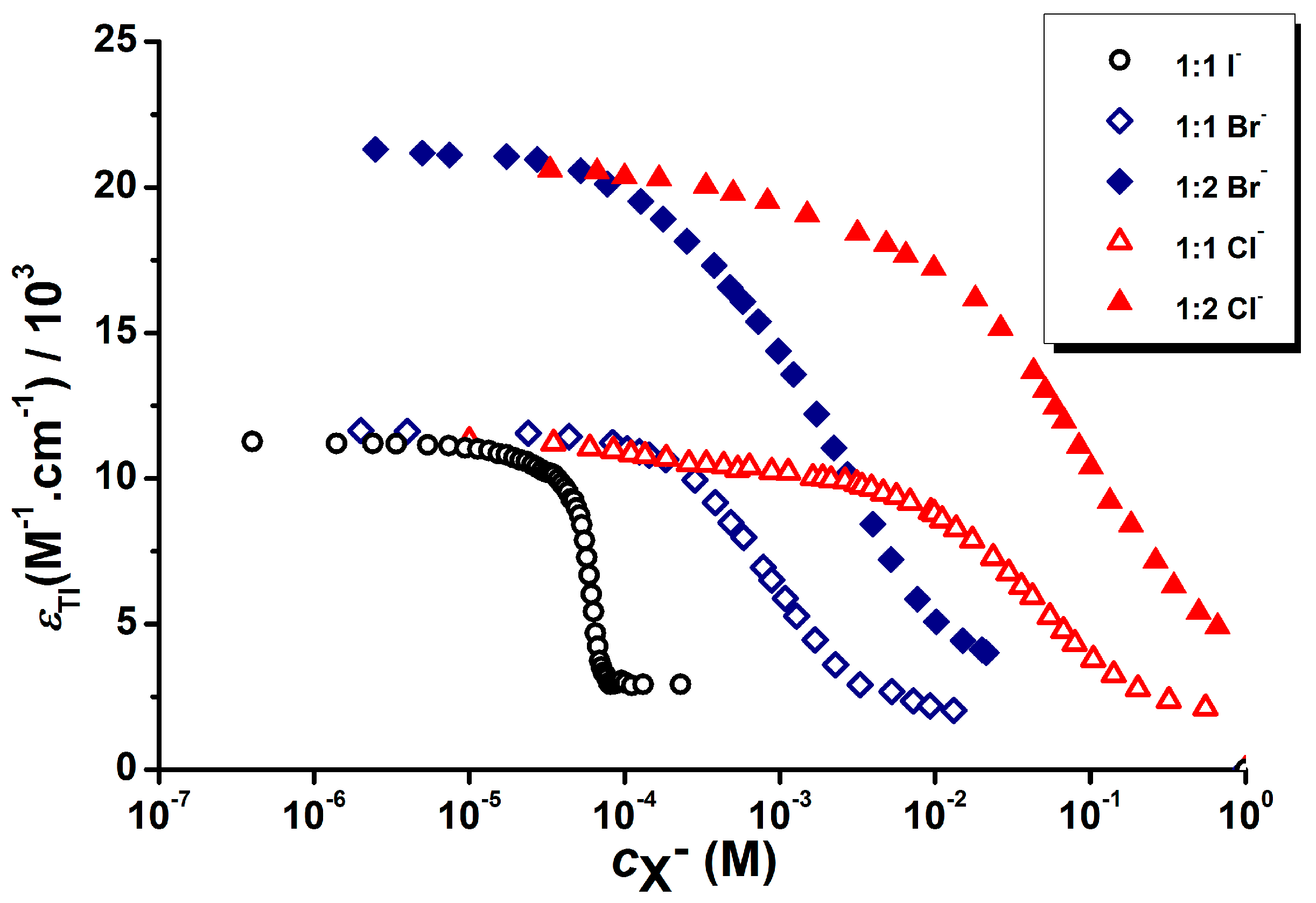
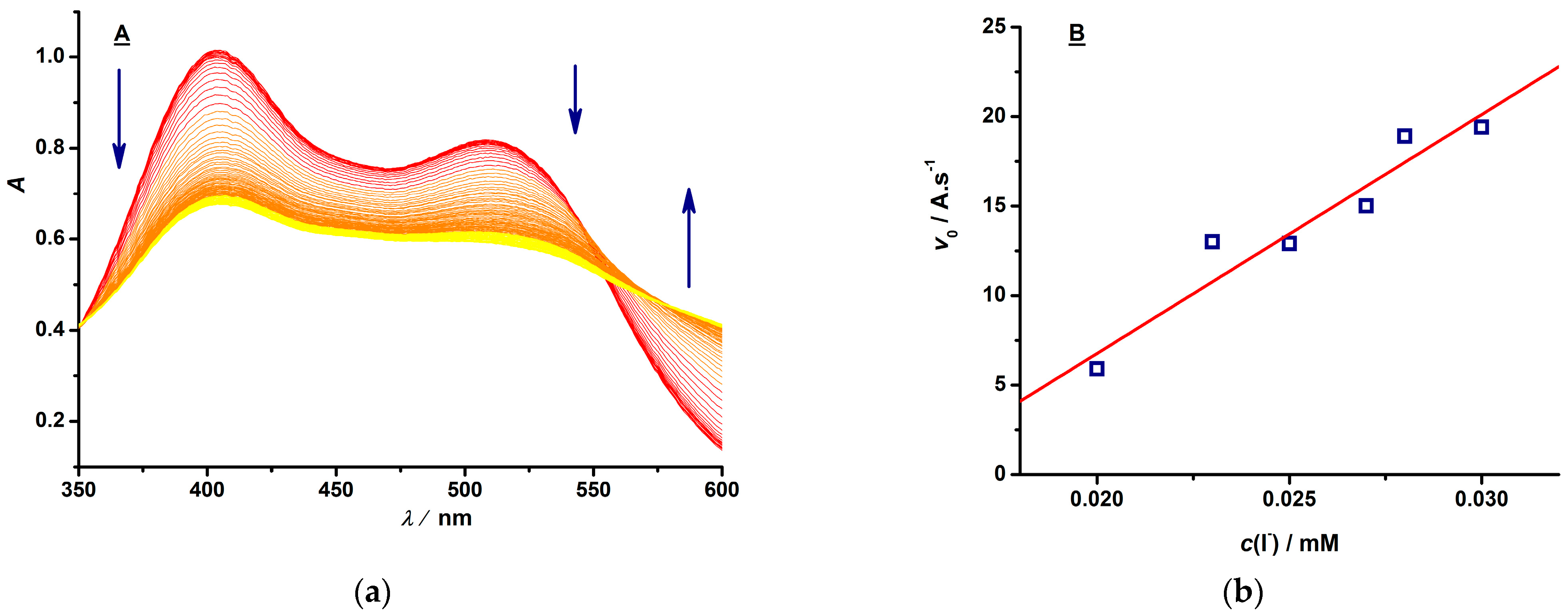
3.1. Development of Halide Sensor
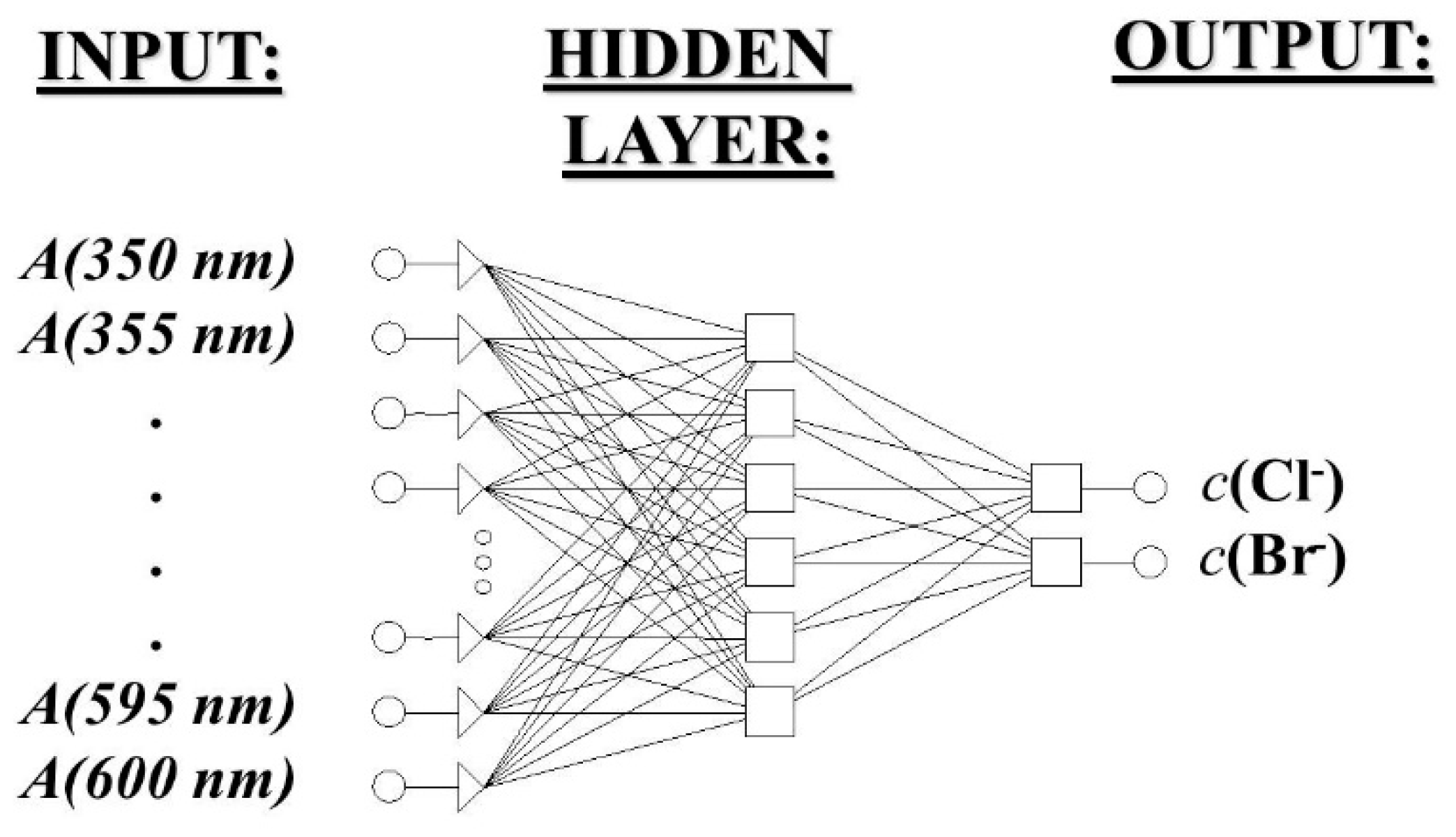
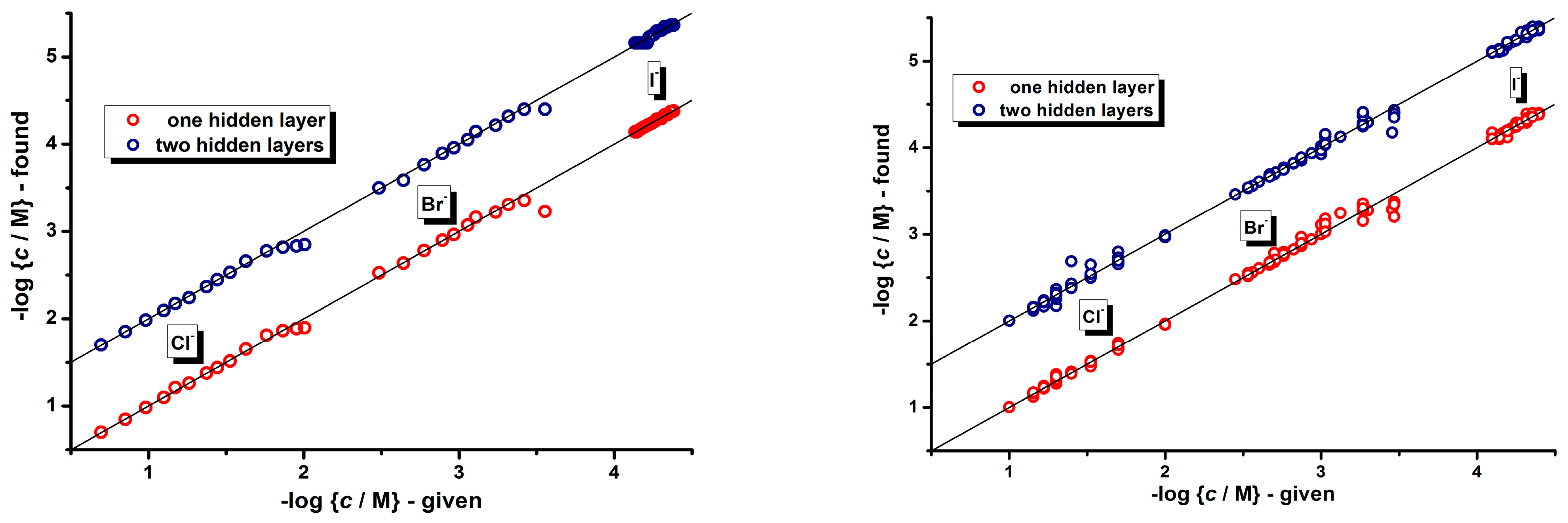
3.2. Indirect Multicomponent Analysis of Halides
3.3. Test Strips for Halides Determination
| Halides Mixture | X− | cI, cBr, cCl/mM | t-Value | |||
|---|---|---|---|---|---|---|
| Given | Found ± s | |||||
| Separate | Simultaneous | Separate | Simultaneous | |||
| Cl−:Br− | Cl | 30.20 | 26.5 ± 4.0 | 28.4 ± 1.8 | 1.59 | 1.64 |
| Br | 1.51 | 1.38 ± 0.14 | 1.51 a | 1.65 | - | |
| Cl−:I− | Cl | 22.91 | 24.8 ± 4.1 | 21.88 | 0.78 | - |
| I | 0.15 | 0.17 ± 0.01 | 0.17 ± 0.02 | 3.46 | 1.73 | |
| Cl | 19.05 | 24.0 ± 4.2 | 21.88 a | 2.07 | - | |
| I | 0.23 | 0.26 ± 0.01 | 0.27 ± 0.02 | 5.20 | 3.46 | |
| Br−: I− | Br | 1.32 | 1.25 ± 0.25 | 1.10 a | 0.50 | - |
| I | 0.15 | 0.16 ± 0.01 | 0.16 ± 0.02 | 1.73 | 0.86 | |
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Halide | log β(TlX4−) | log KTlL,X | log KTlL2,X |
|---|---|---|---|
| Cl− | 18.3 | 4.1 | −8.2 |
| Br− | 23.9 | 9.7 | −2.6 |
| I− | 35.7 | 21.5 | 9.2 |
| Halide | log β(TlX4−) | log KTlL2,X | log β’(TlL2) |
|---|---|---|---|
| Cl− | 18.3 | −4.6 | 22.9 |
| Br− | 23.9 | 1.6 | 22.3 |
| Average value | 22.6 |
References
- Nollet, L.M.L. Handbook of Water Analysis; CRC Press Boca Raton: Boca Raton, Fl, USA, 2007. [Google Scholar]
- Qu, F.; Li, N.B.; Luo, H.Q. Polyethyleneimine-Templated Ag Nanoclusters: A New Fluorescent and Colorimetric Platform for Sensitive and Selective Sensing Halide Ions and High Disturbance-Tolerant Recognitions of Iodide and Bromide in Coexistence with Chloride under Condition of High Ionic Strength. Anal. Chem. 2012, 84, 10373–10379. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Li, H.; Feng, L. Using ratiometric indicator-displacement assays in semi-quantitative colorimetric determination of chloride, bromide, and iodide anions. Analyst 2011, 136, 5025–5029. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, G.H.; Bassett, J.; Mendham, J.; Denney, R.C. Vogel’s Textbook of Quantitative Chemical Analysis; Longman: Essex, UK, 1989. [Google Scholar]
- Janata, J. Principles of Chemical Sensors; Springer: Heidelberg, Germany, 2010. [Google Scholar]
- Banica, F.-G. Chemical Sensors and Biosensors; Wiley: Chichester, UK, 2012. [Google Scholar]
- Boček, P.; Deml, M.; Gebauer, P.; Dolník, V. Analytical Isotachophoresis; VCH Weinheim (FRG): Weinheim, Germany, 1988. [Google Scholar]
- Foret, F.; Křivánková, L.; Boček, P. Capillary Zone Electrophoresis; VCH Weinheim (FRG): Weinheim, Germany, 1993. [Google Scholar]
- Buszewski, B.; Dziubakiewicz, E.; Szumski, M. (Eds.) Electromigration Techniques—Theory and Practice; Springer: Berlin, Germany, 2013. [Google Scholar]
- Wang, B.; Anslyn, E.V. (Eds.) Chemosensors. Principles, Strategies and Applications; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Snowden, T.S.; Anslyn, E.V. Anion recognition: Synthetic receptors for anions and their application in sensors. Current Op. Chem. Biol. 1999, 3, 740–746. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Anslyn, E.V. Indicator-displacement assays. Coord. Chem. Rev. 2006, 250, 3118–3127. [Google Scholar] [CrossRef]
- Anslyn, E.V. Supramolecular analytical chemistry. J. Org. Chem. 2007, 72, 687–699. [Google Scholar] [CrossRef]
- You, L.; Zha, D.; Anslyn, E.V. Recent Advances in Supramolecular Analytical Chemistry Using Optical Sensing. Chem. Rev. 2015, 115, 7840–7892. [Google Scholar] [CrossRef]
- Anzenbacher, P., Jr.; Lubal, P.; Buček, P.; Palacios, M.A.; Kozelkova, M.E. A practical approach to optical cross-reactive sensor arrays. Chem. Soc. Rev. 2010, 39, 3954–3979. [Google Scholar] [CrossRef]
- Palacios, M.A.; Nishiyabu, R.; Marquez, M.; Anzenbacher, P., Jr. Supramolecular Chemistry Approach to the Design of a High-Resolution Sensor Array for Multi-anion Detection in Water. J. Am. Chem. Soc. 2007, 129, 7538–7544. [Google Scholar] [CrossRef] [PubMed]
- Palacios, M.A.; Wang, Z.; Montes, V.A.; Zyryanov, G.V.; Anzenbacher, P., Jr. Rational Design of a Minimal Size Sensor Array for Metal Ion Detection. J. Am. Chem. Soc. 2008, 130, 10307–10314. [Google Scholar] [CrossRef]
- Liu, Y.; Palacios, M.A.; Anzenbacher, P., Jr. The power of the weak: Recognition of ion pairs in water using a simple array sensor. Chem. Comm. 2010, 46, 1860–1862. [Google Scholar] [CrossRef]
- Feng, L.; Li, H.; Li, X.; Chen, L.; Shen, Z.; Guan, Y. Colorimetric sensing of anions in water using ratiometric indicator-displacement assay. Anal. Chim. Acta 2012, 743, 1–8. [Google Scholar] [CrossRef]
- Qian, S.; Lin, H. A facile approach to cross-reactive colorimetric sensor arrays: An application in the recognition of the 20 natural amino acids. RSC Adv. 2014, 4, 29581. [Google Scholar] [CrossRef]
- Li, H.; Jia, M.; Askim, J.R.; Zhang, Y.; Duan, C.; Guana, Y.; Feng, L. An array sensor consisting of a single indicator with multiple concentrations and its application in ion discrimination. Chem. Commun. 2014, 50, 15389–15392. [Google Scholar] [CrossRef]
- Wang, Y.; Huo, D.; Wu, H.; Li, J.; Zhang, Q.; Deng, B.; Zhou, J.; Yang, M.; Hou, C. A visual sensor array based on an indicator displacement assay for the detection of carboxylic acids. Microchim. Acta 2019, 186, 496. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.G.; Topolnicki, I.L.; Zwicker, V.E.; Jolliffe, K.A.; New, E.J. Fluorescent sensing arrays for cations and anions. Analyst 2017, 142, 3549–3563. [Google Scholar] [CrossRef]
- Fan, J.; Ding, L. Single-system based discriminative optical sensors: Different strategies and versatile applications. Analyst 2018, 143, 3775–3788. [Google Scholar] [CrossRef]
- Kangas, M.J.; Burks, R.M.; Atwater, J.; Lukowicz, R.M.; Williams, P.; Holmes, A.E. Colorimetric Sensor Arrays for the Detection and Identification of Chemical Weapons and Explosives. Crit. Rev. Anal. Chem. 2017, 47, 138–153. [Google Scholar] [CrossRef] [PubMed]
- Yawer, M.A.; Havel, V.; Šindelář, V. A Bambusuril Macrocycle that Binds Anions in Water with High Affinity and Selectivity. Angew. Chem. Int. Ed. 2015, 54, 276–279. [Google Scholar] [CrossRef]
- Lisbjerg, M.; Nielsen, B.E.; Milhoj, B.O.; Sauer, S.P.A.; Pittelkow, M. Anion binding by biotin [6]uril in water. Org. Biomol. Chem. 2015, 13, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Sommer, F.; Marcus, Y.; Kubik, S. Effects of Solvent Properties on the Anion Binding of Neutral Water-Soluble Bis(cyclopeptides) in Water and Aqueous Solvent Mixtures. ACS Omega 2017, 2, 3669–3680. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Kaur, G.; Fegade, U.A.; Singh, A.; Sahoo, S.K.; Kuwar, A.S.; Singh, A. Anion sensing with chemosensors having multiple -NH recognition Units. Trends Anal. Chem. 2017, 95, 86–109. [Google Scholar] [CrossRef]
- Amendola, V.; Bergamaschi, G.; Boiocchi, M.; Fabbrizzi, L.; Poggi, A.; Zema, M. Halide ion inclusion into a dicopper(II) bistren cryptate containing ‘active’ 2,5-dimethylfuran spacers: The origin of the bright yellow colour. Inorg. Chim. Acta 2008, 361, 4038–4046. [Google Scholar] [CrossRef]
- Alibrandi, G.; Amendola, V.; Bergamaschi, G.; Dollenz, R.; Fabbrizzi, L.; Licchelli, M.; Lo Vecchio, C. An Automatic Molecular Dispenser of Chloride. Chem. Eur. J. 2013, 19, 3729–3734. [Google Scholar] [CrossRef]
- Bergamaschi, G.; Boiocchi, M.; Perrone, M.L.; Poggi, A.; Viviania, I.; Amendola, V. Mixing the spacers in azacryptands: Effects on halide recognition. Dalton Trans. 2014, 43, 11352–11360. [Google Scholar] [CrossRef]
- Invernici, M.; Ciarrocchi, C.; Dondi, D.; Fabbrizzi, L.; Lazzaroni, S.; Licchelli, M.; Boiocchi, M.; Bonizzoni, M. Bimacrocyclic Effect in Anion Recognition by a Copper(II) Bicyclam Complex. ACS OMEGA 2018, 3, 15692–15701. [Google Scholar] [CrossRef]
- Boiocchi, M.; Bonizzoni, M.; Ciarrocchi, C.; Fabbrizzi, L.; Invernici, M.; Licchelli, M. Anion Recognition in Water, Including Sulfate, by a Bicyclam Bimetallic Receptor: A Process Governed by the Enthalpy/Entropy Compensatory Relationship. Chem. Eur. J. 2018, 24, 5659–5666. [Google Scholar] [CrossRef]
- Marshall, S.R.; Singh, A.; Wagner, J.N.; Busschaert, N. Enhancing the selectivity of optical sensors using synthetic transmembrane ion transporters. Chem. Comm. 2020, 56, 14455–14458. [Google Scholar] [CrossRef]
- Holzbecher, Z.; Diviš, L.; Král, M.; Šůcha, L.; Vláčil, F. Handbook of Organic Reagents in Inorganic Analysis; Ellis Horwood: Chichester, UK, 1976. [Google Scholar]
- Ueno, K.; Imamura, T.; Cheng, K.I. Handbook of Organic Analytical Reagents; CRC Press Boca: Raton, FL, USA, 1992. [Google Scholar]
- Langová-Hniličková, M.; Sommer, L. The Coordination and Analytical Chemsitry of n-Heterocyclic azo Dyes. Folia Fac. Sci. Natur. Univ. Purkynianae Brunensis 1968, 9, 1–131. [Google Scholar]
- Anderson, R.G.; Nickless, G. Heterocyclic Azo Dyestuffs in Analytical Chemistry. A Review. Analyst 1967, 92, 207–238. [Google Scholar] [CrossRef] [PubMed]
- Lubal, P.; Farková, M. Application of artificial neural networks in speciation analysis. In Utilizing of Bio-Electrochemical and Mathematical Methods in Biological Research; Kizek, R., Adam, V., Eds.; Research Signpost: Kerala, India, 2007. [Google Scholar]
- Busev, A.I.; Tiptsova, V.G. Research in thallium(III) analytical chemistry. 5. On complexometric indicators for Tl(III) ion analysis. Zh. Anal. Khim. 1960, 15, 573–580. [Google Scholar]
- Musso, S. Untersuchung von Gleichgewichten in wässriger Lösung und Kristallstrukturbestimmung von Komplex des Thallium(III) mit Chelatliganden. Ph.D. Thesis, ETH Zürich, Rämistrasse, Switzerland, 1993. [Google Scholar]
- Gramlich, V.; Lubal, P.; Musso, S.; Anderegg, G. The stability of metal N,N,N ’,N ’-tetrakis(2-aminoethyl)ethane-1,2-diamine (= penten) complexes and the X-ray crystal structure of [Tl(NO3)(penten)](NO3)2. Helv. Chim. Acta 2001, 84, 623–631. [Google Scholar] [CrossRef]
- Chen, B.; Lubal, P.; Musso, S.; Anderegg, G. Equilibria with the thallium(III)triethylenetetraminehexaacetate anion [Tl(ttha)]3− in aqueous solution. Anal. Chim. Acta 2000, 406, 317–323. [Google Scholar] [CrossRef]
- John Peter, A.L.; Viraraghavan, T. Thallium: A review of public health and environmental concerns. Environ. Int. 2005, 31, 493–501. [Google Scholar] [CrossRef]
- Belzile, N.; Chen, Y.-W. Thallium in the environment: A critical review focused on natural waters, soils, sediments and airborne particles. Appl. Geochem. 2017, 84, 218–243. [Google Scholar] [CrossRef]
- Hniličková, M.; Sommer, L. Spectrophotometric determination of thallium with 4-(2-pyridylazo)resorcinol and 4-(2-thiazolylazo)resorcinol. Talanta 1968, 16, 83–94. [Google Scholar] [CrossRef]
- Sommer, L.; Voznica, P. On the reaction of thallium(III) with heterocyclic azo dyes. Scripta Fac. Sci. Natur. Univ. Purkynianae Brunensis 1980, 10, 81–92. [Google Scholar]
- Biryuk, E.A.; Ravitska, R.V. Interaction of Thallium(III) Ions with Pyridylazonaphthol and Pyridylazoresorcinol. Zh. Anal. Khim. 1971, 26, 1767–1769. [Google Scholar]
- Glaser, J.; Henriksson, U. Thallium-205 Nuclear Magnetic Resonance Study of Thallium(III) Halide Complexes in Aqueous Solutions. J. Am. Chem. Soc. 1981, 103, 6642–6649. [Google Scholar] [CrossRef]
- Johansson, L. The Complex Formation of Thallium(III) with Iodide in Aqueous Solution. Acta Chem. Scand. 1966, 20, 2156–2168. [Google Scholar] [CrossRef]
- Blixt, J.; Györi, B.; Glaser, J. Determination of Stability Constants for Thallium(III) Cyanide Complexes in Aqeous Solution by means of 13C and 205Tl NMR. J. Am. Chem. Soc. 1989, 111, 7784–7791. [Google Scholar] [CrossRef]
- Voznica, P.; Havel, J.; Sommer, L. The reactions of galium, indium and thallium with 2-(2-pyridylazo)-1-naphthol-4-sulfonic acid and their spectrophotometric determination. Collection Czechoslovak Chem. Commun. 1980, 45, 54–79. [Google Scholar] [CrossRef]
- Sommer, L.; Kubáň, V.; Langová, M. Spectrophotometric Investigation of Complexes with Organic Reagents for the Optimization of Spectrophotometric Determinations (UV + VIS) of Inorganic Analytes. Fresenius Z. Anal. Chem. 1982, 310, 51–61. [Google Scholar] [CrossRef]
- Havel, J.; Lubal, P.; Farková, M. Evaluation of chemical equilibria with the use of artificial neural networks. Polyhedron 2002, 21, 1375–1384. [Google Scholar] [CrossRef]
- Harris, D.C. Quantitative Chemical Analysis; W.H. Freeman: New York, NY, USA, 1991. [Google Scholar]
- Biryuk, E.A.; Ravitska, R.V. Mixed-Ligand Complexes of Aluminum and Thallium with 4-(2-Pyridylazo)-Resorcinol and Antipyrine. Zh. Anal. Khim. 1973, 28, 1500–1505. [Google Scholar]
- Funada, R.; Imamura, T.; Fujimoto, M. Rate and Mechanism of the Complex Formation of Thallium(III) with 4-(2-Pyridylazo)resorcinol (PAR) in Aqueous Solution. Bull. Chem. Soc. Japan 1979, 52, 1535–1536. [Google Scholar] [CrossRef]
- Kotrlý, S.; Šůcha, L. Handbook of Chemical Equilibria in Analytical Chemistry; Ellis Horwood: Chichester, UK, 1985. [Google Scholar]
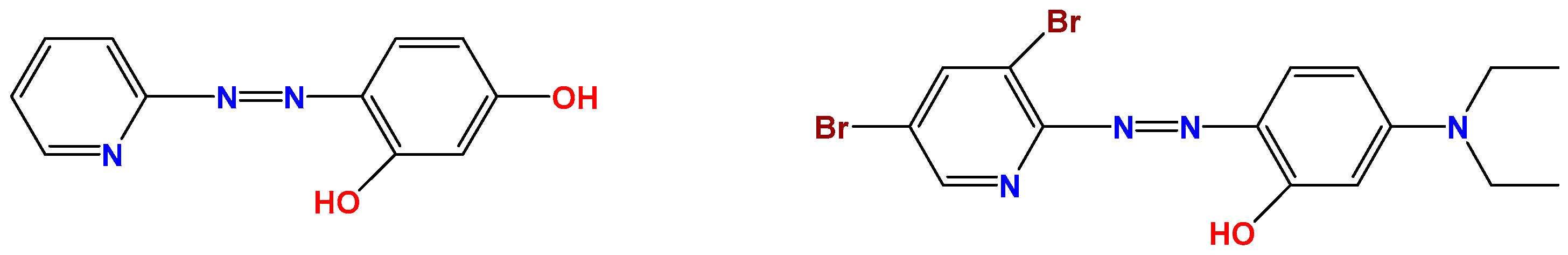
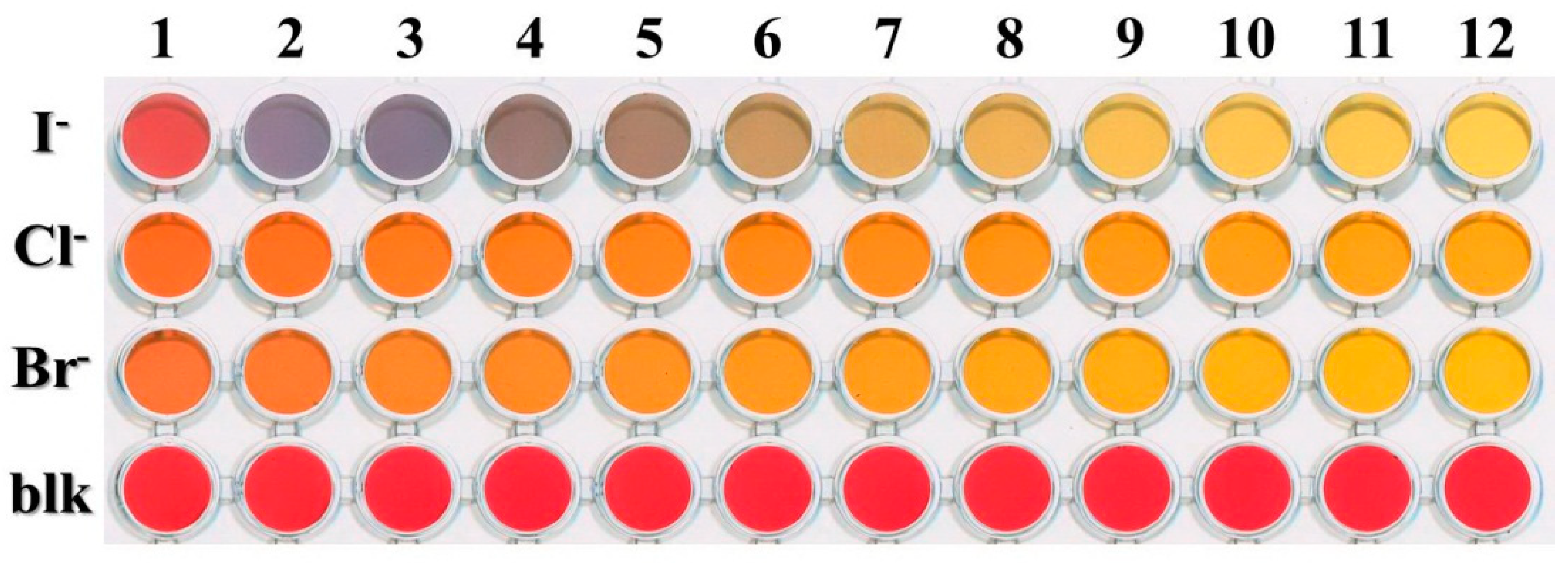

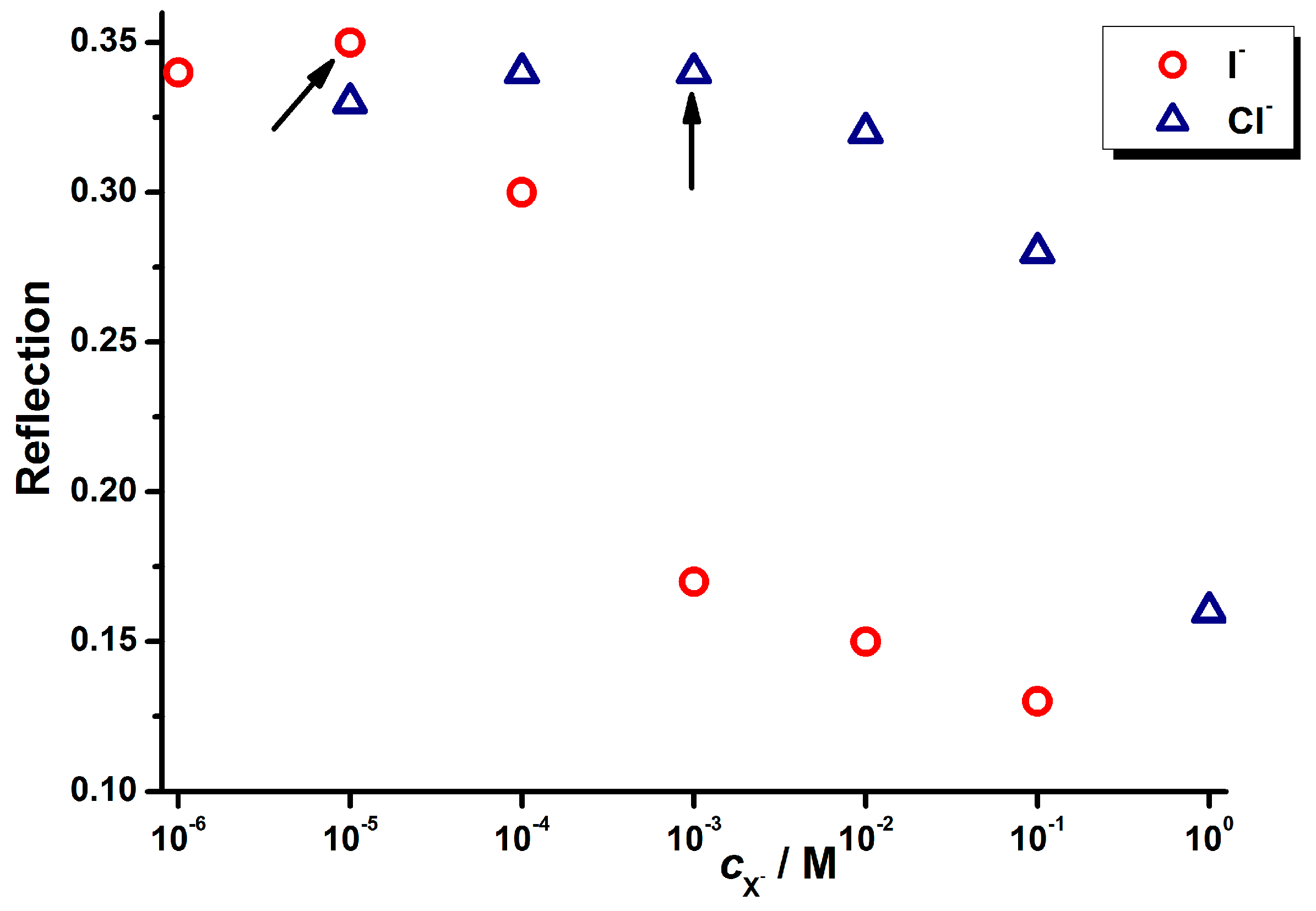
| Eigenvalue Cl− | CTV % | Eigenvalue Br− | CTV % | Eigenvalue I− | CTV % | |
|---|---|---|---|---|---|---|
| 1 | 4.8387 | 96.7734 | 4.7287 | 94.5742 | 2.6591 | 53.1811 |
| 2 | 0.1605 | 99.9843 | 0.2689 | 99.9513 | 1.7812 | 88.8056 |
| 3 | 0.0005 | 99.9936 | 0.0021 | 99.9926 | 0.3424 | 95.6544 |
| 4 | 0.0003 | 99.9994 | 0.0002 | 99.9972 | 0.1934 | 99.5234 |
| 5 | 0.0000 | 100.0000 | 0.0001 | 100.000 | 0.0238 | 100.000 |
| X− | Detection Limit (mM) | Dynamic Range (mM) | Sensitivity ± s (M−1) |
|---|---|---|---|
| Cl− | 6.7 b; 8.7 c | 7–200 b, 9–370 c | 6.5(4) b; 3.2(3)c |
| Br− | 0.2 bc | 0.2–3.0 b, 0.2–12.4 c | 518(20) b; 230(16)c |
| I− | 0.04 b | 0.047–0.073 b, 0.02–0.04 c | 2.92(6) × 104 b; 1.34(2) × 103 ac |
| Anion | Concentration (mM) | Tolerance Ratio |
|---|---|---|
| SO42− | 120 | 1200 |
| NO2− | 1.9 | 19 |
| NO3− | 240 | 2400 |
| CO32− | 24 | 240 |
| HPO4− | 61 | 610 |
| Acetate− | 240 | 2400 |
| Oxalate2− | 30 | 300 |
| F− | 120 | 1200 |
| Halides Mixture | X− | cBr, cCl/mM, cI/µM | t-Value | |||
|---|---|---|---|---|---|---|
| Given | Found ± s | |||||
| Separate | Simultaneous | Separate | Simultaneous | |||
| Cl−:Br− | Cl | 19.20 | 15.6 ± 1.7 | 21.1 ± 1.1 | 3.65 | 3.08 |
| Br | 1.15 | 1.05 ± 0.16 | 1.14 ± 0.13 | 1.09 | 0.16 | |
| Cl−:I− | Cl | 9.80 | 9.42 ± 0.61 | 10.32± 0.49 | 1.00 | 1.94 |
| I | 90.9 | 90.3 ± 17.4 | 96.4 ± 6.7 | 0.09 | 1.34 | |
| Cl | 28.30 | 27.0 ± 5.8 | 31.8 ± 4.3 | 0.36 | 1.48 | |
| I | 74.1 | 78.4 ± 5.5 | 74.3 ± 5.8 | 1.34 | 0.05 | |
| Br−: I− | Br | 0.78 | 1.06 ± 0.52 | 1.04 ± 0.65 | 0.94 | 0.71 |
| I | 38.5 | 50.6 ± 21.9 | 38.5 a | 0.93 | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šídlo, M.; Lubal, P.; Anzenbacher, P., Jr. Colorimetric Chemosensor Array for Determination of Halides. Chemosensors 2021, 9, 39. https://doi.org/10.3390/chemosensors9020039
Šídlo M, Lubal P, Anzenbacher P Jr. Colorimetric Chemosensor Array for Determination of Halides. Chemosensors. 2021; 9(2):39. https://doi.org/10.3390/chemosensors9020039
Chicago/Turabian StyleŠídlo, Michal, Přemysl Lubal, and Pavel Anzenbacher, Jr. 2021. "Colorimetric Chemosensor Array for Determination of Halides" Chemosensors 9, no. 2: 39. https://doi.org/10.3390/chemosensors9020039
APA StyleŠídlo, M., Lubal, P., & Anzenbacher, P., Jr. (2021). Colorimetric Chemosensor Array for Determination of Halides. Chemosensors, 9(2), 39. https://doi.org/10.3390/chemosensors9020039







