Colorimetric Sensing of Benzoyl Peroxide Based on the Emission Wavelength-Shift of CsPbBr3 Perovskite Nanocrystals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Instruments
2.3. Preparation of Materials
2.3.1. Preparation of Cesium Oleate
2.3.2. Preparation and Purification of CsPbBr3 NCs
2.3.3. Synthesis of Oleylammonium Iodide
2.3.4. Fluorescence Wavelength-Shift with the Different OLAM-I Concentration
2.3.5. BPO Concentration and the Corresponding Wavelength-Shift
3. Results and Discussion
3.1. Spectroscopy and Structural Characterization of CsPbBr3 PNCs
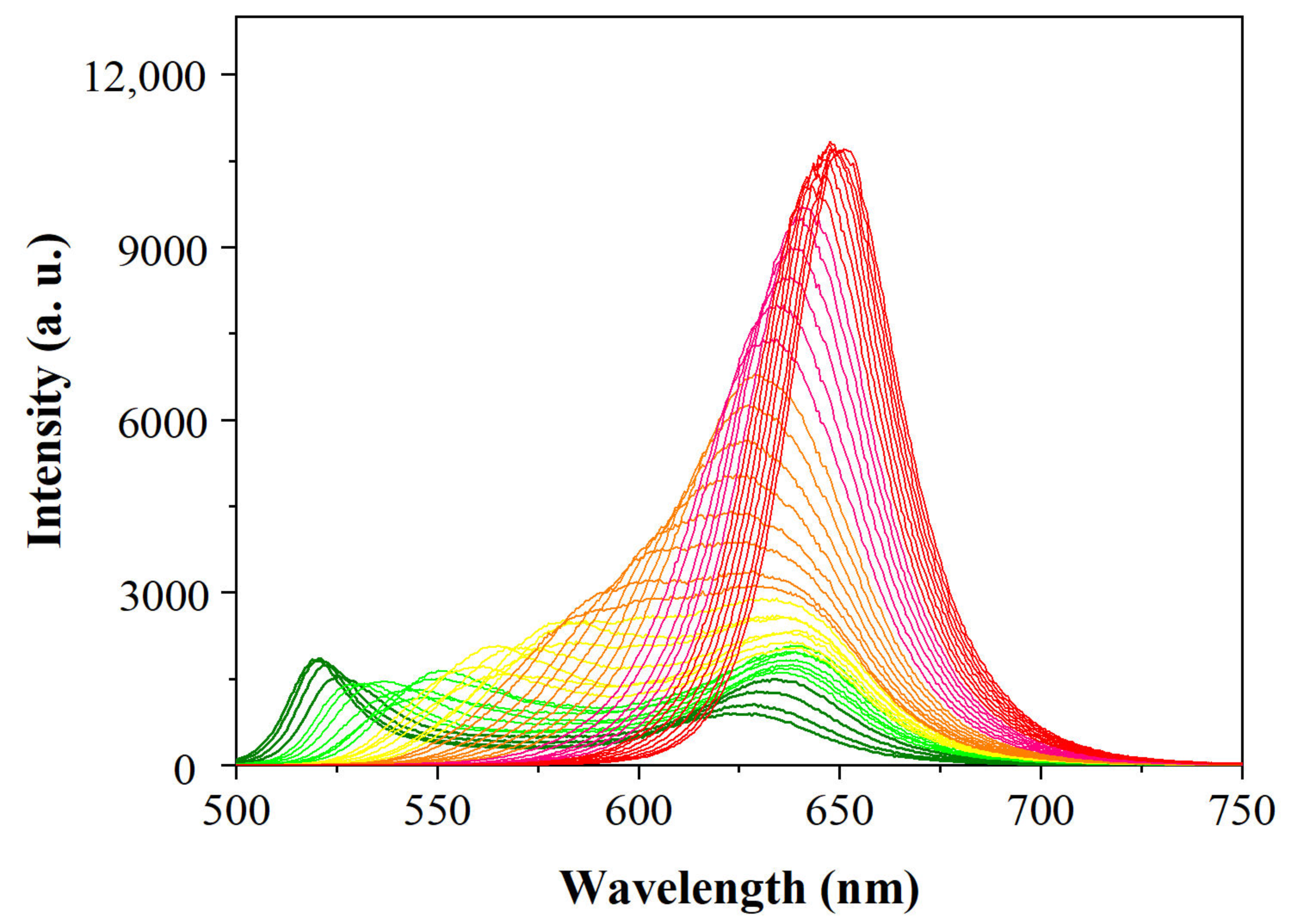
3.2. Fluorescence Wavelength-Shift in the Halogen Exchanges of CsPbBr3 PNCs
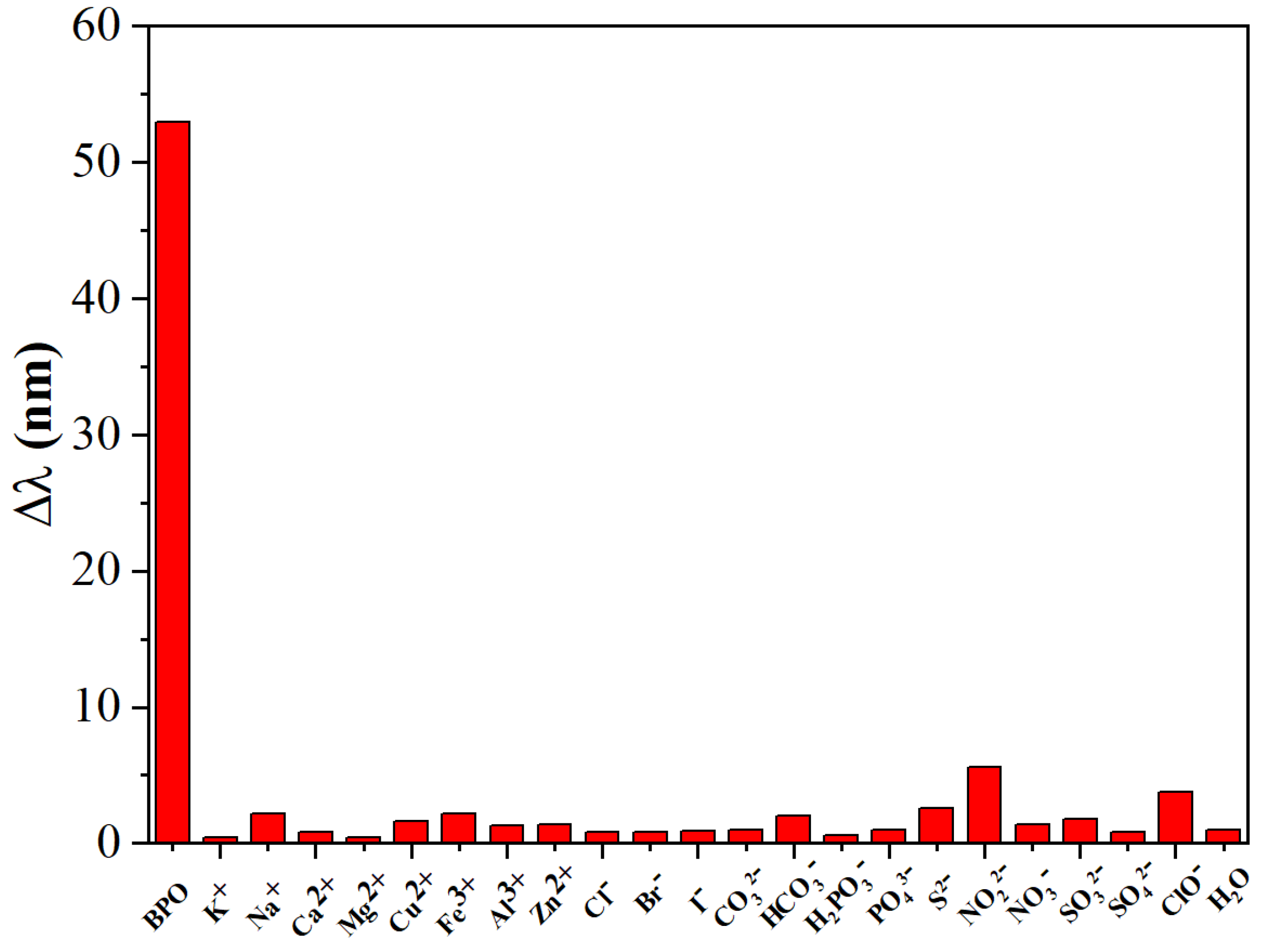
3.3. Analytical Performance of the Sensing Approach
3.4. Determination of BPO in Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Møller, C.K.N. Crystal Structure and Photoconductivity of Cæsium Plumbohalides. Nat. Cell Biol. 1958, 182, 1436. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Lin, K.; Xing, J.; Quan, L.N.; de Arquer, F.P.G.; Gong, X.; Lu, J.; Xie, L.; Zhao, W.; Zhang, D.; Yan, C.; et al. Perovskite light-emitting diodes with external quantum efficiency exceeding 20 percent. Nature 2018, 562, 245–248. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, J.; Ou, X.; Huang, B.; Almutlaq, J.; Zhumekenov, A.A.; Guan, X.; Han, S.; Liang, L.; Yi, Z.; et al. All-inorganic perovskite nanocrystal scintillators. Nat. Cell Biol. 2018, 561, 88–93. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, R.; Ou, X.; Fu, K.; Chen, Q.; Ding, Y.; Xu, L.-J.; Liu, L.; Han, Y.; Malko, A.V.; et al. Metal Halide Perovskite Nanosheet for X-ray High-Resolution Scintillation Imaging Screens. ACS Nano 2019, 13, 2520–2525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yakunin, S.; Chaaban, J.; Benin, B.M.; Cherniukh, I.; Bernasconi, C.; Landuyt, A.; Shynkarenko, Y.; Bolat, S.; Hofer, C.; Romanyuk, Y.E.; et al. Radiative lifetime-encoded unicolour security tags using perovskite nanocrystals. Nature Comm. 2021, 12, 981. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Wang, Q.; Liu, X.; Mei, E.; Liang, X.; Xiang, W. The promotion of TiO2 induction for finely tunable self-crystallized CsPbX3 (X = Cl, Br and I) nanocrystal glasses for LED backlighting display. Chem. Eng. J. 2022, 429, 132391. [Google Scholar] [CrossRef]
- Song, J.; Li, J.; Li, X.; Xu, L.; Dong, Y.; Zeng, H. Quantum Dot Light-Emitting Diodes Based on Inorganic Perovskite Cesium Lead Halides (CsPbX3). Adv. Mater. 2015, 27, 7162–7167. [Google Scholar] [CrossRef]
- Fu, Y.; Zhu, H.; Stoumpos, C.; Ding, Q.; Wang, J.; Kanatzidis, M.G.; Zhu, X.; Jin, S. Broad Wavelength Tunable Robust Lasing from Single-Crystal Nanowires of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, I). ACS Nano 2016, 10, 7963–7972. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Sandanayaka, A.S.D.; Zhao, C.; Matsushima, T.; Zhang, D.; Fujihara, T.; Adachi, C. Stable room-temperature continuous-wave lasing in quasi-2D perovskite films. Nat. Cell Biol. 2020, 585, 53–57. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, F.; Huang, Y.; Lin, F.; Chen, X. Wavelength-Shift-Based Colorimetric Sensing for Peroxide Number of Edible Oil Using CsPbBr3 Perovskite Nanocrystals. Anal. Chem. 2019, 91, 14183–14187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.; Sun, Q.; Zhang, Z.; Dai, J.; Xing, G.; Li, S.; Huang, X.; Huang, W. Metal halide perovskites: Stability and sensing-ability. J. Mater. Chem. C 2018, 6, 10121–10137. [Google Scholar] [CrossRef]
- Huang, S.; Guo, M.; Tan, J.; Geng, Y.; Wu, J.; Tang, Y.; Su, C.; Lin, C.C.; Liang, Y. Novel Fluorescence Sensor Based on All-Inorganic Perovskite Quantum Dots Coated with Molecularly Imprinted Polymers for Highly Selective and Sensitive Detection of Omethoate. ACS Appl. Mater. Interfaces 2018, 10, 39056–39063. [Google Scholar] [CrossRef]
- Shamsi, J.; Urban, A.S.; Imran, M.; De Trizio, L.; Manna, L. Metal Halide Perovskite Nanocrystals: Synthesis, Post-Synthesis Modifications, and Their Optical Properties. Chem. Rev. 2019, 119, 3296–3348. [Google Scholar] [CrossRef]
- Schmidt, L.; Pertegás, A.; Gonzalez-Carrero, S.; Malinkiewicz, O.; Agouram, S.; Espallargas, G.M.; Bolink, H.; Galian, R.E.; Pérez-Prieto, J. Nontemplate Synthesis of CH3NH3PbBr3 Perovskite Nanoparticles. J. Am. Chem. Soc. 2014, 136, 850–853. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akkerman, Q.; D’Innocenzo, V.; Accornero, S.; Scarpellini, A.; Petrozza, A.; Prato, M.; Manna, L. Tuning the Optical Properties of Cesium Lead Halide Perovskite Nanocrystals by Anion Exchange Reactions. J. Am. Chem. Soc. 2015, 137, 10276–10281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, L.-Q.; Tan, T.; Tian, X.-K.; Li, Y.; Deng, P. Visual and sensitive fluorescent sensing for ultratrace mercury ions by perovskite quantum dots. Anal. Chim. Acta 2017, 986, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Li, F.; Cai, Z.; Wang, Y.; Luo, F.; Chen, X. An ultrasensitive and reversible fluorescence sensor of humidity using perovskite CH3NH3PbBr3. J. Mater. Chem. C 2016, 4, 9651–9655. [Google Scholar] [CrossRef]
- Chen, C.; Cai, Q.; Luo, F.; Dong, N.; Guo, L.; Qiu, B.; Lin, Z. Sensitive Fluorescent Sensor for Hydrogen Sulfide in Rat Brain Microdialysis via CsPbBr3 Quantum Dots. Anal. Chem. 2019, 91, 15915–15921. [Google Scholar] [CrossRef]
- Rodà, C.; Abdelhady, A.L.; Shamsi, J.; Lorenzon, M.; Pinchetti, V.; Gandini, M.; Meinardi, F.; Manna, L.; Brovelli, S. O2 as a molecular probe for nonradiative surface defects in CsPbBr3 perovskite nanostructures and single crystals. Nanoscale 2019, 11, 7613–7623. [Google Scholar] [CrossRef]
- Lin, F.; Li, F.; Lai, Z.; Cai, Z.; Wang, Y.; Wolfbeis, O.S.; Chen, X. MnII-Doped Cesium Lead Chloride Perovskite Nanocrystals: Demonstration of Oxygen Sensing Capability Based on Luminescent Dopants and Host-Dopant Energy Transfer. ACS Appl. Mater. Interfaces 2018, 10, 23335–23343. [Google Scholar] [CrossRef]
- Li, F.; Feng, Y.; Huang, Y.; Yao, Q.; Huang, G.; Zhu, Y.; Chen, X. Colorimetric sensing of chloride in sweat based on fluorescence wavelength shift via halide exchange of CsPbBr3 perovskite nanocrystals. Microchim. Acta 2021, 188, 1–8. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, S.; Zhu, Y.; Li, F.; Jin, J.; Dong, J.; Lin, F.; Wang, Y.; Chen, X. Dual-Mode of Fluorescence Turn-On and Wavelength-Shift for Methylamine Gas Sensing Based on Space-Confined Growth of Methylammonium Lead Tribromide Perovskite Nanocrystals. Anal. Chem. 2020, 92, 5661–5665. [Google Scholar] [CrossRef] [Green Version]
- Yin, W.; Li, H.; Chesman, A.S.R.; Tadgell, B.; Scully, A.D.; Wang, M.; Huang, W.; McNeill, C.R.; Wong, W.W.H.; Medhekar, N.V.; et al. Detection of Halomethanes Using Cesium Lead Halide Perovskite Nanocrystals. ACS Nano 2021, 15, 1454–1464. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Kang, S.-M.; Lee, G.W.; Kwak, C.H.; Rethinasabapathy, M.; Huh, Y.S. Fabrication of CsPbBr3 Perovskite Quantum Dots/Cellulose-Based Colorimetric Sensor: Dual-Responsive On-Site Detection of Chloride and Iodide Ions. Ind. Eng. Chem. Res. 2019, 59, 793–801. [Google Scholar] [CrossRef]
- Ministry of Health; Ministry of Industry and Information Technology; Ministry of Commerce; The State Administration of Industry and Commerce; General Administration of Quality Supervision; State Grain Administration; State Food and Drug Administration. Announcement of Ministry of Health and Other Seven Departments on Revoking Benzoyl Peroxide and Calcium Peroxide as Food Additives; Chinese Journal of Food Hygiene: Beijing, China, 2011.
- Zhang, S. Detection of benzoyl peroxide in food by gas chromatography. J. Food Saf. Qual. 2016, 7, 353–356. [Google Scholar]
- The General Administration of Quality Supervision; Inspection and Quarantine of the People’s Republic of China; The Standardization Administration of China. Determination of Benzoyl Peroxide in Wheat Flour by High Performance Liquid Chromatography (GB/T 22325-2008); Standards Press of China: Beijing, China, 2008. [Google Scholar]
- Saiz, A.I.; Manrique, G.D.; Fritz, R. Determination of benzoyl peroxide and benzoic acid levels by HPLC during wheat flour bleaching process. J. Agric. Food Chem. 2001, 49, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Abe-Onishi, Y.; Yomota, C.; Sugimoto, N.; Kubota, H.; Tanamoto, K. Determination of benzoyl peroxide and benzoic acid in wheat flour by high-performance liquid chromatography and its identification by high-performance liquid chromatography–mass spectrometry. J. Chromatogr. A 2004, 1040, 209–214. [Google Scholar] [CrossRef]
- Mu, G.; Liu, H.; Gao, Y.; Luan, F. Determination of benzoyl peroxide, as benzoic acid, in wheat flour by capillary electrophoresis compared with HPLC. J. Sci. Food Agric. 2011, 92, 960–964. [Google Scholar] [CrossRef] [PubMed]
- Kozan, J.; Silva, R.; Serrano, S.; Lima, A.; Angnes, L. Amperometric detection of benzoyl peroxide in pharmaceutical preparations using carbon paste electrodes with peroxidases naturally immobilized on coconut fibers. Biosens. Bioelectron. 2010, 25, 1143–1148. [Google Scholar] [CrossRef]
- Wang, C.; Hu, X. Determination of Benzoyl Peroxide Levels in Wheat Flour and Pharmaceutical Preparations by Differential Pulse Voltammetry in Nonaqueous Media. Anal. Lett. 2005, 38, 2175–2187. [Google Scholar] [CrossRef]
- Bowyer, J.; Spurlin, S. Chemiluminescence method for determination of benzoyl peroxide in solution. Anal. Chim. Acta 1987, 192, 289–292. [Google Scholar] [CrossRef]
- Yang, W.-P.; Zhang, Z.-J.; Hun, X. A novel capillary microliter droplet sample injection–chemiluminescence detector and its application to the determination of benzoyl peroxide in wheat flour. Talanta 2003, 62, 661–666. [Google Scholar] [CrossRef]
- Wang, S.P.; Liu, X. The clinical features, CT and MRI diagnosis of Wallenberg syndrome. J. Taishan. Med. Colle. 2010, 31, 446–447. [Google Scholar]
- Hu, Q.; Li, W.; Qin, C.; Zeng, L.; Hou, J.-T. Rapid and Visual Detection of Benzoyl Peroxide in Food by a Colorimetric and Ratiometric Fluorescent Probe. J. Agric. Food Chem. 2018, 66, 10913–10920. [Google Scholar] [CrossRef]
- Zeng, L.; Wu, X.; Hu, Q.; Yuan, H.-Q.; Bao, G.-M. A single fluorescent chemosensor for discriminative detection of bisulfite and benzoyl peroxide in food with different emission. Sensors Actuators B Chem. 2019, 299, 126994. [Google Scholar] [CrossRef]
- Wu, X.; Zeng, L.; Chen, B.-Q.; Zhang, M.; Rodrigues, J.; Sheng, R.; Bao, G.-M. A selective cascade reaction-based probe for colorimetric and ratiometric fluorescence detection of benzoyl peroxide in food and living cells. J. Mater. Chem. B 2019, 7, 5775–5781. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Zhang, M.; Xu, F.; Wang, X.; Xu, Z.; Guo, L. Colorimetric detection of benzoyl peroxide based on the etching of silver nanoshells of Au@Ag nanorods. Sensors Actuators B Chem. 2018, 261, 379–384. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; Martínez-Sarti, L.; Goldoni, L.; Imran, M.; Baranov, D.; Bolink, H.J.; Palazon, F.; Manna, L. Molecular Iodine for a General Synthesis of Binary and Ternary Inorganic and Hybrid Organic–Inorganic Iodide Nanocrystals. Chem. Mater. 2018, 30, 6915–6921. [Google Scholar] [CrossRef]
- Hoffman, J.B.; Schleper, A.L.; Kamat, P.V. Transformation of Sintered CsPbBr3 Nanocrystals to Cubic CsPbI3 and Gradient CsPbBrxI3–x through Halide Exchange. J. Am. Chem. Soc. 2016, 138, 8603–8611. [Google Scholar] [CrossRef] [PubMed]







| Sample | BPO (μmol L−1) | Spiked (μmol L−1) | Found (μmol L−1) | RSD (%, n = 6) | Recovery (%) |
|---|---|---|---|---|---|
| Flour 1 | ND | 5.0 10.0 | 5.2 ± 0.2 10.6 ± 0.5 | 3.8 4.7 | 104.0 106.0 |
| Flour 2 | ND | 5.0 10.0 | 5.4 ± 0.2 11.2 ± 0.4 | 4.0 3.5 | 108.0 112.0 |
| Flour 3 | ND | 5.0 10.0 | 5.5 ± 0.3 10.2 ± 0.4 | 5.5 4.2 | 110.0 102.0 |
| Noodle 1 | ND | 5.0 10.0 | 4.9 ± 0.2 9.8 ± 0.5 | 4.3 4.7 | 98.0 97.0 |
| Noodle 2 | ND | 5.0 10.0 | 4.8 ± 0.2 10.1 ± 0.3 | 4.2 2.8 | 96.0 101.0 |
| Noodle 3 | ND | 5.0 10.0 | 4.7 ± 0.2 10.3 ± 0.4 | 4.1 3.9 | 94.0 103.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Zhu, Y.; Li, F.; Zhang, L.; You, L.; Guo, Z.; Huang, Y.; Zhao, L.; Chen, X. Colorimetric Sensing of Benzoyl Peroxide Based on the Emission Wavelength-Shift of CsPbBr3 Perovskite Nanocrystals. Chemosensors 2021, 9, 319. https://doi.org/10.3390/chemosensors9110319
Zhang L, Zhu Y, Li F, Zhang L, You L, Guo Z, Huang Y, Zhao L, Chen X. Colorimetric Sensing of Benzoyl Peroxide Based on the Emission Wavelength-Shift of CsPbBr3 Perovskite Nanocrystals. Chemosensors. 2021; 9(11):319. https://doi.org/10.3390/chemosensors9110319
Chicago/Turabian StyleZhang, Li, Yimeng Zhu, Feiming Li, Linchun Zhang, Longjie You, Zhiyong Guo, Yaning Huang, Li Zhao, and Xi Chen. 2021. "Colorimetric Sensing of Benzoyl Peroxide Based on the Emission Wavelength-Shift of CsPbBr3 Perovskite Nanocrystals" Chemosensors 9, no. 11: 319. https://doi.org/10.3390/chemosensors9110319
APA StyleZhang, L., Zhu, Y., Li, F., Zhang, L., You, L., Guo, Z., Huang, Y., Zhao, L., & Chen, X. (2021). Colorimetric Sensing of Benzoyl Peroxide Based on the Emission Wavelength-Shift of CsPbBr3 Perovskite Nanocrystals. Chemosensors, 9(11), 319. https://doi.org/10.3390/chemosensors9110319








