Decoration of Ag Nanoparticle on ZnO Nanowire by Intense Pulsed Light and Enhanced UV Photodetector
Abstract
:1. Introduction
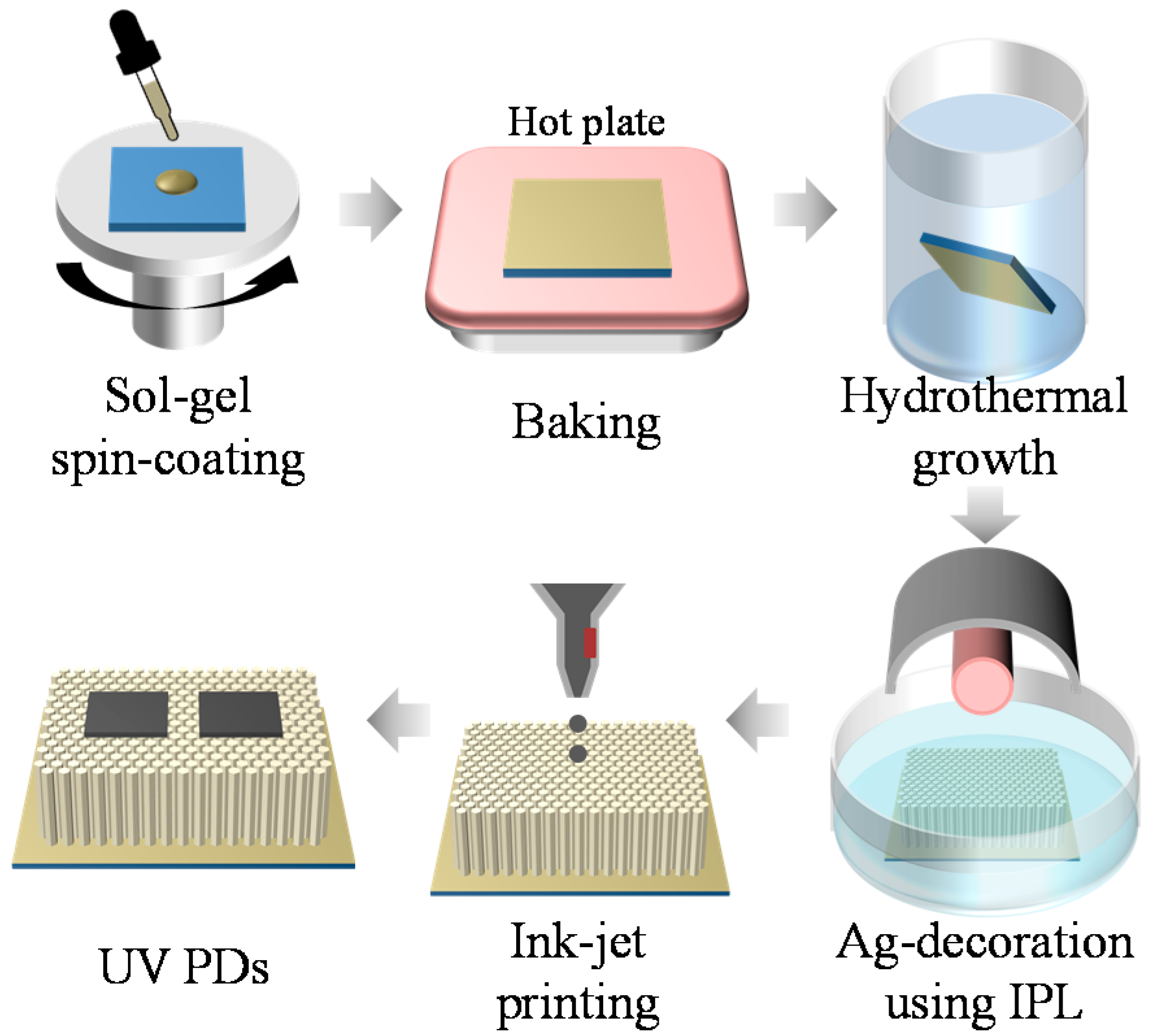
2. Materials and Methods
2.1. Synthesis of ZnO NWs
2.2. Ag Decoration on as-Grown ZnO NWs
2.3. Fabrication of UV PDs
2.4. Characterization
3. Results and Discussion
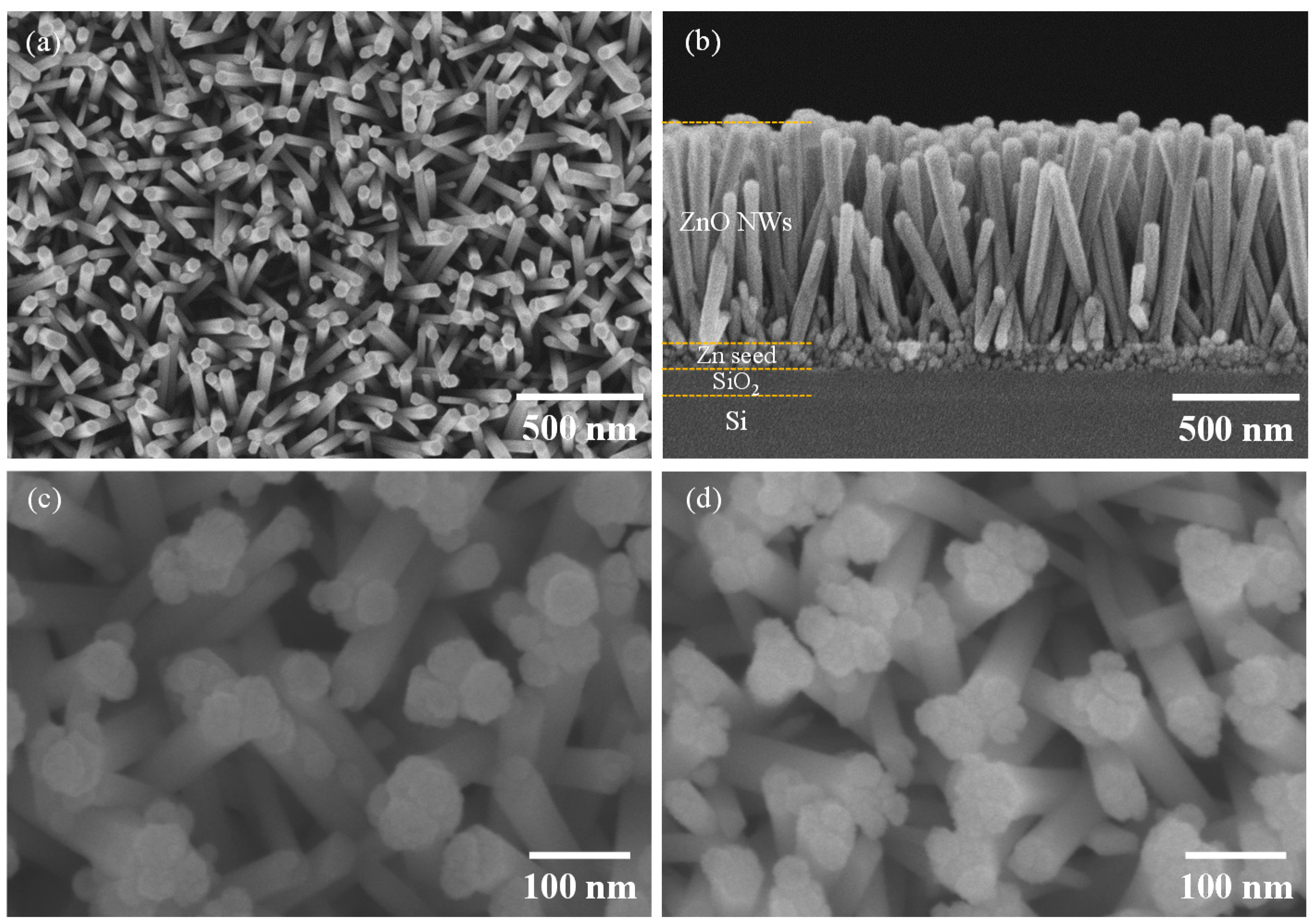
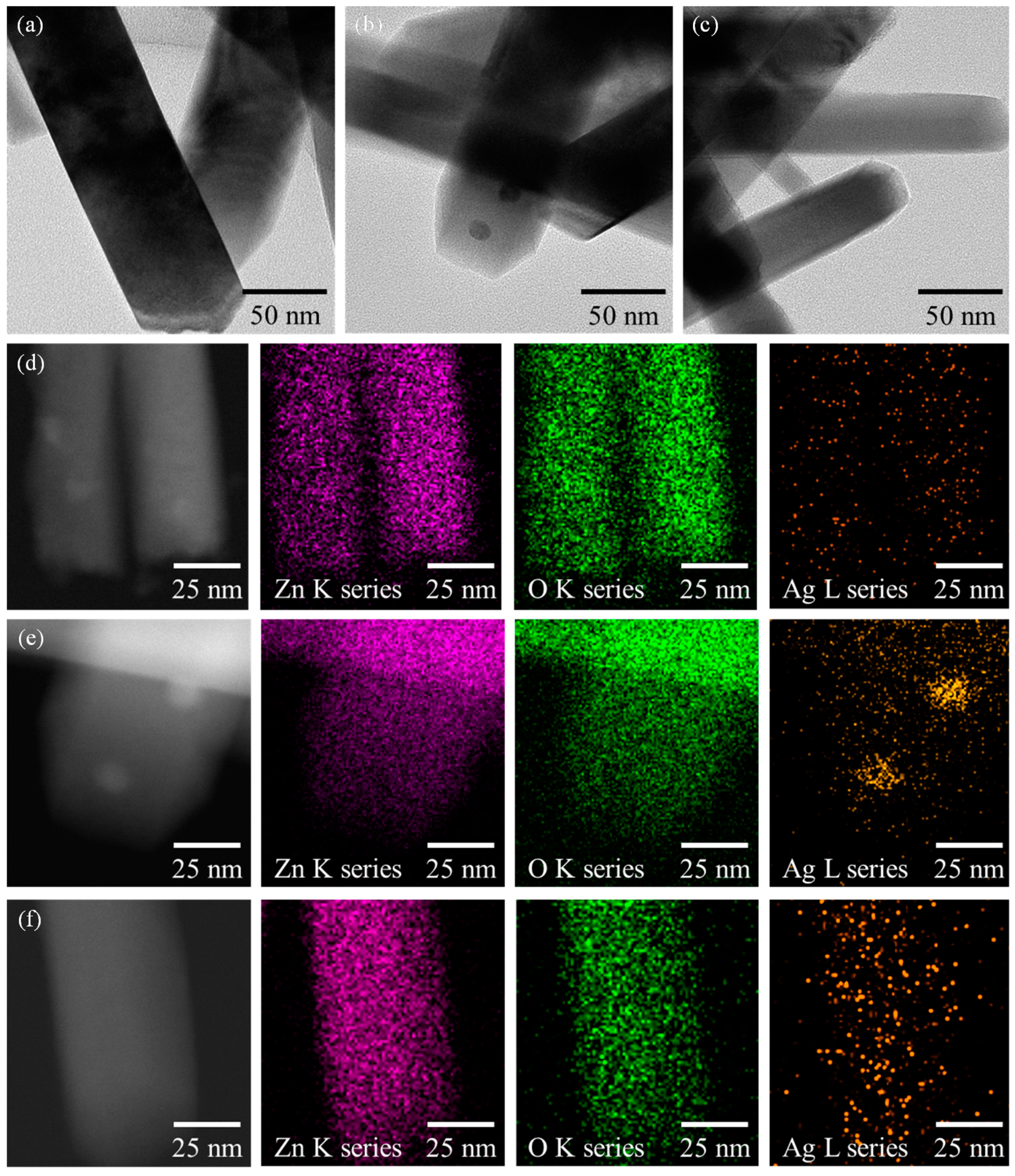
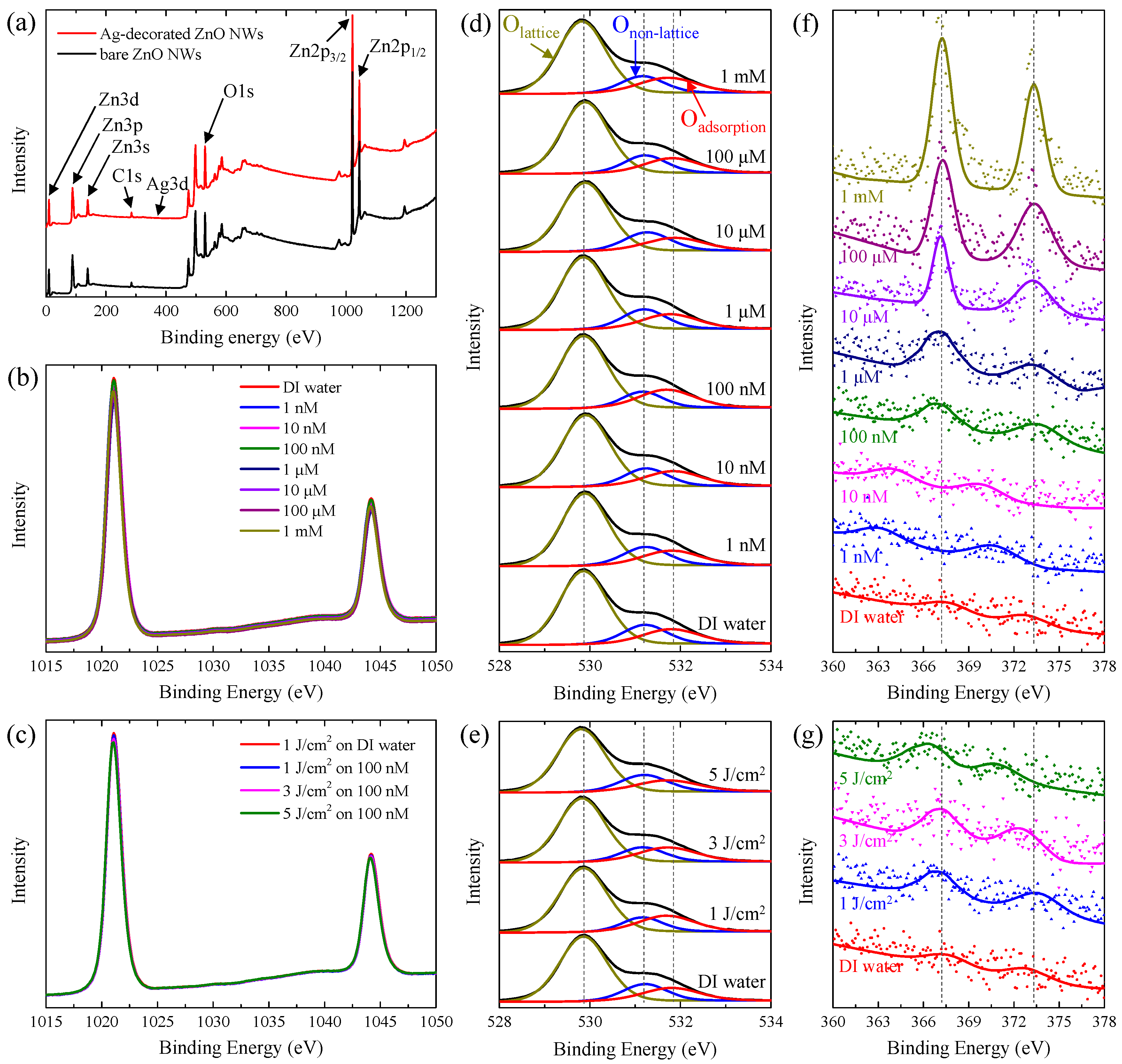
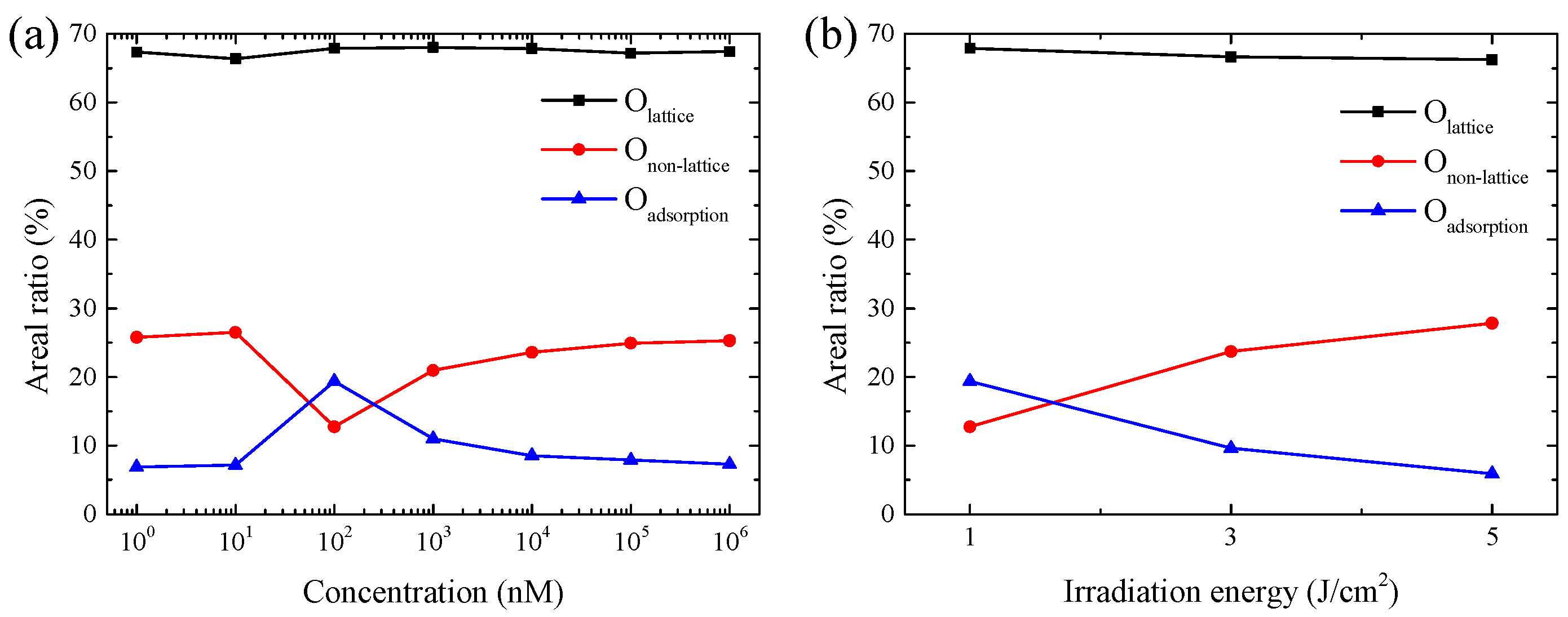
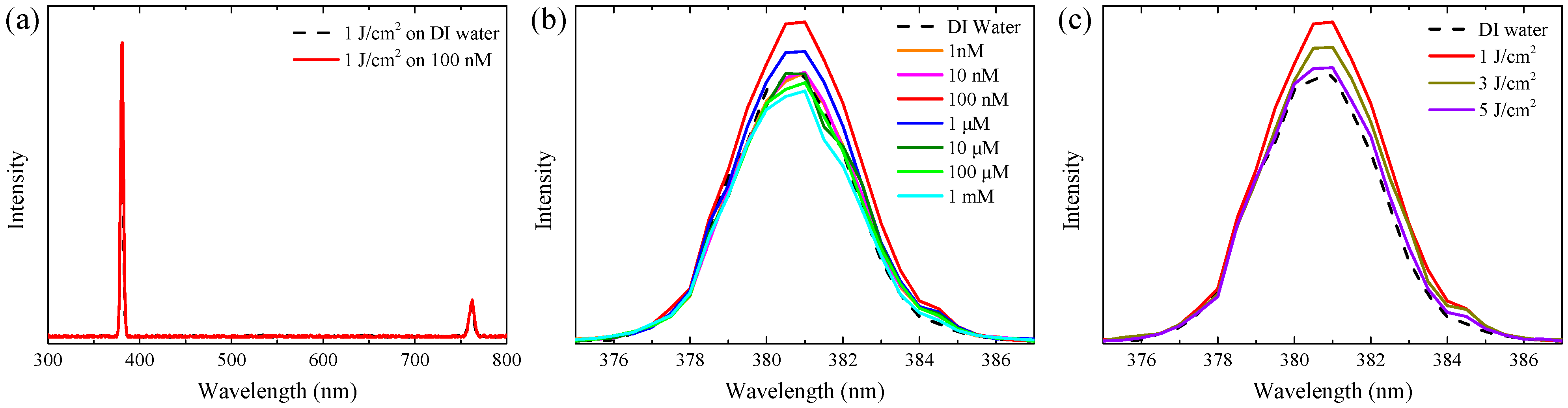
3.1. Ag-Decorated ZnO NWs
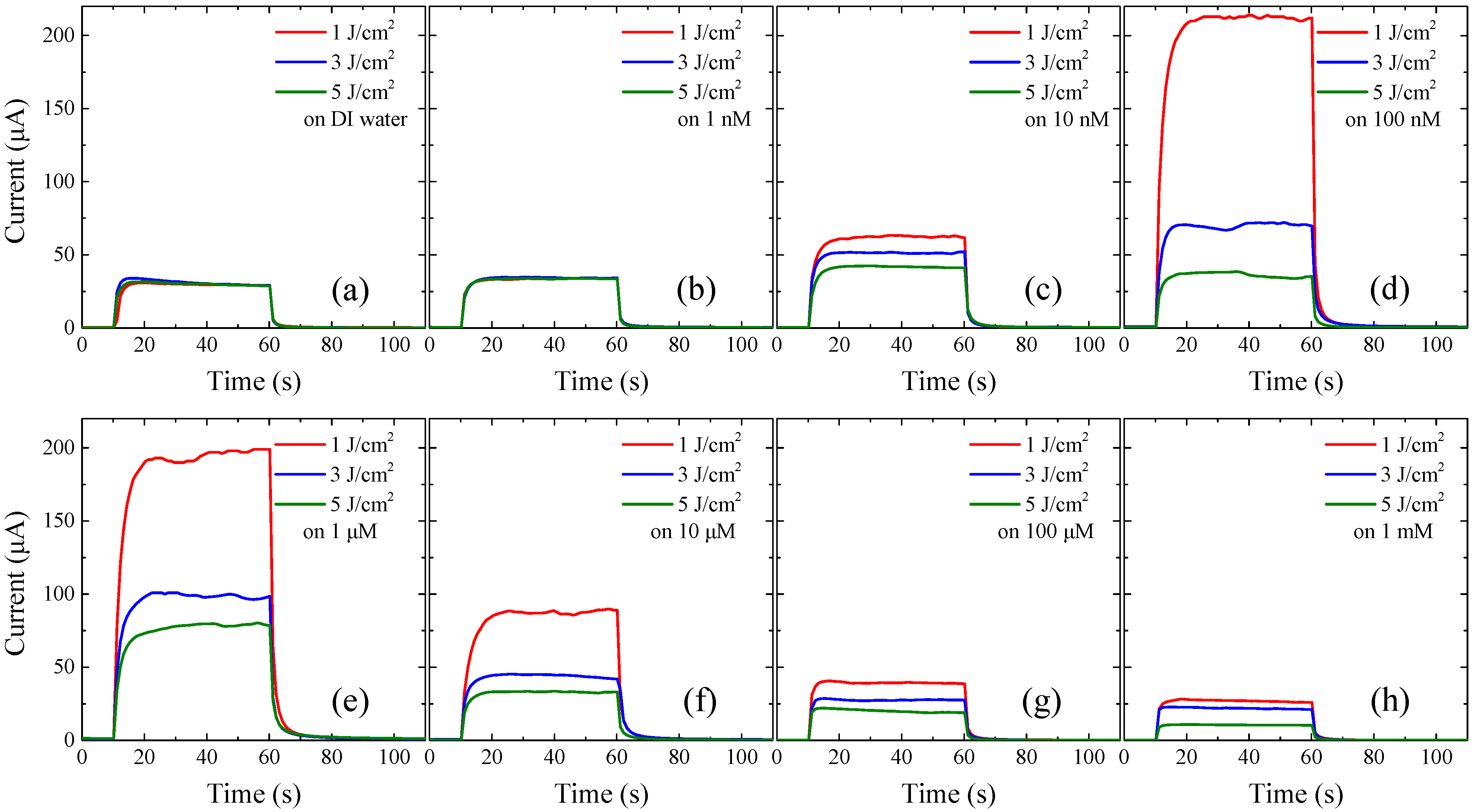
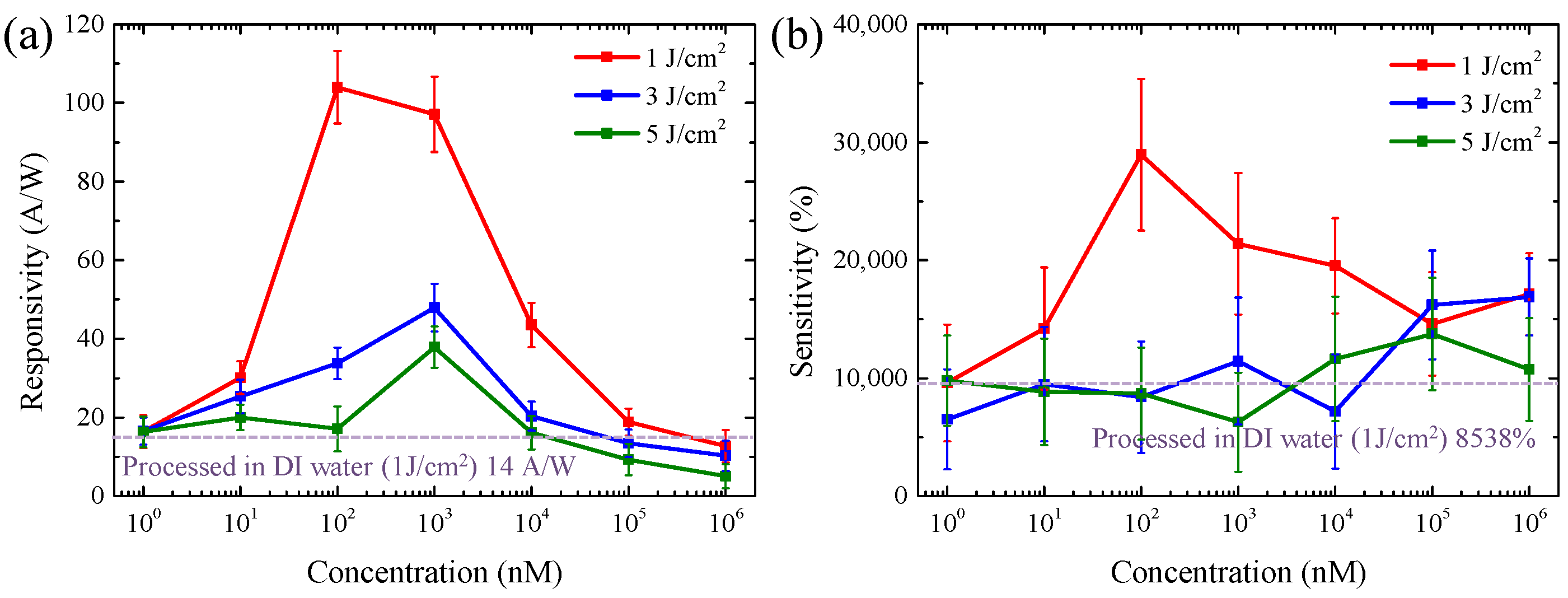
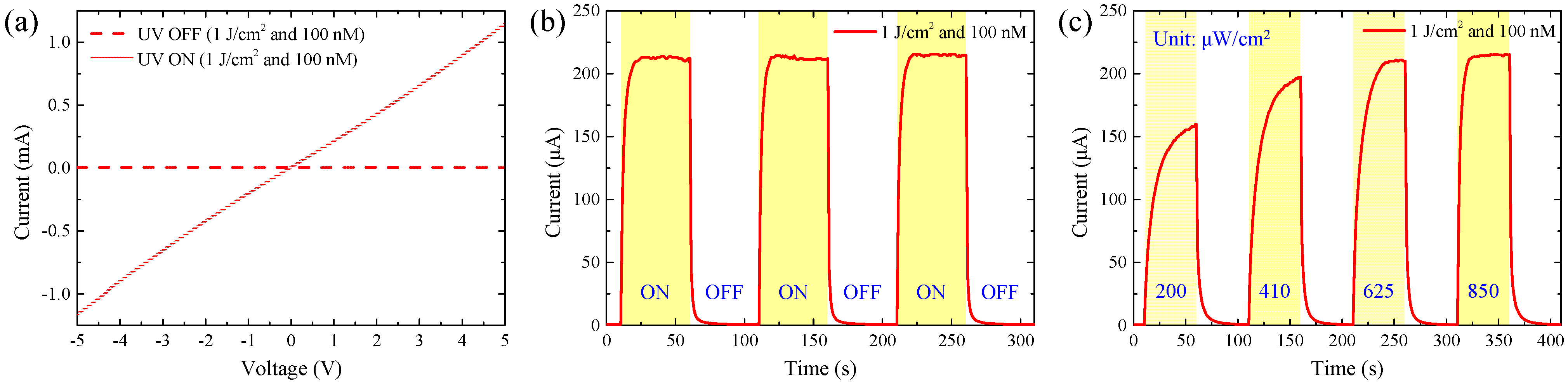
3.2. The Performance of Ag-Decorated ZnO NW UV PDs
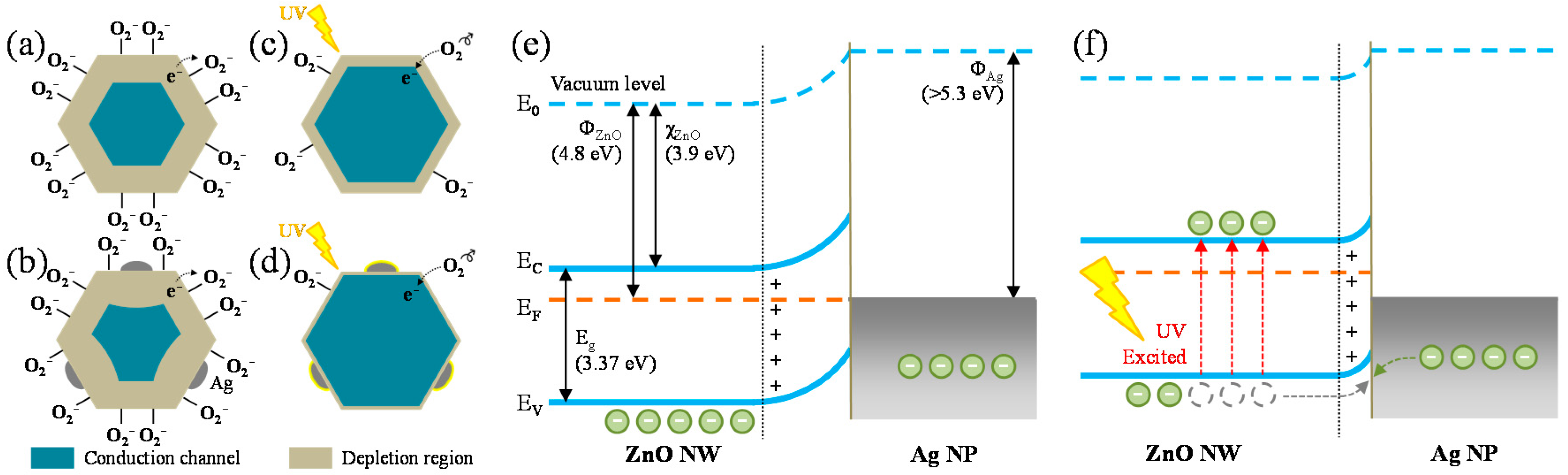
3.3. UV-Detection Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schmidt-Mende, L.; MacManus-Driscoll, J.L. ZnO—nanostructures, defects, and devices. Mater. Today 2007, 10, 40–48. [Google Scholar] [CrossRef]
- Kong, Y.C.; Yu, D.P.; Zhang, B.; Fang, W.; Feng, S.Q. Ultraviolet-emitting ZnO nanowires synthesized by a physical vapor deposition approach. Appl. Phys. Lett. 2001, 78, 407–409. [Google Scholar] [CrossRef]
- Florica, C.; Preda, N.; Costas, A.; Zgura, I.; Enculescu, I. ZnO nanowires grown directly on zinc foils by thermal oxidation in air: Wetting and water adhesion properties. Mater. Lett. 2016, 170, 156–159. [Google Scholar] [CrossRef]
- Greene, L.E.; Law, M.; Goldberger, J.; Kim, F.; Johnson, J.C.; Zhang, Y.; Saykally, R.J.; Yang, P. Low-Temperature Wafer-Scale Production of ZnO Nanowire Arrays. Angew. Chem. Int. Ed. 2003, 42, 3031–3034. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, L. Hydrothermal synthesis and photoluminescence properties of ZnO nanowires. Solid State Commun. 2004, 132, 269–271. [Google Scholar] [CrossRef]
- Rühle, S.; van Vugt, L.K.; Li, H.Y.; Keizer, N.A.; Kuipers, L.; Vanmaekelbergh, D. Nature of Sub-Band Gap Luminescent Eigenmodes in a ZnO Nanowire. Nano Lett. 2008, 8, 119–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Lee, J.; Noh, Y.; Shin, K.-H.; Lee, D. Flexible ultraviolet photodetectors with ZnO nanowire networks fabricated by large area controlled roll-to-roll processing. J. Mater. Chem. C 2016, 4, 7948–7958. [Google Scholar] [CrossRef]
- Nam, D.; Park, J.; Park, S.; Min, Y.; Noh, Y.; Lee, D. High-density hydrothermal growth of zinc-oxide nanowires using printed resistive heater. Mater. Lett. 2015, 153, 29–32. [Google Scholar] [CrossRef]
- Lee, D.; Kim, T.; Park, S.; Lee, S.S.; Ko, S.H. Zinc Oxide Nanowire Forest for Pool Boiling Heat Transfer. Jpn. J. Appl. Phys. 2012, 51, 11PE11. [Google Scholar] [CrossRef]
- Song, K.; Noh, J.; Jun, T.; Jung, Y.; Kang, H.-Y.; Moon, J. Fully Flexible Solution-Deposited ZnO Thin-Film Transistors. Adv. Mater. 2010, 22, 4308–4312. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, J.; Sun, B.; Blakesley, J.C.; Greenham, N.C. Solution-Processed Ultraviolet Photodetectors Based on Colloidal ZnO Nanoparticles. Nano Lett. 2008, 8, 1649–1653. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Das, P.P.; Maity, S.; Ghosh, P.; Devi, P.S. Solution grown ZnO rods: Synthesis, characterization and defect mediated photocatalytic activity. Appl. Catal. B Environ. 2015, 165, 128–138. [Google Scholar] [CrossRef]
- Kakiuchi, K.; Hosono, E.; Kimura, T.; Imai, H.; Fujihara, S. Fabrication of mesoporous ZnO nanosheets from precursor templates grown in aqueous solutions. J. Sol-Gel Sci. Technol. 2006, 39, 63–72. [Google Scholar] [CrossRef]
- Xi, Y.; Hu, C.G.; Han, X.Y.; Xiong, Y.F.; Gao, P.X.; Liu, G.B. Hydrothermal synthesis of ZnO nanobelts and gas sensitivity property. Solid State Commun. 2007, 141, 506–509. [Google Scholar] [CrossRef]
- Xi, Y.; Song, J.; Xu, S.; Yang, R.; Gao, Z.; Hu, C.; Wang, Z.L. Growth of ZnO nanotube arrays and nanotube based piezoelectric nanogenerators. J. Mater. Chem. 2009, 19, 9260–9264. [Google Scholar] [CrossRef]
- Akhtar, N.; Metkar, S.K.; Girigoswami, A.; Girigoswami, K. ZnO nanoflower based sensitive nano-biosensor for amyloid detection. Mater. Sci. Eng. C 2017, 78, 960–968. [Google Scholar] [CrossRef]
- Al-Hardan, N.H.; Jalar, A.; Abdul Hamid, M.A.; Keng, L.K.; Ahmed, N.M.; Shamsudin, R. A wide-band UV photodiode based on n-ZnO/p-Si heterojunctions. Sens. Actuator A-Phys. 2014, 207, 61–66. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, Q.; Wu, A.; Jiang, M.; Liang, Z.; Jiang, B.; Fu, H. Cost-effective large-scale synthesis of ZnO photocatalyst with excellent performance for dye photodegradation. Chem. Commun. 2012, 48, 2858–2860. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Kang, Y.; Liu, X.; Zhang, S.; Huang, W.; Wang, S. ZnO nanorod gas sensor for ethanol detection. Sens. Actuator B-Chem. 2012, 162, 237–243. [Google Scholar] [CrossRef]
- Hatamie, A.; Khan, A.; Golabi, M.; Turner, A.P.F.; Beni, V.; Mak, W.C.; Sadollahkhani, A.; Alnoor, H.; Zargar, B.; Bano, S.; et al. Zinc Oxide Nanostructure-Modified Textile and Its Application to Biosensing, Photocatalysis, and as Antibacterial Material. Langmuir 2015, 31, 10913–10921. [Google Scholar] [CrossRef]
- Lu, M.-P.; Song, J.; Lu, M.-Y.; Chen, M.-T.; Gao, Y.; Chen, L.-J.; Wang, Z.L. Piezoelectric Nanogenerator Using p-Type ZnO Nanowire Arrays. Nano Lett. 2009, 9, 1223–1227. [Google Scholar] [CrossRef] [PubMed]
- Djurišić, A.B.; Leung, Y.H. Optical Properties of ZnO Nanostructures. Small 2006, 2, 944–961. [Google Scholar] [CrossRef] [PubMed]
- Klingshirn, C. ZnO: From basics towards applications. Phys. Status Solidi B 2007, 244, 3027–3073. [Google Scholar] [CrossRef]
- Dhara, S.; Giri, P.K. Enhanced UV photosensitivity from rapid thermal annealed vertically aligned ZnO nanowires. Nanoscale Res. Lett. 2011, 6, 504. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Que, W. Preparation and characterization of sol–gel Al-doped ZnO thin films and ZnO nanowire arrays grown on Al-doped ZnO seed layer by hydrothermal method. Sol. Energy Mater. Sol. Cells 2010, 94, 2181–2186. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, S.; Liang, L.; Lu, W. Oxygen plasma assisted enhanced photoresponse of ZnO nanowires fabricated by catalyst-free chemical vapor deposition. RSC Adv. 2018, 8, 28928–28933. [Google Scholar] [CrossRef] [Green Version]
- Wagata, H.; Ohashi, N.; Katsumata, K.-i.; Segawa, H.; Wada, Y.; Yoshikawa, H.; Ueda, S.; Okada, K.; Matsushita, N. An aqueous solution process and subsequent UV treatment for highly transparent conductive ZnO films. J. Mater. Chem. 2012, 22, 20706–20712. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, J.; Wang, S.; Dai, H.; Sun, X. Shape-dependent localized surface plasmon enhanced photocatalytic effect of ZnO nanorods decorated with Ag. Int. J. Hydrogen Energy 2014, 39, 8238–8245. [Google Scholar] [CrossRef]
- Xiang, Q.; Meng, G.; Zhang, Y.; Xu, J.; Xu, P.; Pan, Q.; Yu, W. Ag nanoparticle embedded-ZnO nanorods synthesized via a photochemical method and its gas-sensing properties. Sens. Actuator B-Chem. 2010, 143, 635–640. [Google Scholar] [CrossRef]
- Ko, Y.H.; Yu, J.S. Silver nanoparticle decorated ZnO nanorod arrays on AZO films for light absorption enhancement. Phys. Status Solidi A 2012, 209, 297–301. [Google Scholar] [CrossRef]
- Tang, H.; Meng, G.; Huang, Q.; Zhang, Z.; Huang, Z.; Zhu, C. Arrays of Cone-Shaped ZnO Nanorods Decorated with Ag Nanoparticles as 3D Surface-Enhanced Raman Scattering Substrates for Rapid Detection of Trace Polychlorinated Biphenyls. Adv. Funct. Mater. 2012, 22, 218–224. [Google Scholar] [CrossRef]
- Slimi, B.; Ben Assaker, I.; Kriaa, A.; Marí, B.; Chtourou, R. One-step electrodeposition of Ag-decorated ZnO nanowires. J. Solid State Electrochem. 2017, 21, 1253–1261. [Google Scholar] [CrossRef]
- Tzeng, S.-K.; Hon, M.-H.; Leu, I.-C. Improving the Performance of a Zinc Oxide Nanowire Ultraviolet Photodetector by Adding Silver Nanoparticles. J. Electrochem. Soc. 2012, 159, H440–H443. [Google Scholar] [CrossRef]
- Han, Z.; Li, S.; Chu, J.; Chen, Y. Controlled growth of well-aligned ZnO nanowire arrays using the improved hydrothermal method. J. Semicond. 2013, 34, 063002. [Google Scholar] [CrossRef]
- Zhang, Z.; Lv, Y.; Yan, J.; Hui, D.; Yun, J.; Zhai, C.; Zhao, W. Uniform ZnO nanowire arrays: Hydrothermal synthesis, formation mechanism and field emission performance. J. Alloys Compd. 2015, 650, 374–380. [Google Scholar] [CrossRef]
- Chang, C.W.; Wu, H.T.; Huang, S.H.; Chen, C.K.; Un, I.W.; Yen, T.J. Single-crystalline heterostructure of ZnO nanowire arrays on large Ag microplates and its photocatalytic activity. Acta Mater. 2013, 61, 6993–6999. [Google Scholar] [CrossRef]
- Chen, K.J.; Hung, F.Y.; Chang, S.J.; Young, S.J. Optoelectronic characteristics of UV photodetector based on ZnO nanowire thin films. J. Alloys Compd. 2009, 479, 674–677. [Google Scholar] [CrossRef]
- Lai, Y.; Xin, P.; Cheng, S.; Yu, J.; Zheng, Q. Plasma enhanced multistate storage capability of single ZnO nanowire based memory. Appl. Phys. Lett. 2015, 106, 031603. [Google Scholar] [CrossRef]
- Yan, S.; Li, Z.; Li, H.; Wu, Z.; Wang, J.; Shen, W.; Fu, Y.Q. Ultra-sensitive room-temperature H2S sensor using Ag–In2O3 nanorod composites. J. Mater. Sci. 2018, 53, 16331–16344. [Google Scholar] [CrossRef]
- Chen, M.; Wang, H.; Hu, J.; Zhang, Y.; Li, K.; Zhang, D.; Zhou, S.; Zhang, J.; Zhu, Z.; Liu, Q. Near-Room-Temperature Ethanol Gas Sensor Based on Mesoporous Ag/Zn–LaFeO3 Nanocomposite. Adv. Mater. Interfaces 2019, 6, 1801453. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Song, D.; Liu, Q.; Chen, R.; Hou, J.; Liu, J.; Zhang, H.; Yu, J.; Liu, P.; Wang, J. Designed synthesis of Ag-functionalized Ni-doped In2O3 nanorods with enhanced formaldehyde gas sensing properties. J. Mater. Chem. C 2019, 7, 7219–7229. [Google Scholar] [CrossRef]
- Xu, L.; Xing, R.; Song, J.; Xu, W.; Song, H. ZnO–SnO2 nanotubes surface engineered by Ag nanoparticles: Synthesis, characterization, and highly enhanced HCHO gas sensing properties. J. Mater. Chem. C 2013, 1, 2174–2182. [Google Scholar] [CrossRef]
- Li, F.; Wu, S.; Zhang, L.; Li, Z. Ag nanoparticles decorated ZnO nanoarrays with enhanced surface-enhanced Raman scattering and field emission property. J. Mater. Sci. Mater. Electron. 2017, 28, 16233–16238. [Google Scholar] [CrossRef]
- Liu, H.; Liu, H.; Yang, J.; Zhai, H.; Liu, X.; Jia, H. Microwave-assisted one-pot synthesis of Ag decorated flower-like ZnO composites photocatalysts for dye degradation and NO removal. Ceram. Int. 2019, 45, 20133–20140. [Google Scholar] [CrossRef]
- Chen, C.; Zheng, Y.; Zhan, Y.; Lin, X.; Zheng, Q.; Wei, K. Enhanced Raman scattering and photocatalytic activity of Ag/ZnO heterojunction nanocrystals. Dalton Trans. 2011, 40, 9566–9570. [Google Scholar] [CrossRef]
- Zheng, Y.; Zheng, L.; Zhan, Y.; Lin, X.; Zheng, Q.; Wei, K. Ag/ZnO Heterostructure Nanocrystals: Synthesis, Characterization, and Photocatalysis. Lnorg. Chem. 2007, 46, 6980–6986. [Google Scholar] [CrossRef] [PubMed]
- Musa, I.; Qamhieh, N.; Mahmoud, S.T. Synthesis and length dependent photoluminescence property of zinc oxide nanorods. Results Phys. 2017, 7, 3552–3556. [Google Scholar] [CrossRef]
- Khan, W.; Khan, F.; Ajmal, H.M.; Huda, N.U.; Kim, J.H.; Kim, S.-D. Evolution of Structural and Optical Properties of ZnO Nanorods Grown on Vacuum Annealed Seed Crystallites. Nanomaterials 2018, 8, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jane, P.; Mariano, G.; Ilaria, R.; Luca De, S.; Ivo, R. Photoluminescence characterization of ZnO nanowires functionalization. In Proceedings of the SPIE Optics + Optoelectronics, Prague, Czech Republic, 13–16 April 2015. [Google Scholar]
- Liang, Y.-C.; Lo, Y.-R.; Wang, C.-C.; Xu, N.-C. Shell Layer Thickness-Dependent Photocatalytic Activity of Sputtering Synthesized Hexagonally Structured ZnO-ZnS Composite Nanorods. Materials 2018, 11, 87. [Google Scholar] [CrossRef] [Green Version]
- Abdulrahman, A.F.; Ahmed, S.M.; Barzinjy, A.A.; Hamad, S.M.; Ahmed, N.M.; Almessiere, M.A. Fabrication and Characterization of High-Quality UV Photodetectors Based ZnO Nanorods Using Traditional and Modified Chemical Bath Deposition Methods. Nanomaterials 2021, 11, 677. [Google Scholar] [CrossRef]
- Li, M.-Y.; Yu, M.; Su, D.; Zhang, J.; Jiang, S.; Wu, J.; Wang, Q.; Liu, S. Ultrahigh Responsivity UV Photodetector Based on Cu Nanostructure/ZnO QD Hybrid Architectures. Small 2019, 15, 1901606. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Su, J.; Li, H.; Zhang, Q.; Gao, Y. Ag nanoparticles@ZnO nanowire composite arrays: An absorption enhanced UV photodetector. Opt. Express 2014, 22, 30148–30155. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, F.; Shi, L.; Wang, Y.; Zhao, H.; Zhao, D. Performance enhancement in ZnO nanowire based double Schottky-barrier photodetector by applying optimized Ag nanoparticles. RSC Adv. 2016, 6, 4634–4639. [Google Scholar] [CrossRef]
- Yang, C.; Yu, H.; Su, Y.; Chuang, M.; Hsiao, C.; Kao, T. Noise Properties of Ag Nanoparticle-Decorated ZnO Nanorod UV Photodetectors. IEEE Photonics Technol. Lett. 2016, 28, 379–382. [Google Scholar] [CrossRef]
- Dinh, T.M.; Huynh, H.Q.; Mai, T.M.N.; Truong, H.S.; Luong, H.N.; Nguyen, N.P.; Tran, C.K.; Phan, B.T.; Dang, V.Q. Enhancing the performance of photodetectors based on ZnO nanorods decorated with Ag nanoparticles. Semicond. Sci. Technol. 2021, 36, 045009. [Google Scholar] [CrossRef]
- Wang, X.; Liu, K.; Chen, X.; Li, B.; Jiang, M.; Zhang, Z.; Zhao, H.; Shen, D. Highly Wavelength-Selective Enhancement of Responsivity in Ag Nanoparticle-Modified ZnO UV Photodetector. ACS Appl. Mater. Interfaces 2017, 9, 5574–5579. [Google Scholar] [CrossRef]
- Wang, H.-C.; Hong, Y.; Chen, Z.; Lao, C.; Lu, Y.; Yang, Z.; Zhu, Y.; Liu, X. ZnO UV Photodetectors Modified by Ag Nanoparticles Using All-Inkjet-Printing. Nanoscale Res. Lett. 2020, 15, 176. [Google Scholar] [CrossRef]
- Khan, R.; Uthirakumar, P.; Kim, T.H.; Lee, I.-H. Enhanced photocurrent performance of partially decorated Au nanoparticles on ZnO nanorods based UV photodetector. Mater. Res. Bull. 2019, 115, 176–181. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Geng, X.; Huang, Y.; Li, Y.; Liu, H.; Liu, Y.; Li, Y.; Wang, X.; Tian, H.; et al. Au Nanoparticles-Decorated Surface Plasmon Enhanced ZnO Nanorods Ultraviolet Photodetector on Flexible Transparent Mica Substrate. IEEE J. Electron Devices Soc. 2019, 7, 196–202. [Google Scholar] [CrossRef]
- Gogurla, N.; Sinha, A.K.; Santra, S.; Manna, S.; Ray, S.K. Multifunctional Au-ZnO Plasmonic Nanostructures for Enhanced UV Photodetector and Room Temperature NO Sensing Devices. Sci. Rep. 2014, 4, 6483. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Xu, C.; Dai, J.; Li, J.; Wang, Y.; Lin, Y.; Li, P. Improved UV photoresponse of ZnO nanorod arrays by resonant coupling with surface plasmons of Al nanoparticles. Nanoscale 2015, 7, 3396–3403. [Google Scholar] [CrossRef] [PubMed]
- Jayalakshmi, G.; Saravanan, K. High-performance UV surface photodetector based on plasmonic Ni nanoparticles-decorated hexagonal-faceted ZnO nanorod arrays architecture. J. Mater. Sci. Mater. Electron. 2020, 31, 5710–5720. [Google Scholar] [CrossRef]
- Subha, P.P.; Jayaraj, M.K. Enhanced room temperature gas sensing properties of low temperature solution processed ZnO/CuO heterojunction. BMC Chem. 2019, 13, 4. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Chen, Y.; Kumar, A.; Mahmoud, A.; Nychka, J.A.; Chung, H.-J. Sponge-Templated Macroporous Graphene Network for Piezoelectric ZnO Nanogenerator. ACS Appl. Mater. Interfaces 2015, 7, 20753–20760. [Google Scholar] [CrossRef] [PubMed]
- Schnippering, M.; Carrara, M.; Foelske, A.; Kötz, R.; Fermín, D.J. Electronic properties of Ag nanoparticle arrays. A Kelvin probe and high resolution XPS study. PCCP 2007, 9, 725–730. [Google Scholar] [CrossRef]
- Kumatani, A.; Li, Y.; Darmawan, P.; Minari, T.; Tsukagoshi, K. On Practical Charge Injection at the Metal/Organic Semiconductor Interface. Sci. Rep. 2013, 3, 1026. [Google Scholar] [CrossRef]
- Yang, F.; Zheng, M.; Zhao, L.; Guo, J.; Zhang, B.; Gu, G.; Cheng, G.; Du, Z. The high-speed ultraviolet photodetector of ZnO nanowire Schottky barrier based on the triboelectric-nanogenerator-powered surface-ionic-gate. Nano Energy 2019, 60, 680–688. [Google Scholar] [CrossRef]
- Liu, K.; Sakurai, M.; Liao, M.; Aono, M. Giant Improvement of the Performance of ZnO Nanowire Photodetectors by Au Nanoparticles. J. Phys. Chem. C 2010, 114, 19835–19839. [Google Scholar] [CrossRef]
- Atwater, H.A.; Polman, A. Plasmonics for improved photovoltaic devices. Nat. Mater. 2010, 9, 205–213. [Google Scholar] [CrossRef]
| Materials | Bias Voltage (V) | Wavelength (nm) | Responsivity (A/W) | Sensitivity (%) | Response/ Recovery Time (s) | Ref. |
|---|---|---|---|---|---|---|
| ZnO NWs | 1 | 365 | 14 | 8538 | 4.3/3.2 | This work |
| Ag NPs-ZnO NWs | 1 | 365 | 104 | 28,951 | 5.6/2.2 | This work |
| Ag NPs-ZnO NWs | 5 | 365 | - | ~8970 | 1.02/15.5 | [53] |
| Ag NPs-ZnO NWs | 0.5 | 365 | - | 239,992 | 4/36 | [33] |
| Ag NPs-ZnO NWs | 5 | 365 | 4.91 × 106 | - | 6.3/26.3 | [54] |
| Ag NPs-ZnO NRs | 0.2 | 380 | 12.4 | - | - | [55] |
| Ag NPs-ZnO NRs | 5 | 400 | 6.52 × 10−3 | - | -/157.77 | [56] |
| Ag NPs-ZnO TFs | 5 | 380 | 2.86 | - | 15/330 | [57] |
| Ag NPs-ZnO TFs | 20 | 365 | ~0.04 | - | 3.89/4.38 | [58] |
| Au NPs-ZnO NRs | 3 | 365 | - | ~4385 | - | [59] |
| Au NPs-ZnO NRs | 1 | 365 | 70 | - | - | [60] |
| Au NPs-ZnO NSs | 5 | 325 | ~60 | - | - | [61] |
| Al NPs-ZnO NRs | 5 | 325 | ~1.65 | - | 0.03/0.035 | [62] |
| Ni NPs-ZnO NRs | 1 | 325 | 0.124 | - | Prompt rise/rapid fall | [63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noh, Y.; Shin, J.; Lee, H.; Kim, G.Y.; Kumar, M.; Lee, D. Decoration of Ag Nanoparticle on ZnO Nanowire by Intense Pulsed Light and Enhanced UV Photodetector. Chemosensors 2021, 9, 321. https://doi.org/10.3390/chemosensors9110321
Noh Y, Shin J, Lee H, Kim GY, Kumar M, Lee D. Decoration of Ag Nanoparticle on ZnO Nanowire by Intense Pulsed Light and Enhanced UV Photodetector. Chemosensors. 2021; 9(11):321. https://doi.org/10.3390/chemosensors9110321
Chicago/Turabian StyleNoh, Youngwook, Jaehak Shin, Horim Lee, Gyu Young Kim, Manoj Kumar, and Dongjin Lee. 2021. "Decoration of Ag Nanoparticle on ZnO Nanowire by Intense Pulsed Light and Enhanced UV Photodetector" Chemosensors 9, no. 11: 321. https://doi.org/10.3390/chemosensors9110321
APA StyleNoh, Y., Shin, J., Lee, H., Kim, G. Y., Kumar, M., & Lee, D. (2021). Decoration of Ag Nanoparticle on ZnO Nanowire by Intense Pulsed Light and Enhanced UV Photodetector. Chemosensors, 9(11), 321. https://doi.org/10.3390/chemosensors9110321






