Fluorescent AgNCs Formed on Bifunctional DNA Template for Potassium Ion Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. DNA-Ag Nanoclusters Synthesis
2.2.2. Fluorescence Spectroscopy
- C12-AgNCs: Emission spectra of C12-AgNCs were collected at 3 excitation wavelengths: λex = 260 nm, λex = 470 nm and λex = 580 nm. Excitation spectra were collected at emission wavelength λem = 630 nm.
- Tel22C12-AgNCs: Emission spectra of Tel22C12-AgNCs were collected at 3 excitation wavelengths: λex = 260 nm, λex = 470 nm and λex = 570 nm. Excitation spectra were collected at 2 emission wavelengths: λem = 550 nm and λem = 620 nm.
- TBAC12-AgNCs: Emission spectra of TBAC12-AgNCs were collected at 3 excitation wavelengths: λex = 260 nm, λex = 470 nm and λex = 560 nm. Excitation spectra were collected at 2 emission wavelengths: λem = 550 nm and λem = 610 nm.
2.2.3. Circular Dichroism (CD) Spectroscopy
2.2.4. UV-Vis Absorption Spectroscopy
2.2.5. Transmission Electron Microscopy (TEM)
2.2.6. Studies upon Metal Cation Effect
3. Results
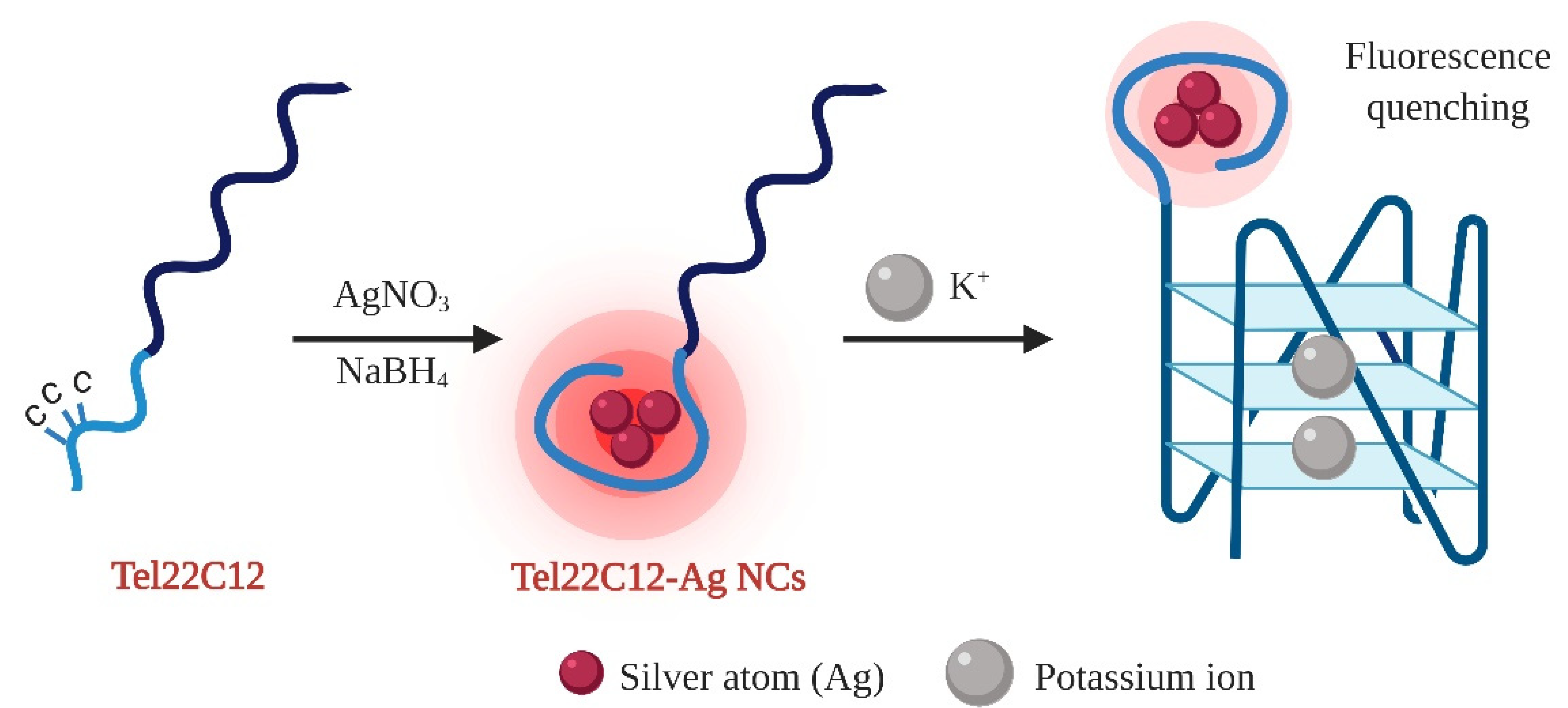
3.1. The Prinicple Working Idea
3.2. Rationalization of AgNCs Templated on C12 Integrated with GQ
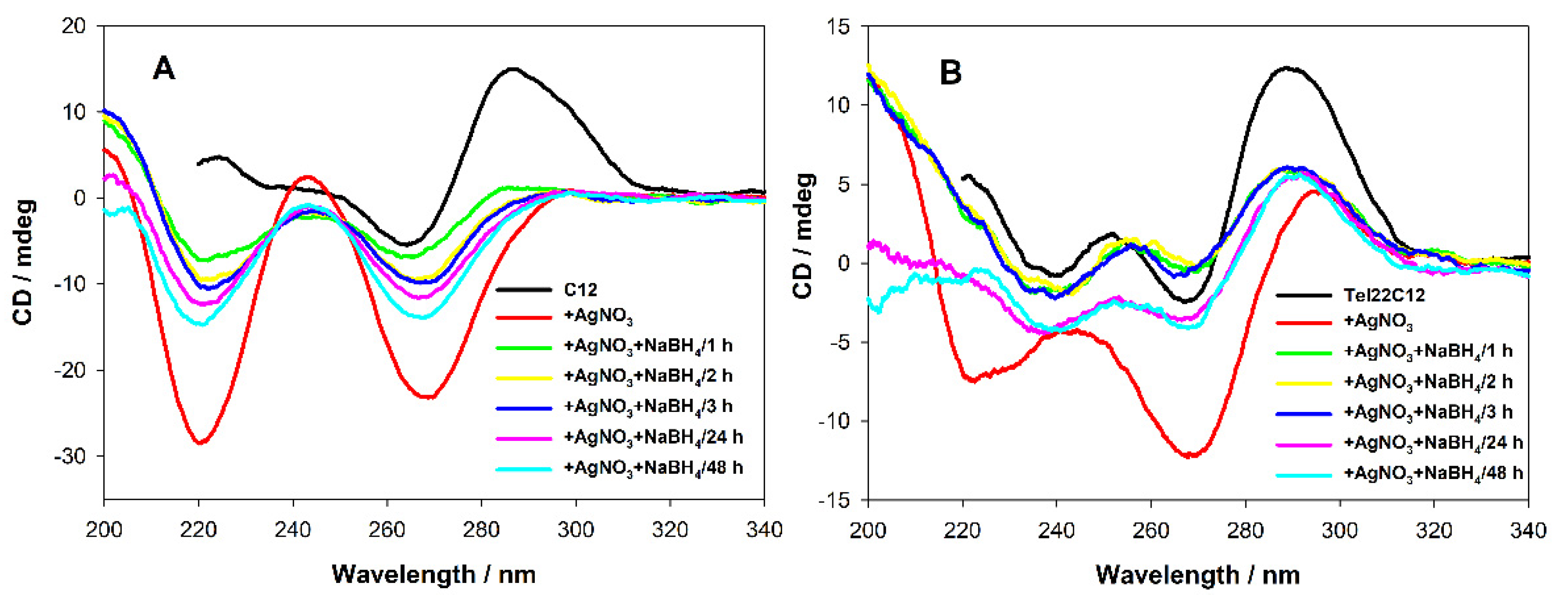
3.3. Circular Dichroism Studies upon AgNCs Templated on C12 Integrated with Tel22
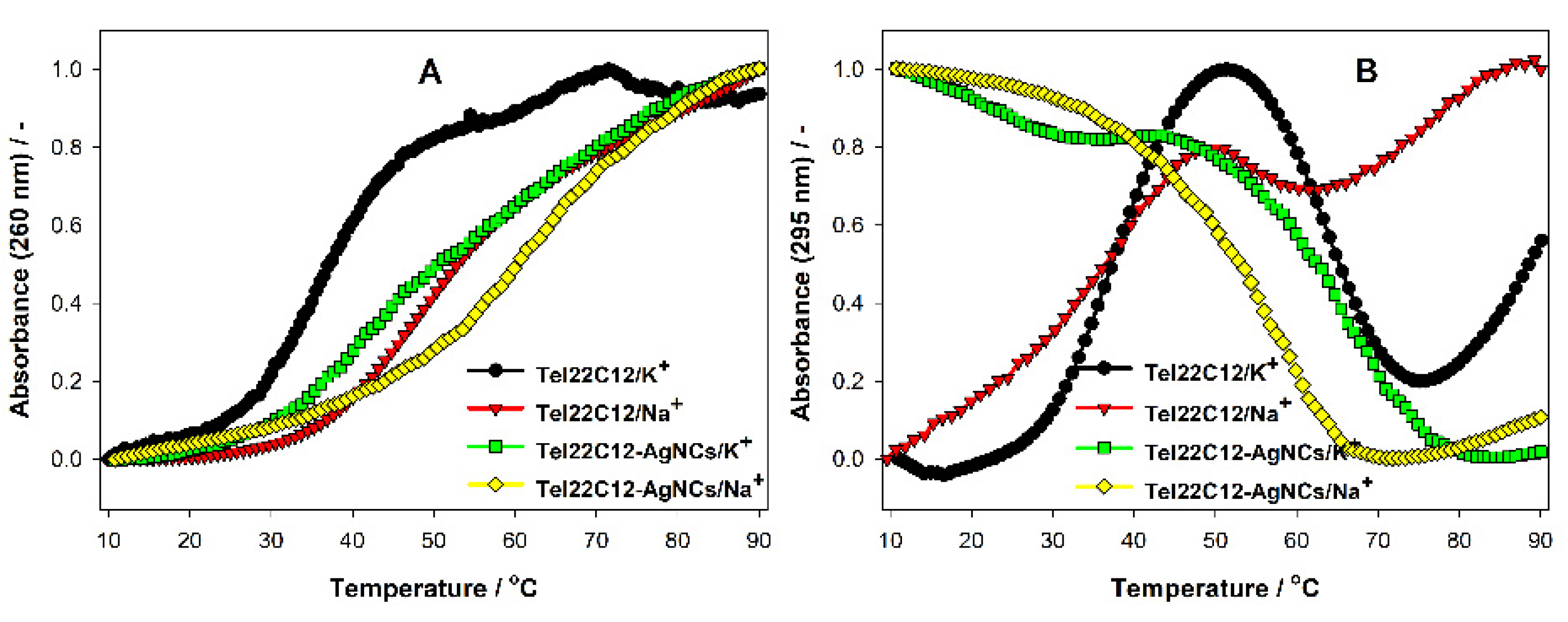
3.4. Thermodynamic Stability of GQ Integrated with C12 Scaffold for AgNCs
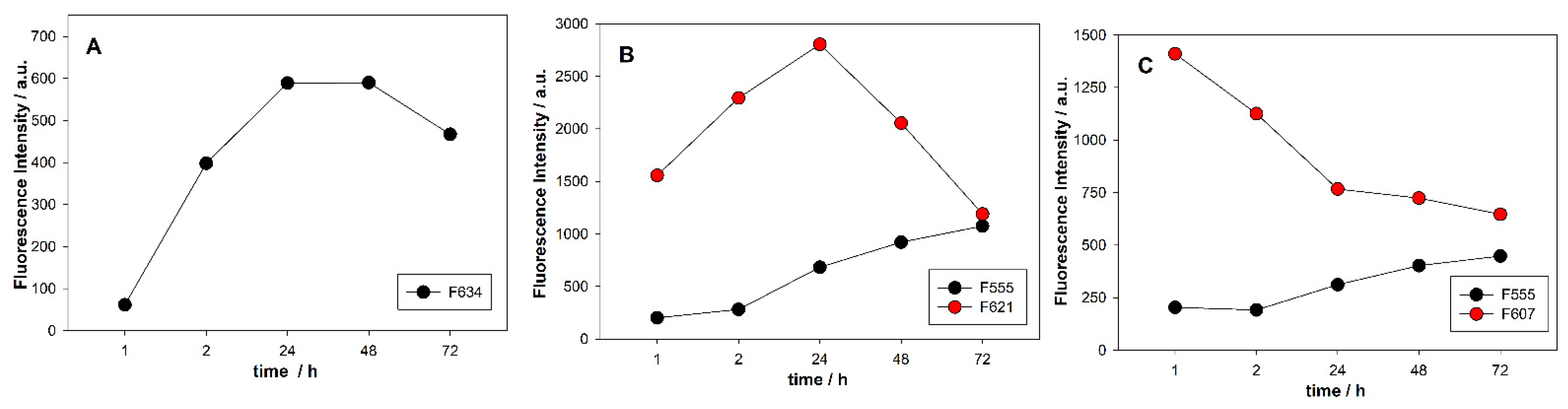
3.5. Temporal Stability of Fluorescent AgNCs Templated on C12 Integrated with GQ
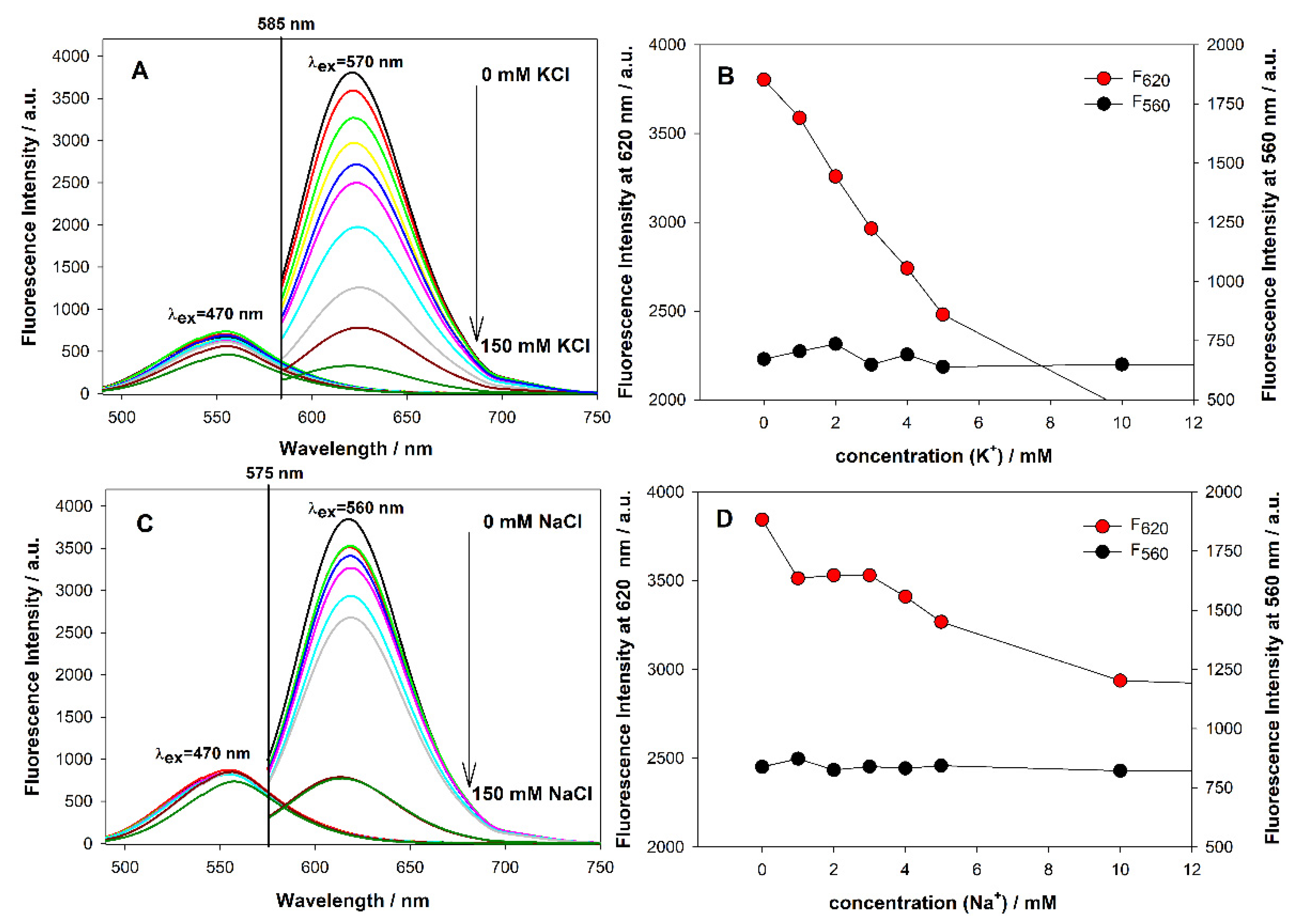
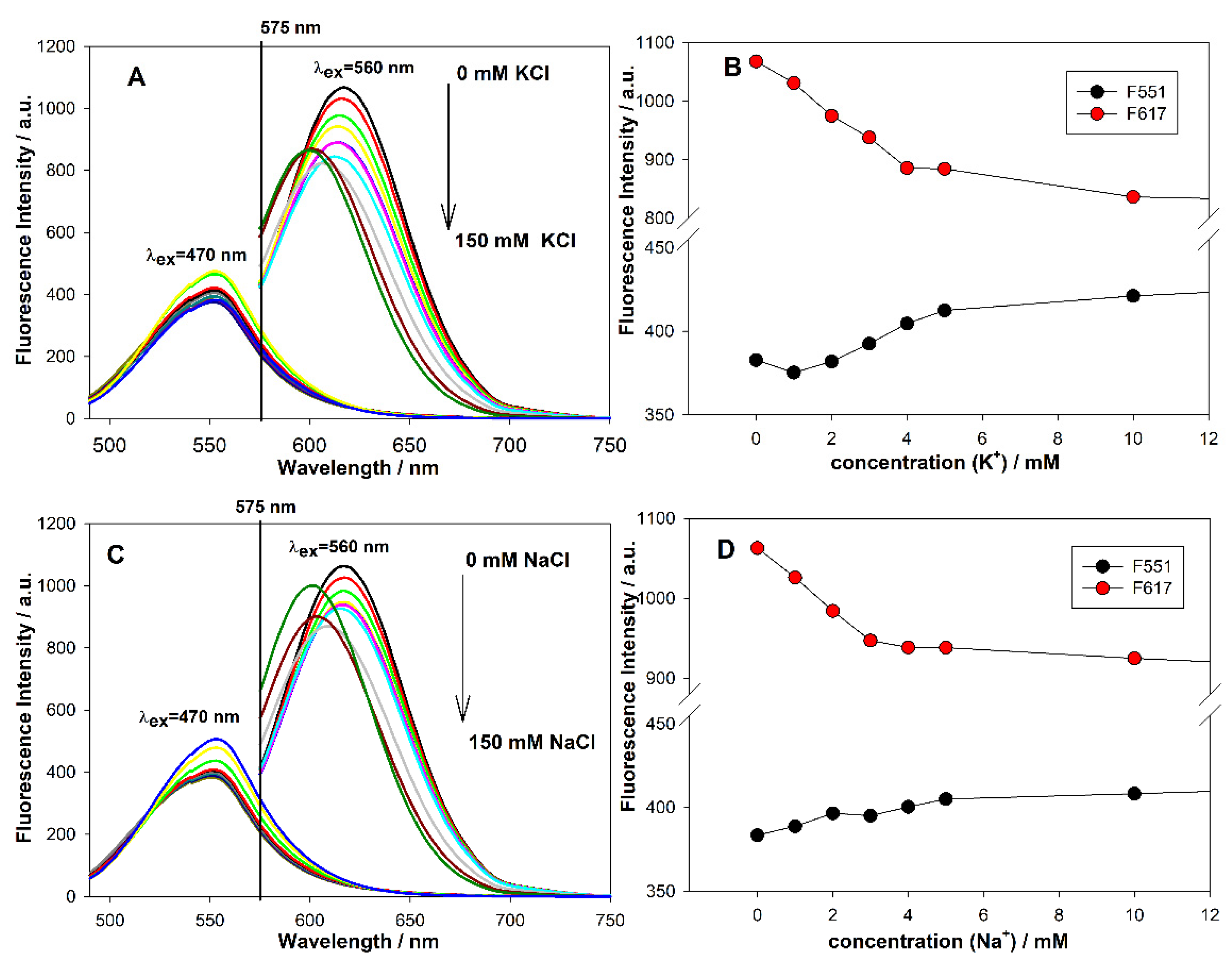
3.6. Effect of Potassium and Sodium Ions on AgNCs Templated on C12 Integrated with GQ
3.7. Feasibility of Proposed Systems to Detect K+ Ions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Q.; Yang, M.; Zhu, Y.; Mao, C. Metallic Nanoclusters for Cancer Imaging and Therapy. Curr. Med. Chem. 2018, 25, 1379–1396. [Google Scholar] [CrossRef] [PubMed]
- Ono, A.; Cao, S.; Togashi, H.; Tashiro, M.; Fujimoto, T.; Machinami, T.; Oda, S.; Miyake, Y.; Okamoto, I.; Tanaka, Y. Specific Interactions between Silver (i) Ions and Cytosine–Cytosine Pairs in DNA Duplexes. Chem. Commun. 2008, 39, 4825–4827. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Fu, Y.; He, Z.; Han, Y.; Zheng, L.; Zhang, J.; Li, W. Growth Mechanisms of Fluorescent Silver Clusters Regulated by Polymorphic DNA Templates: A DFT Study. J. Phys. Chem. B 2012, 116, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Petty, J.T.; Zheng, J.; Hud, N.V.; Dickson, R.M. DNA-Templated Ag Nanocluster Formation. J. Am. Chem. Soc. 2004, 126, 5207–5212. [Google Scholar] [CrossRef]
- Berti, L.; Burley, G.A. Nucleic acid and nucleotide-mediated synthesis of inorganic nanoparticles. Nat. Nanotechnol. 2008, 3, 81–87. [Google Scholar] [CrossRef]
- Shukla, S.; Sastry, M. Probing Differential Ag+–Nucleobase Interactions with Isothermal Titration Calorimetry (ITC): Towards Patterned DNA Metallization. Nanoscale 2009, 1, 122–127. [Google Scholar] [CrossRef]
- Li, W.; Liu, L.; Fu, Y.; Sun, Y.; Zhang, J.; Zhang, R. Effects of Polymorphic DNA on the Fluorescent Properties of Silver Nanoclusters. Photochem. Photobiol. Sci. 2013, 12, 1864–1872. [Google Scholar] [CrossRef]
- Chen, Y.; Phipps, L.M.; Werner, J.H.; Chakraborty, S.; Martinez, J.S. DNA Templated Metal Nanoclusters: From Emergent Properties to Unique Applications. Acc. Chem. Res. 2008, 51, 12756–12763. [Google Scholar] [CrossRef] [PubMed]
- New, S.Y.; Lee, S.T.; Su, X.D. DNA-templated silver nanoclusters: Structural correlation and fluorescence modulation. Nanoscale 2016, 8, 17729–17746. [Google Scholar] [CrossRef]
- Zhou, Z.; Du, Y.; Dong, S. DNA-Ag nanoclusters as fluorescence probe for turn-on aptamer sensor of small molecules. Biosens. Bioelectron. 2011, 28, 33–37. [Google Scholar] [CrossRef]
- Alipour, M.; Jalili, S.; Shirzad, H.; Dezfouli, E.A.; Fouani, M.H.; Sadeghan, A.A.; Bardania, H.; Hosseinkhani, S. Development of dual-emission cluster of Ag atoms for genetically modified organisms detection. Mikrochim. Acta 2020, 187, 628, Erratum in 2020, 187, 662. [Google Scholar] [CrossRef]
- Han, G.M.; Jia, Z.Z.; Zhu, Y.J.; Jiao, J.J.; Kong, D.M.; Feng, X.Z. Biostable L-DNA-Templated Aptamer-Silver Nanoclusters for Cell-Type-Specific Imaging at Physiological Temperature. Anal. Chem. 2016, 88, 10800–10804. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Reyes, E.M.; Teng, Y.; Bates, P. A new paradigm for aptamer therapeutic AS1411 action: Uptake by macropinocytosis and its stimulation by a nucleolin-dependent mechanism. J. Cancer Res. 2010, 70, 8617–8629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soundararajan, S.; Chen, W.; Spicer, E.K.; Courtenay-Luck, N.; Fernandes, D. The nucleolin targeting aptamer AS1411 destabilizes Bcl-2 messenger RNA in human breast cancer cells. J. Cancer Res. 2008, 68, 2358–2365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, J.; Yeh, H.-C.; Yoo, H.; Werner, J.H.; Martinez, J.S. DNA Silver nanocluster aptamers: In situ generation of intrinsically fluorescent recognition ligands for protein detection. ChemComm 2011, 47, 2294–2296. [Google Scholar]
- Luu, K.N.; Phan, A.T.; Kuryavyi, V.; Lacroix, L.; Patel, D.J. Structure of the human telomere in K⁺ solution: An intramolecular (3+1) G-quadruplex scaffold. J. Am. Chem. Soc. 2006, 128, 9963–9970. [Google Scholar] [CrossRef] [Green Version]
- Phan, A.T.; Kuryavyi, V.; Luu, K.N.; Patel, D.J. Structure of two intramolecular G-quadruplexes formed by natural human telomere sequences in K⁺ solution. Nucleic Acids Res. 2007, 35, 6517–6525. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Patel, D.J. Solution structure of the human telomeric repeat d[AG(3)(T2AG3)3] G-tetraplex. Structure 1993, 1, 263–282. [Google Scholar] [CrossRef]
- Bock, L.C.; Griffin, L.C.; Latham, J.A.; Vermaas, E.H.; Toole, J.J. Selection of single-stranded-DNA molecules that bind and inhibit human thrombin. Nature 1992, 355, 564–566. [Google Scholar] [CrossRef]
- Schultze, P.; Macaya, R.F.; Feigon, J. 3-Dimensional solution structure of the thrombin-binding DNA aptamer d (GGTTGGTGTGGTTGG). J. Mol. Biol. 1994, 235, 1532–1547. [Google Scholar] [CrossRef]
- Wang, K.Y.; Krawczyk, S.H.; Bischofberger, N.; Swaminathan, S.; Bolton, P.H. The tertiary structure of a DNA aptamer which binds to and inhibits thrombin determines activity. Biochemistry 1993, 32, 11285–11292. [Google Scholar] [CrossRef] [PubMed]
- Nagatoishi, S.; Nojima, T.; Juskowiak, B.; Takenaka, S. A Pyrene-labeled G-quadruplex oligonucleotide as a fluorescence probe for potassium ions detection in biological applications. Angew. Chem. 2005, 44, 5067–5070. [Google Scholar] [CrossRef]
- He, F.; Tang, Y.; Wang, S.; Li, Y.; Zhu, D. Fluorescent Amplifying Recognition for DNA G-Quadruplex Folding with a Cationic Conjugated Polymer: A Platform for Homogeneous Potassium Detection. J. Am. Chem. Soc. 2005, 127, 12343–12346. [Google Scholar] [CrossRef] [PubMed]
- Nagatoishi, S.; Nojima, T.; Galezowska, E.; Juskowiak, B.; Takenaka, S. G-quadruplex-based FRET probes with the thrombin-binding aptamer (TBA) sequence designed for the efficient fluorometric detection of the potassium ion. ChemBioChem 2006, 7, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Guo, J.; Yang, W.; Ma, Y.; Shen, H. Crystal violet-G-quadruplex complexes as fluorescent sensors for homogeneous detection of potassium ion. Biosens. Bioelectron. 2009, 25, 88–93. [Google Scholar] [CrossRef]
- Ohtsuka, S.K.; Sato, S.; Sato, Y.; Sota, K.; Ohzawa, S.; Matsuda, T.; Takemoto, K.; Takamune, N.; Juskowiak, B.; Nagaib, T.; et al. Fluorescence imaging of potassium ions in living cells using a fluorescent probe based on a thrombin binding aptamer–peptide conjugate. Chem. Commun. 2012, 48, 4740–4742. [Google Scholar] [CrossRef] [PubMed]
- Switalska, A.; Dembska, A.; Fedoruk-Wyszomirska, A.; Juskowiak, B. Cholesterol-Bearing Fluorescent G-Quadruplex Potassium Probes for Anchoring at the Langmuir Monolayer and Cell Membrane. Sensors 2018, 18, 2201–2219. [Google Scholar] [CrossRef] [Green Version]
- Takenaka, S. Detection of tetraplex DNA and detection by tetraplex DNA. Anal. Sci. 2021, 37, 9–15. [Google Scholar] [CrossRef]
- Tietz, N.W. Fundamentals of Clinical Chemistry; W.B. Saunders Company: Philadelphia, PA, USA, 1976; pp. 875–877. [Google Scholar]
- Ritchie, C.M.; Johnsen, K.R.; Kiser, J.R.; Antoku, Y.; Dickson, R.M.; Petty, J.T. Ag Nanocluster Formation Using a Cytosine Oligonucleotide Template. J. Phys. Chem. C 2007, 111, 175–181. [Google Scholar] [CrossRef] [Green Version]
- Dembska, A.; Switalska, A.; Fedoruk-Wyszomirska, A.; Juskowiak, B. Development of fluorescence oligonucleotide probes based on cytosine- and guanine-rich sequences. Sci. Rep. 2020, 10, 11006–11018. [Google Scholar] [CrossRef]
- Richards, C.I.; Choi, S.; Hsiang, J.C.; Antoku, Y.; Vosch, T.; Bongiorno, A.; Tzeng, Y.L.; Dickson, R.M. Oligonucleotide stabilized Ag nanocluster fluorophore. J. Am. Chem. Soc. 2008, 130, 5038–5039. [Google Scholar] [CrossRef] [Green Version]
- Daune, M.; Dekker, C.A.; Schachman, H.K. Complexes of Silver Ion with Natural and Synthetic Polynucleotides. Biopolymers 1966, 4, 51–76. [Google Scholar] [CrossRef]
- Petty, J.T.; Story, S.P.; Hsiang, J.C.; Dickson, R.M. DNA-Templated Molecular Silver Fluorophores. J. Phys. Chem. Lett. 2013, 4, 1148–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soto-Verdugo, V.; Metiu, H.; Gwinn, E. The Properties of Small Ag Clusters Bound to DNA Bases. J. Chem. Phys. 2010, 132, 195102. [Google Scholar] [CrossRef] [PubMed]
- Manzini, G.; Yathindra, N.; Xodo, L.E. Evidence for intramolecularly folded i-DNA structures in biologically relevant CCCrepeat sequences. Nucleic Acids Res. 1994, 22, 4634–4640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simonsson, T.; Pribylova, M.; Vorlickova, M. A Nuclease Hypersensitive Element in the Human c-myc Promoter Adopts Several Distinct i-Tetraplex Structures. Biochem. Biophys. Res. Commun. 2000, 278, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Pagano, B.; Martino, L.; Randazzo, A.; Giancola, C. Stability and binding properties of a modified thrombin binding aptamer. Biophys. J. 2008, 94, 562–569. [Google Scholar] [CrossRef] [Green Version]
- Nagatoishi, S.; Isono, N.; Tsumoto, K.; Sugimoto, N. Loop residues of thrombin-binding DNA aptamer impact G-quadruplex stability and thrombin binding. Biochimie 2011, 93, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Sacca, B. The Effect of Chemical Modifications on the Thermal Stability of Different G-Quadruplex-Forming Oligonucleotides. Nucleic Acids Res. 2005, 33, 1182–1192. [Google Scholar] [CrossRef] [Green Version]
- Tao, Y.; Li, M.; Ren, J.; Qu, X. Metal nanoclusters: Novel probes for diagnostic and therapeutic applications. Chem. Soc. Rev. 2015, 44, 8636–8663. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Tan, L.; Hu, L.; Luan, Y. Superior fluorescent probe for detection of potassium ion. Talanta 2015, 144, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Rastegar, A. Serum Potassium. In Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990; Chapter 195. Available online: https://www.ncbi.nlm.nih.gov/books/NBK307/ (accessed on 30 October 2021).
- Gwinn, E.G.; O’Neill, P.; Guerrero, A.J.; Bouwmeester, D.; Fygenson, D.K. Sequence-Dependent Fluorescence of DNA-Hosted Silver Nanoclusters. Adv. Mater. 2008, 20, 279–283. [Google Scholar] [CrossRef]
- Sengupta, B.; Ritchie, C.M.; Buckman, J.G.; Johnsen, K.R.; Goodwin, P.M.; Petty, J.T. Base-Directed Formation of Fluorescent Silver Clusters. J. Phys. Chem. C 2008, 112, 18776–18782. [Google Scholar] [CrossRef] [PubMed]


| Name | Oligonucleotide Sequence | Company |
|---|---|---|
| C12 | 5’-CCC CCC CCC CCC-3’ | IBA |
| Tel22C12 TBAC12 | 5’-AGG GTT AGG GTT AGG GTT AGG G CC CCC CCC CCC C-3’ 5’-GGT TGG TGT GGT TGG CCC CCC CCC CCC-3’ | Genomed Genomed |
| Abbreviation of Probes | Tm/K+ (°C) | Tm/Na+ (°C) |
|---|---|---|
| Tel22 | 62.0 1 | 55.0 1 |
| Tel22C12 | 37.5/65.0 | 36.0/55.0 |
| Tel22C12-AgNCs | 64.5 | 56.0 |
| TBA | 53.0 1 | 24.0 1 |
| TBAC12 | 18.2/52.0 | - |
| TBA C12-AgNCs | 51.0 | 42.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filipczuk, P.; Świtalska, A.; Kosman, J.; Nowaczyk, G.; Dembska, A. Fluorescent AgNCs Formed on Bifunctional DNA Template for Potassium Ion Detection. Chemosensors 2021, 9, 349. https://doi.org/10.3390/chemosensors9120349
Filipczuk P, Świtalska A, Kosman J, Nowaczyk G, Dembska A. Fluorescent AgNCs Formed on Bifunctional DNA Template for Potassium Ion Detection. Chemosensors. 2021; 9(12):349. https://doi.org/10.3390/chemosensors9120349
Chicago/Turabian StyleFilipczuk, Patrycja, Angelika Świtalska, Joanna Kosman, Grzegorz Nowaczyk, and Anna Dembska. 2021. "Fluorescent AgNCs Formed on Bifunctional DNA Template for Potassium Ion Detection" Chemosensors 9, no. 12: 349. https://doi.org/10.3390/chemosensors9120349
APA StyleFilipczuk, P., Świtalska, A., Kosman, J., Nowaczyk, G., & Dembska, A. (2021). Fluorescent AgNCs Formed on Bifunctional DNA Template for Potassium Ion Detection. Chemosensors, 9(12), 349. https://doi.org/10.3390/chemosensors9120349








