An Enzymatic Multiplexed Impedimetric Sensor Based on α-MnO2/GQD Nano-Composite for the Detection of Diabetes and Diabetic Foot Ulcer Using Micro-Fluidic Platform
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of Graphene Quantum Dots (GQDs)
2.3. Synthesis of α-MnO2/GQD Nanocomposite
2.4. Characterization of α-MnO2/GQD Nanocomposites
2.5. Sensor Fabrication
3. Results
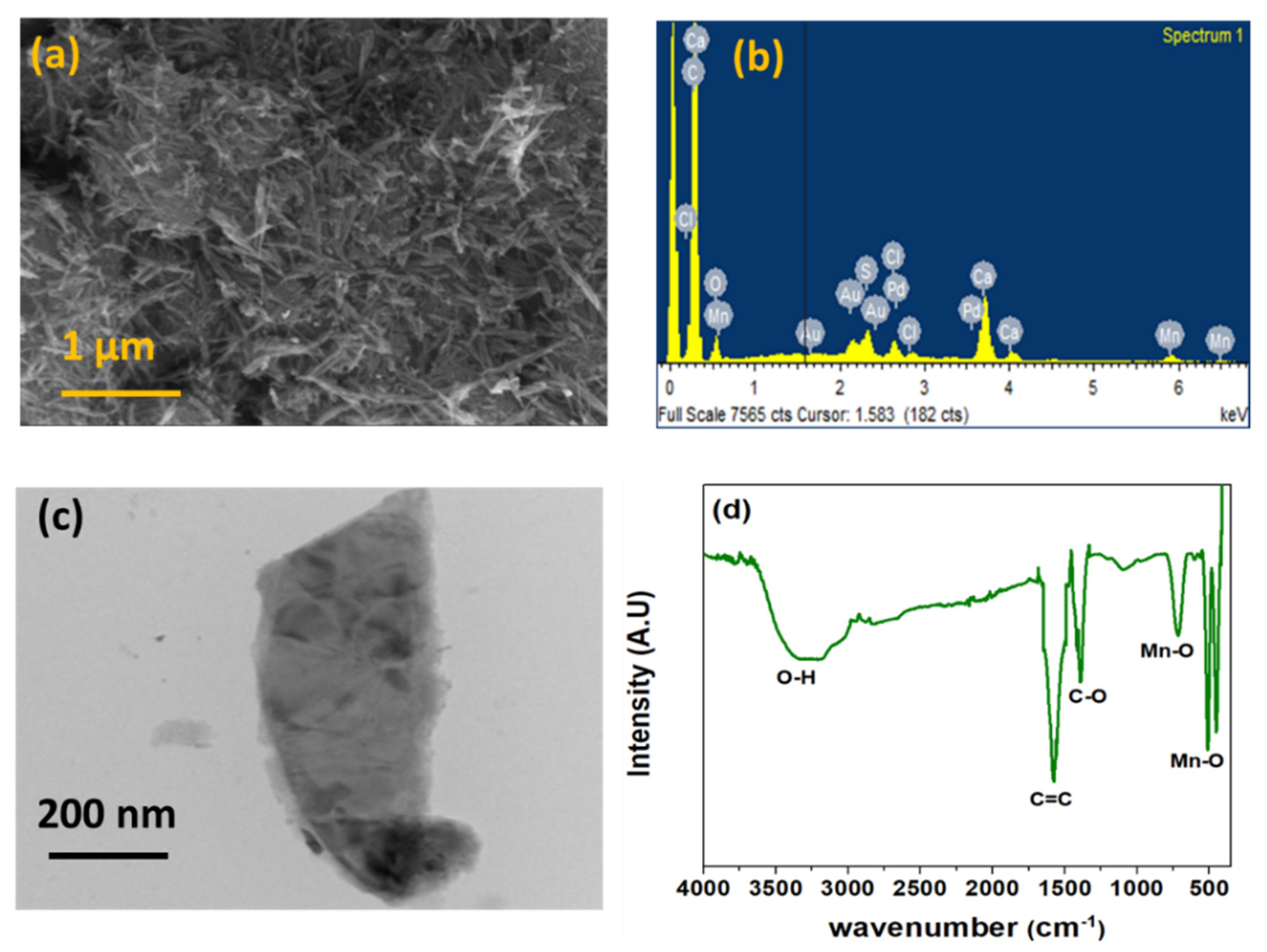
3.1. Surface Characterization of α-MnO2/GQD Nanocomposites
3.2. Electrochemical Multiplexed Monitoring of Glucose and Tyrosine
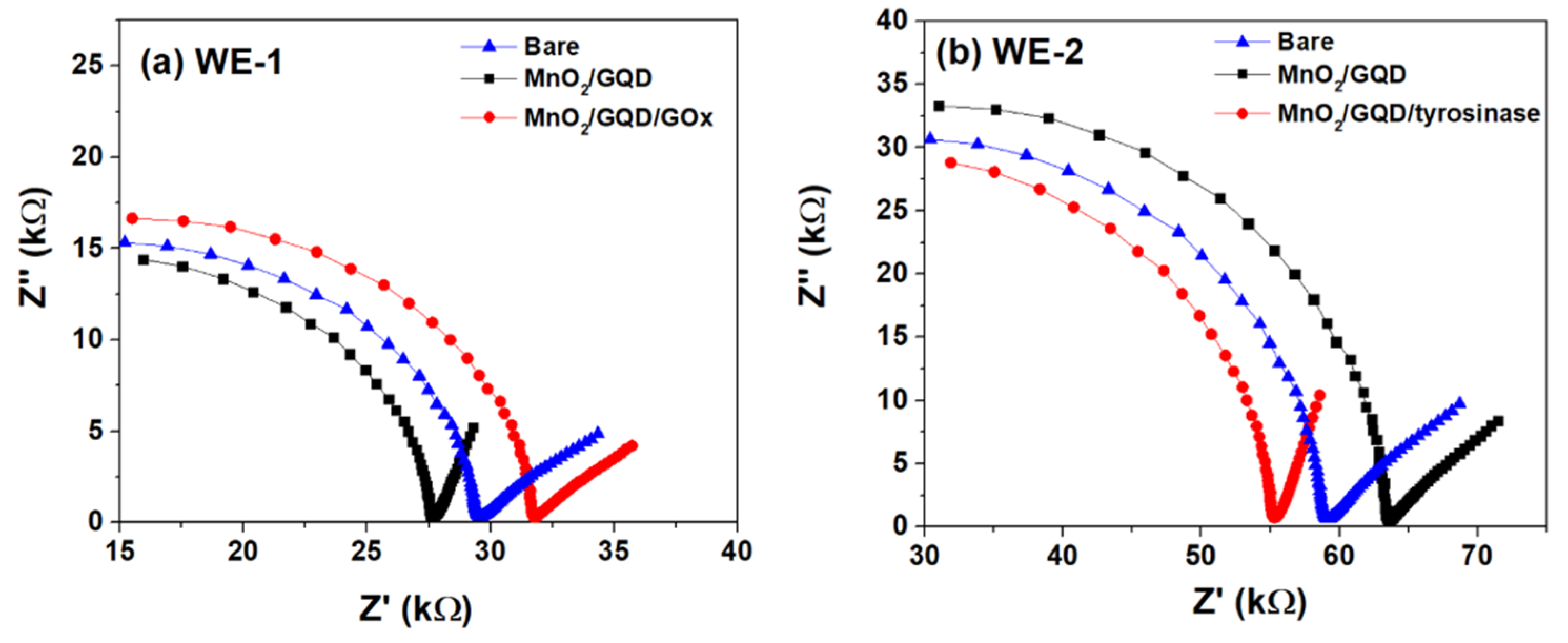
3.2.1. Sensor Stages Response
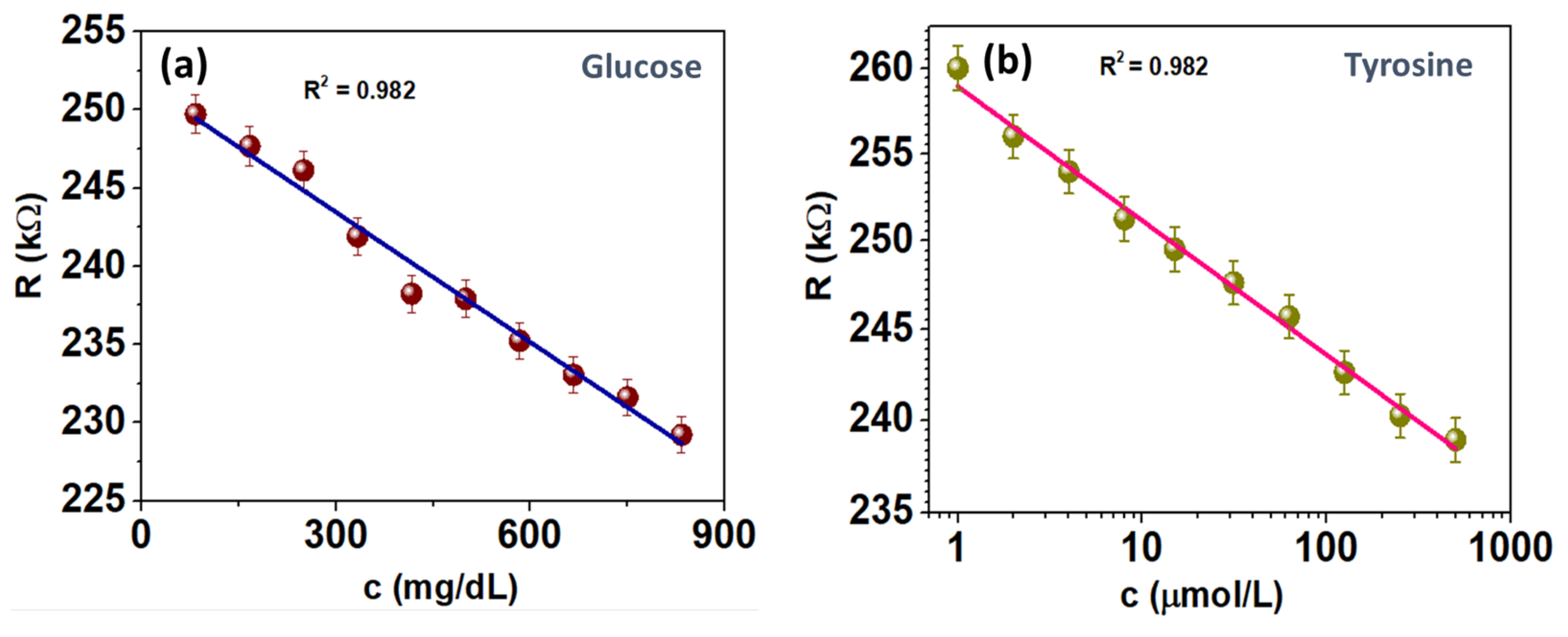
3.2.2. Analytical Performance of Multiplexed Sensor: Benchtop EIS Calibration
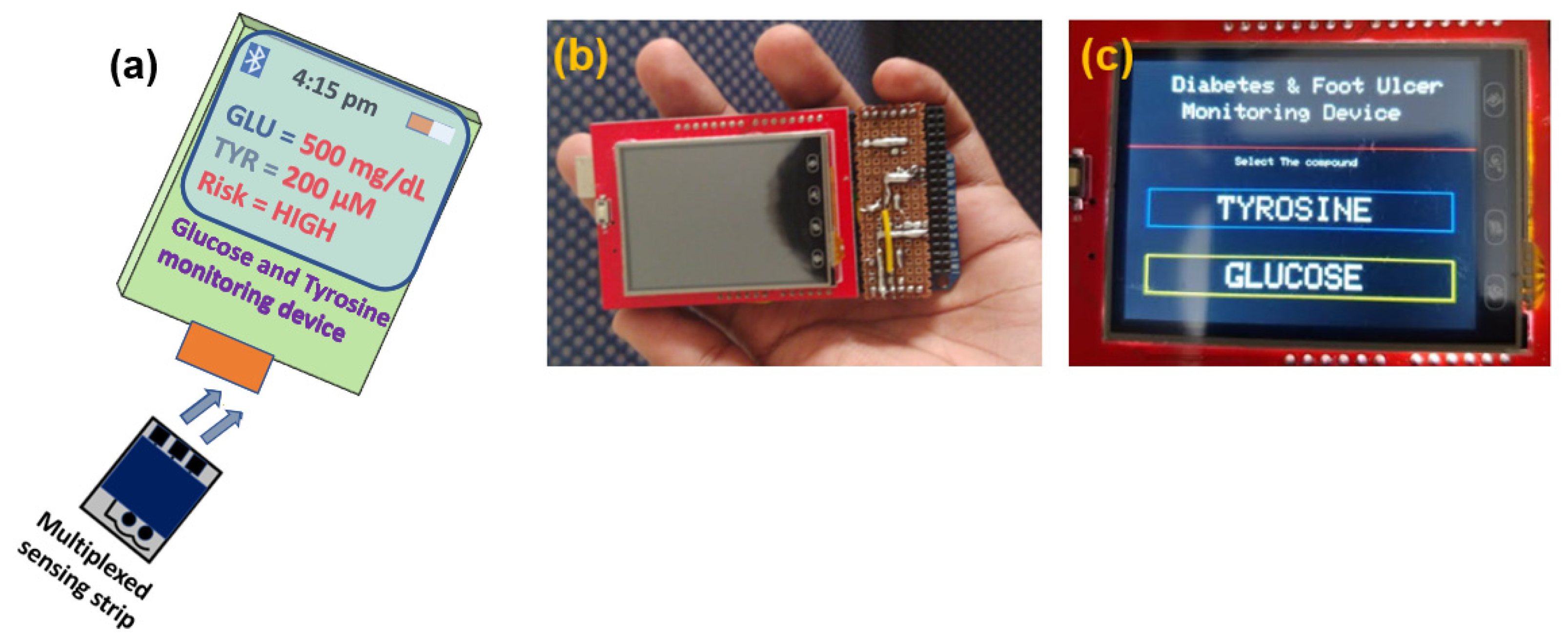
3.2.3. Implementation of Multiplexed Sensing Strip towards Portable Device Applications
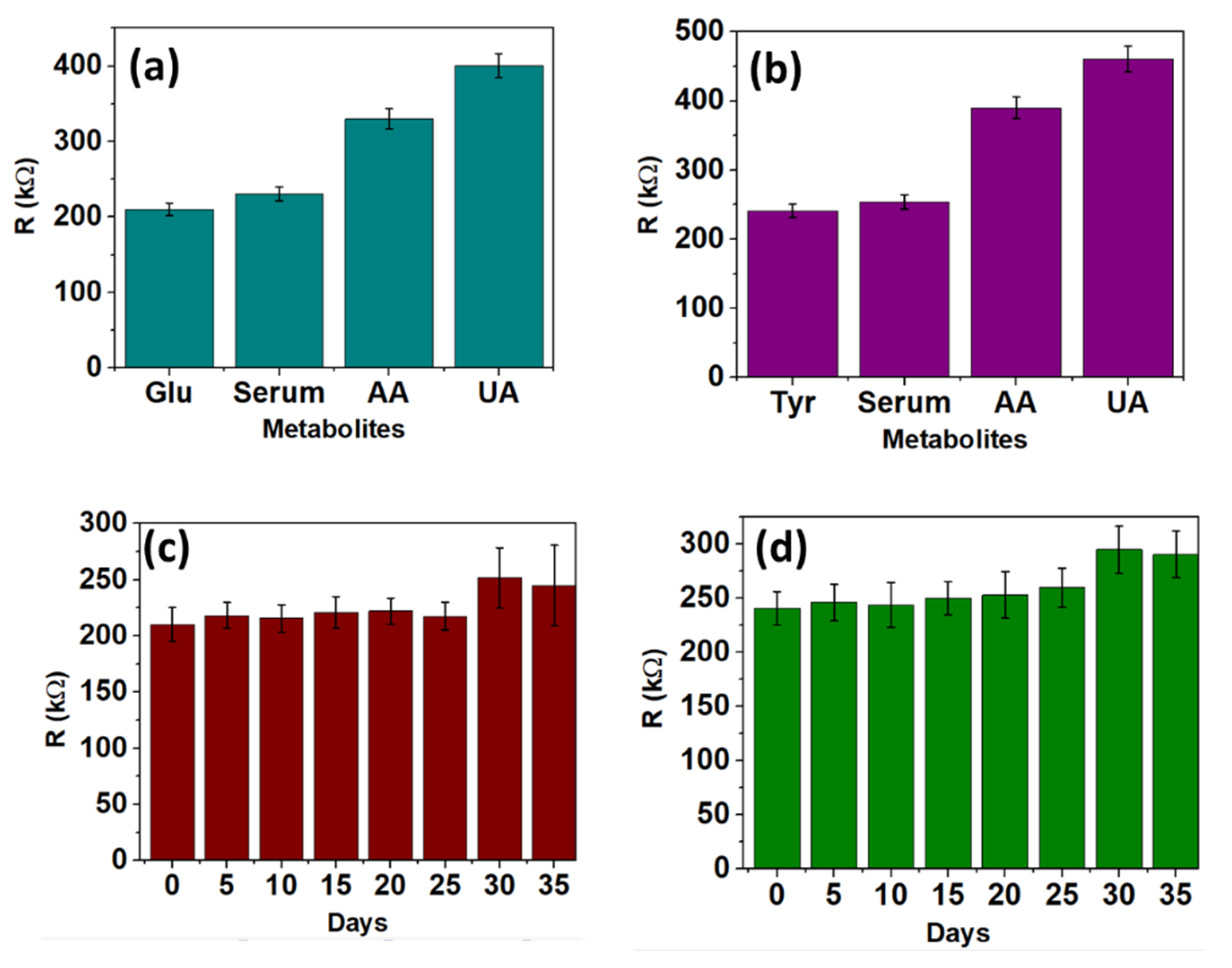
3.2.4. Selectivity and Shelf-Life Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Li, J.; Wang, Z.; Deng, A. Wound exudate CXCL6: A potential biomarker for wound healing of diabetic foot ulcers. Biomark. Med. 2019, 13, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; John, A.; Nagabooshanam, S.; Mishra, A.; Wadhwa, S.; Mathur, A.; Narang, J.; Singh, J.; Dilawar, N.; Davis, J. Self-aligned TiO2—Photo reduced graphene oxide hybrid surface for smart bandage application. Appl. Surf. Sci. 2019, 488, 261–268. [Google Scholar] [CrossRef]
- Kundu, Z.S.; Tanwar, M.; Singh, K.; Singh, B. Clinical Assessment, Risk Factors, and Classification of Diabetic Foot: An Overview. Clin. Assess. 2017, 4, 35–39. [Google Scholar] [CrossRef]
- Sheahan, H.; Canning, K.; Refausse, N.; Kinnear, E.M.; Jorgensen, G.; Walsh, J.R.; Lazzarini, P.A. Differences in the daily activity of patients with diabetic foot ulcers compared to controls in their free-living environments. Int. Wound J. 2017, 14, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Maluf, K.S.; Mueller, M.J. Comparison of physical activity and cumulative plantar tissue stress among subjects with and without diabetes mellitus and a history of recurrent plantar ulcers. Clin. Biomech. 2003, 18, 567–575. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Abu-Rumman, P.L.; Nixon, B.P.; Boulton, A.J.M. Continuous activity monitoring in persons at high risk for diabetes-related lower-extremity amputation. J. Am. Podiatr. Med. Assoc. 2001, 91, 451–455. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Lavery, L.A.; Holtz-Neiderer, K.; Mohler, M.J.; Wendel, C.S.; Nixon, B.P.; Boulton, A.J. Variability in activity may precede diabetic foot ulceration. Diabetes Care 2004, 27, 1980–1984. [Google Scholar] [CrossRef] [Green Version]
- Kanade, R.V.; van Deursen, R.W.M.; Harding, K.; Price, P. Walking performance in people with diabetic neuropathy: Benefits and threats. Diabetology 2006, 49, 1747–1754. [Google Scholar] [CrossRef]
- Crews, R.T.; Schneider, K.L.; Yalla, S.V.; Reeves, N.D.; Vileikyte, L. Physiological and psychological challenges of increasing physical activity and exercise in patients at risk of diabetic foot ulcers: A critical review. Diabetes. Metab. Res. Rev. 2016, 32, 791–804. [Google Scholar] [CrossRef] [Green Version]
- Luhar, S.; Kondal, D.; Jones, R.; Anjana, R.M.; Patel, S.A.; Kinra, S.; Clarke, L.; Ali, M.K.; Prabhakaran, D.; Kadir, M.M.; et al. Lifetime risk of diabetes in metropolitan cities in India. Diabetology 2020, 64, 521–529. [Google Scholar] [CrossRef]
- Oyibo, S.O.; Jude, E.B.; Tarawneh, I.; Nguyen, H.C.; Armstrong, D.G.; Harkless, L.B.; Boulton, A.J. The effects of ulcer size and site, patient’s age, sex and type and duration of diabetes on the outcome of diabetic foot ulcers. Diabet. Med. 2001, 18, 133–138. [Google Scholar] [CrossRef]
- Nagase, T.; Sanada, H.; Takehara, K.; Oe, M.; Iizaka, S.; Ohashi, Y.; Oba, M.; Kadowaki, T.; Nakagami, G. Variations of plantar thermographic patterns in normal controls and non-ulcer diabetic patients: Novel classification using angiosome concept. J. Plast. Reconstr. Aesthetic Surg. 2011, 64, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Alexovic, M.; Sabo, J.; Longuespee, R. Microproteomic sample preparation. Protemics 2021, 21, 2000318. [Google Scholar] [CrossRef]
- Debats, I.B.; Booi, D.; Deutz, N.E.; Buurman, W.A.; Boeckx, W.D.; van der Hulst, R.R. Infected chronic wounds show different local and systemic arginine conversion compared with acute wounds. J. Surg. Res. 2006, 134, 205–214. [Google Scholar] [CrossRef]
- Felitsyn, N.M.; Henderson, G.N.; James, M.O.; Stacpoole, P.W. Liquid chromatography-tandem mass spectrometry method for the simultaneous determination of δ-ALA, tyrosine and creatinine in biological fluids. Clin. Chim. Acta 2004, 350, 219–230. [Google Scholar] [CrossRef]
- Ishii, Y.; Iijima, M.; Umemura, T.; Nishikawa, A.; Iwasaki, Y.; Ito, R.; Saito, K.; Hirose, M.; Nakazawa, H. Determination of nitrotyrosine and tyrosine by high-performance liquid chromatography with tandem mass spectrometry and immunohistochemical analysis in livers of mice administered acetaminophen. J. Pharm. Biomed. Anal. 2006, 41, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Latorre, R.M.; Saurina, J.; Hernández-Cassou, S. Determination of amino acids in overlapped capillary electrophoresis peaks by means of partial least-squares regression. J. Chromatogr. A 2000, 871, 331–340. [Google Scholar] [CrossRef]
- Roy, A.K.; Nisha, V.S.; Dhand, C.; Malhotra, B.D. Molecularly imprinted polyaniline film for ascorbic acid detection. J. Mol. Recognit. 2011, 24, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.Y.; Li, X.R.; Zhao, W.W.; Xu, J.J.; Chen, H.Y. Graphene oxide–thionine–Au nanostructure composites: Preparation and applications in non-enzymatic glucose sensing. Electrochem. Commun. 2012, 14, 59–62. [Google Scholar] [CrossRef]
- Singhal, C.; Dubey, A.; Mathur, A.; Pundir, C.S.; Narang, J. Paper based DNA biosensor for detection of chikungunya virus using gold shells coated magnetic nanocubes. Process Biochem. 2018, 74, 35–42. [Google Scholar] [CrossRef]
- Apetrei, I.M.; Apetrei, C. Development of a Novel Biosensor Based on Tyrosinase/Platinum Nanoparticles/Chitosan/Graphene Nanostructured Layer with Applicability in Bioanalysis. Materials 2019, 12, 1009. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Cheng, H. Recent Developments of Flexible and Stretchable Electrochemical Biosensors. Micromachines 2020, 11, 243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Economou, A.; Kokkinos, C.; Prodromidis, M. Flexible plastic, paper and textile lab-on-a chip platforms for electrochemical biosensing. Lab Chip 2018, 13, 1812–1830. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Cai, Y.; Lin, J.; Liang, H.; Qi, J.; Cao, J.; Feng, J.; Fei, W. Heterostructural Graphene Quantum Dot/MnO2 Nanosheets toward High-Potential Window Electrodes for High-Performance Supercapacitors. Adv. Sci. 2018, 5, 1700887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, X.; Song, Y.; Zhu, C.; Song, J.; Du, D.; Su, X.; Lin, Y. Graphene Quantum Dot-MnO2 Nanosheet-Based Optical Sensing Platform: A Sensitive Fluorescence “Turn Off-On” Nanosensor for Glutathione Detection and Intracellular Imaging. Appl. Mater. Interfaces 2016, 8, 21990–21996. [Google Scholar] [CrossRef]
- Dong, Y.; Shao, J.; Chen, C.; Li, H.; Wang, R.; Chi, Y.; Lin, X.; Chen, G. Blue luminescent graphene quantum dots and graphene oxide prepared by tuning the carbonization degree of citric acid. Carbon 2012, 50, 4738–4743. [Google Scholar] [CrossRef]
- Dubey, M.; Challagulla, N.V.; Wadhwa, S.; Kumar, R. Ultrasound assisted synthesis of magnetic Fe3O4/α-MnO2 nanocomposite for photodegradation of organic dye. Colloids Surf. A Physicochem. Eng. Asp. 2021, 609, 125720. [Google Scholar] [CrossRef]
- Kumar, R.; Sithambaram, S.; Suib, S.L. Cyclohexane oxidation catalyzed by manganese oxide octahedral molecular sieves-Effect of acidity of the catalyst. J. Catal. 2009, 262, 304–313. [Google Scholar] [CrossRef]
- Roy, S.; Nagabooshanam, S.; Wadhwa, S.; Kumar, R.; Mathur, A.; Dubey, A.K. A label-free impedimetric sensor based on αmnO2/tyrosinase hybrid for monitoring of diabetic foot ulcers. In Proceedings of the 2020 7th International Conference on Signal Processing and Integrated Networks (SPIN), Noida, India, 27–28 February 2020; pp. 1157–1161. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Z.; Qian, D.; Li, Y.; Zhang, W. Single-crystal α-MnO2 nanorods: Synthesis and electrochemical properties. Nanotechnology 2007, 18, 115616. [Google Scholar] [CrossRef]
- Choudhary, R.P.; Shukla, S.; Vaibhav, K.; Pawar, P.B.; Saxena, S. Optical properties of few layered graphene quantum dots. Mater. Res. Express 2015, 2, 095024. [Google Scholar] [CrossRef]
- Rana, A.; Killa, M.; Yadav, N.; Mishra, A.; Mathur, A.; Kumar, A.; Khanuja, M.; Narang, J.; Pilloton, R. Graphitic Carbon Nitride as an Amplification Platform on an Electrochemical Paper-Based Device for the Detection of Norovirus-Specific DNA. Sensors 2020, 20, 2070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, Y.H.; Zhang, H.; Xu, J.J.; Chen, H.Y. Relationship between nanostructure and electrochemical/biosensing properties of MnO2 nanomaterials for H2O 2/choline. J. Phys. Chem. C 2008, 112, 18984–18990. [Google Scholar] [CrossRef]
- Lvovich, V.F. Fundamentals of Electrochemical Impedance Spectroscopy. Impedance Spectrosc. 2012, 1–21. [Google Scholar] [CrossRef]
- Katz, E.; Willner, I. Probing biomolecular interactions at conductive and semiconductive surfaces by impedance spectroscopy: Routes to impedimetric immunosensors, DNA-sensors, and enzyme biosensors. Electroanalysis 2003, 15, 913–947. [Google Scholar] [CrossRef]
- Rakhi, R.B.; Nayak, P.; Xia, C.; Alshareef, H.N. Novel amperometric glucose biosensor based on MXene nanocomposite. Sci. Rep. 2016, 6, 36422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.K.; Khanna, M.; Roy, S.; Pankaj; Nagabooshanam, S.; Kumar, R.; Wadhwa, S.; Mathur, A. Design and development of a portable resistive sensor based on α-MnO2/GQD nanocomposites for trace quantification of Pb(II) in water. IET Nanobiotechnol. 2021, 15, 505–511. [Google Scholar] [CrossRef]
- Lee, S.; Chon, H.; Lee, J.; Ko, J.; Chung, B.H.; Lim, D.W.; Choo, J. Rapid and sensitive phenotypic marker detection on breast cancer cells using surface-enhanced Raman scattering (SERS) imaging. Biosens. Bioelectron. 2014, 51, 238–243. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, L.; Zhang, R.; Gao, M.; Zhang, X. Facilely synthesized polydopamine encapsulated surface-enhanced Raman scattering (SERS) probes for multiplex tumor associated cell surface antigen detection using SERS imaging. RSC Adv. 2015, 5, 72369–72372. [Google Scholar] [CrossRef]
- Guo, Z.; Hao, T.; Du, S.; Chen, B.; Wang, Z.; Li, X.; Wang, S. Multiplex electrochemiluminescence immunoassay of two tumor markers using multicolor quantum dots as labels and graphene asconductingbridge. Biosens. Bioelectron. 2013, 44, 101–107. [Google Scholar] [CrossRef]
- Babamiri, B.; Hallaj, R.; Salimi, A. Ultrasensitive electrochemiluminescence immunoassay for simultaneous determination of CA125 and CA15-3 tumor markers based on PAMAM-sulfanilic acid-Ru(bpy)32+ and PAMAM-CdTe@CdS nanocomposite. Biosens. Bioelectron. 2018, 99, 353–360. [Google Scholar] [CrossRef]
- Jiang, Y.; Tang, Y.; Miao, P. Polydopamine nanosphere@silver nanoclusters for fluorescence detection of multiplex tumor markers. Nanoscale 2019, 11, 8119–8123. [Google Scholar] [CrossRef] [PubMed]


| Analyte | LoD | Sensitivity |
|---|---|---|
| Glucose | 58.30 mg/dL | 13.11 kΩ/mg dL−1/mm2 |
| Tyrosine | 0.31 µmol/L | 0.71 kΩ/µmol L−1/mm2 |
| Analyte | LoD | Sensitivity |
|---|---|---|
| Glucose | 55.51 mg/dL | 0.29 kΩ/mg dL−1/mm2 |
| Tyrosine | 0.87 µmol/L | 2.21 kΩ/µmol L−1/mm2 |
| Sensor Surface | Detection Technique | Remarks | Reference |
|---|---|---|---|
| Silica encapsulated Au nanospheres | SERS | Benchtop calibration, image analysis | [38] |
| Polydopamine encapsulated Au nanoparticles | SERS | Benchtop calibration, image analysis | [39] |
| Streptavidin-CdSe/ZnS Quantum dots | ECL | Benchtop calibration, longer wait time/ incubation time | [40] |
| PAMAM quantum dots | ECL | Benchtop calibration, high applied potential | [41] |
| Gold nanoparticles | FC | Benchtop calibration, image analysis | [42] |
| α-MnO2/GQD | EC | Device-level calibration, faster response, cost- effective tests, scaled till TRL-5 | Current Work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathur, A.; Nayak, H.C.; Rajput, S.; Roy, S.; Nagabooshanam, S.; Wadhwa, S.; Kumar, R. An Enzymatic Multiplexed Impedimetric Sensor Based on α-MnO2/GQD Nano-Composite for the Detection of Diabetes and Diabetic Foot Ulcer Using Micro-Fluidic Platform. Chemosensors 2021, 9, 339. https://doi.org/10.3390/chemosensors9120339
Mathur A, Nayak HC, Rajput S, Roy S, Nagabooshanam S, Wadhwa S, Kumar R. An Enzymatic Multiplexed Impedimetric Sensor Based on α-MnO2/GQD Nano-Composite for the Detection of Diabetes and Diabetic Foot Ulcer Using Micro-Fluidic Platform. Chemosensors. 2021; 9(12):339. https://doi.org/10.3390/chemosensors9120339
Chicago/Turabian StyleMathur, Ashish, Hari Chandra Nayak, Shailendra Rajput, Souradeep Roy, Shalini Nagabooshanam, Shikha Wadhwa, and Ranjit Kumar. 2021. "An Enzymatic Multiplexed Impedimetric Sensor Based on α-MnO2/GQD Nano-Composite for the Detection of Diabetes and Diabetic Foot Ulcer Using Micro-Fluidic Platform" Chemosensors 9, no. 12: 339. https://doi.org/10.3390/chemosensors9120339
APA StyleMathur, A., Nayak, H. C., Rajput, S., Roy, S., Nagabooshanam, S., Wadhwa, S., & Kumar, R. (2021). An Enzymatic Multiplexed Impedimetric Sensor Based on α-MnO2/GQD Nano-Composite for the Detection of Diabetes and Diabetic Foot Ulcer Using Micro-Fluidic Platform. Chemosensors, 9(12), 339. https://doi.org/10.3390/chemosensors9120339








