A Novel Multi-Ionophore Approach for Potentiometric Analysis of Lanthanide Mixtures
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Membrane Compositions
2.3. Potentiometric Measurements
3. Results and Discussion
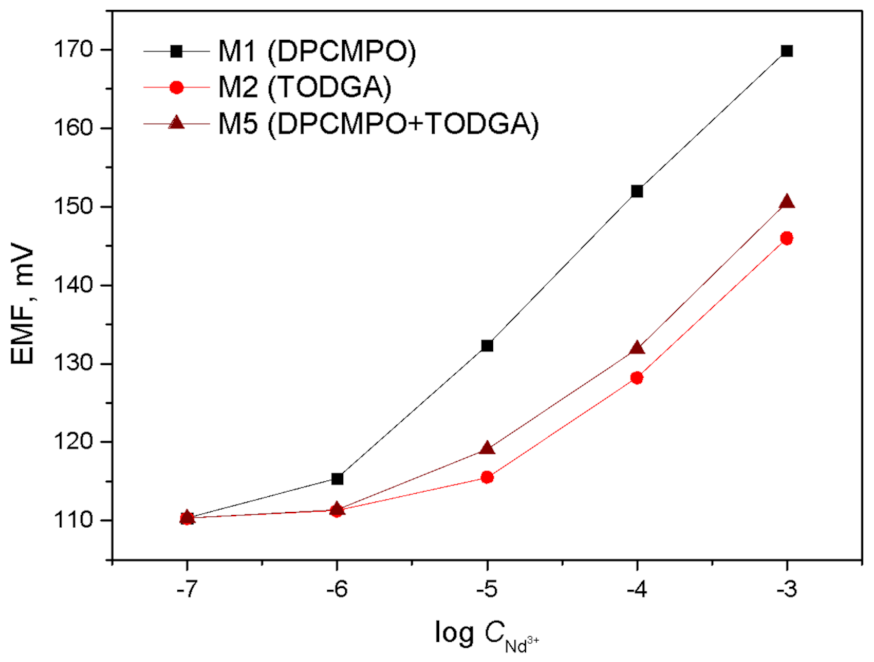
3.1. Sensor Sensitivity
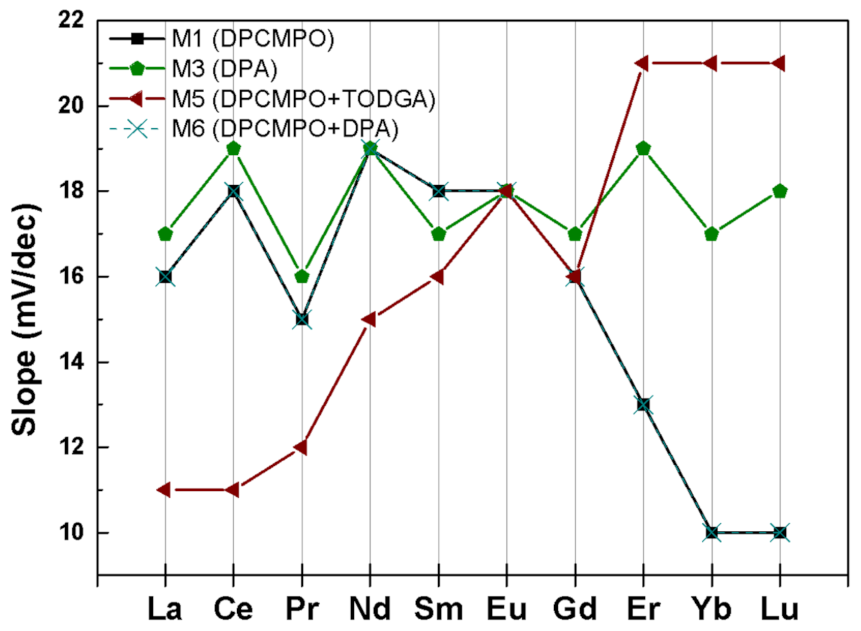
3.2. Selectivity of Ln3+ Determination
3.3. Binary Mixtures Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crespo, G.A. Recent Advances in Ion-selective membrane electrodes for in situ environmental water analysis. Electrochim. Acta 2017, 245, 1023–1034. [Google Scholar] [CrossRef]
- Gallardo, J.; Alegret, S.; Del Valle, M. Application of a potentiometric electronic tongue as a classification tool in food analysis. Talanta 2005, 66, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Oleneva, E.; Savosina, J.; Agafonova-Moroz, M.; Lumpov, A.; Babain, V.; Jahatspanian, I.; Legin, A.; Kirsanov, D. Potentiometric multisensor system for tetra- and hexavalent actinide quantification in complex rare earth metal mixtures related to spent nuclear fuel reprocessing. Sens. Actuators B Chem. 2019, 288, 155–162. [Google Scholar] [CrossRef]
- Chen, Q.; Ma, X.; Zhang, X.; Liu, Y.; Yu, M. Extraction of rare earth ions from phosphate leach solution using emulsion liquid membrane in concentrated nitric acid medium. J. Rare Earths 2018, 36, 1190–1197. [Google Scholar] [CrossRef]
- He, J.; Li, Y.; Xue, X.; Ru, H.; Huang, X.; Yang, H. A novel Ce(IV) ion-selective polyvinyl chloride membrane electrode based on HDEHP and HEH/EHP. J. Rare Earths 2017, 35, 934–940. [Google Scholar] [CrossRef]
- Kirsanov, D.; Khaydukova, M.; Tkachenko, L.; Legin, A.; Babain, V. Potentiometric Sensor Array for Analysis of Complex Rare Earth Mixtures. Electroanalysis 2012, 24, 121–130. [Google Scholar] [CrossRef]
- Hoh, Y.C.; Nevarez, M.; Bautista, R.G. A Predictive Thermodynamic Model for the Distribution Coefficients of Neodymium in the Nd(NO3)3-HNO3-H2O-1 M HDEHP-Amsco Liquid-Liquid Extraction System. Ind. Eng. Chem. Process Des. Dev. 1978, 17, 88–91. [Google Scholar] [CrossRef]
- Bakker, E.; Simon, W. Selectivity of ion-sensitive bulk optodes. Anal. Chem. 1992, 64, 1805–1812. [Google Scholar] [CrossRef]
- Alyapyshev, M.; Babain, V.; Borisova, N.; Eliseev, I.; Kirsanov, D.; Kostin, A.; Legin, A.; Reshetova, M.; Smirnova, Z. 2,2′-Dipyridyl-6,6′-dicarboxylic acid diamides: Synthesis, complexation and extraction properties. Polyhedron 2010, 29, 1998–2005. [Google Scholar] [CrossRef]
- Alyapyshev, M.Y.; Babain, V.A.; Boyko, V.I.; Eliseev, I.I.; Kirsanov, D.O.; Klimchuk, O.V.; Legin, A.V.; Mikhailina, E.S.; Rodik, R.V.; Smirnov, I.V. Calixarenes functionalized with phosphine oxide and diamide functions as extractants and ionofores for rare-earth metals. J. Incl. Phenom. Macrocycl. Chem. 2010, 67, 117–126. [Google Scholar] [CrossRef]
- Legin, A.V.; Babain, V.A.; Kirsanov, D.O.; Mednova, O.V. Cross-sensitive rare earth metal ion sensors based on extraction systems. Sens. Actuators B Chem. 2008, 131, 29–36. [Google Scholar] [CrossRef]
- Khaydukova, M.; Militsyn, D.; Karnaukh, M.; Grüner, B.; Selucký, P.; Babain, V.; Wilden, A.; Kirsanov, D.; Legin, A. Modified Diamide and Phosphine Oxide Extracting Compounds as Membrane Components for Cross-Sensitive Chemical Sensors. Chemosensors 2019, 7, 41. [Google Scholar] [CrossRef]
- Babain, V.A.; Legin, A.V.; Kirsanov, D.O.; Rudnitskaya, A.M.; Tatuev, Y.M.; Baulin, V.E. New chemical sensors based on extraction systems for stable fission products analysis. Radiochim. Acta 2009, 97, 479–484. [Google Scholar] [CrossRef]
- Kirsanov, D.O.; Borisova, N.E.; Reshetova, M.D.; Ivanov, A.V.; Korotkov, L.A.; Eliseev, I.I.; Alyapyshev, M.Y.; Spiridonov, I.G.; Legin, A.V.; Vlasov, Y.G.; et al. Novel diamides of 2,2′-dipyridyl-6,6′-dicarboxylic acid: Synthesis, coordination properties, and possibilities of use in electrochemical sensors and liquid extraction. Russ. Chem. Bull. 2013, 61, 881–890. [Google Scholar] [CrossRef]
- Kirsanov, D.O.; Legin, A.V.; Babain, V.A.; Vlasov, Y.G. Polymeric sensors based on extraction systems for determination of rare-earth metals. Russ. J. Appl. Chem. 2005, 78, 568–573. [Google Scholar] [CrossRef]
- Turanov, A.N.; Karandashev, V.K. Synergistic Solvent Extraction of Lanthanides (III) with Mixtures of Tetraphenylmethylenediphosphine Dioxide and Picrolonic Acid from HCl Solutions. Solvent Extr. Ion Exch. 2017, 35, 104–116. [Google Scholar] [CrossRef]
- Lumetta, G.J.; Carter, J.C.; Gelis, A.V.; Vandegrift, G.F. Combining Octyl(phenyl)-N,N-diisobutylcarbamoylmethylphosphine oxide and bis-(2-ethylhexyl)phosphoric acid extractants for recovering transuranic elements from irradiated nuclear fuel. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2010; Volume 1046, pp. 107–118. [Google Scholar]
- Tkac, P.; Vandegrift, G.F.; Lumetta, G.J.; Gelis, A.V. Study of the Interaction between HDEHP and CMPO and Its Effect on the Extraction of Selected Lanthanides. Ind. Eng. Chem. Res. 2012, 51, 10433–10444. [Google Scholar] [CrossRef]
- Borisova, N.E.; Eroshkina, E.A.; Korotkov, L.A.; Ustynyuk, Y.A.; Alyapyshev, M.Y.; Eliseev, I.I.; Babain, V.A. Actinide-Lanthanide Separation by Bipyridyl-Based Ligands: DFT Calculations and Experimental Results. In Proceedings of the 10th International Conference Toward and over the Fukushima Daiichi accident, Chiba, Japan, 11–16 December 2011. [Google Scholar]
- Hyun Han, S.; Shin Lee, K.; Sig Cha, G.; Liu, D.; Trojanowicz, M. Potentiometric detection in ion chromatography using multi-ionophore membrane electrodes. J. Chromatogr. A 1993, 648, 283–288. [Google Scholar] [CrossRef][Green Version]
- Cuartero, M.; Crespo, G.A.; Bakker, E. Ionophore-Based Voltammetric Ion Activity Sensing with Thin Layer Membranes. Anal. Chem. 2016, 88, 1654–1660. [Google Scholar] [CrossRef]
- Jadhav, S.; Bakker, E. Selectivity Behavior and Multianalyte Detection Capability of Voltammetric Ionophore-Based Plasticized Polymeric Membrane Sensors. Anal. Chem. 2001, 73, 80–90. [Google Scholar] [CrossRef]
- Crespo, G.A.; Cuartero, M.; Bakker, E. Thin Layer Ionophore-Based Membrane for Multianalyte Ion Activity Detection. Anal. Chem. 2015, 87, 7729–7737. [Google Scholar] [CrossRef] [PubMed]
- Murphy, L.; Slater, J.M. Multi-Ionophore Membrane Electrode. U.S. Patent No. DE60311517T2, 28 November 2002. [Google Scholar]
- Bakker, E.; Pretsch, E. Ion-Selective Electrodes Based on Two Competitive Ionophores for Determining Effective Stability Constants of Ion−Carrier Complexes in Solvent Polymeric Membranes. Anal. Chem 1998, 70, 295–302. [Google Scholar] [CrossRef]
- Legin, A.V.; Kirsanov, D.O.; Babain, V.A.; Borovoy, A.V.; Herbst, R.S. Cross-sensitive rare-earth metal sensors based on bidentate neutral organophosphorus compounds and chlorinated cobalt dicarbollide. Anal. Chim. Acta 2006, 572, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Horwltz, E.P.; Martin, K.A.; Diamond, H.; Kaplan, L. Extraction of Am from Nitric Acid by Carbamoyl-Phosphoryl Extractants: The Influence of Substituents on The Selectivity of Am Over Fe and Selected Fission Products. Solvent Extr. Ion Exch. 1986, 4, 449–494. [Google Scholar] [CrossRef]
- Babain, V.A.; Alyapyshev, M.Y.; Kiseleva, R.N. Metal extraction by N,N′-dialkyl-N,N′-diaryl-dipicolinamides from nitric acid solutions. Radiochim. Acta 2007, 95, 217–223. [Google Scholar] [CrossRef]
- Richards, E.; Bessant, C.; Saini, S. Multivariate data analysis in electroanalytical chemistry. Electroanalysis 2002, 14, 1533–1542. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Ciosek, P.; Mamińska, R.; Dybko, A.; Wróblewski, W. Potentiometric electronic tongue based on integrated array of microelectrodes. Sens. Actuators B Chem. 2007, 127, 8–14. [Google Scholar] [CrossRef]
- Bakker, E.; Pretsch, E.; Bühlmann, P. Selectivity of potentiometric ion sensors. Anal. Chem. 2000, 72, 1127–1133. [Google Scholar] [CrossRef]
- Kirsanov, D.; Panchuk, V.; Agafonova-Moroz, M.; Khaydukova, M.; Lumpov, A.; Semenov, V.; Legin, A. A sample-effective calibration design for multiple components. Analyst 2014, 139, 4303–4309. [Google Scholar] [CrossRef]
- Suzuki, H.; Naganawa, H.; Tachimori, S. Role of hydrophobic counteranions in the ion pair extraction of lanthanides(III) with an electrically neutral extractant. Phys. Chem. Chem. Phys. 2003, 5, 726–733. [Google Scholar] [CrossRef]
- Lumetta, G.J.; Gelis, A.V.; Braley, J.C.; Carter, J.C.; Pittman, J.W.; Warner, M.G.; Vandegrift, G.F. The TRUSPEAK Concept: Combining CMPO and HDEHP for Separating Trivalent Lanthanides from the Transuranic Elements. Solvent Extr. Ion Exch. 2013, 31, 223–236. [Google Scholar] [CrossRef]
- Alyapyshev, M.Y.; Babain, V.A.; Tkachenko, L.I.; Eliseev, I.I.; Didenko, A.V.; Petrov, M.L. Dependence of Extraction Properties of 2,6-Dicarboxypyridine Diamides on Extractant Structure. Solvent Extr. Ion Exch.e 2011, 29, 619–636. [Google Scholar] [CrossRef]
- Faber, K.; Kowalski, B.R. Propagation of measurement errors for the validation of predictions obtained by principal component regression and partial least squares. J. Chemom. 1997, 11, 181–238. [Google Scholar] [CrossRef]

| # | mionophore, mg | mplasticizer | mPVC | mlipophilic additive | ||
|---|---|---|---|---|---|---|
| DPCMPO | TODGA | DPA | ||||
| M1 | 5.6 | - | - | 194.5 | 97.2 | 1.5 |
| M2 | - | 8.7 | - | 192.4 | 96.2 | 1.5 |
| M3 | - | - | 6.1 | 194.1 | 97.1 | 1.5 |
| M4 | 1.8 | 2.9 | 2.0 | 193.7 | 96.8 | 1.5 |
| M5 | 2.8 | 4.4 | - | 193.4 | 96.7 | 1.5 |
| M6 | 2.8 | - | 3.1 | 194.3 | 97.1 | 1.5 |
| M7 | - | 4.4 | 3.1 | 193.2 | 96.6 | 1.5 |
| Primary Ln3+ion/Sensor no. | M1 | M2 | M3 | M4 | M5 | M6 | M7 |
|---|---|---|---|---|---|---|---|
| Ce | 0.4 | 3.2 | 0.4 | 0.8 | 1.6 | 0.4 | 0.4 |
| Pr | 0.8 | >>10 | 1.6 | >>10 | >>10 | 3.2 | 10.0 |
| Nd | 0.3 | 0.2 | 0.6 | 0.3 | 0.5 | 0.5 | 0.3 |
| Sm | 1.6 | 0.3 | 1.0 | 0.6 | 0.6 | 1.3 | 0.6 |
| Eu | 0.5 | 0.1 | 0.2 | 0.1 | 0.1 | 1.0 | 0.1 |
| Gd | 1.0 | 0.3 | 0.3 | 0.2 | 0.2 | 1.0 | 0.2 |
| Er | 3.9 | 0.4 | 1.0 | 0.4 | 0.2 | 5.0 | 0.1 |
| Sensor Set | La3+ | Nd3+ | ||||||
|---|---|---|---|---|---|---|---|---|
| Slope | RMSEP (logC) | R2 | N | Slope | RMSEP (logC) | R2 | N | |
| Complete array (M1–M7) | 0.69 | 0.12 | 0.85 | 4 | 1.14 | 0.15 | 0.89 | 2 |
| light-sensitive (M1 + M3 + M6) | 0.84 | 0.09 | 0.90 | 3 | 0.31 | 0.35 | 0.40 | 3 |
| heavy-sensitive (M2 + M4 + M5 + M7) | 0.37 | 0.24 | 0.55 | 2 | 1.06 | 0.13 | 0.91 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashina, J.; Babain, V.; Kirsanov, D.; Legin, A. A Novel Multi-Ionophore Approach for Potentiometric Analysis of Lanthanide Mixtures. Chemosensors 2021, 9, 23. https://doi.org/10.3390/chemosensors9020023
Ashina J, Babain V, Kirsanov D, Legin A. A Novel Multi-Ionophore Approach for Potentiometric Analysis of Lanthanide Mixtures. Chemosensors. 2021; 9(2):23. https://doi.org/10.3390/chemosensors9020023
Chicago/Turabian StyleAshina, Julia, Vasily Babain, Dmitry Kirsanov, and Andrey Legin. 2021. "A Novel Multi-Ionophore Approach for Potentiometric Analysis of Lanthanide Mixtures" Chemosensors 9, no. 2: 23. https://doi.org/10.3390/chemosensors9020023
APA StyleAshina, J., Babain, V., Kirsanov, D., & Legin, A. (2021). A Novel Multi-Ionophore Approach for Potentiometric Analysis of Lanthanide Mixtures. Chemosensors, 9(2), 23. https://doi.org/10.3390/chemosensors9020023






