Fabrication of Zinc Protoporphyrin-Modified Gold Electrode for Sensitive and Fast Detection of Vascular Endothelial Growth Factor
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Characterizations of ZnPP
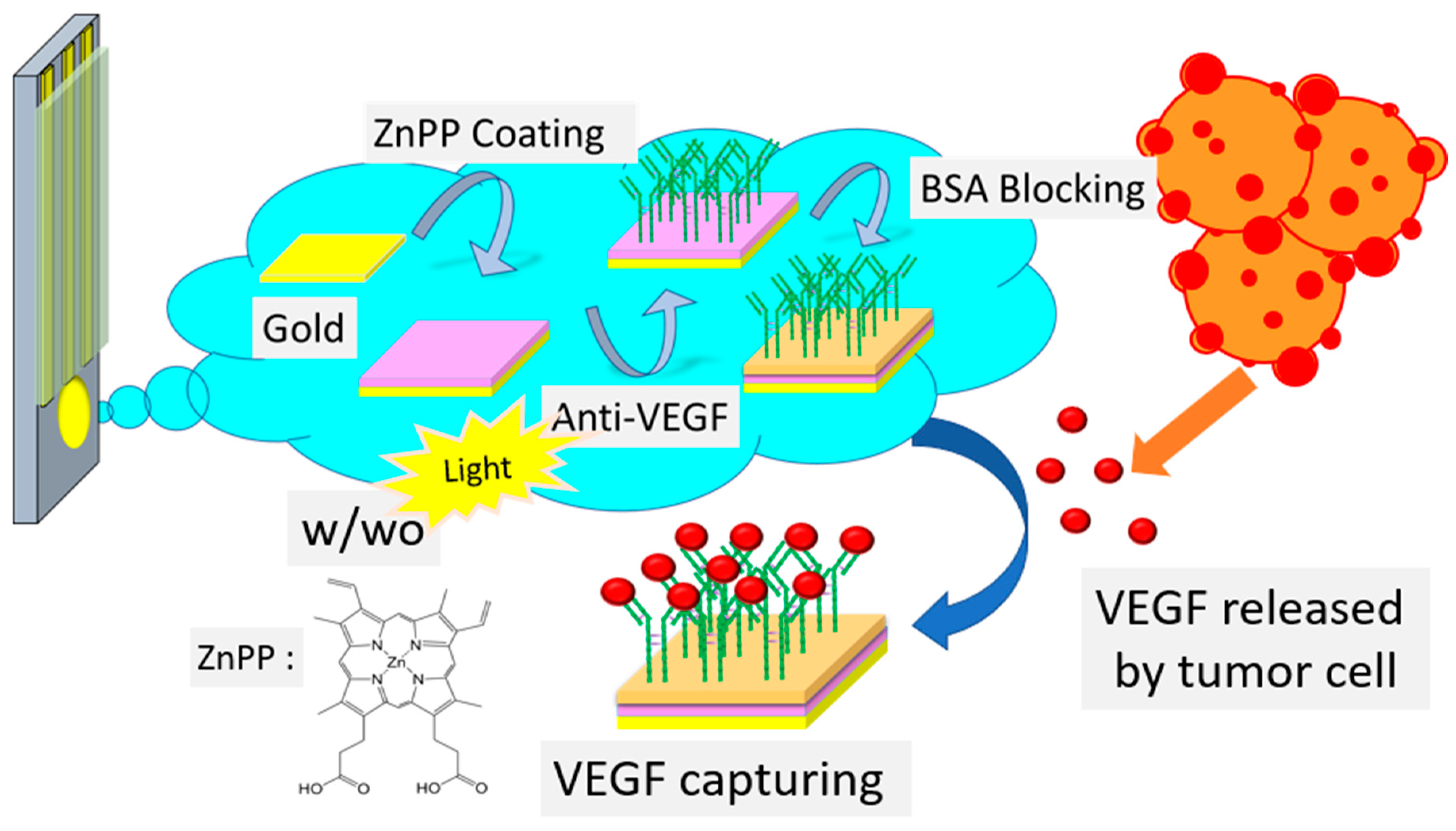
2.3. Fabrication of VEGF Biosensors with and without Light Treatment
2.4. Detection of VEGF and Selectivity Tests
3. Results and Discussion
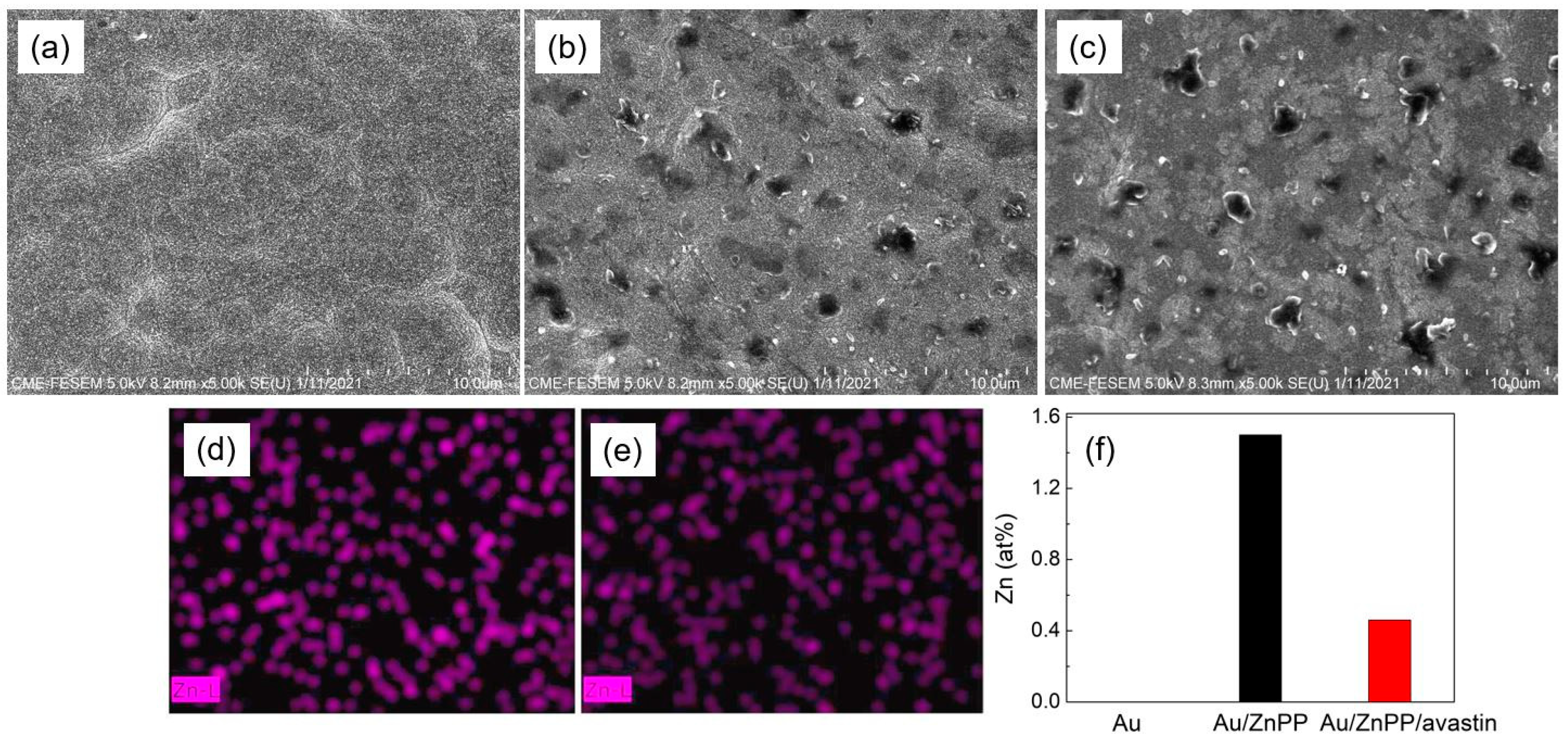
3.1. FESEM Image and EDX Mapping
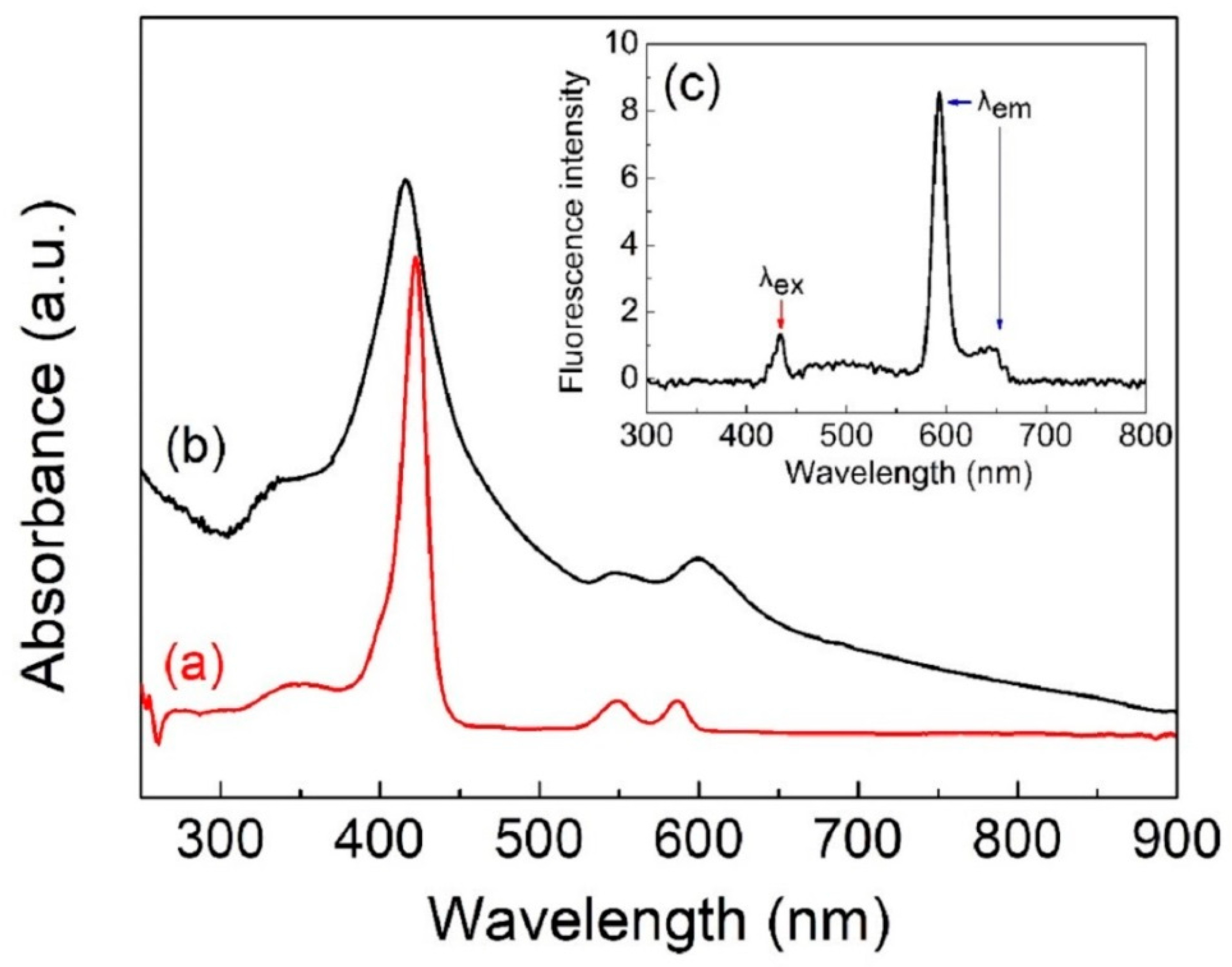
3.2. UV/Vis Near-IR and PL Spectroscopy FESEM Image
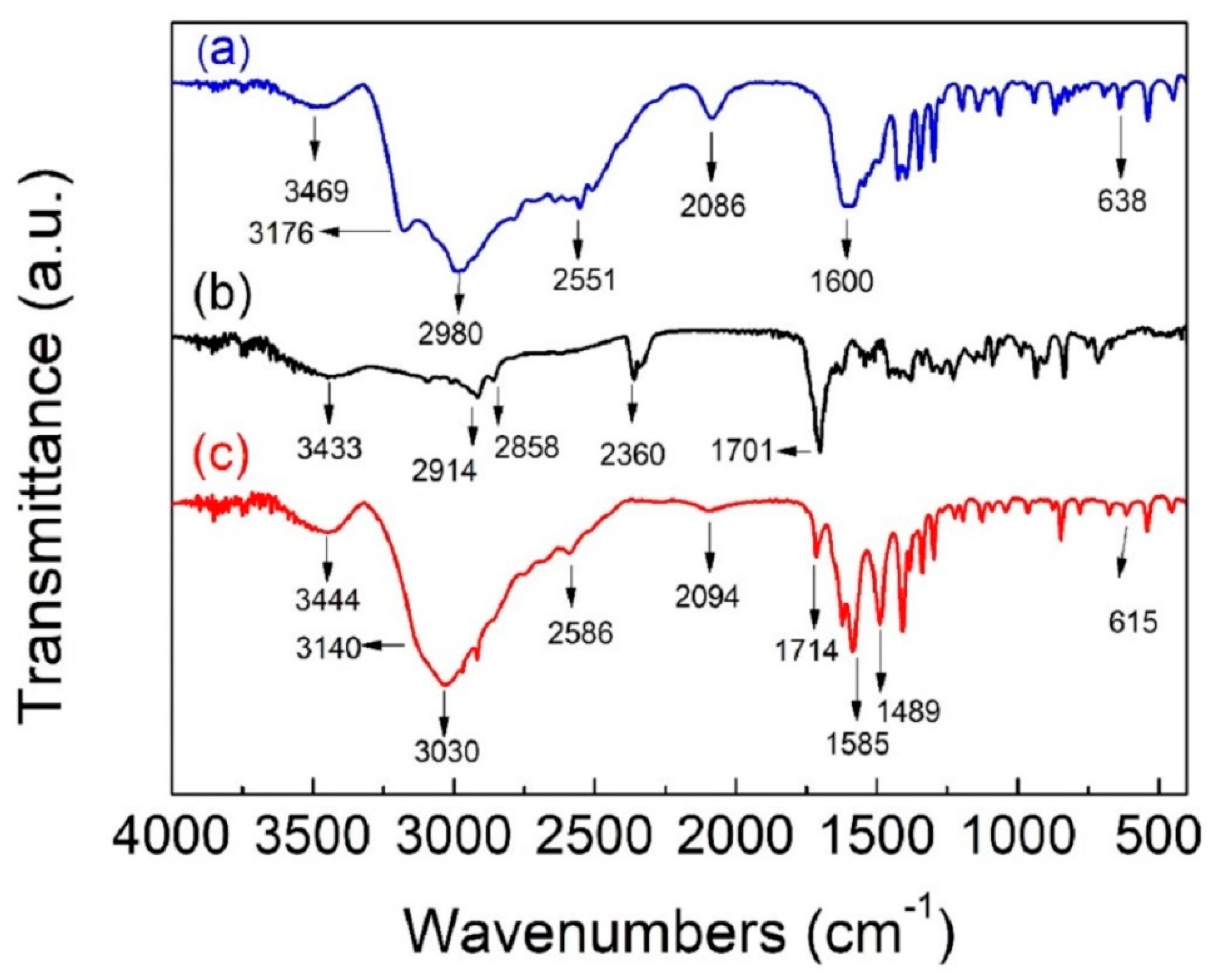
3.3. FTIR Spectroscopy
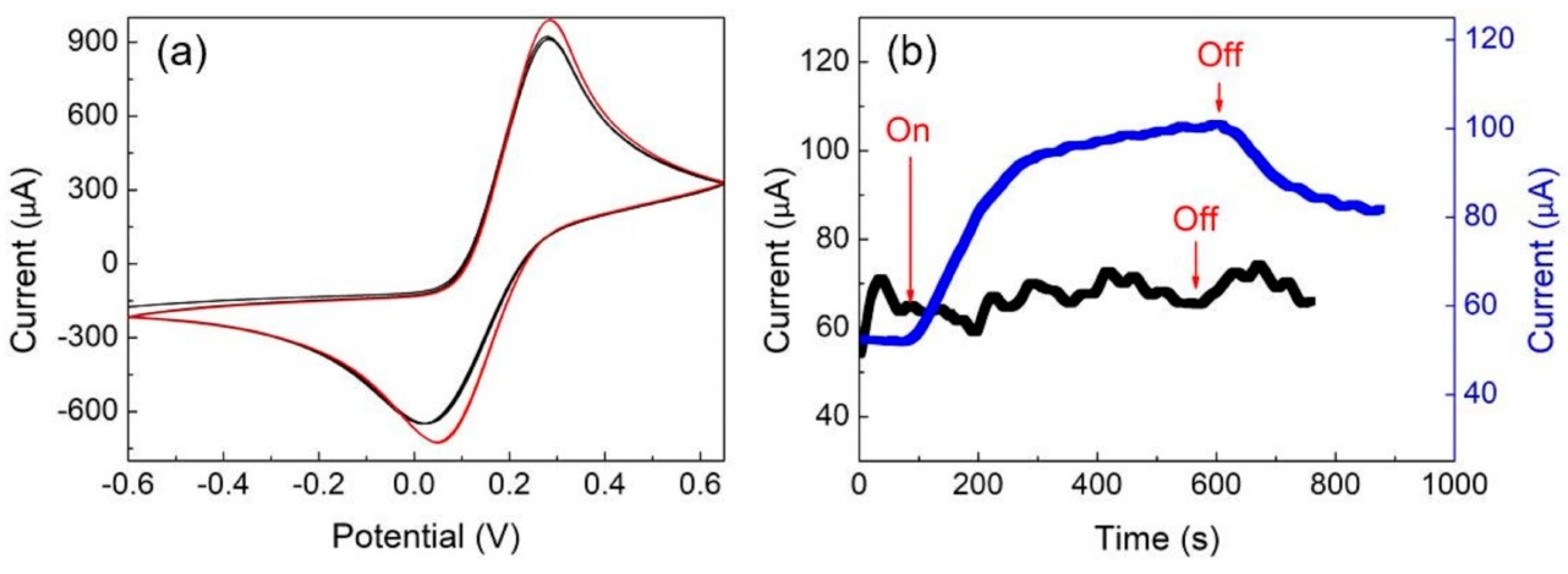
3.4. Electrochemical Phenomena of Au/ZnPP Electrodes with and without Light Treatment
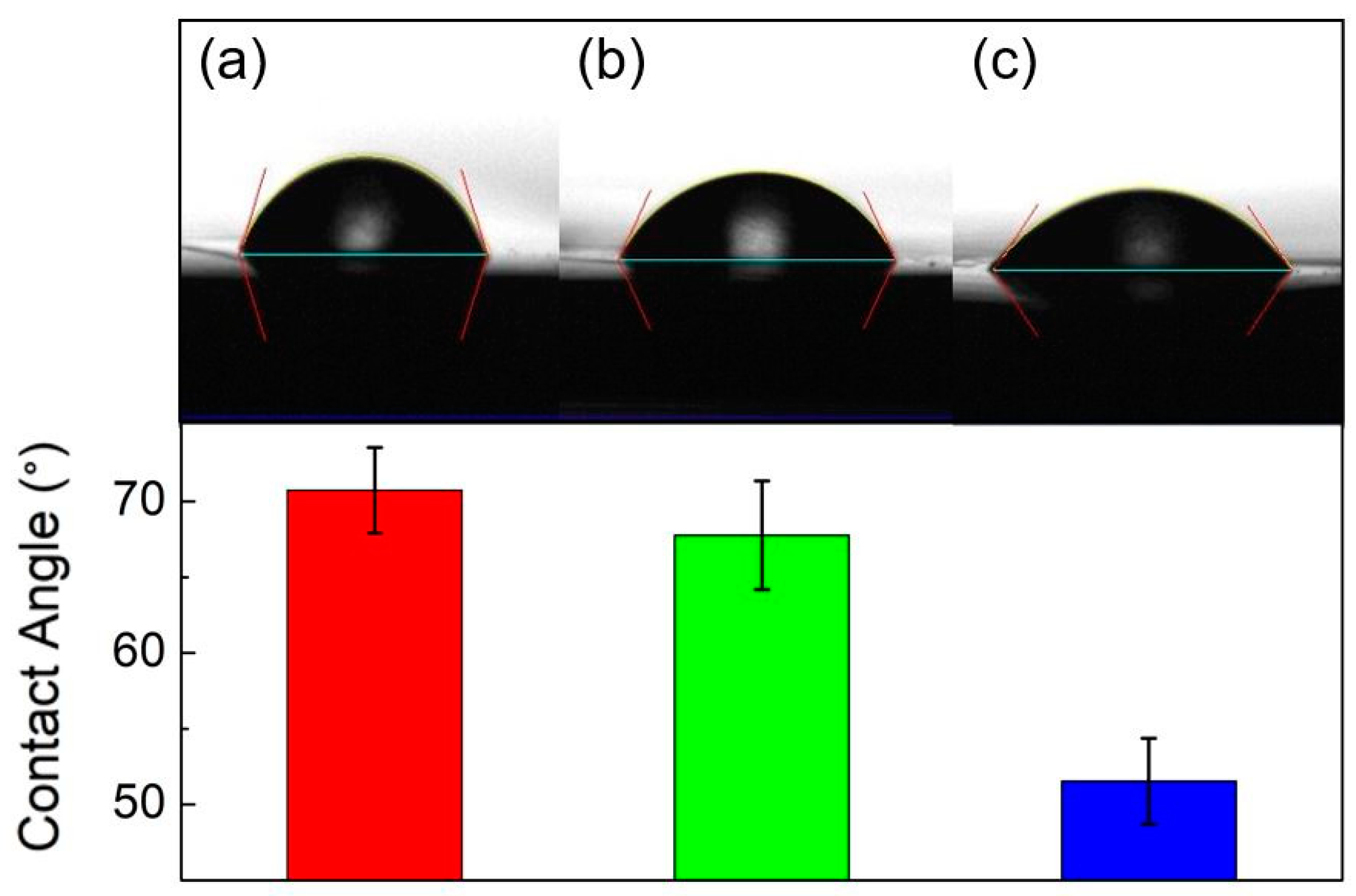
3.5. Contact Angles and Avastin Immobilization
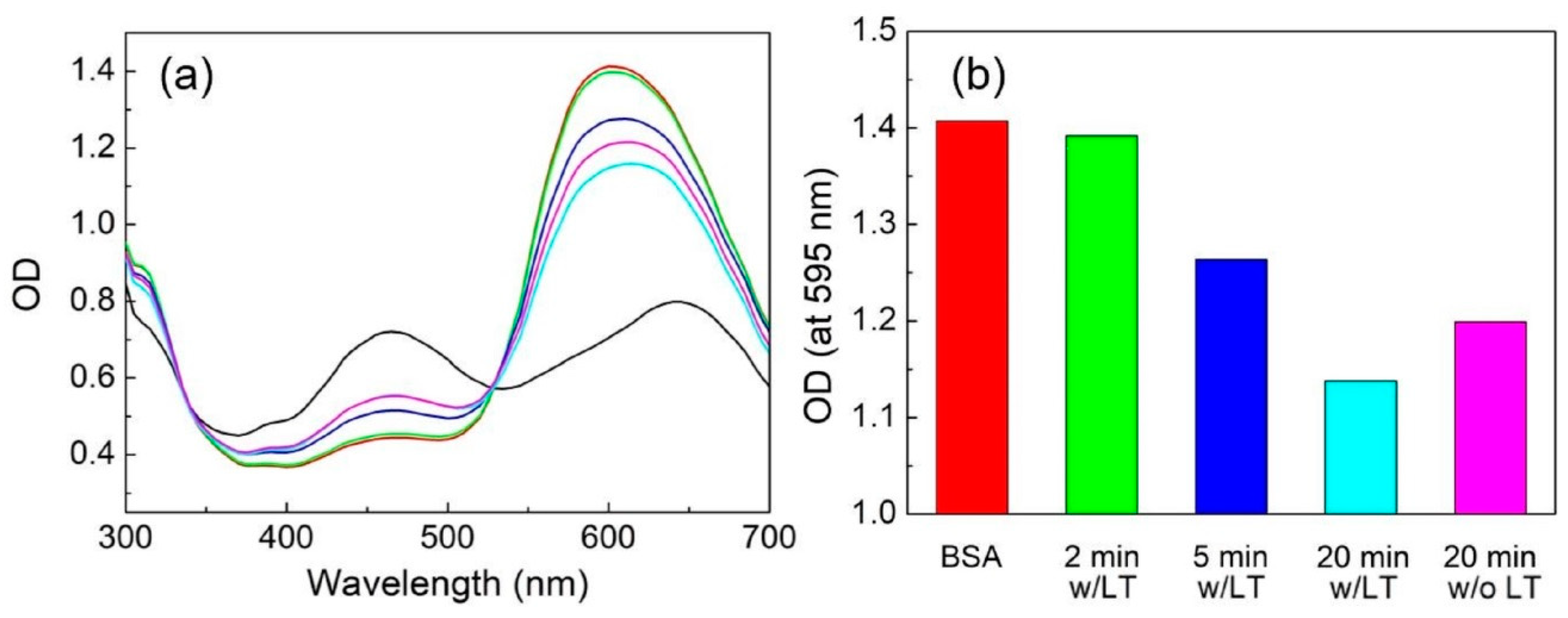
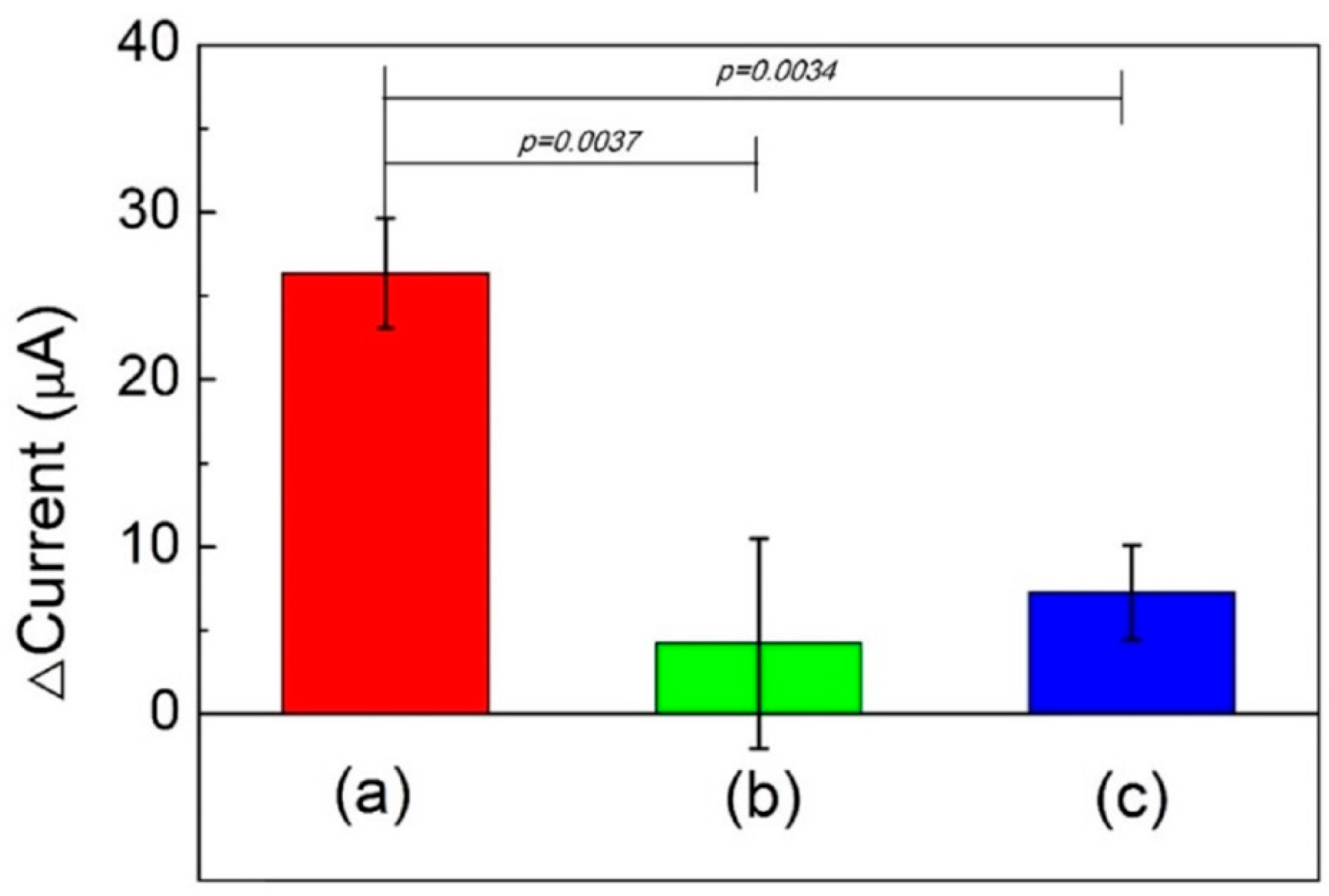
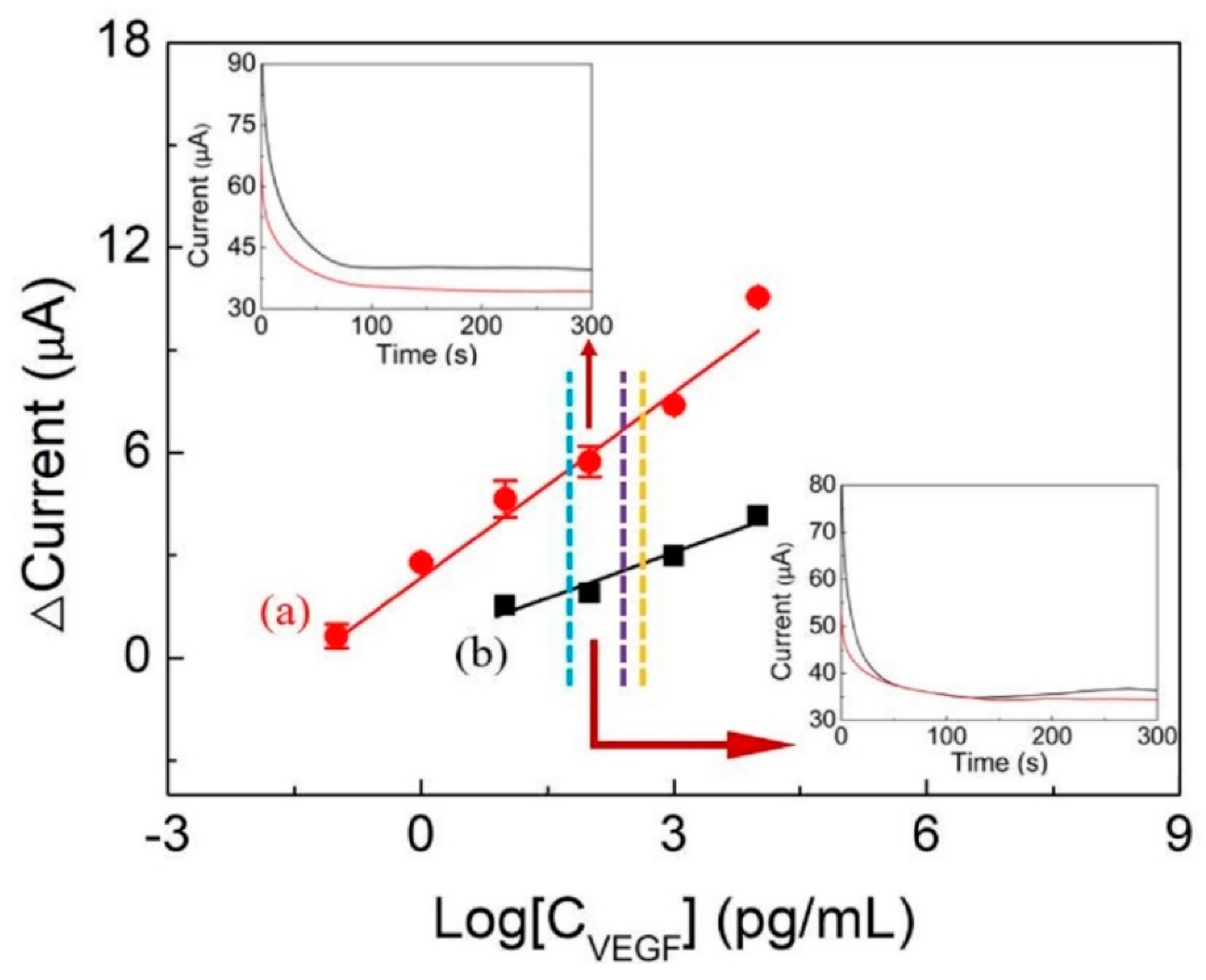
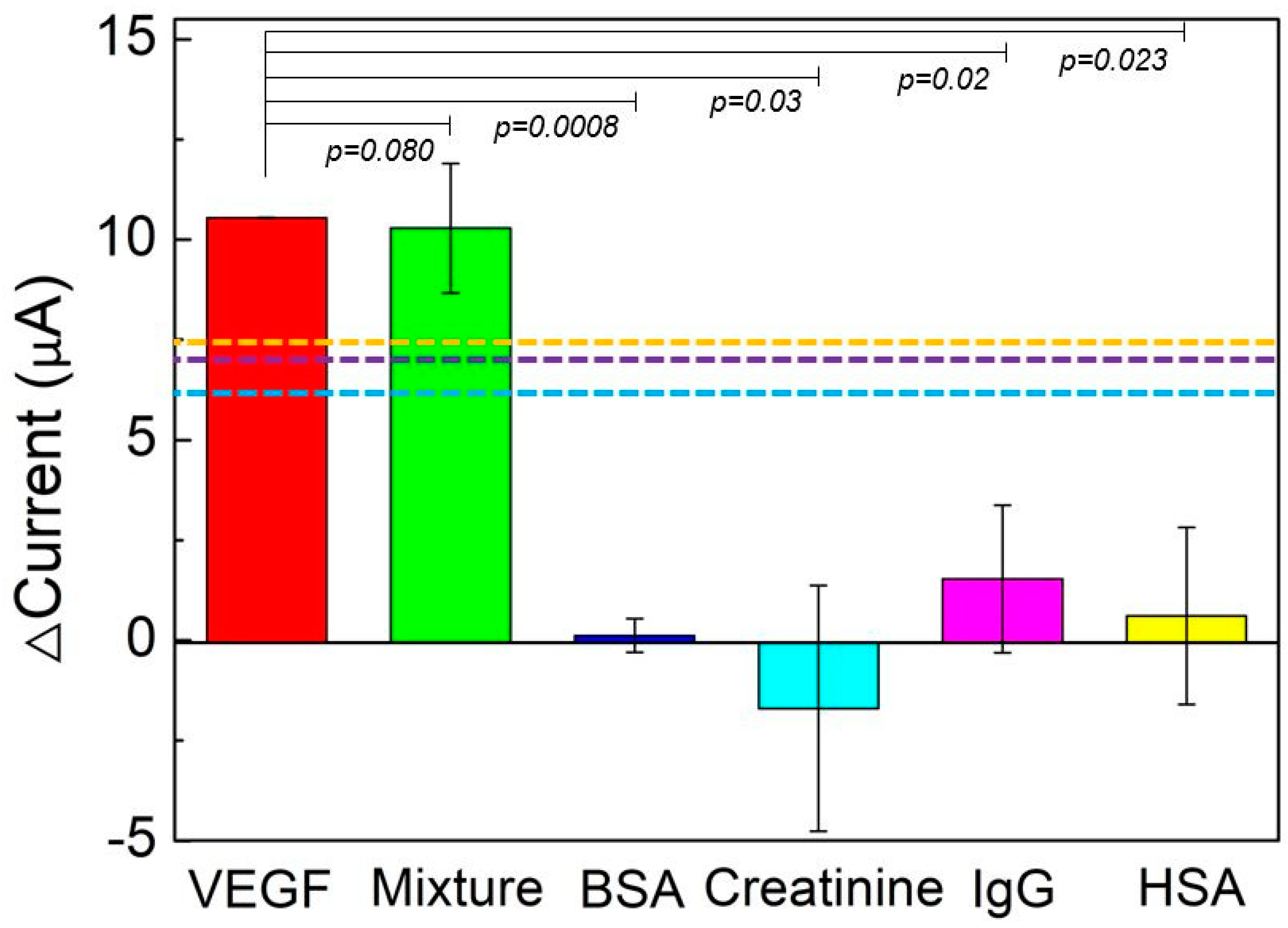
3.6. VEGF Detection and Reproducibility, Stability, and Specificity Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, K.M. Comprehensive Coordination Chemistry II, 3rd ed.; Pergamon Press: Oxford, UK, 2003; pp. 493–506. [Google Scholar]
- Bryden, F.; Boyle, R.W. Advances in Inorganic Chemistry, 1st ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 141–221. [Google Scholar]
- Mashima, T.; Oohora, K.; Hayashi, T. Successive energy transfer within multiple photosensitizers assembled in a hexameric hemoprotein scaffold. Phys. Chem. Chem. Phys. 2018, 20, 3200–3209. [Google Scholar] [CrossRef] [PubMed]
- Hennig, G.; Homann, C.; Teksan, I.; Hasbargen, U.; Hasmüller, S.; Holdt, L.M.; Khaled, N.; Sroka, R.; Stauch, T.; Stepp, H.; et al. Non-invasive detection of iron deficiency by fluorescence measurement of erythrocyte zinc protoporphyrin in the lip. Nat. Commun. 2016, 7, 10776. [Google Scholar] [CrossRef] [PubMed]
- Broll, R.; Erdmann, H.; Duchrow, M.; Oevermann, E.; Schwandner, O.; Markert, U.; Bruch, H.P.; Windhövel, U. Vascular endothelial growth factor (VEGF)—A valuable serum tumour marker in patients with colorectal cancer? Eur. J. Surg. Oncol. 2001, 27, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Luo, J.; Fan, X.; Li, L.; Li, S.; Wen, K.; Feng, J.; Wu, G. Elevated Gab2 induces tumor growth and angiogenesis in colorectal cancer through upregulating VEGF levels. J. Exp. Clin. Cancer Res. 2017, 36, 56. [Google Scholar] [CrossRef]
- Kido, S.; Kitadai, Y.; Hattori, N.; Haruma, K.; Kido, T.; Ohta, M.; Tanaka, S.; Yoshihara, M.; Sumii, K.; Ohmoto, Y.; et al. Interleukin 8 and Vascular Endothelial Growth Factor—Prognostic Factors in Human Gastric Carcinomas? Eur. J. Cancer 2001, 37, 1482–1487. [Google Scholar] [CrossRef]
- Chen, H.C.; Qiu, J.T.; Yang, F.L.; Liu, Y.C.; Chen, M.C.; Tsai, R.Y.; Yang, H.W.; Lin, C.Y.; Lin, C.C.; Wu, T.S.; et al. Magnetic-composite-modified polycrystalline-silicon nanowire field-effect transistor for vascular endothelial growth factor detection and cancer diagnosis. Anal. Chem. 2014, 86, 9443–9450. [Google Scholar] [CrossRef]
- Yang, H.W.; Tsai, R.Y.; Chen, J.P.; Ju, S.P.; Liao, J.F.; Wei, K.C.; Zou, W.L.; Hua, M.Y. Fabrication of a nano-gold-dot array for rapid and sensitive detection of vascular endothelial growth factor in human serum. ACS Appl. Mater. Interfaces 2016, 8, 30845–30852. [Google Scholar] [CrossRef]
- Taiwan Cancer Clinic Foundation. Available online: http://www.tccf.org.tw/old/medecine/nm_29.htm (accessed on 10 January 2021).
- Napoleone, F.; Kenneth, J.H.; William, N.T. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem. Biophys. Res. Commun. 2005, 333, 328–335. [Google Scholar] [CrossRef]
- Kim, M.; Lezzi, R., Jr.; Shim, B.S.; Martin, D.C. Impedimetric Biosensors for Detecting Vascular Endothelial Growth Factor (VEGF) Based on Poly(3,4-ethylene dioxythiophene) (PEDOT)/Gold Nanoparticle (Au NP) Composites. Front. Chem. 2019, 7, 234–245. [Google Scholar] [CrossRef]
- Dehghani, S.; Nosrati, R.; Yousefi, M.; Nezami, A.; Soltani, F.; Taghdisi, S.M.; Abnous, K.; Alinolandi, M.; Ramezani, M. Aptamer-based biosensors and nanosensors for the detection of vascularendothelial growth factor (VEGF): A review. Biosens. Bioelectron. 2018, 110, 23–37. [Google Scholar] [CrossRef]
- Jorge, R.; Costa, R.A.; Calucci, D.; Cintra, L.P.; Scott, I.U. Intravitreal bevacizumab (Avastin) for persistent new vessels in diabetic retinopathy. Retina 2006, 26, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Hu, T.; Zhong, X.; Zhu, M.; Chai, Y.; Yuan, R. Highly Sensitive Photoelectrochemical Biosensor Based on Quantum Dots Sensitizing Bi2Te3 Nanosheets and DNA-Amplifying Strategies. ACS Appl. Mater. Interfaces 2020, 12, 22624–22629. [Google Scholar] [CrossRef]
- Fan, B.; Fan, Q.; Cui, M.; Wu, T.; Wang, J.; Ma, H.; Wei, Q. Photoelectrochemical Biosensor for Sensitive Detection of Soluble CD44 Based on the Facile Construction of a Poly(ethylene glycol)/Hyaluronic Acid Hybrid Antifouling Interface. ACS Appl. Mater. Interfaces 2019, 11, 24764–24770. [Google Scholar] [CrossRef] [PubMed]
- Gai, P.; Gu, C.; Li, H.; Sun, X.; Li, F. Ultrasensitive Ratiometric Homogeneous Electrochemical MicroRNA Biosensing via Target-Triggered Ru(III) Release and Redox Recycling. Anal. Chem. 2017, 89, 12293–12298. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Wang, X.; Wang, J.; Li, H.; Li, F. Nucleic Acid-Functionalized Metal–Organic Framework-Based Homogeneous Electrochemical Biosensor for Simultaneous Detection of Multiple Tumor Biomarkers. Anal. Chem. 2019, 91, 3604–3610. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Xu, N.; Wang, W.; Ge, L.; Li, F. Truly Immobilization-Free Diffusivity-Mediated Photoelectrochemical Biosensing Strategy for Facile and Highly Sensitive MicroRNA Assay. Anal. Chem. 2018, 90, 9591–9597. [Google Scholar] [CrossRef]
- Hou, T.; Zhang, L.; Sun, X.; Li, F. Biphasic photoelectrochemical sensing strategy based on in situ formation of CdS quantum dots for highly sensitive detection of acetylcholinesterase activity and inhibition. Biosens. Bioelectron. 2016, 75, 359–364. [Google Scholar] [CrossRef]
- Xiong, E.; Yan, X.; Zhang, X.; Li, Y.; Yang, R.; Meng, L.; Chen, J. A new photoelectrochemical biosensor for ultrasensitive determination of nucleic acids based on a three-stage cascade signal amplification strategy. Analyst 2018, 12, 2799–2806. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Zhao, G.C. A Novel Photoelectrochemical Biosensor for Tyrosinase and Thrombin Detection. Sensors 2016, 16, 135. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Li, S.; Zhang, S. Photoelectrochemical biosensor for detection of adenosine triphosphate in the extracts of cancer cells. Chem. Commun. 2010, 48, 9173–9175. [Google Scholar] [CrossRef]
- Chen, H.C.; Chen, Y.T.; Tsai, R.Y.; Chen, M.C.; Chen, S.L.; Xiao, M.C.; Chen, C.L.; Hua, M.Y. A sensitive and selective magnetic graphene composite-modified polycrystalline-silicon nanowire field-effect transistor for bladder Cancer diagnosis. Biosens. Bioelectron. 2015, 66, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Jeyachandran, Y.L.; Mielczarski, J.A.; Mielczarski, E.; Rai, B. Efficiency of blocking of non-specific interaction of different proteins by BSA adsorbed on hydrophobic and hydrophilic surfaces. J. Colloid Interface Sci. 2010, 341, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Hideshima, S.; Sato, R.; Inoue, S.; Kuroiwa, S.; Osaka, T. Detection of tumor marker in blood serum using antibody-modified field effect transistor with optimized BSA blocking. Sens. Actuators B: Chem. 2012, 161, 146–150. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, R.; Liu, Z. Formation of a Porphyrin Monolayer Film by Axial Ligation of Protoporphyrin IX Zinc to an Amino-Terminated Silanized Glass Surface. Langmuir 2000, 16, 1158–1162. [Google Scholar] [CrossRef]
- Science Online. Available online: https://highscope.ch.ntu.edu.tw/wordpress/?p=58362 (accessed on 10 December 2020).
- Latunde-Dada, G.O. Encyclopedia of Food and Health, 1st ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 452–460. [Google Scholar]
- Coates, J. Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2000; pp. 10815–10837. [Google Scholar] [CrossRef]
- IR Spectrum Table & Chart. Available online: https://www.sigmaaldrich.com/technical-documents/articles/biology/ir-spectrum-table.html (accessed on 28 November 2020).
- Li, L.; Liao, L.; Ding, Y.; Zeng, H. Dithizone-etched CdTe nanoparticles-based fluorescence sensor for the off–on detection of cadmium ion in aqueous media. RSC Adv. 2017, 7, 10361–10368. [Google Scholar] [CrossRef]
- Onoda, A.; Kakikura, Y.; Uematsu, T.; Kuwabata, S.; Hayashi, T. Photocurrent Generation from Hierarchical Zinc-Substituted Hemoprotein Assemblies Immobilized on a Gold Electrode. Angew. Commun. 2011, 51, 2628–2631. [Google Scholar] [CrossRef]
- Onoda, A.; Kakikura, Y.; Hayashi, T. Cathodic photocurrent generation from zinc-substituted cytochrome b562 assemblies immobilized on an apocytochrome b562-modified gold electrode. Dalton Trans. 2013, 42, 16102–16107. [Google Scholar] [CrossRef]
- Zhang, H.; Ji, J.; Gonzalez, A.A.; Choi, J.H. Tailoring photoelectrochemical properties of semiconducting transition metal dichalcogenide nanolayers with porphyrin functionalization. J. Mater. Chem. C 2017, 5, 11233–11238. [Google Scholar] [CrossRef]
- Walker, J.M. The Protein Protocols Handbook, 2nd ed.; Humana Press: Totowa, NJ, USA, 2002; pp. 15–243. [Google Scholar] [CrossRef]
- Compton, S.J.; Jones, C.G. Mechanism of dye response and interference in the Bradford protein assay. Anal. Biochem. 1985, 151, 369–374. [Google Scholar] [CrossRef]
- Congdon, R.W.; Muth, G.W.; Splittgerber, A.G. The binding interaction of Coomassie Blue with proteins. Anal. Biochem. 1993, 213, 407–413. [Google Scholar] [CrossRef]
- Hamdy, M.N.; Shaheen, K.Y.; Awad, M.A.; Barakat, E.M.; Shalaby, S.; Gupta, N.; Gupta, V. Vascular endothelial growth factor (VEGF) as a biochemical marker for the diagnosis of hepatocellular carcinoma (HCC). Clin. Pract. 2020, 17, 1441–1453. [Google Scholar]
- Zajkowska, M.; Głażewska, E.K.; Będkowska, G.E.; Chorąży, P.; Szmitkowski, M.; Ławicki, S. Diagnostic Power of Vascular Endothelial Growth Factor and Macrophage Colony-Stimulating Factor in Breast Cancer Patients Based on ROC Analysis. Mediat. Inflamm. 2016, 2016, 5962946. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, B. “p < 0.05” Might Not Mean What You Think: American Statistical Association Clarifies P Values. J. Natl. Cancer Inst. 2016, 108, djw194. [Google Scholar] [CrossRef]
- Andrade, C. The P Value and Statistical Significance: Misunderstandings, Explanations, Challenges, and Alternatives. Indian J. Psychol. Med. 2019, 41, 210–215. [Google Scholar] [CrossRef] [PubMed]
| Temperature (°C) | Time (h) | Mean (%) | Standard Deviation (%) | RSD a (%) | p Value b |
|---|---|---|---|---|---|
| 4 | 0 | 100.00 | |||
| 8 | 105.07 | 7.23 | 6.88 | 0.43 | |
| 16 | 101.29 | 7.26 | 7.16 | 0.82 | |
| 24 | 97.71 | 12.86 | 13.16 | 0.82 | |
| 48 | 95.65 | 8.43 | 8.81 | 0.54 | |
| 64 | 94.57 | 13.70 | 14.49 | 0.63 | |
| 20 | 0 | 100.00 | |||
| 8 | 110.25 | 1.12 | 1.00 | 0.07 | |
| 16 | 102.64 | 1.01 | 0.97 | 0.07 | |
| 24 | 105.52 | 3.95 | 3.74 | 0.19 | |
| 48 | 94.92 | 3.69 | 3.89 | 0.19 | |
| 64 | 94.92 | 3.69 | 3.89 | 0.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.-Y.; Liao, C.-C.; Hua, M.-Y. Fabrication of Zinc Protoporphyrin-Modified Gold Electrode for Sensitive and Fast Detection of Vascular Endothelial Growth Factor. Chemosensors 2021, 9, 21. https://doi.org/10.3390/chemosensors9020021
Lin H-Y, Liao C-C, Hua M-Y. Fabrication of Zinc Protoporphyrin-Modified Gold Electrode for Sensitive and Fast Detection of Vascular Endothelial Growth Factor. Chemosensors. 2021; 9(2):21. https://doi.org/10.3390/chemosensors9020021
Chicago/Turabian StyleLin, Hung-Yu, Chin-Cheng Liao, and Mu-Yi Hua. 2021. "Fabrication of Zinc Protoporphyrin-Modified Gold Electrode for Sensitive and Fast Detection of Vascular Endothelial Growth Factor" Chemosensors 9, no. 2: 21. https://doi.org/10.3390/chemosensors9020021
APA StyleLin, H.-Y., Liao, C.-C., & Hua, M.-Y. (2021). Fabrication of Zinc Protoporphyrin-Modified Gold Electrode for Sensitive and Fast Detection of Vascular Endothelial Growth Factor. Chemosensors, 9(2), 21. https://doi.org/10.3390/chemosensors9020021




