Reconfigurable Modular Platform for Prolonged Sensing of Toxic Gases in Particle Polluted Environments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Spiral Microfluidics for In-Line Filtration
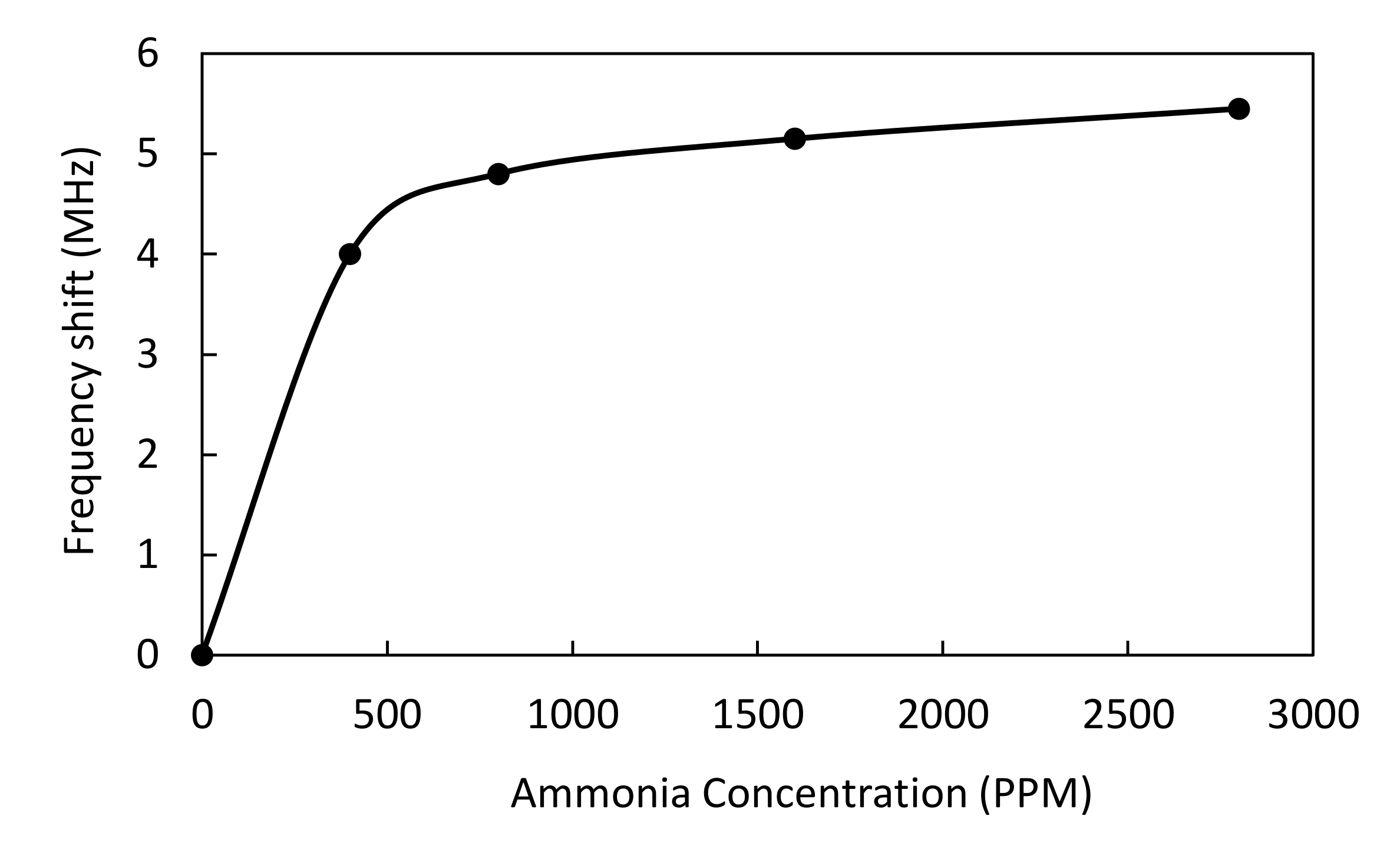
2.2. Ammonia Adsorption
2.3. D Microwave Sensing
2.3.1. Design of Microwave Resonator
2.3.2. Simulation
3. Results and Discussions
3.1. Particle Separation in Microfluidics
3.2. Effect of Ammonia on Permittivity of Adsorbent
3.3. Microwave Simulation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Calculation of Relative Permittivity of Zeolite-Y at 3 GHz
| Frequency (GHz) | Saturated Dielectric Response K’R |
|---|---|
| 60 | 1.94 |
| 70 | 2.30 |
| 80 | 3.02 |
| 90 | 3.53 |

| Ammonia Concentration | Calculated K’R |
|---|---|
| 400 ppm | 0.60 |
| 800 ppm | 1.50 |
| 1600 ppm | 4.20 |
| 2800 ppm | 5.00 |
| Frequency (GHz) | Relative Permittivity (K’g) |
|---|---|
| 3.0 | 7.17 (extrapolated) |
| 60 | 3.73 |
| 70 | 3.15 |
| 80 | 2.84 |
| 90 | 2.60 |
| Ammonia Concentration | Relative Permittivity K’ |
|---|---|
| 0 ppm (zeolite Y) | 7.17 |
| 400 ppm | 7.13 |
| 800 ppm | 7.06 |
| 1600 ppm | 6.87 |
| 2800 ppm | 6.81 |
References
- Tang, N.; Zhou, C.; Xu, L.; Jiang, Y.; Qu, H.; Duan, X. A fully integrated wireless flexible ammonia sensor fabricated by soft nano-lithography. ACS Sens. 2019, 4, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liao, Z.; Meng, F.; Yuan, Z. Theoretical and experimental research on ammonia sensing properties of sulfur-doped graphene oxide. Chemosensors 2021, 9, 220. [Google Scholar] [CrossRef]
- Madhaiyan, G.; Sun, A.-T.; Zan, H.-W.; Meng, H.-F.; Horng, S.-F.; Chen, L.-Y.; Hung, H.-W. Solution-processed chloroaluminum phthalocyanine (ClAlPc) ammonia gas sensor with vertical organic porous diodes. Sensors 2021, 21, 5783. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wu, H.; Hua, C.; Zheng, Y. Ammonia adsorption-induced change in permittivity of zeolite Y in millimeter-wave band. Sens. Actuators A Phys. 2020, 303, 111852. [Google Scholar] [CrossRef]
- Sadabadi, H.; Bostani, A.; Esmaeili, A. Detection of toxic gas in dust-filled environment using integrated microwave-microfluidics. In Proceedings of the 3 International Conference on Microelectronic Devices and Technologies (MicDAT ′2020), Canary Island, Spain, 22–23 October 2020; pp. 42–45. [Google Scholar]
- Bourrounet, B.; Talou, T.; Gaset, A. Application of a multi-gas-sensor device in the meat industry for boar-taint detection. Sens. Actuators B Chem. 1995, 27, 250–254. [Google Scholar] [CrossRef]
- Vargas, A.P.; Gámez, F.; Roales, J.; Lopes-Costa, T.; Pedrosa, J.M. A paper-based ultrasensitive optical sensor for the selective detection of H2S vapors. Chemosensors 2021, 9, 40. [Google Scholar] [CrossRef]
- Hsieh, L.-T.; Chen, T.-C. Characteristics of ambient ammonia levels measured in three different industrial parks in southern Taiwan. Aerosol Air Qual. Res. 2010, 10, 596–608. [Google Scholar] [CrossRef]
- The National Institute for Occupational Safety and Health (NIOSH). Immediately Dangerous to Life or Health Concentrations of Chemicals, Ammonia; CAS number: 7664-41-7; U.S. Department of Health & Human Services, CDC: Washington, DC, USA, 1994. Available online: https://www.cdc.gov/niosh/idlh/7664417.html (accessed on 11 November 2021).
- Kendler, S.; Zuck, A. The Challenges of Prolonged Gas Sensing in the Modern Urban Environment. Sensors 2020, 20, 5189. [Google Scholar] [CrossRef]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal oxide semi-conductor gas sensors in environmental monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef] [Green Version]
- Zuck, A.; Sharabi, H.; Kendler, S. Detection of hazardous vapours in a dusty environment–development of a protective module for chemical sensor using a laboratory setup for systematically simulating realistic conditions. Int. J. Environ. Anal. Chem. 2020, 100, 134–151. [Google Scholar] [CrossRef]
- Wagner, F.; Bortoli, D.; Pereira, S.; Costa, M.J.; Maria Silva, A.; Weinzierl, B.; Esselborn, M.; Petzold, A.; Rasp, K.; Heinold, B. Properties of dust aerosol particles transported to Portugal from the Sahara Desert. Tellus B Chem. Phys. Meteorol. 2009, 61, 297–306. [Google Scholar] [CrossRef] [Green Version]
- Al-Attar, I.; Wakeman, R.J.; Tarleton, E.; Husain, A. Physical and Chemical Characterization of Kuwaiti Atmospheric Dust and Synthetic Dusts: Effects on the Pressure Drop and Fractional Efficiency of HEPA Filters; A&M University: College Station, TX, USA, 2010; Available online: https://hdl.handle.net/1969.1961/94141 (accessed on 11 November 2021).
- Lee, J.; Arrigan, D.W.; Silvester, D.S. Achievement of prolonged oxygen detection in room-temperature ionic liquids on mechanically polished platinum screen-printed electrodes. Anal. Chem. 2016, 88, 5104–5111. [Google Scholar] [CrossRef] [Green Version]
- Erdem, K.; Ahmadi, V.E.; Kosar, A.; Kuddusi, L. Differential sorting of microparticles using spiral microchannels with elliptic configurations. Micromachines 2020, 11, 412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuntaegowdanahalli, S.S.; Bhagat, A.A.S.; Kumar, G.; Papautsky, I. Inertial microfluidics for continuous particle separation in spiral microchannels. Lab Chip 2009, 9, 2973–2980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarifi, M.H.; Sadabadi, H.; Hejazi, S.H.; Daneshmand, M.; Sanati-Nezhad, A. Noncontact and nonintrusive microwave-microfluidic flow sensor for energy and biomedical engineering. Sci. Rep. 2018, 8, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.-L.; Chen, Y.; Jiang, H.-L.; Qiu, X.-B.; Yu, D.-L. Smartphone-based microfluidic colorimetric sensor for gaseous formaldehyde determination with high sensitivity and selectivity. Sensors 2018, 18, 3141. [Google Scholar] [CrossRef] [Green Version]
- Montazeri, M.M.; O’Brien, A.; Hoorfar, M. Understanding microfluidic-based gas detectors: A numerical model to investigate fundamental sensor operation, influencing phenomena and optimum geometries. Sens. Actuators B Chem. 2019, 300, 126904. [Google Scholar] [CrossRef]
- Gradov, O.V.; Gradova, M.A. Microwave enthrakometric labs-on-a-chip and on-chip enthrakometric catalymetry: From non-conventional chemotronics towards microwave-assisted chemosensors. Chemosensors 2019, 7, 48. [Google Scholar] [CrossRef] [Green Version]
- Zarifi, M.H.; Shariaty, P.; Hashisho, Z.; Daneshmand, M. A non-contact microwave sensor for monitoring the interaction of zeolite 13X with CO2 and CH4 in gaseous streams. Sens. Actuators B Chem. 2017, 238, 1240–1247. [Google Scholar] [CrossRef]
- Salim, A.; Ghosh, S.; Lim, S. Low-cost and lightweight 3D-printed split-ring resonator for chemical sensing applications. Sensors 2018, 18, 3049. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.C.; Hsiao, C.T.; Sun, S.; Yang, K.-Y.; Wu, P.C.; Chen, W.T.; Tang, Y.H.; Chau, Y.-F.; Plum, E.; Guo, G.-Y. Fabrication of three dimensional split ring resonators by stress-driven assembly method. Opt. Express 2012, 20, 9415–9420. [Google Scholar] [CrossRef]
- Keller, J.U.; Staudt, R. Gas Adsorption Equilibria: Experimental Methods and Adsorptive Isotherms; Springer Science & Business Media: Berlin/Heildelberg, Germany, 2005. [Google Scholar]
- Yavari, F.; Chen, Z.; Thomas, A.V.; Ren, W.; Cheng, H.-M.; Koratkar, N. High sensitivity gas detection using a macroscopic three-dimensional graphene foam network. Sci. Rep. 2011, 1, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.-K.; Kang, T.-G.; Kim, B.-H.; Lee, H.-J.; Choi, H.H.; Yook, J.-G. Real-time humidity sensor based on microwave resonator coupled with PEDOT: PSS conducting polymer film. Sci. Rep. 2018, 8, 439. [Google Scholar]
- Brochocka, A.; Nowak, A.; Zajączkowska, H.; Sieradzka, M. Chemosensitive thin films active to ammonia vapours. Sensors 2021, 21, 2948. [Google Scholar] [CrossRef] [PubMed]
- Sadabadi, H.; Sanati Nezhad, A. Nanofluids for performance improvement of heavy machinery journal bearings: A simulation study. Nanomaterials 2020, 10, 2120. [Google Scholar] [CrossRef]
- Francis, D.; Alshamsi, N.; Cuesta, J.; Gokcen Isik, A.; Dundar, C. Cyclogenesis and density currents in the Middle East and the associated dust activity in September 2015. Geosciences 2019, 9, 376. [Google Scholar] [CrossRef] [Green Version]
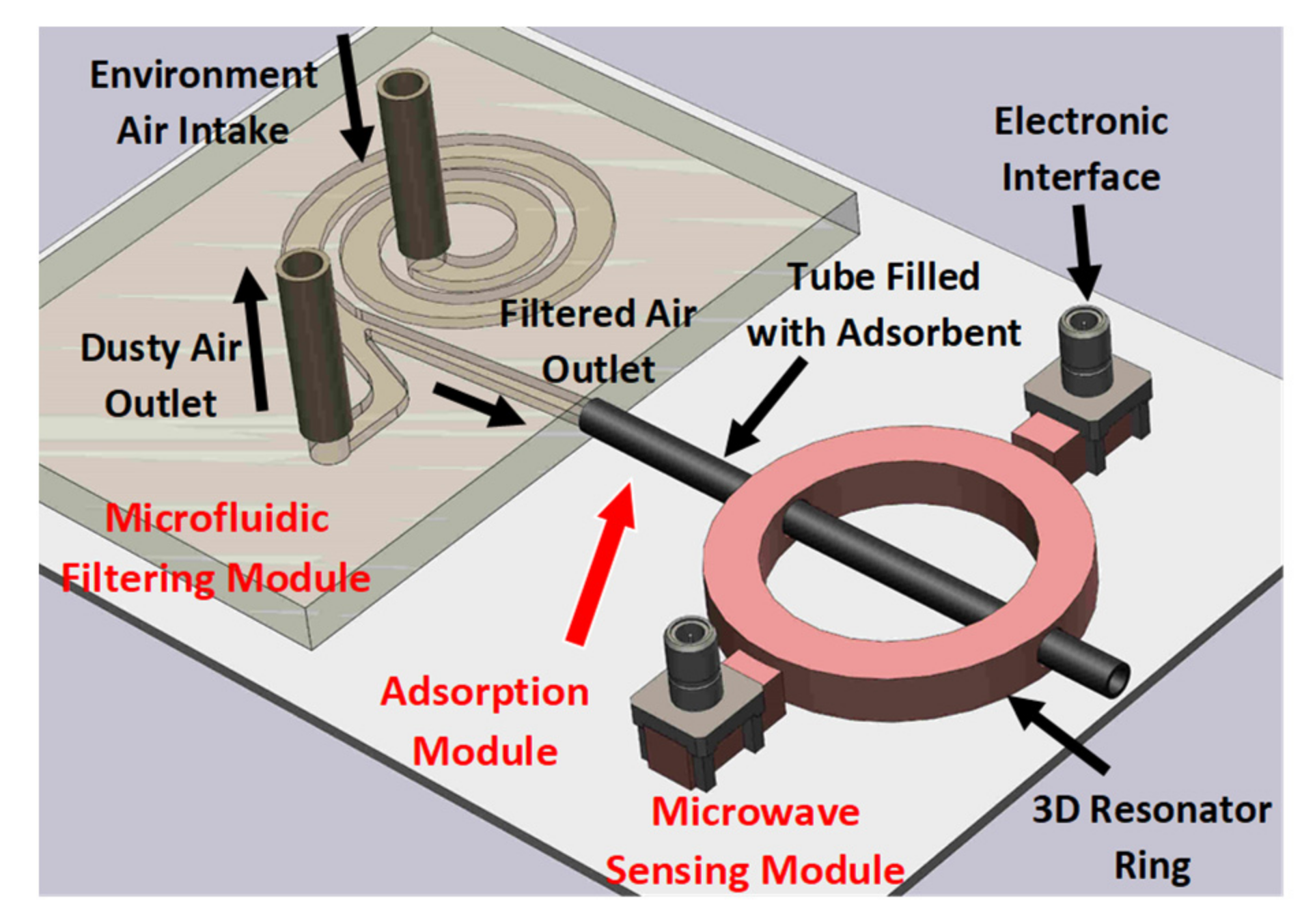
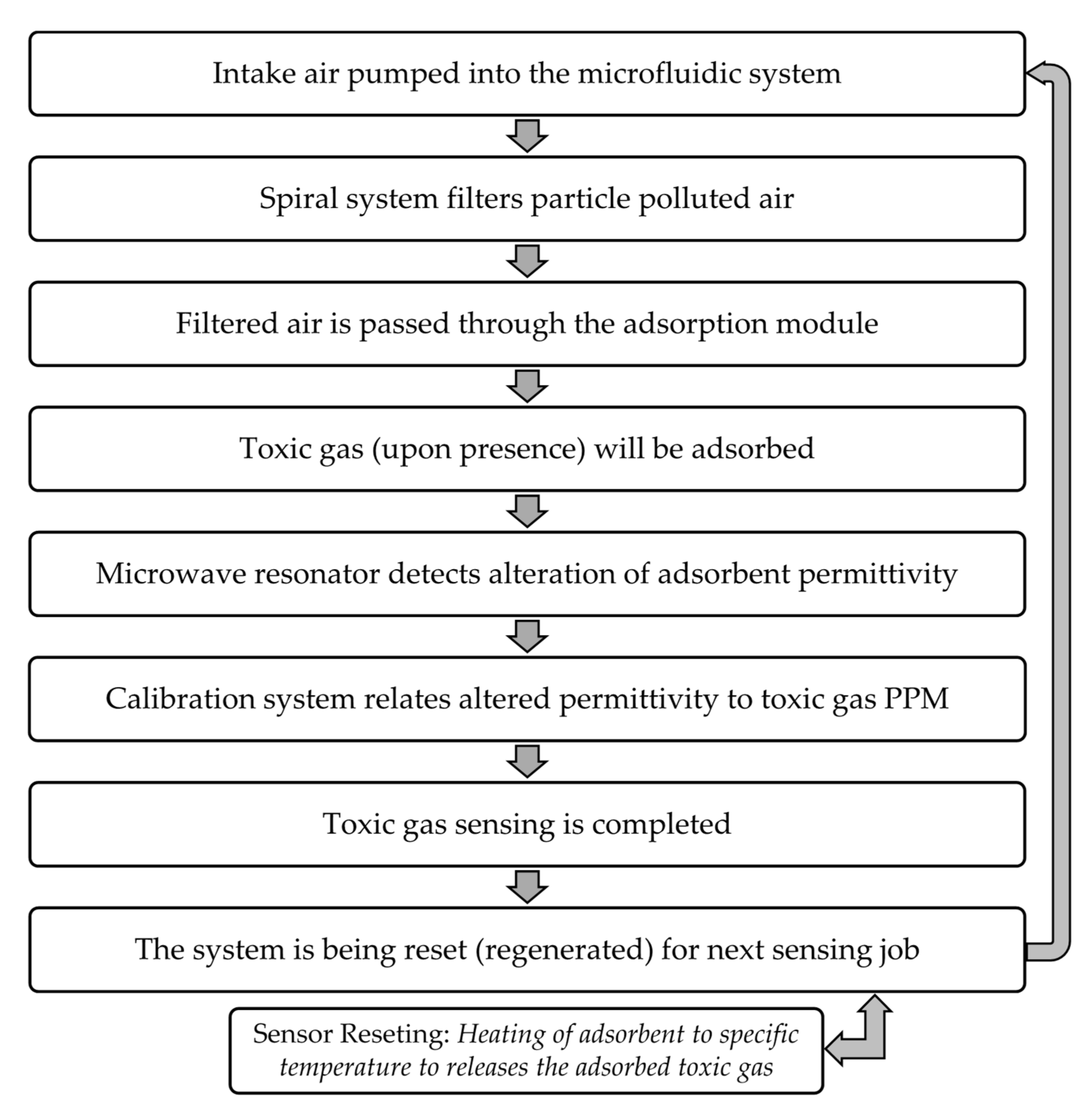
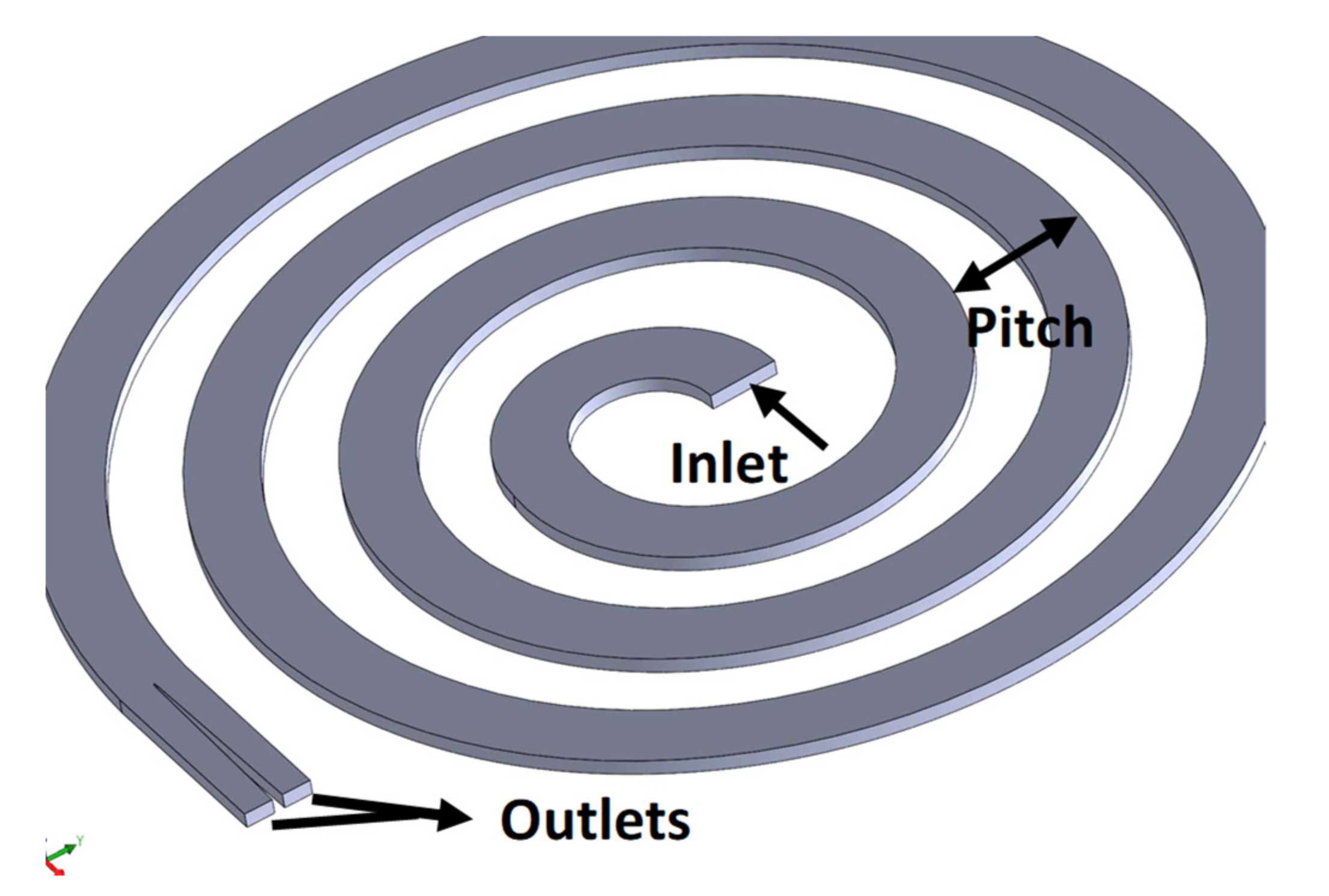
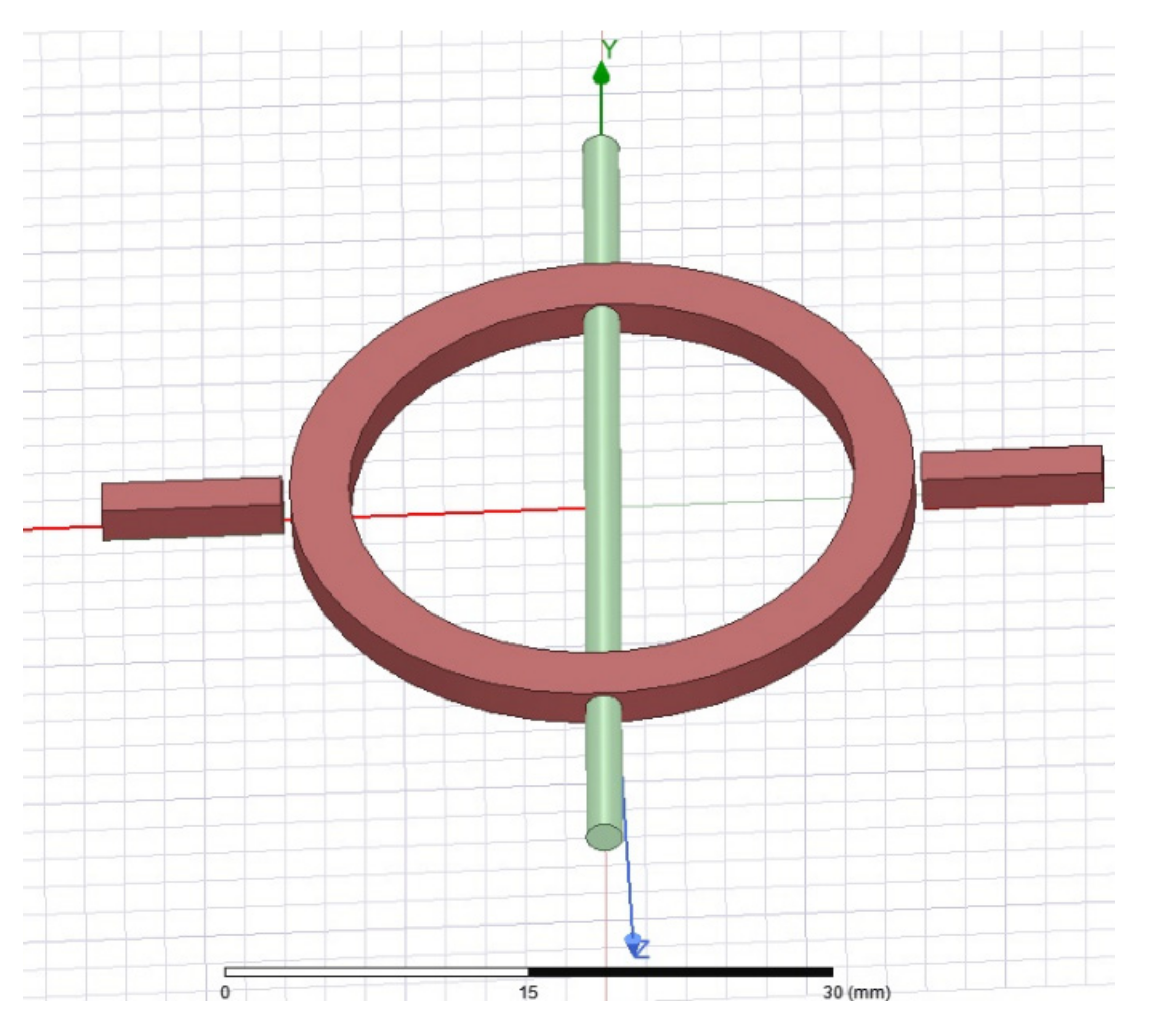
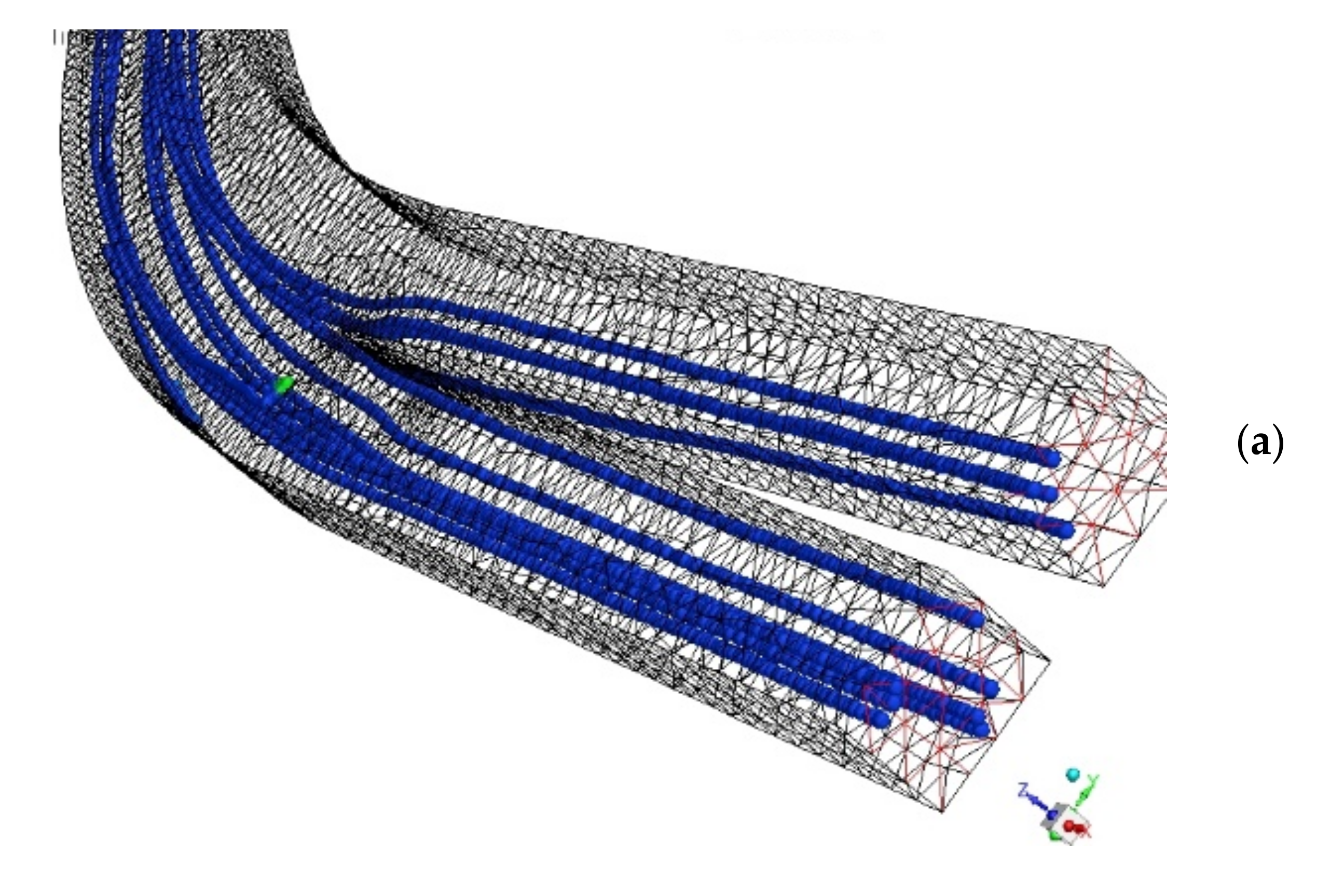
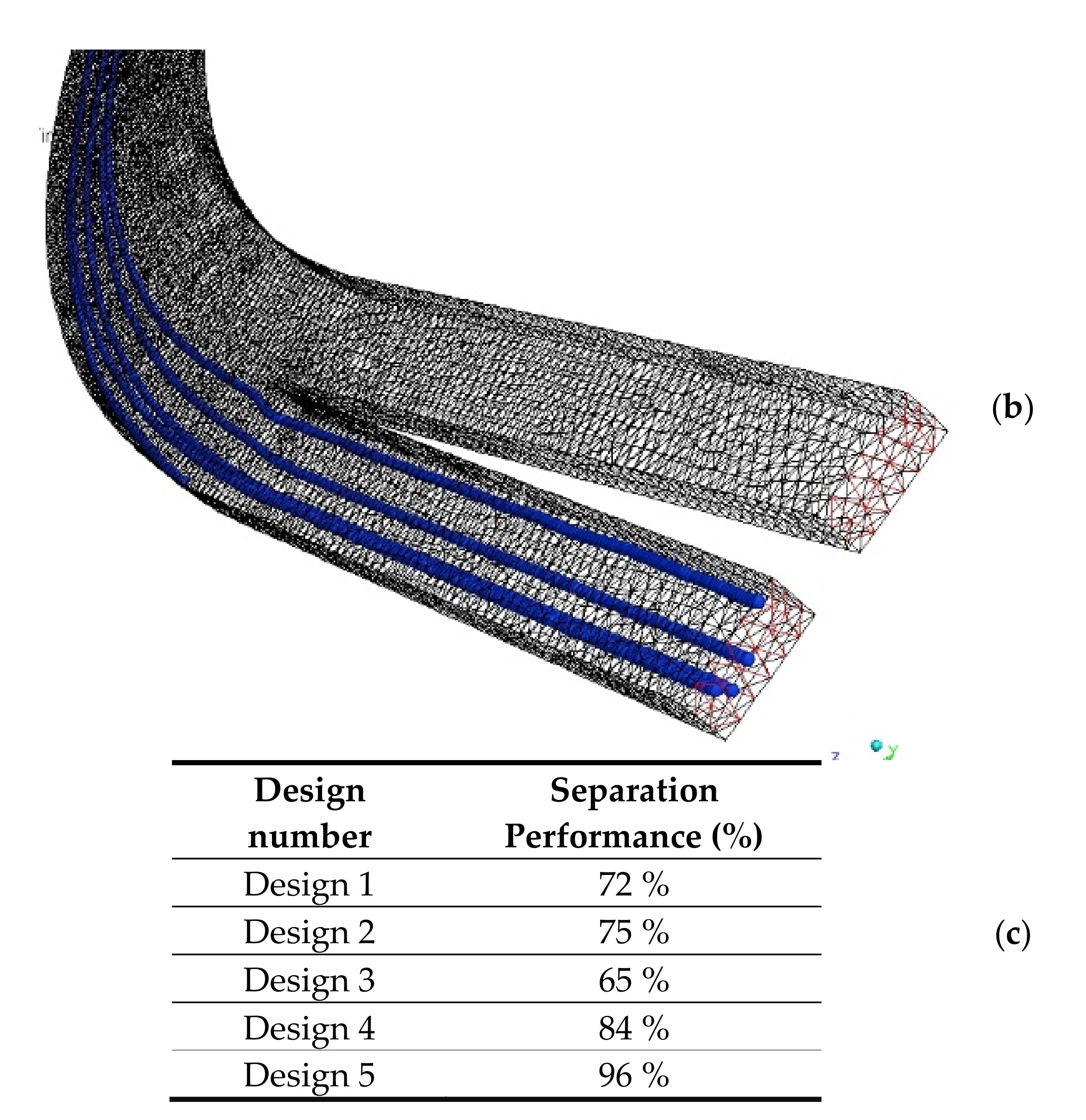
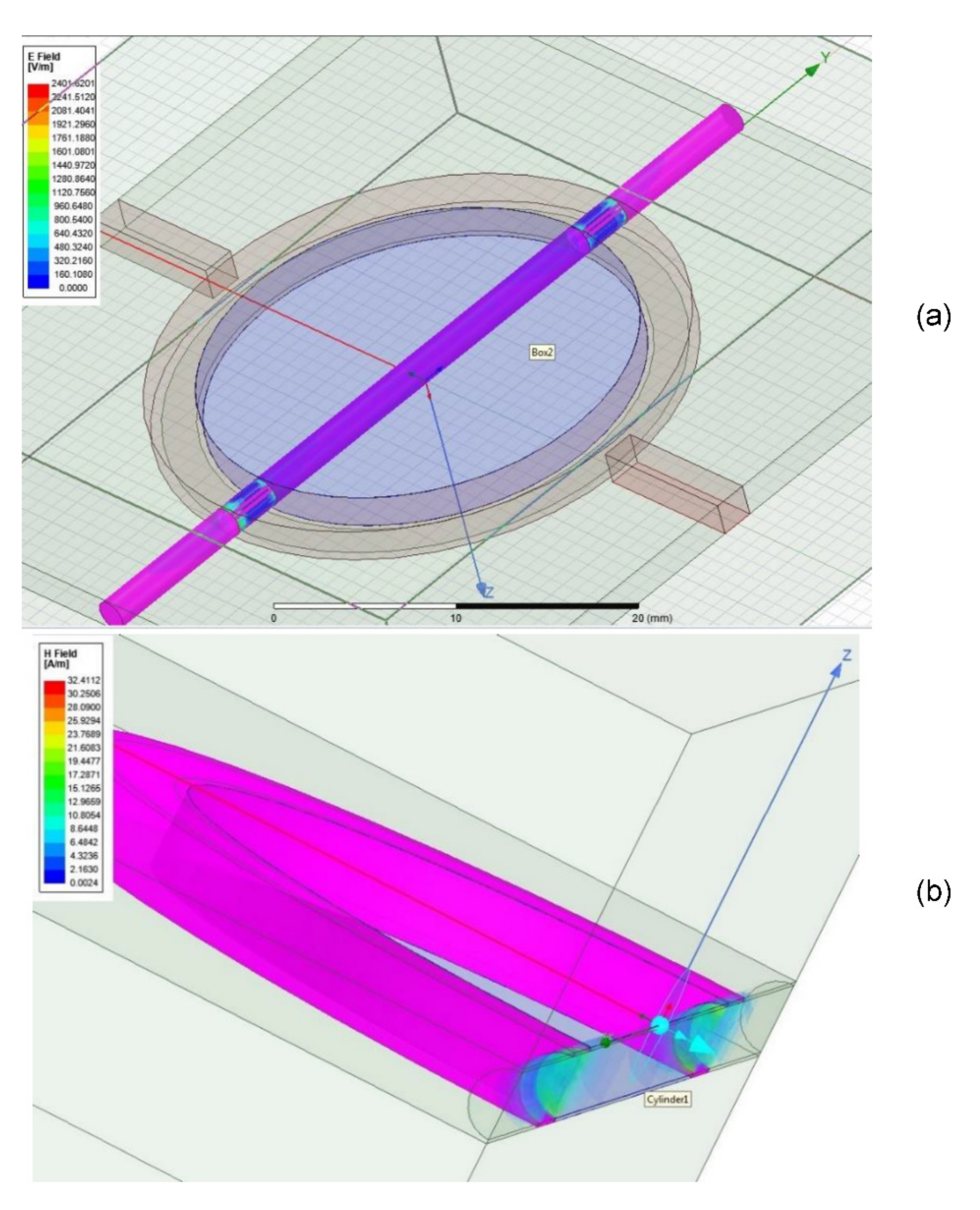
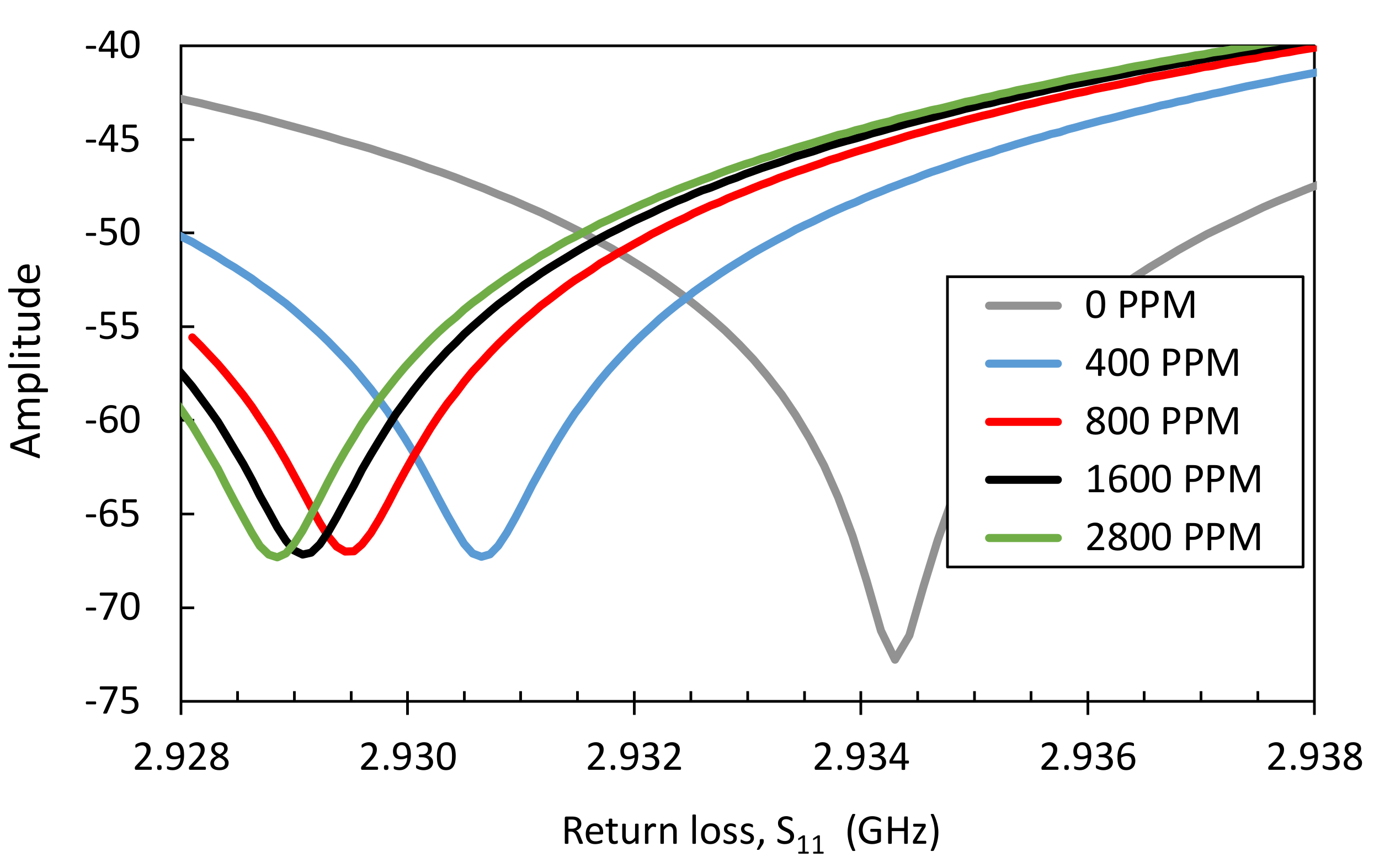
| Design # | Channel Cross-Section (W × H) | Pitch (mm) |
|---|---|---|
| Design 1 | 3 mm × 1 mm | 4 mm |
| Design 2 | 3 mm × 1 mm | 6 mm |
| Design 3 | 3 mm × 1 mm | 8 mm |
| Design 4 | 4 mm × 1 mm | 8 mm |
| Design 5 | 3 mm × 0.5 mm | 8 mm |
| Flow Simulation Parameter | Value |
|---|---|
| Intake air density | ρ = 1.225 kg/m3 |
| Particle pollution average size | D = 6 µm |
| Intake flow velocity | V = 0.25 m/s |
| Air viscosity (@15 °C) | µ = 1.8 × 10−5 Pa·s |
| Concentration of Adsorbed Ammonia in Zeolite Y | Relative Permittivity (K’) |
|---|---|
| 0 ppm (bare zeolite Y) | 7.17 |
| 400 ppm | 7.13 |
| 800 ppm | 7.06 |
| 1600 ppm | 6.87 |
| 2800 ppm | 6.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadabadi, H.; Bostani, A.; Esmaeili, A.S. Reconfigurable Modular Platform for Prolonged Sensing of Toxic Gases in Particle Polluted Environments. Chemosensors 2021, 9, 328. https://doi.org/10.3390/chemosensors9110328
Sadabadi H, Bostani A, Esmaeili AS. Reconfigurable Modular Platform for Prolonged Sensing of Toxic Gases in Particle Polluted Environments. Chemosensors. 2021; 9(11):328. https://doi.org/10.3390/chemosensors9110328
Chicago/Turabian StyleSadabadi, Hamid, Ali Bostani, and Amin S. Esmaeili. 2021. "Reconfigurable Modular Platform for Prolonged Sensing of Toxic Gases in Particle Polluted Environments" Chemosensors 9, no. 11: 328. https://doi.org/10.3390/chemosensors9110328
APA StyleSadabadi, H., Bostani, A., & Esmaeili, A. S. (2021). Reconfigurable Modular Platform for Prolonged Sensing of Toxic Gases in Particle Polluted Environments. Chemosensors, 9(11), 328. https://doi.org/10.3390/chemosensors9110328








