Histidine Functionalized Gold Nanoparticles for Screening Aminoglycosides and Nanomolar Level Detection of Streptomycin in Water, Milk, and Whey
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Biosynthesis and Characterization of His@AuNPs
2.3. Effect of Dilution and Ionic Strength on His@AuNPs
2.4. Selectivity of His@AuNPs
2.5. Real-Time Response of His@AuNPs
2.6. Sensitivity of His@AuNPs
2.7. Practical Application of His@AuNPs
3. Results and Discussion
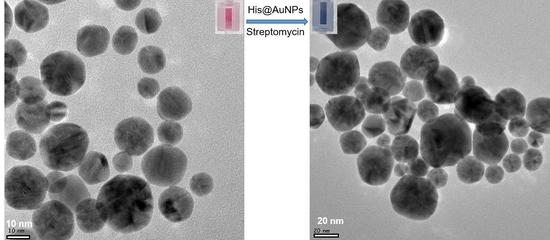
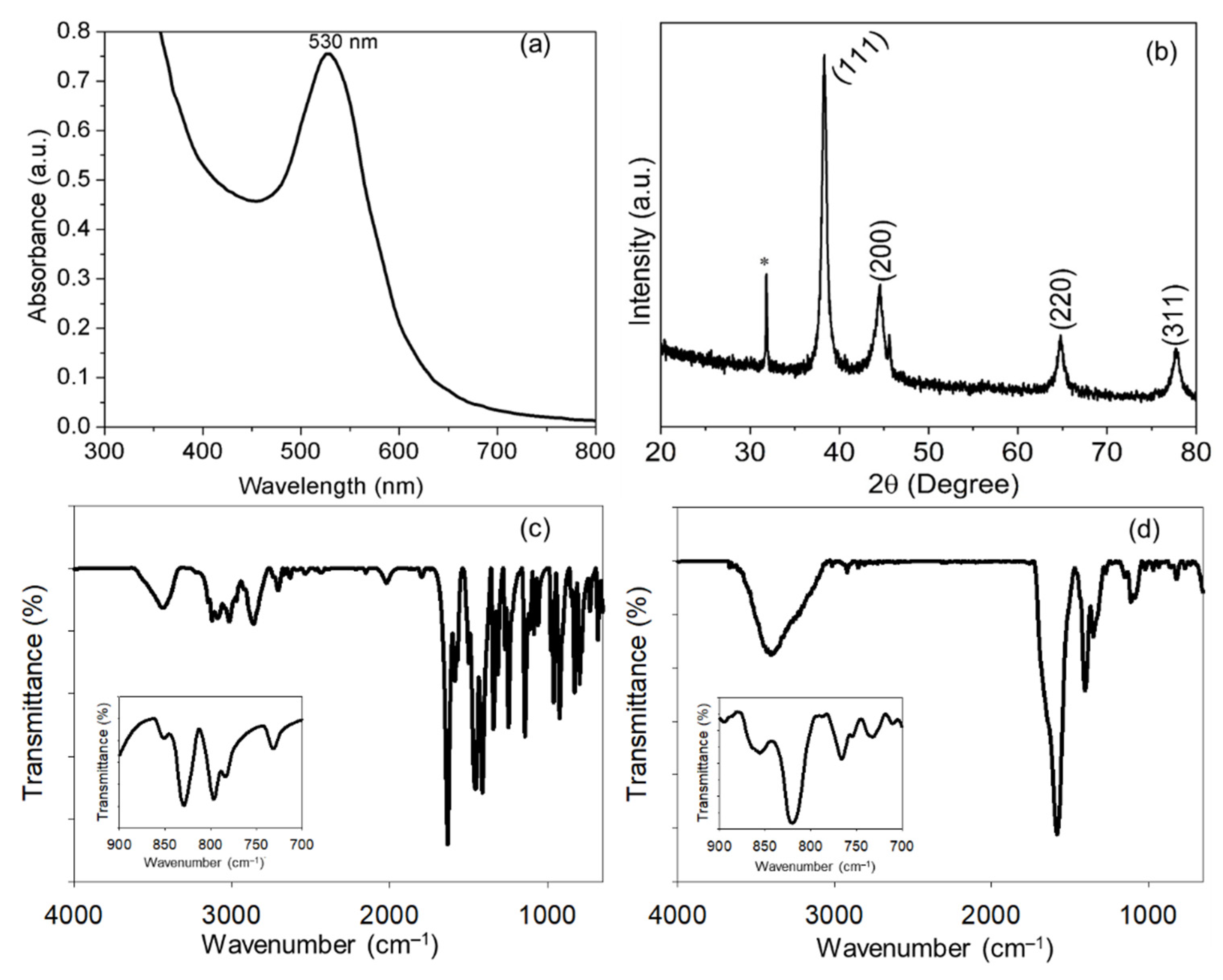
3.1. Biosynthesis and Characterization of His@AuNPs
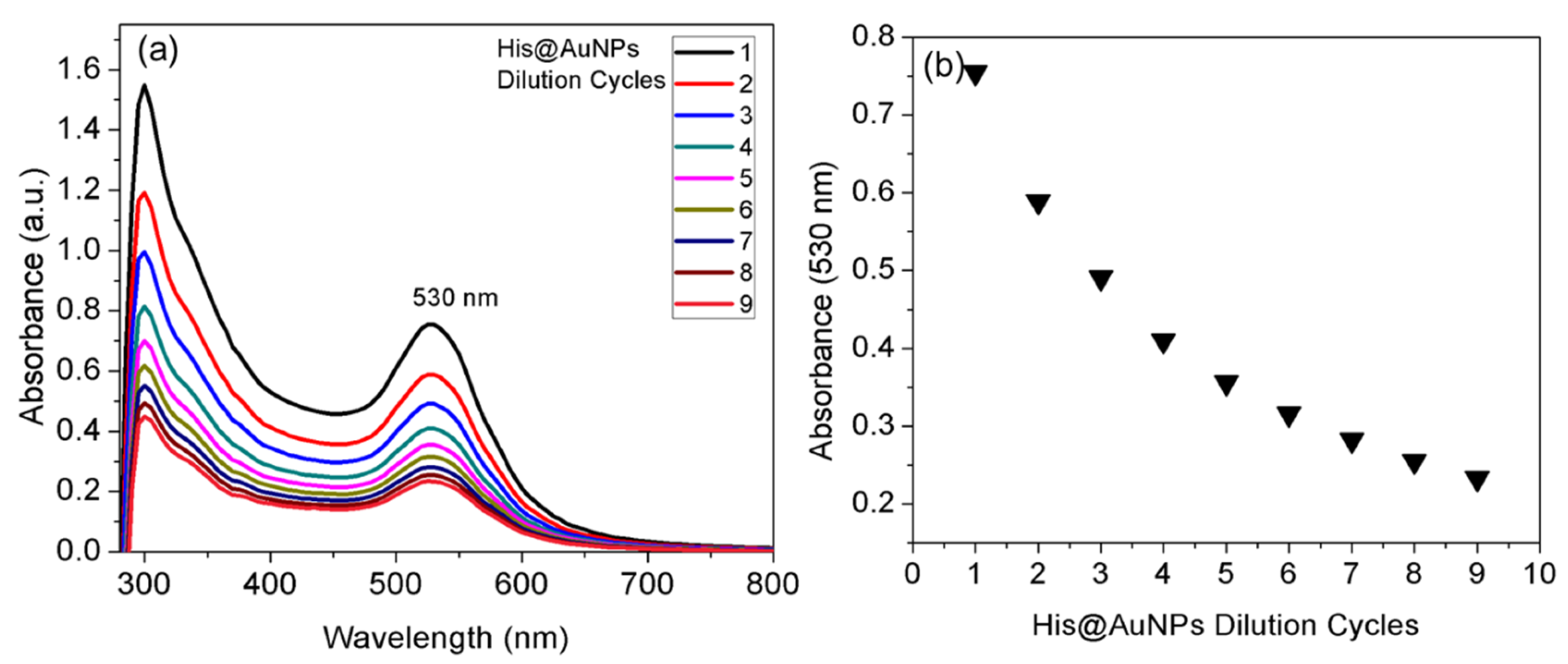
3.2. Purification and Stability of His@AuNPs
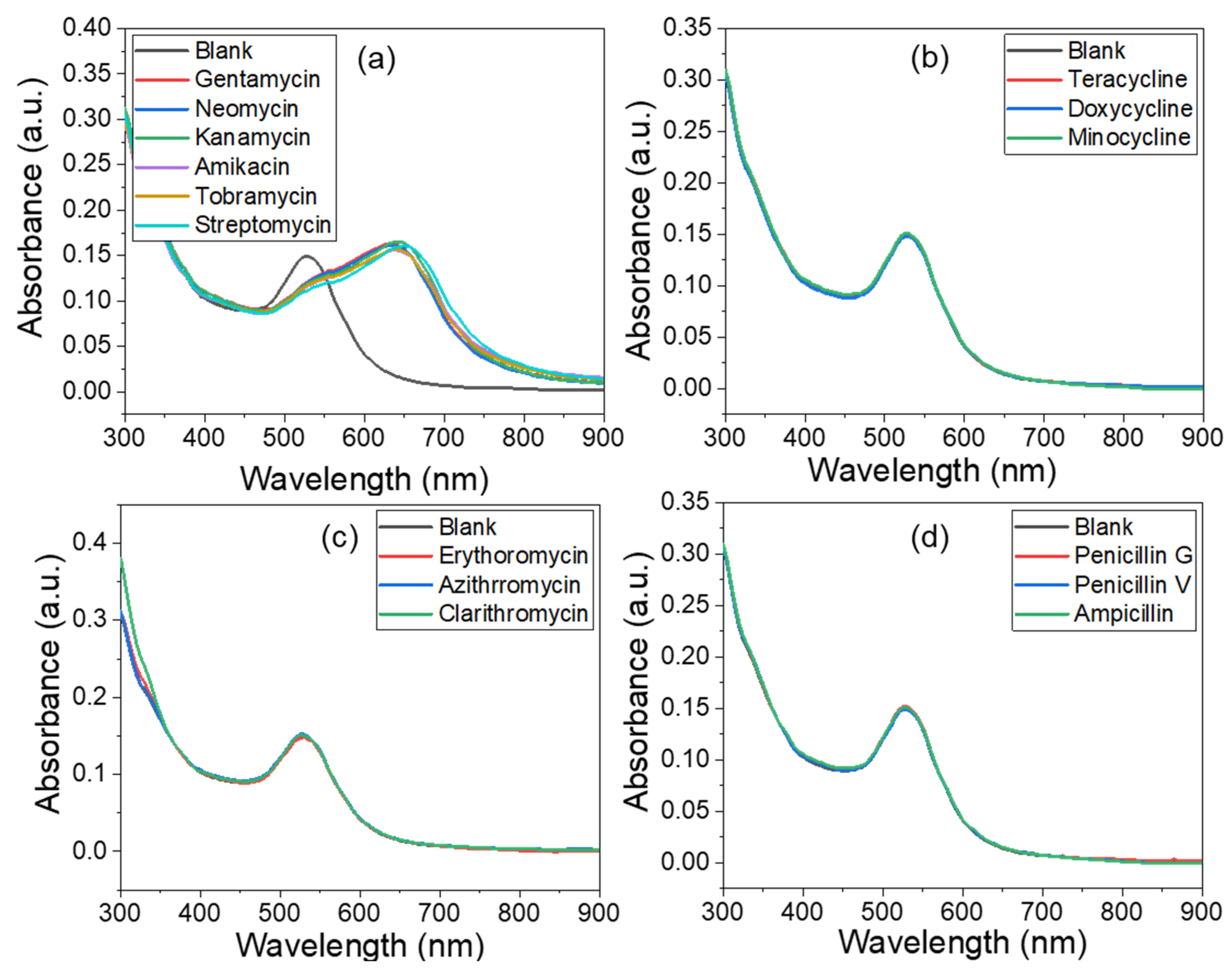
3.3. Selectivity of His@AuNPs
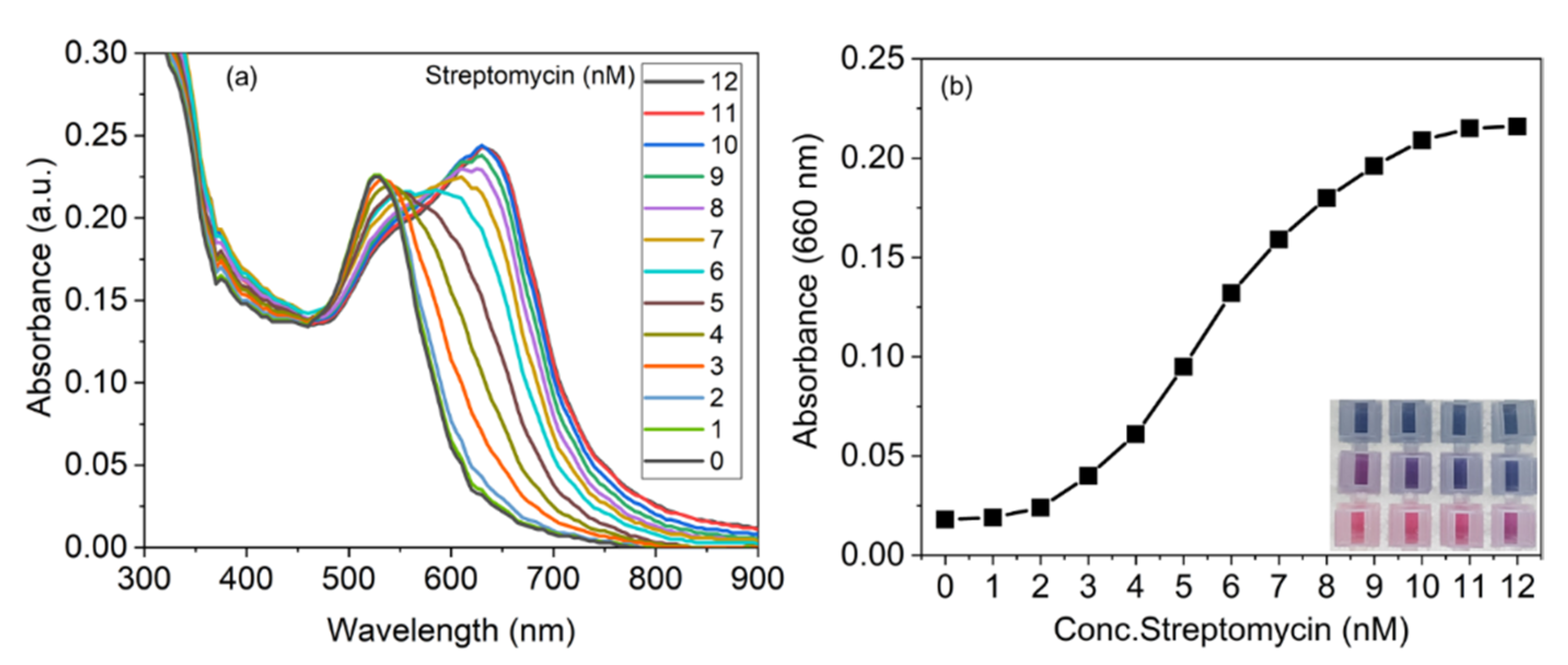
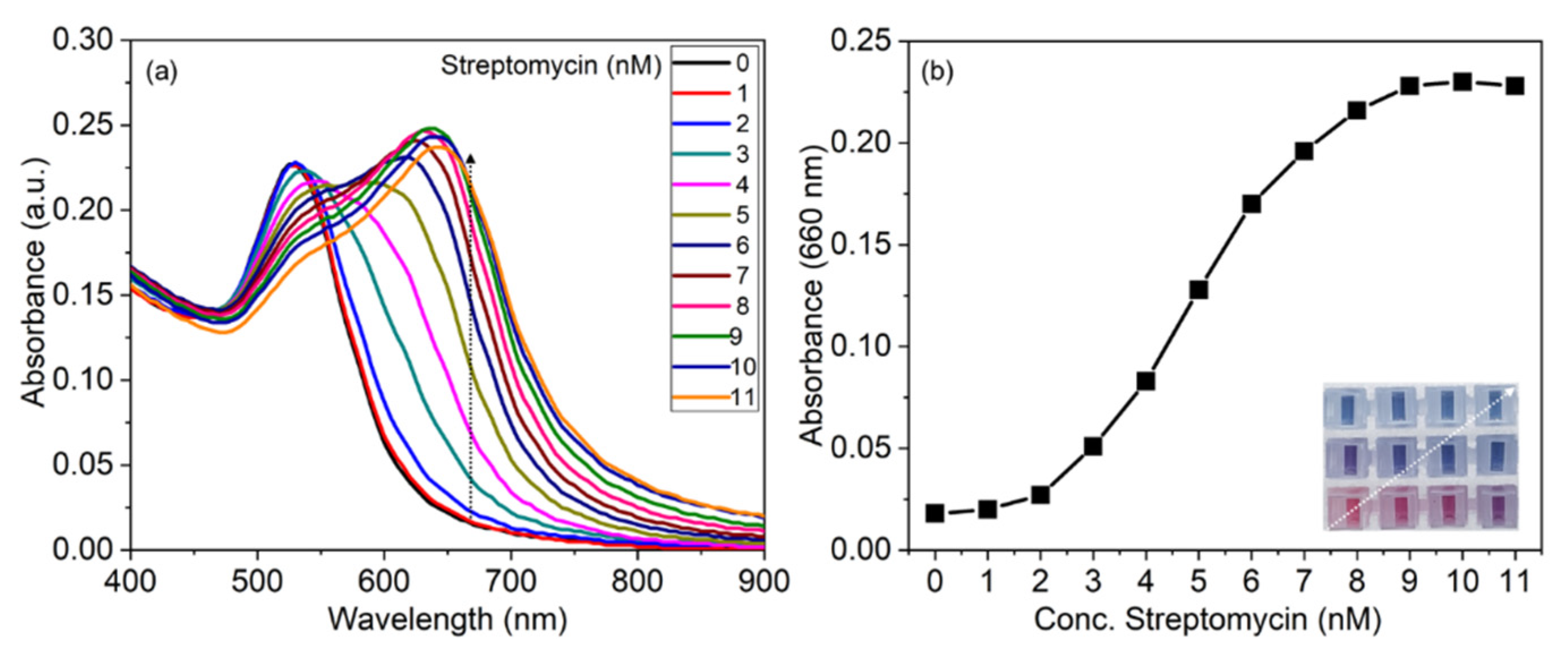
3.4. Sensitivity of His@AuNPs
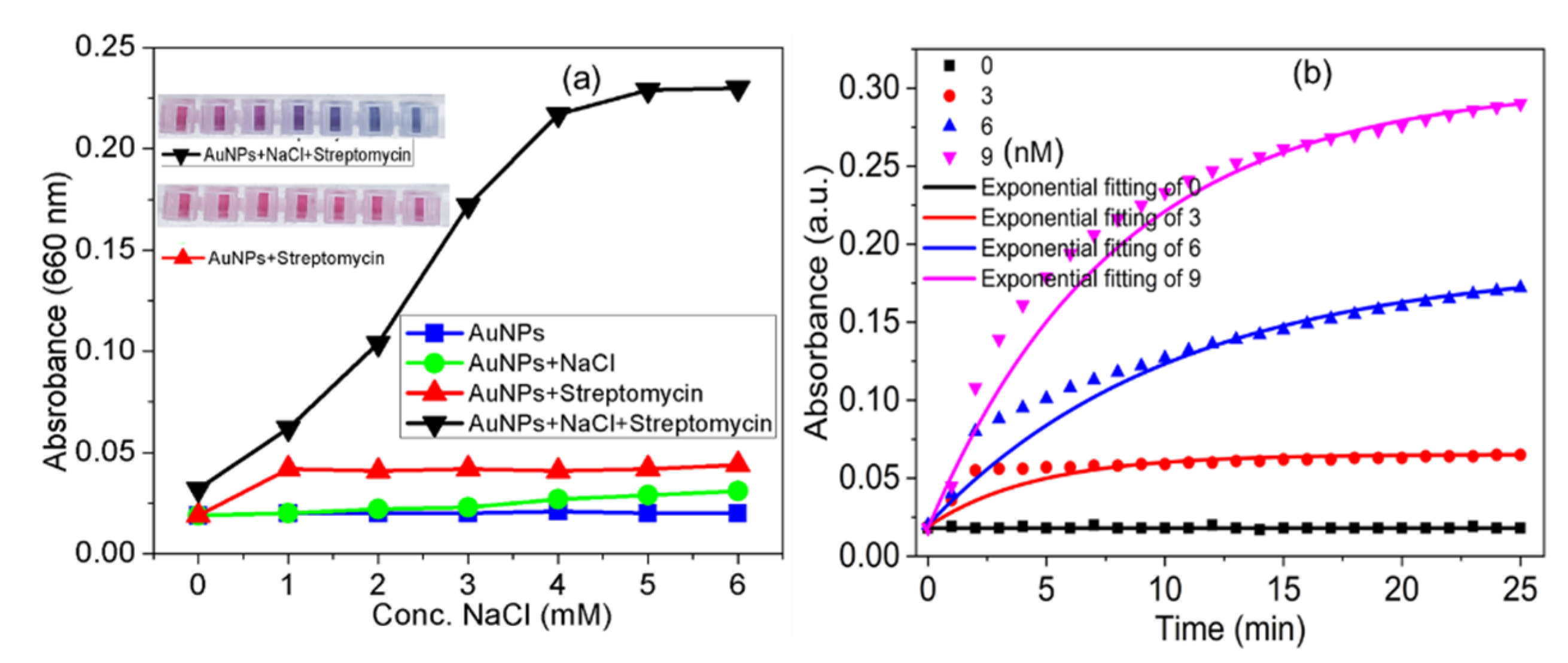
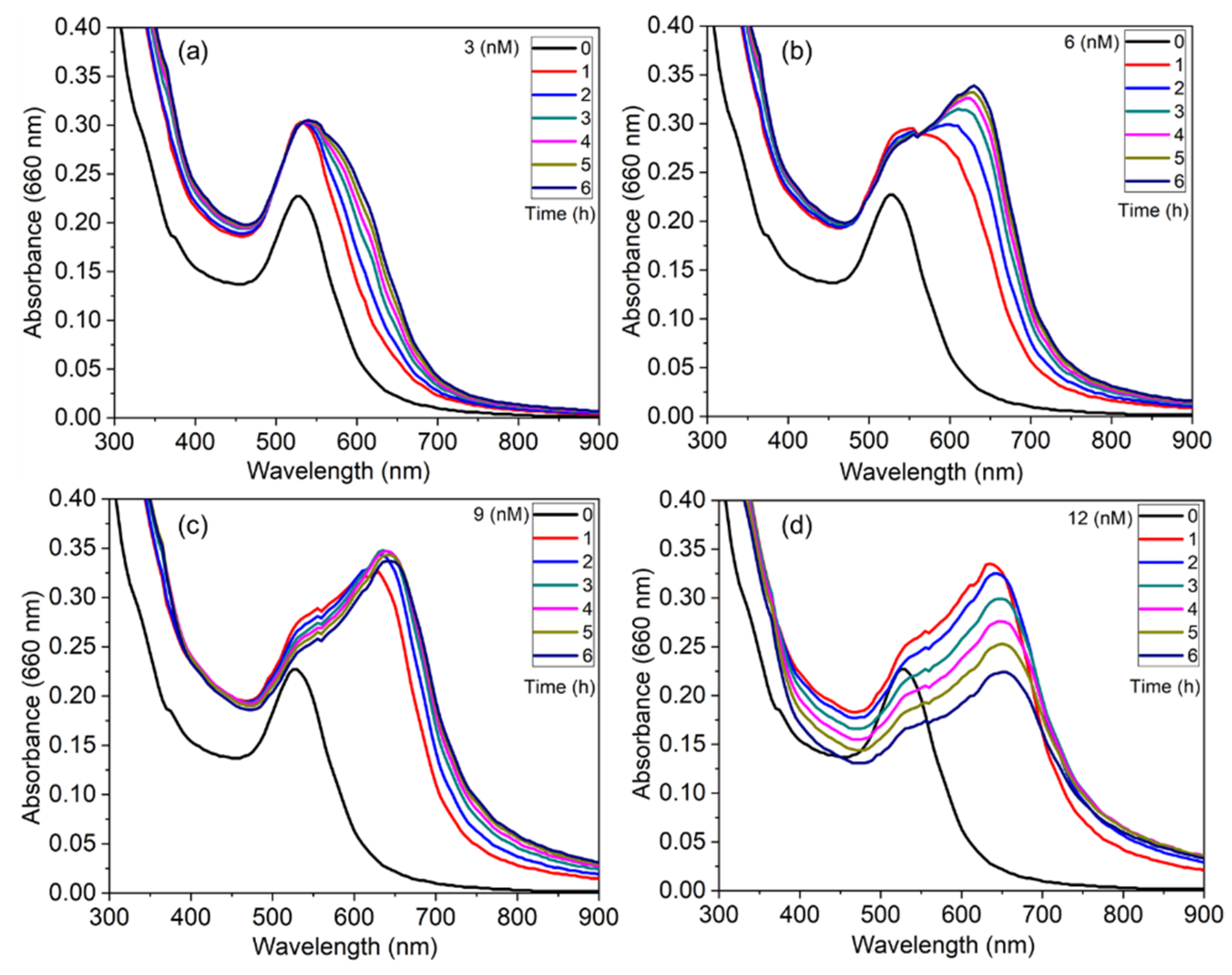
3.5. Effect of Ionic Strength and Real-Time Response
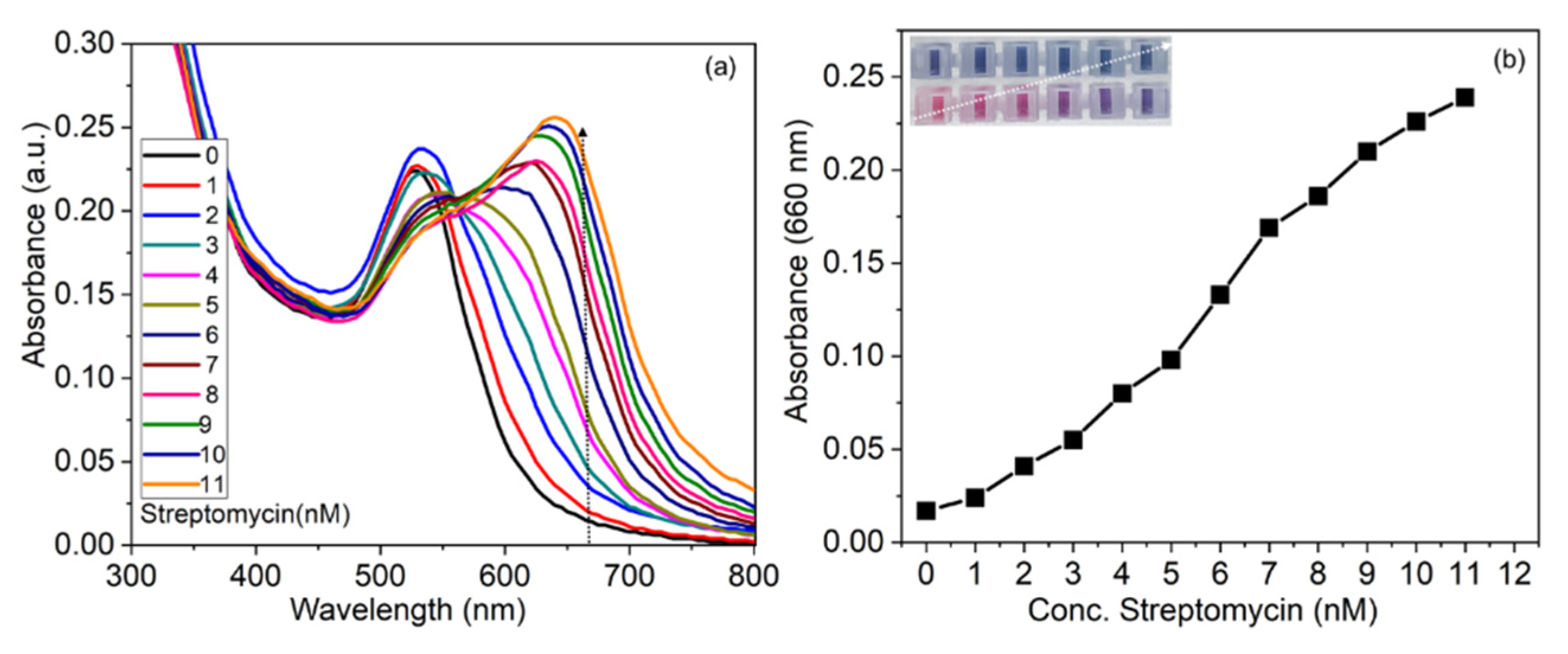
3.6. Applicability of the Detection System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eibl, C.; Bexiga, R.; Viora, L.; Guyot, H.; Félix, J.; Wilms, J.; Tichy, A.; Hund, A. The Antibiotic Treatment of Calf Diarrhea in Four European Countries: A Survey. Antibiotics 2021, 10, 910. [Google Scholar] [CrossRef] [PubMed]
- Little, S.; Woodward, A.; Browning, G.; Billman-Jacobe, H. In-Water Antibiotic Dosing Practices on Pig Farms. Antibiotics 2021, 10, 169. [Google Scholar] [CrossRef]
- De Briyne, N.; Atkinson, J.; Pokludová, L.; Borriello, S.P. Antibiotics used most commonly to treat animals in Europe. Vet. Rec. 2014, 175, 325. [Google Scholar] [CrossRef]
- Almeida, M.P.; Rezende, C.P.; Souza, L.F.; Brito, R.B. Validation of a quantitative and confirmatory method for residue analysis of aminoglycoside antibiotics in poultry, bovine, equine and swine kidney through liquid chromatography-tandem mass spectrometry. Food Addit. Contam. Part A 2012, 29, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Caglayan, M.O. Aptamer-based ellipsometric sensor for ultrasensitive determination of aminoglycoside group antibiotics from dairy products. J. Sci. Food Agric. 2020, 100, 3386–3393. [Google Scholar] [CrossRef]
- Tan, X.; Jiang, Y.-W.; Huang, Y.-J.; Hu, S.-H. Persistence of gentamicin residues in milk after the intramammary treatment of lactating cows for mastitis. J. Zhejiang Univ. Sci. B 2009, 10, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Childs-Kean, L.M.; Shaeer, K.M.; Varghese Gupta, S.; Cho, J.C. Aminoglycoside Allergic Reactions. Pharmacy 2019, 7, 124. [Google Scholar] [CrossRef] [PubMed]
- Peloquin, C.A.; Berning, S.E.; Nitta, A.T.; Simone, P.M.; Goble, M.; Huitt, G.A.; Iseman, M.D.; Cook, J.L.; Curran-Everett, D. Aminoglycoside Toxicity: Daily versus Thrice-Weekly Dosing for Treatment of Mycobacterial Diseases. Clin. Infect. Dis. 2004, 38, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Karasawa, T.; Steyger, P.S. Intracellular mechanisms of aminoglycoside-induced cytotoxicity. Integr. Biol. 2011, 3, 879–886. [Google Scholar] [CrossRef]
- Swan, S.K. Aminoglycoside nephrotoxicity. Semin. Nephrol. 1997, 17, 27–33. [Google Scholar]
- Zuo, P.; Yu, P.; Alvarez, P.J.J.; Dozois, C.M. Aminoglycosides Antagonize Bacteriophage Proliferation, Attenuating Phage Suppression of Bacterial Growth, Biofilm Formation, and Antibiotic Resistance. Appl. Environ. Microbiol. 2021, 87. [Google Scholar] [CrossRef] [PubMed]
- Obayiuwana, A.; Ibekwe, A.M. Antibiotic Resistance Genes Occurrence in Wastewaters from Selected Pharmaceutical Facilities in Nigeria. Water 2020, 12, 1897. [Google Scholar] [CrossRef]
- Garneau-Tsodikova, S.; Labby, K.J. Mechanisms of Resistance to Aminoglycoside Antibiotics: Overview and Perspectives. MedChemComm 2016, 7, 11–27. [Google Scholar] [CrossRef]
- Li, J.H.; Yousif, M.H.; Li, Z.Q.; Wu, Z.H.; Li, S.L.; Yang, H.J.; Wang, Y.J.; Cao, Z.J. Effects of antibiotic residues in milk on growth, ruminal fermentation, and microbial community of preweaning dairy calves. J. Dairy Sci. 2019, 102, 2298–2307. [Google Scholar] [CrossRef] [PubMed]
- Woodward, K.N. Antibiotics and Drugs|Uses in Food Production. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, MS, USA, 2003; pp. 249–254. [Google Scholar]
- Kim, S.; Lee, H.J. Gold Nanostar Enhanced Surface Plasmon Resonance Detection of an Antibiotic at Attomolar Concentrations via an Aptamer-Antibody Sandwich Assay. Anal. Chem. 2017, 89, 6624–6630. [Google Scholar] [CrossRef] [PubMed]
- Cappi, G.; Spiga, F.M.; Moncada, Y.; Ferretti, A.; Beyeler, M.; Bianchessi, M.; Decosterd, L.; Buclin, T.; Guiducci, C. Label-Free Detection of Tobramycin in Serum by Transmission-Localized Surface Plasmon Resonance. Anal. Chem. 2015, 87, 5278–5285. [Google Scholar] [CrossRef]
- McKeating, K.S.; Couture, M.; Dinel, M.-P.; Garneau-Tsodikova, S.; Masson, J.-F. High throughput LSPR and SERS analysis of aminoglycoside antibiotics. Analyst 2016, 141, 5120–5126. [Google Scholar] [CrossRef]
- Gfeller, K.Y.; Nugaeva, N.; Hegner, M. Rapid biosensor for detection of antibiotic-selective growth of Escherichia coli. Appl. Environ. Microbiol. 2005, 71, 2626–2631. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.C.; Nadaraja, A.V.; Narang, R.; Zarifi, M.H. Rapid and real-time monitoring of bacterial growth against antibiotics in solid growth medium using a contactless planar microwave resonator sensor. Sci. Rep. 2021, 11, 14775. [Google Scholar] [CrossRef]
- Derbyshire, N.; White, S.J.; Bunka, D.H.J.; Song, L.; Stead, S.; Tarbin, J.; Sharman, M.; Zhou, D.; Stockley, P.G. Toggled RNA Aptamers Against Aminoglycosides Allowing Facile Detection of Antibiotics Using Gold Nanoparticle Assays. Anal. Chem. 2012, 84, 6595–6602. [Google Scholar] [CrossRef]
- Schoukroun-Barnes, L.R.; Wagan, S.; White, R.J. Correction to Enhancing the Analytical Performance of Electrochemical RNA Aptamer-Based Sensors for Sensitive Detection of Aminoglycoside Antibiotics. Anal. Chem. 2014, 86, 5188. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Deng, S.; Yuan, Z.; Li, C.; Lu, Y.; He, Q.; Zhou, M.; Deng, R. Engineering Multivalence Aptamer Probes for Amplified and Label-Free Detection of Antibiotics in Aquatic Products. J. Agric. Food Chem. 2020, 68, 2554–2561. [Google Scholar] [CrossRef] [PubMed]
- Frasconi, M.; Tel-Vered, R.; Riskin, M.; Willner, I. Surface Plasmon Resonance Analysis of Antibiotics Using Imprinted Boronic Acid-Functionalized Au Nanoparticle Composites. Anal. Chem. 2010, 82, 2512–2519. [Google Scholar] [CrossRef] [PubMed]
- Zakharenkova, S.A.; Dobrovolskii, A.A.; Garshev, A.V.; Statkus, M.A.; Beklemishev, M.K. Chlorophyll-Based Self-Assembled Nanostructures for Fluorescent Sensing of Aminoglycoside Antibiotics. ACS Sustain. Chem. Eng. 2021, 9, 3408–3415. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, F.; Zhu, Y.; Xie, S.; Chen, M.; Xiong, Y.; Liu, Q.; Yang, H.; Chen, X. Intelligent Platform for Simultaneous Detection of Multiple Aminoglycosides Based on a Ratiometric Paper-Based Device with Digital Fluorescence Detector Readout. ACS Sens. 2019, 4, 3283–3290. [Google Scholar] [CrossRef]
- Ahmed, S.; Ning, J.; Cheng, G.; Ahmad, I.; Li, J.; Mingyue, L.; Qu, W.; Iqbal, M.; Shabbir, M.A.B.; Yuan, Z. Receptor-based screening assays for the detection of antibiotics residues—A review. Talanta 2017, 166, 176–186. [Google Scholar] [CrossRef]
- Écija-Arenas, Á.; Román-Pizarro, V.; Fernández-Romero, J.M. Usefulness of Hybrid Magnetoliposomes for Aminoglycoside Antibiotic Residues Determination in Food Using an Integrated Microfluidic System with Fluorometric Detection. J. Agric. Food Chem. 2021, 69, 6888–6896. [Google Scholar] [CrossRef] [PubMed]
- Bobbitt, D.R.; Ng, K.W. Chromatographic analysis of antibiotic materials in food. J. Chromatogr. 1992, 624, 153–170. [Google Scholar] [CrossRef]
- Liu, Q.; Li, J.; Song, X.; Zhang, M.; Li, E.; Gao, F.; He, L. Simultaneous determination of aminoglycoside antibiotics in feeds using high performance liquid chromatography with evaporative light scattering detection. RSC Adv. 2017, 7, 1251–1259. [Google Scholar] [CrossRef]
- Ahmed, S.; Ning, J.; Peng, D.; Chen, T.; Ahmad, I.; Ali, A.; Lei, Z.; Abu bakr Shabbir, M.; Cheng, G.; Yuan, Z. Current advances in immunoassays for the detection of antibiotics residues: A review. Food Agric. Immunol. 2020, 31, 268–290. [Google Scholar] [CrossRef]
- Jung, S.; Kaar, J.L.; Stoykovich, M.P. Design and functionalization of responsive hydrogels for photonic crystal biosensors. Mol. Syst. Des. Eng. 2016, 1, 225–241. [Google Scholar] [CrossRef]
- Feriotto, G.; Corradini, R.; Sforza, S.; Bianchi, N.; Mischiati, C.; Marchelli, R.; Gambari, R. Peptide Nucleic Acids and Biosensor Technology for Real-Time Detection of the Cystic Fibrosis W1282X Mutation by Surface Plasmon Resonance. Lab. Investig. 2001, 81, 1415–1427. [Google Scholar] [CrossRef][Green Version]
- Vello, T.P.; da Silva, L.M.B.; Silva, G.O.; de Camargo, D.H.S.; Corrêa, C.C.; Bof Bufon, C.C. Hybrid organic/inorganic interfaces as reversible label-free platform for direct monitoring of biochemical interactions. Biosens. Bioelectron. 2017, 87, 209–215. [Google Scholar] [CrossRef]
- Kim, H.-M.; Park, J.-H.; Jeong, D.H.; Lee, H.-Y.; Lee, S.-K. Real-time detection of prostate-specific antigens using a highly reliable fiber-optic localized surface plasmon resonance sensor combined with micro fluidic channel. Sens. Actuators B Chem. 2018, 273, 891–898. [Google Scholar] [CrossRef]
- Yang, S.; Wu, T.; Zhao, X.; Li, X.; Tan, W. The Optical Property of Core-Shell Nanosensors and Detection of Atrazine Based on Localized Surface Plasmon Resonance (LSPR) Sensing. Sensors 2014, 14, 13273–13284. [Google Scholar] [CrossRef] [PubMed]
- Homola, J. Present and future of surface plasmon resonance biosensors. Anal. Bioanal. Chem. 2003, 377, 528–539. [Google Scholar] [CrossRef]
- Jain, P.K.; El-Sayed, M.A. Plasmonic coupling in noble metal nanostructures. Chem. Phys. Lett. 2010, 487, 153–164. [Google Scholar] [CrossRef]
- Langer, J.; Novikov, S.M.; Liz-Marzán, L.M. Sensing using plasmonic nanostructures and nanoparticles. Nanotechnology 2015, 26, 322001. [Google Scholar] [CrossRef]
- Willets, K.A.; Duyne, R.P.V. Localized Surface Plasmon Resonance Spectroscopy and Sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-H.; Lee, S.-W.; Jung, J.-A.; Ahn, J.; Kim, M.-G.; Shin, Y.-B. Signal Amplification by Enzymatic Reaction in an Immunosensor Based on Localized Surface Plasmon Resonance (LSPR). Sensors 2010, 10, 2045–2053. [Google Scholar] [CrossRef]
- Hu, Y.; Song, Y.; Wang, Y.; Di, J. Electrochemical synthesis of gold nanoparticles onto indium tin oxide glass and application in biosensors. Thin Solid Films 2011, 519, 6605–6609. [Google Scholar] [CrossRef]
- Tu, M.H.; Sun, T.; Grattan, K.T.V. LSPR optical fibre sensors based on hollow gold nanostructures. Sens. Actuators B Chem. 2014, 191, 37–44. [Google Scholar] [CrossRef]
- Chen, H.; Kou, X.; Yang, Z.; Ni, W.; Wang, J. Shape- and Size-Dependent Refractive Index Sensitivity of Gold Nanoparticles. Langmuir 2008, 24, 5233–5237. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Sun, T.; Grattan, K.T.V. Gold nanorod-based localized surface plasmon resonance biosensors: A review. Sens. Actuators B Chem. 2014, 195, 332–351. [Google Scholar] [CrossRef]
- Deng, J.; Song, Y.; Wang, Y.; Di, J. Label-free optical biosensor based on localized surface plasmon resonance of twin-linked gold nanoparticles electrodeposited on ITO glass. Biosens. Bioelectron. 2010, 26, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated Absorption and Scattering Properties of Gold Nanoparticles of Different Size, Shape, and Composition: Applications in Biological Imaging and Biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef]
- Do, P.Q.T.; Huong, V.T.; Phuong, N.T.T.; Nguyen, T.-H.; Ta, H.K.T.; Ju, H.; Phan, T.B.; Phung, V.-D.; Trinh, K.T.L.; Tran, N.H.T. The highly sensitive determination of serotonin by using gold nanoparticles (Au NPs) with a localized surface plasmon resonance (LSPR) absorption wavelength in the visible region. RSC Adv. 2020, 10, 30858–30869. [Google Scholar] [CrossRef]
- Liu, B.; Huang, R.; Yu, Y.; Su, R.; Qi, W.; He, Z. Gold Nanoparticle-Aptamer-Based LSPR Sensing of Ochratoxin A at a Widened Detection Range by Double Calibration Curve Method. Front. Chem. 2018, 6, 94. [Google Scholar] [CrossRef]
- Iarossi, M.; Schiattarella, C.; Rea, I.; De Stefano, L.; Fittipaldi, R.; Vecchione, A.; Velotta, R.; Ventura, B.D. Colorimetric Immunosensor by Aggregation of Photochemically Functionalized Gold Nanoparticles. ACS Omega 2018, 3, 3805–3812. [Google Scholar] [CrossRef]
- Guo, L.; Xu, Y.; Ferhan, A.R.; Chen, G.; Kim, D.-H. Oriented Gold Nanoparticle Aggregation for Colorimetric Sensors with Surprisingly High Analytical Figures of Merit. J. Am. Chem. Soc. 2013, 135, 12338–12345. [Google Scholar] [CrossRef]
- Mahmood, H.Z.; Jilani, A.; Farooq, S.; Javed, Y.; Jamil, Y.; Iqbal, J.; Ullah, S.; Wageh, S. Plasmon-Based Label-Free Biosensor Using Gold Nanosphere for Dengue Detection. Crystals 2021, 11, 1340. [Google Scholar] [CrossRef]
- Gong, X.; Tang, J.; Ji, Y.; Wu, B.; Wu, H.; Liu, A. Adjustable plasmonic optical properties of hollow gold nanospheres monolayers and LSPR-dependent surface-enhanced Raman scattering of hollow gold nanosphere/graphene oxide hybrids. RSC Adv. 2015, 5, 42653–42662. [Google Scholar] [CrossRef]
- Chen, J.; Shi, S.; Su, R.; Qi, W.; Huang, R.; Wang, M.; Wang, L.; He, Z. Optimization and Application of Reflective LSPR Optical Fiber Biosensors Based on Silver Nanoparticles. Sensors 2015, 15, 12205–12217. [Google Scholar] [CrossRef]
- Sepúlveda, B.; Angelomé, P.C.; Lechuga, L.M.; Liz-Marzán, L.M. LSPR-based nanobiosensors. Nano Today 2009, 4, 244–251. [Google Scholar] [CrossRef]
- Shen, Y.; Zhou, J.; Liu, T.; Tao, Y.; Jiang, R.; Liu, M.; Xiao, G.; Zhu, J.; Zhou, Z.K.; Wang, X.; et al. Plasmonic gold mushroom arrays with refractive index sensing figures of merit approaching the theoretical limit. Nat. Commun. 2013, 4, 2381. [Google Scholar] [CrossRef]
- Reder-Christ, K.; Bendas, G. Biosensor Applications in the Field of Antibiotic Research—A Review of Recent Developments. Sensors 2011, 11, 9450–9466. [Google Scholar] [CrossRef]
- Altintas, Z. Surface plasmon resonance based sensor for the detection of glycopeptide antibiotics in milk using rationally designed nanoMIPs. Sci. Rep. 2018, 8, 11222. [Google Scholar] [CrossRef] [PubMed]
- Helmerhorst, E.; Chandler, D.J.; Nussio, M.; Mamotte, C.D. Real-time and Label-free Bio-sensing of Molecular Interactions by Surface Plasmon Resonance: A Laboratory Medicine Perspective. Clin. Biochem. Rev. 2012, 33, 161–173. [Google Scholar] [PubMed]
- Agrawal, A.; Cho, S.H.; Zandi, O.; Ghosh, S.; Johns, R.W.; Milliron, D.J. Localized Surface Plasmon Resonance in Semiconductor Nanocrystals. Chem. Rev. 2018, 118, 3121–3207. [Google Scholar] [CrossRef] [PubMed]
- Jakhmola, A.; Celentano, M.; Vecchione, R.; Manikas, A.; Battista, E.; Calcagno, V.; Netti, P.A. Self-assembly of gold nanowire networks into gold foams: Production, ultrastructure and applications. Inorg. Chem. Front. 2017, 4, 1033–1041. [Google Scholar] [CrossRef]
- Zhou, Y.; Ji, Y.; Cao, Z. Recent Advances in Optical Detection of Aminoglycosides. Appl. Sci. 2020, 10, 6579. [Google Scholar] [CrossRef]
- Xu, N.; Qu, C.; Ma, W.; Xu, L.; Xu, L.; Liu, L.; Kuang, H.; Xu, C. Development and application of one-step ELISA for the detection of neomycin in milk. Food Agric. Immunol. 2011, 22, 259–269. [Google Scholar] [CrossRef]
- Yue, F.; Li, F.; Kong, Q.; Guo, Y.; Sun, X. Recent advances in aptamer-based sensors for aminoglycoside antibiotics detection and their applications. Sci. Total Environ. 2021, 762, 143129. [Google Scholar] [CrossRef] [PubMed]
- Nikolaus, N.; Strehlitz, B. DNA-Aptamers Binding Aminoglycoside Antibiotics. Sensors 2014, 14, 3737–3755. [Google Scholar] [CrossRef]
- Yan, S.; Lai, X.; Du, G.; Xiang, Y. Identification of aminoglycoside antibiotics in milk matrix with a colorimetric sensor array and pattern recognition methods. Anal. Chim. Acta 2018, 1034, 153–160. [Google Scholar] [CrossRef]
- Haasnoot, W.; Stouten, P.; Cazemier, G.; Lommen, A.; Nouws, J.F.; Keukens, H.J. Immunochemical detection of aminoglycosides in milk and kidney. Analyst 1999, 124, 301–305. [Google Scholar] [CrossRef]
- Nivedhini Iswarya, C.; Kiruba Daniel, S.C.G.; Sivakumar, M. Studies on l-histidine capped Ag and Au nanoparticles for dopamine detection. Mater. Sci. Eng. C 2017, 75, 393–401. [Google Scholar] [CrossRef]
- Shukla, R.; Nune, S.K.; Chanda, N.; Katti, K.; Mekapothula, S.; Kulkarni, R.R.; Welshons, W.V.; Kannan, R.; Katti, K.V. Soybeans as a phytochemical reservoir for the production and stabilization of biocompatible gold nanoparticles. Small 2008, 4, 1425–1436. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-Y.; Shinde, S.; Ghodake, G. Colorimetric detection of magnesium (II) ions using tryptophan functionalized gold nanoparticles. Sci. Rep. 2017, 7, 3966. [Google Scholar] [CrossRef]
- Sachi, S.; Ferdous, J.; Sikder, M.H.; Azizul Karim Hussani, S.M. Antibiotic residues in milk: Past, present, and future. J Adv. Vet. Anim. Res. 2019, 6, 315–332. [Google Scholar] [CrossRef]
- Tan, H.; Chen, Y. Silver nanoparticle enhanced fluorescence of europium (III) for detection of tetracycline in milk. Sens. Actuators B Chem. 2012, 173, 262–267. [Google Scholar] [CrossRef]
- Mukha, I.; Vityuk, N.; Severynovska, O.; Eremenko, A.; Smirnova, N. The pH-Dependent Stucture and Properties of Au and Ag Nanoparticles Produced by Tryptophan Reduction. Nanoscale Res. Lett. 2016, 11, 101. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Kim, M.; Shinde, S.; Saratale, R.G.; Sung, J.-S.; Ghodake, G. Temperature Dependent Synthesis of Tryptophan-Functionalized Gold Nanoparticles and Their Application in Imaging Human Neuronal Cells. ACS Sustain. Chem. Eng. 2017, 5, 7678–7689. [Google Scholar] [CrossRef]
- Guan, J.; Jiang, L.; Li, J.; Yang, W. pH-Dependent Aggregation of Histidine-Functionalized Au Nanoparticles Induced by Fe3+ Ions. J. Phys. Chem. C 2008, 112, 3267–3271. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Q.; Paau, M.C.; Xie, S.; Gao, P.; Chan, W.; Choi, M.M.F. Probing Histidine-Stabilized Gold Nanoclusters Product by High-Performance Liquid Chromatography and Mass Spectrometry. J. Phys. Chem. C 2013, 117, 18697–18708. [Google Scholar] [CrossRef]
- Liu, Z.; Zu, Y.; Fu, Y.; Meng, R.; Guo, S.; Xing, Z.; Tan, S. Hydrothermal synthesis of histidine-functionalized single-crystalline gold nanoparticles and their pH-dependent UV absorption characteristic. Colloids Surf. B Biointerfaces 2010, 76, 311–316. [Google Scholar] [CrossRef]
- Siva, C.; Iswarya, C.N.; Baraneedharan, P.; Sivakumar, M. L-Cysteine assisted formation of mesh like Ag2S and Ag3AuS2 nanocrystals through hydrogen bonds. Mater. Lett. 2014, 134, 56–59. [Google Scholar] [CrossRef]
- Barth, A. The infrared absorption of amino acid side chains. Prog. Biophys. Mol. Biol. 2000, 74, 141–173. [Google Scholar] [CrossRef]
- Liu, Z.; Xing, Z.; Zu, Y.; Tan, S.; Zhao, L.; Zhou, Z.; Sun, T. Synthesis and characterization of L-histidine capped silver nanoparticles. Mater. Sci. Eng. C 2012, 32, 811–816. [Google Scholar] [CrossRef]
- Alharbi, R.; Irannejad, M.; Yavuz, M. A Short Review on the Role of the Metal-Graphene Hybrid Nanostructure in Promoting the Localized Surface Plasmon Resonance Sensor Performance. Sensors 2019, 19, 862. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Wang, N.; Li, C.; Guo, X.; Lu, A. Advances in the Application of Aptamer Biosensors to the Detection of Aminoglycoside Antibiotics. Antibiotics 2020, 9, 787. [Google Scholar] [CrossRef]
- Caglayan, M.G.; Onur, F. A metal-enhanced fluorescence study of primary amines: Determination of aminoglycosides with europium and gold nanoparticles. Anal. Methods 2015, 7, 1407–1414. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Shao, H.; Wang, Z.; Wang, X.; Jiang, X. Label-Free Colorimetric Detection of Cadmium Ions in Rice Samples Using Gold Nanoparticles. Anal. Chem. 2014, 86, 8530–8534. [Google Scholar] [CrossRef] [PubMed]
- Meienberg, K.; Malinina, T.; Nguyen, Z.; Park, C.S.; Glaser, M.A.; Clark, N.A.; Maclennan, J.E. Nanoparticle Aggregation and Fractal Growth in Fluid Smectic Membranes. Mol. Cryst. Liq. Cryst. 2015, 611, 14–20. [Google Scholar] [CrossRef]
- Lu, G.; Chen, Q.; Li, Y.; Liu, Y.; Zhang, Y.; Huang, Y.; Zhu, L. Status of antibiotic residues and detection techniques used in Chinese milk: A systematic review based on cross-sectional surveillance data. Food Res. Int. 2021, 147, 110450. [Google Scholar] [CrossRef]
- Layada, S.; Benouareth, D.-E.; Coucke, W.; Andjelkovic, M. Assessment of antibiotic residues in commercial and farm milk collected in the region of Guelma (Algeria). Int. J. Food Contam. 2016, 3, 19. [Google Scholar] [CrossRef]
- Rahman, M.S.; Hassan, M.M.; Chowdhury, S. Determination of antibiotic residues in milk and assessment of human health risk in Bangladesh. Heliyon 2021, 7, e07739. [Google Scholar] [CrossRef]
- Moghadam, M.M.; Amiri, M.; Riabi, H.R.A.; Riabi, H.R.A. Evaluation of Antibiotic Residues in Pasteurized and Raw Milk Distributed in the South of Khorasan-e Razavi Province, Iran. J. Clin. Diagn. Res. JCDR 2016, 10, FC31–FC35. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, L.M.; Di Cesare, F.; Nobile, M.; Villa, R.; Decastelli, L.; Martucci, F.; Fontana, M.; Pavlovic, R.; Arioli, F.; Panseri, S. Antibiotics and Non-Targeted Metabolite Residues Detection as a Comprehensive Approach toward Food Safety in Raw Milk. Foods 2021, 10, 544. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Sharma, G.; Leahy, E.; Shome, B.R.; Bandyopadhyay, S.; Deka, R.P.; Shome, R.; Dey, T.K.; Lindahl, J.F. Understanding Antibiotic Usage on Small-Scale Dairy Farms in the Indian States of Assam and Haryana Using a Mixed-Methods Approach—Outcomes and Challenges. Antibiotics 2021, 10, 1124. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shinde, S.K.; Kim, D.-Y.; Saratale, R.G.; Kadam, A.A.; Saratale, G.D.; Syed, A.; Bahkali, A.H.; Ghodake, G.S. Histidine Functionalized Gold Nanoparticles for Screening Aminoglycosides and Nanomolar Level Detection of Streptomycin in Water, Milk, and Whey. Chemosensors 2021, 9, 358. https://doi.org/10.3390/chemosensors9120358
Shinde SK, Kim D-Y, Saratale RG, Kadam AA, Saratale GD, Syed A, Bahkali AH, Ghodake GS. Histidine Functionalized Gold Nanoparticles for Screening Aminoglycosides and Nanomolar Level Detection of Streptomycin in Water, Milk, and Whey. Chemosensors. 2021; 9(12):358. https://doi.org/10.3390/chemosensors9120358
Chicago/Turabian StyleShinde, Surendra Krushna, Dae-Young Kim, Rijuta Ganesh Saratale, Avinash Ashok Kadam, Ganesh Dattatraya Saratale, Asad Syed, Ali H. Bahkali, and Gajanan Sampatrao Ghodake. 2021. "Histidine Functionalized Gold Nanoparticles for Screening Aminoglycosides and Nanomolar Level Detection of Streptomycin in Water, Milk, and Whey" Chemosensors 9, no. 12: 358. https://doi.org/10.3390/chemosensors9120358
APA StyleShinde, S. K., Kim, D.-Y., Saratale, R. G., Kadam, A. A., Saratale, G. D., Syed, A., Bahkali, A. H., & Ghodake, G. S. (2021). Histidine Functionalized Gold Nanoparticles for Screening Aminoglycosides and Nanomolar Level Detection of Streptomycin in Water, Milk, and Whey. Chemosensors, 9(12), 358. https://doi.org/10.3390/chemosensors9120358