Rapid Screening of Butyl Paraben Additive in Toner Sample by Molecularly Imprinted Photonic Crystal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
2.3. Experimental Methods
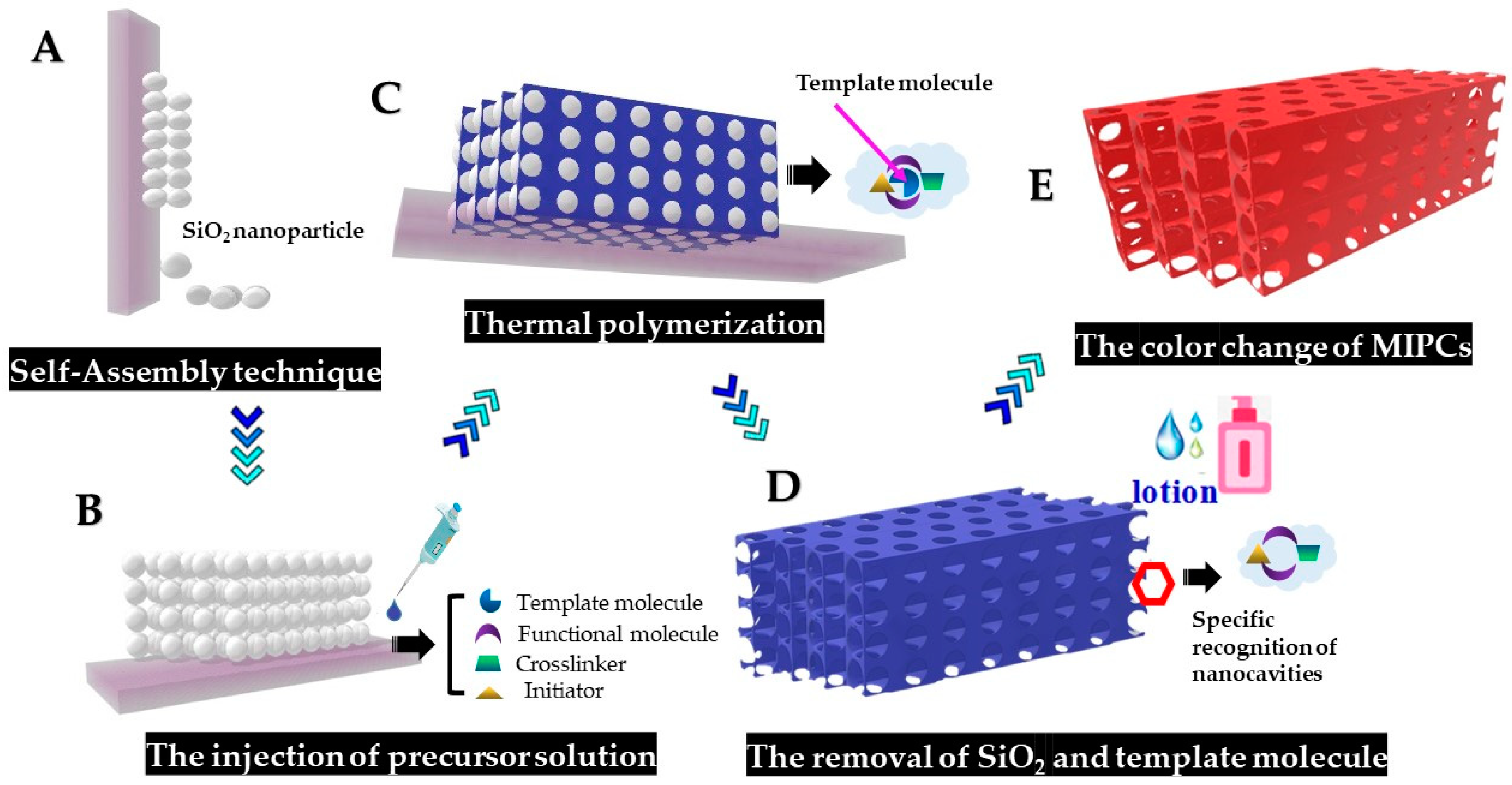
2.3.1. Preparation and Self-Assembly of Silica Colloidal Microspheres
2.3.2. Fabrication of the Molecularly Imprinted Photonic Crystal (MIPC)
2.4. Investigation of the Analytical Performance of the MIPCs
2.5. The Application of MIPCs
3. Results
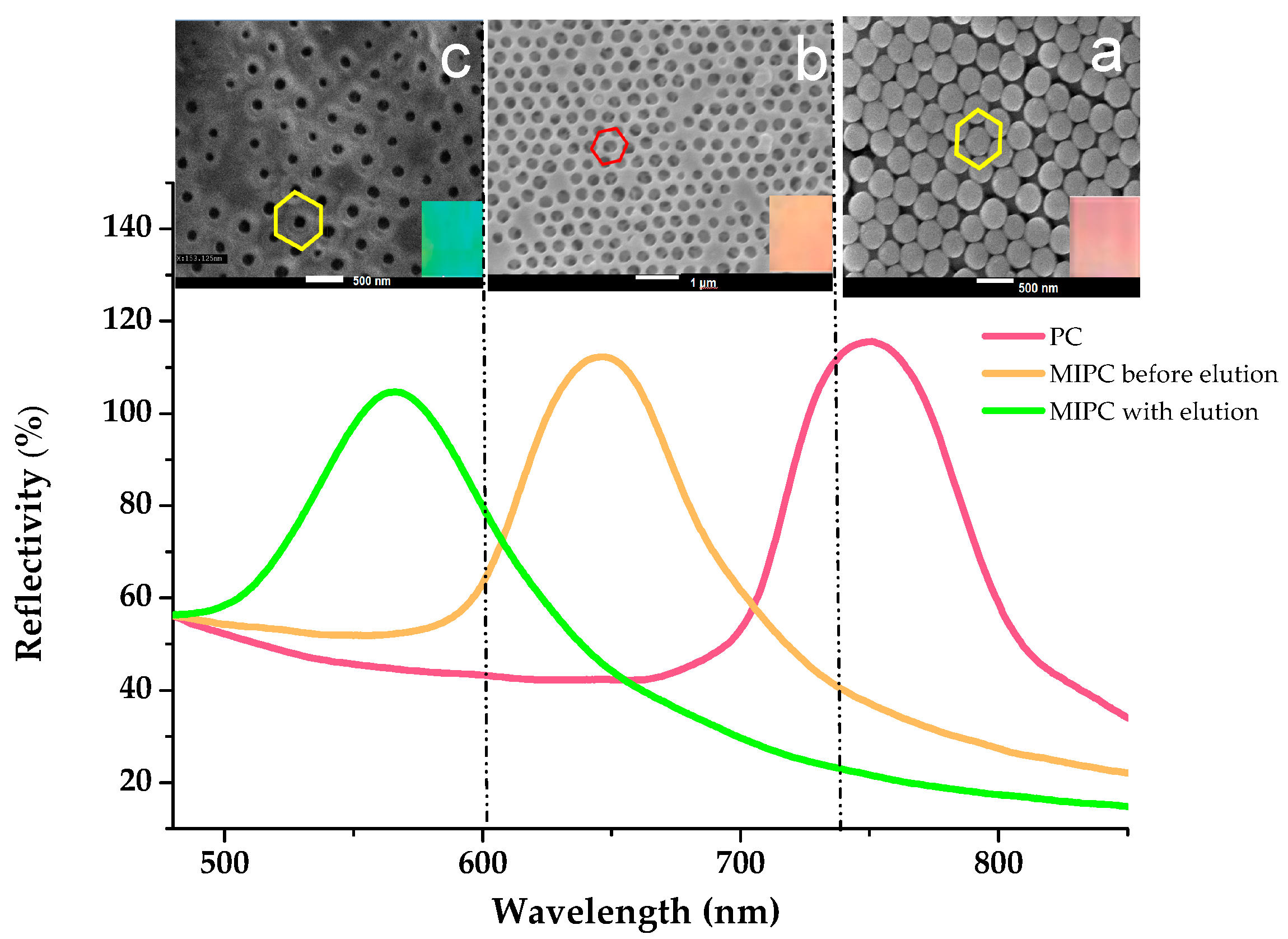
3.1. The Fabrication of PC Template and MIPC Sensor
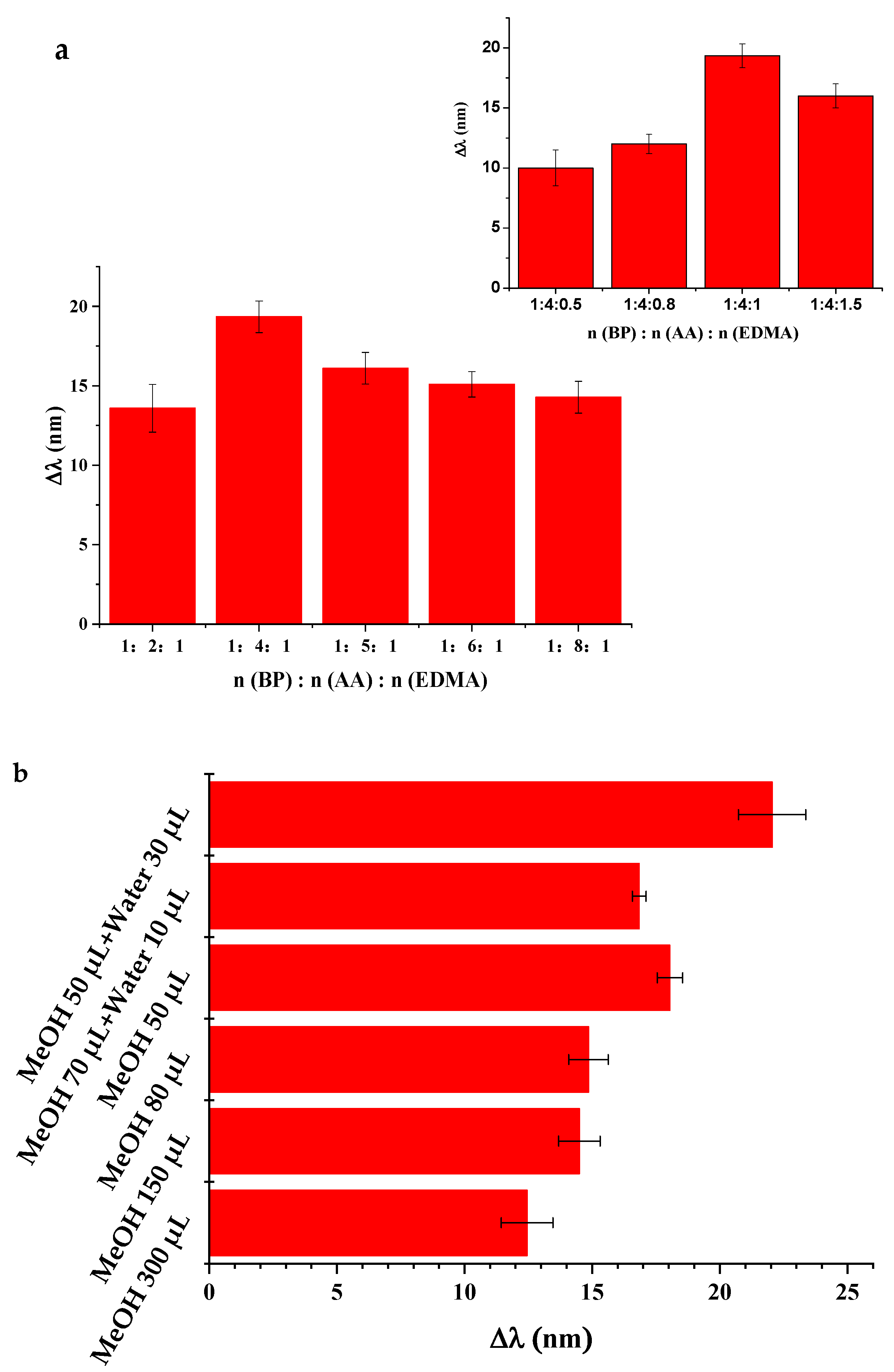
3.2. Analytical Performance for Sensing BPs
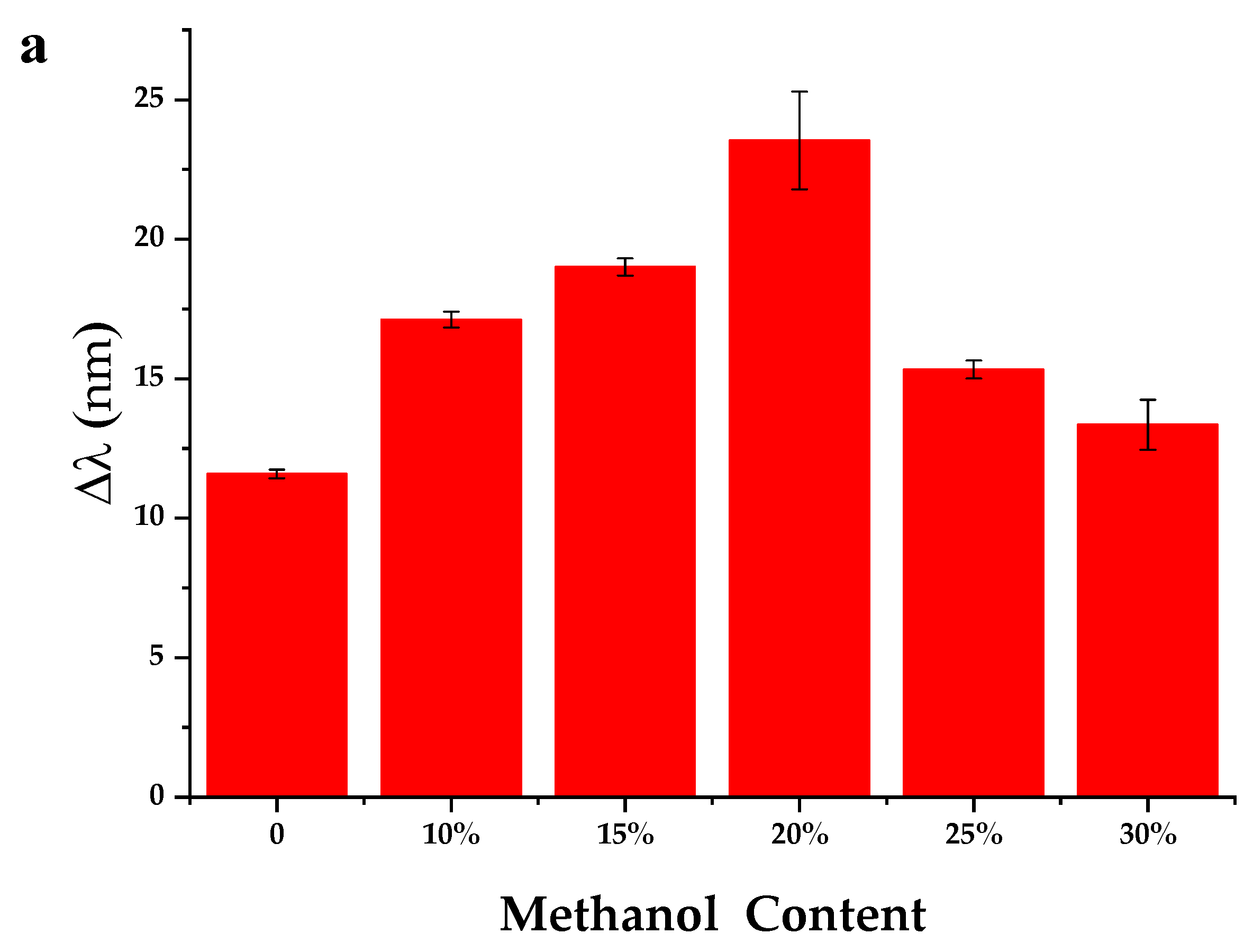
3.2.1. Effect of the Methanol Content in the Assayed Sample Solution
3.2.2. The Effect of pH on the Performance of MIPC
3.2.3. The Effect of Temperature on the Performance of MIPCs
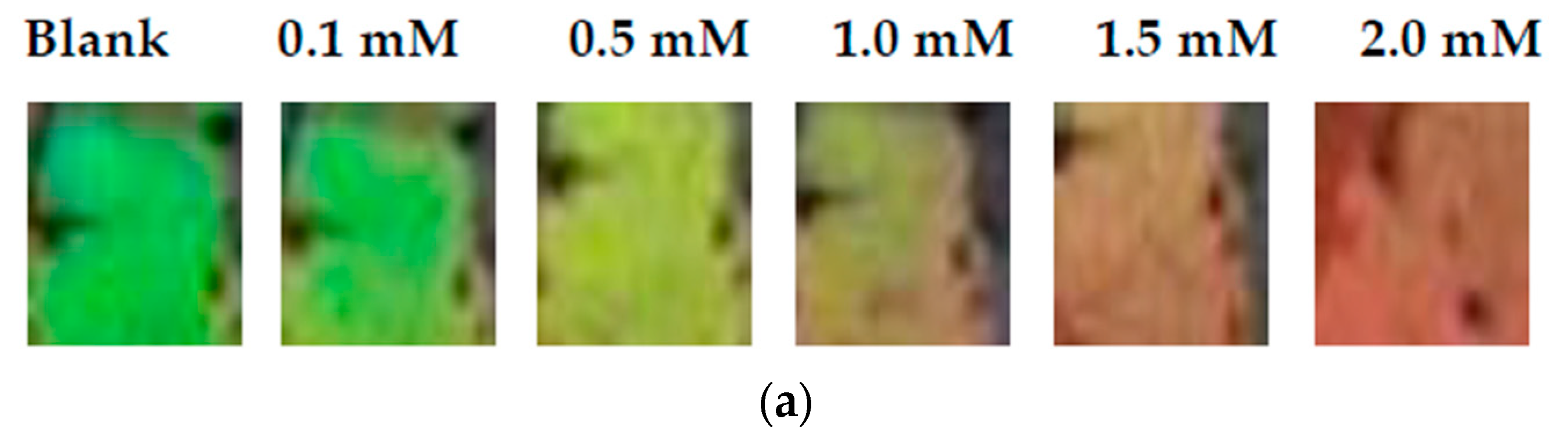
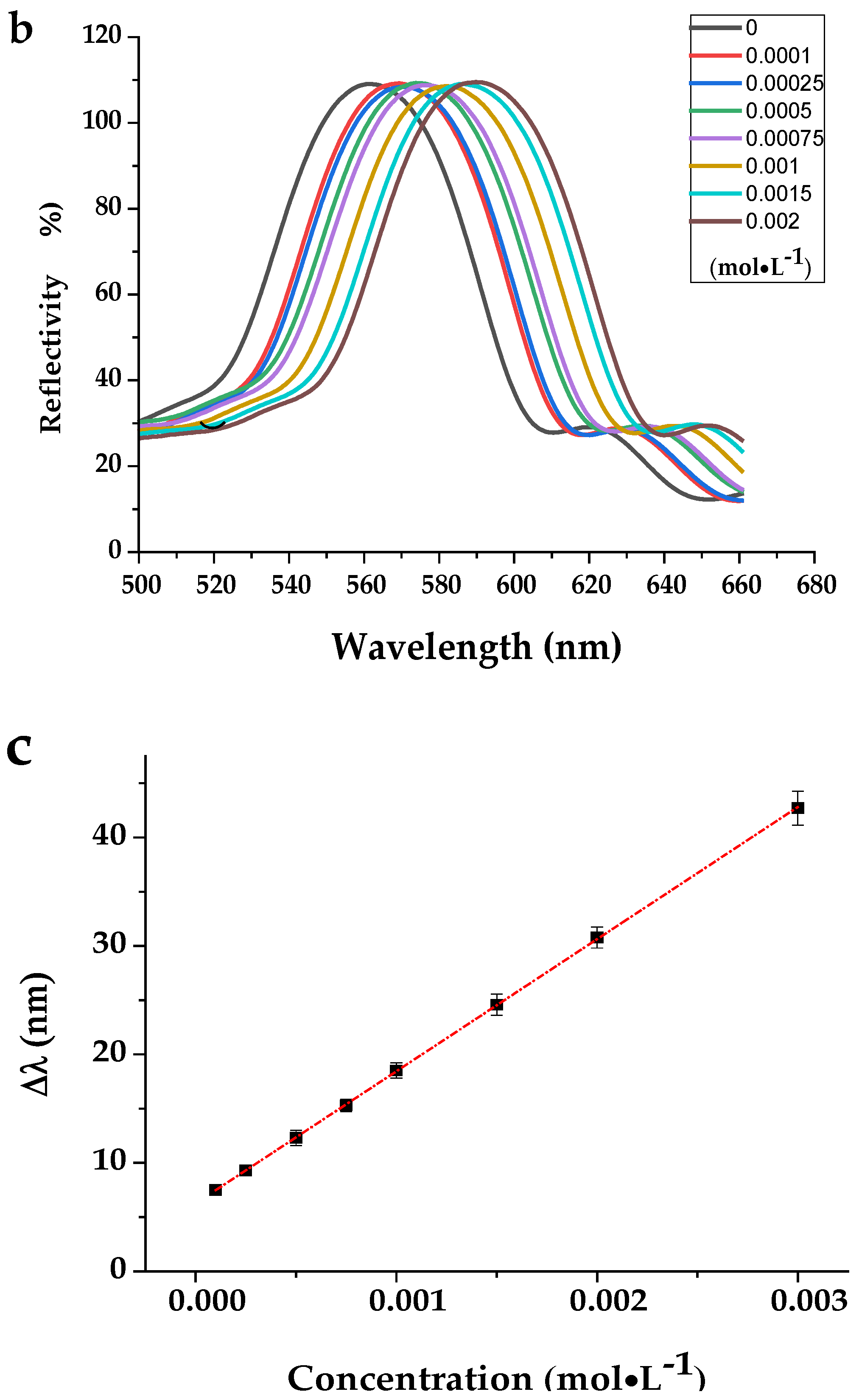
3.2.4. The Analytical Performance of MIPCs to BP
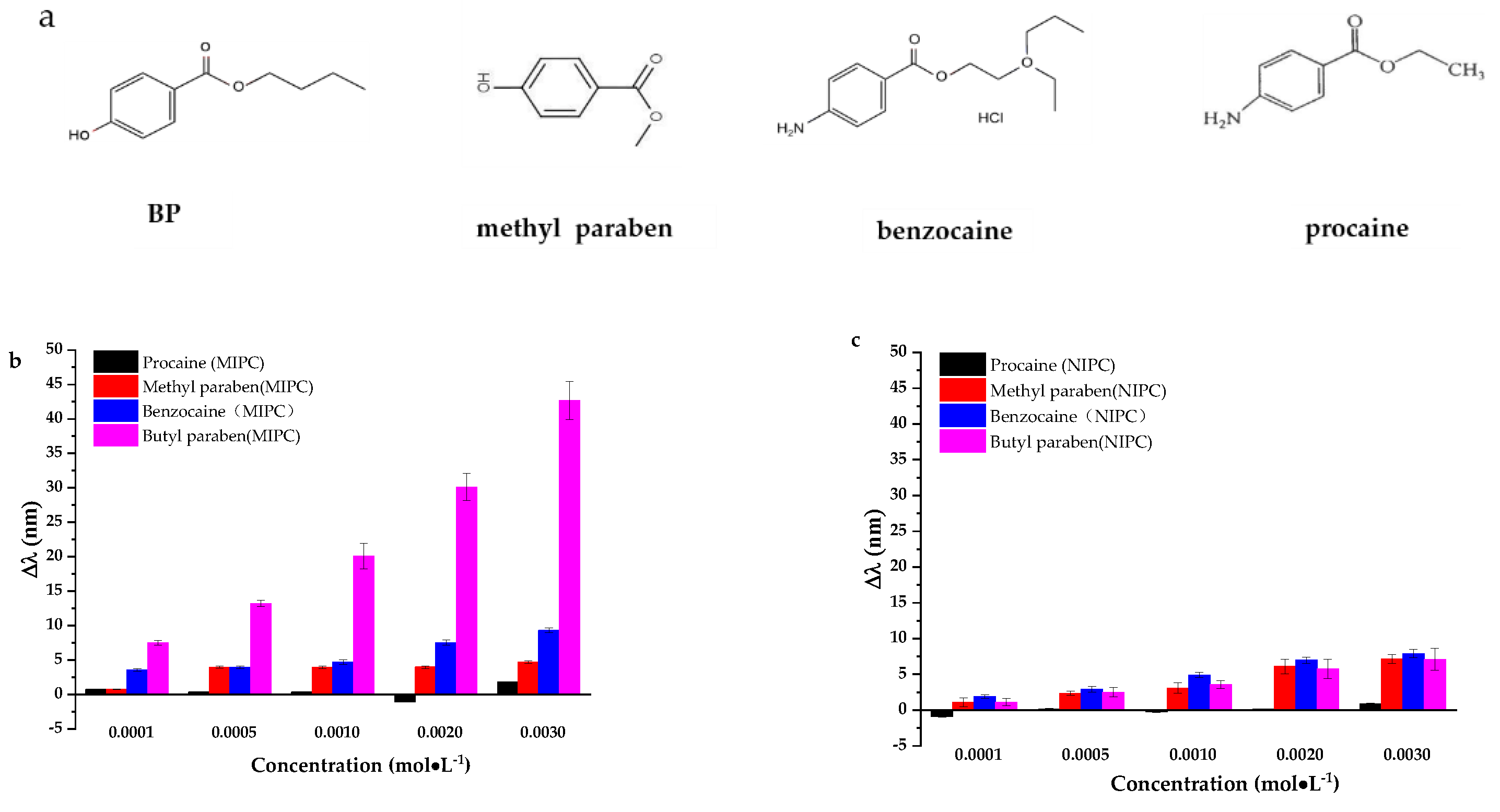
3.2.5. The Selectivity of MIPCs Film to BP
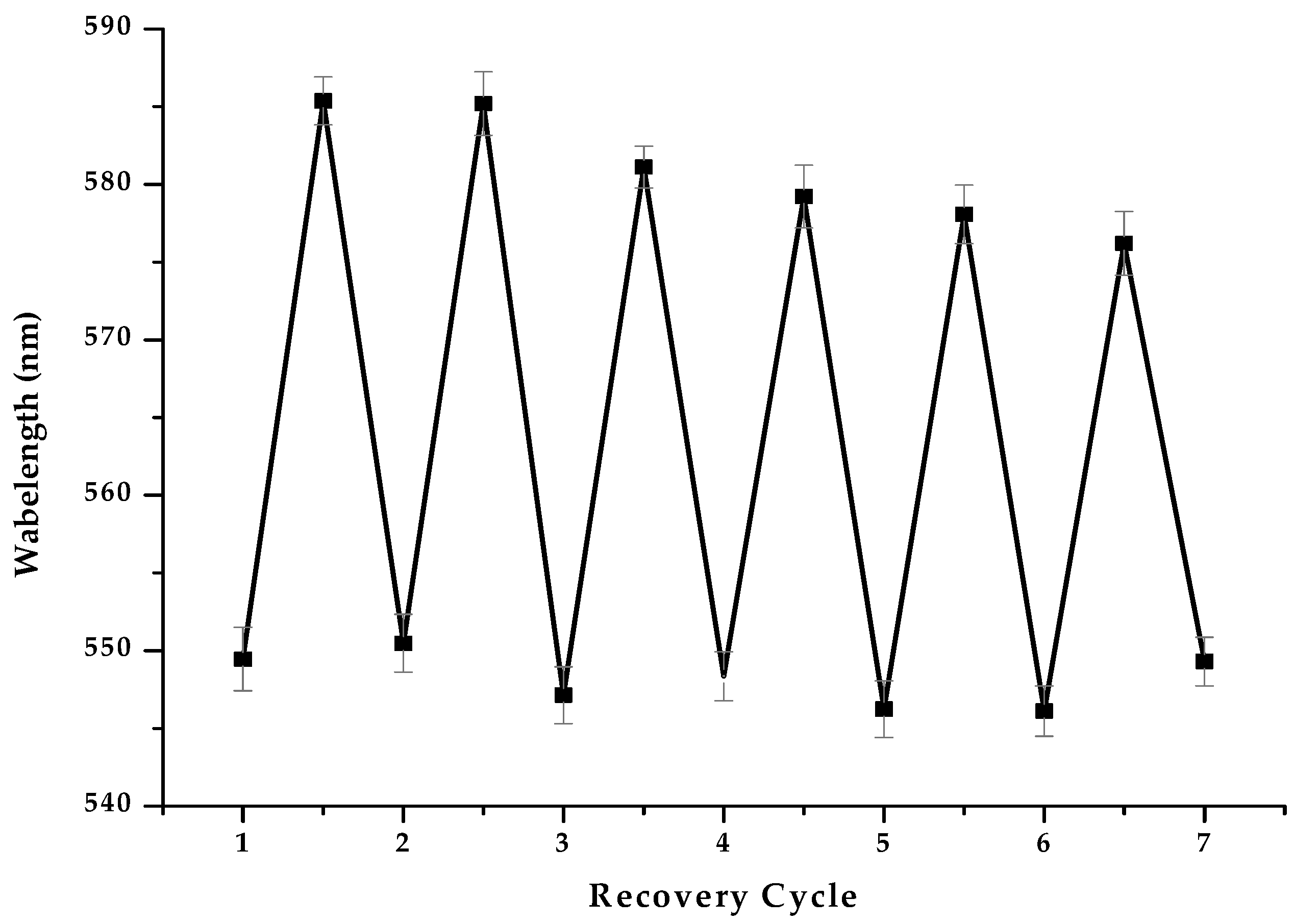
3.2.6. The Reusability of MIPCs Film
4. Application
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peinado, F.M.; Lendinez, I.; Sotelo, R.; Iribarne-Duran, L.M.; Fernandez-Parra, J.; Vela-Soria, F.; Olea, N.; Fernandez, M.F.; Freire, C.; Leon, J.; et al. Association of Urinary Levels of Bisphenols A, F, and S with Endometriosis Risk: Preliminary Results of the EndEA Study. Int. J. Environ. Res. Public Health. 2020, 17, 1194. [Google Scholar] [CrossRef] [Green Version]
- Gao, C.J.; Kannan, K. Phthalates, bisphenols, parabens, and triclocarban in feminine hygiene products from the United States and their implications for human exposure. Environ. Int. 2020, 136, 105465. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cui, X.Y.; Chen, Y.; Liao, C.Y. Paraben concentrations in human fingernail and its association with personal care product use. Ecotoxicol. Environ. Saf. 2020, 202, 110933. [Google Scholar] [CrossRef]
- Oliveira, M.M.; Martins, F.; Silva, M.G.; Correia, E.; Videira, R.; Peixoto, F. Use of Parabens (Methyl and Butyl) during the Gestation Period: Mitochondrial Bioenergetics of the Testes and Antioxidant Capacity Alterations in Testes and Other Vital Organs of the F1 Generation. Antioxidants 2020, 9, 1302. [Google Scholar] [CrossRef] [PubMed]
- Bledzka, D.; Gromadzinska, J.; Wasowicz, W. Parabens. From environmental studies to human health. Environ. Int. 2014, 67, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.; Dwivedi, A.K.; Alvarado, L.; Kupesic-Plavsic, S. Exposure of U.S. population to endocrine disruptive chemicals (Parabens, Benzophenone-3, Bisphenol-A and Triclosan) and their associations with female infertility. Environ. Pollut. 2020, 265, 114763. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, Y.; Liu, S.; Yang, D.; Ma, H.; Zhu, Z.; Kang, L.; Lu, S. Exposure of Chinese adult females to parabens from personal care products: Estimation of intake via dermal contact and health risks. Environ. Pollut. 2021, 272, 116043. [Google Scholar] [CrossRef]
- Bayulken, D.G.; Tuylu, B.A. In vitro genotoxic and cytotoxic effects of some paraben esters on human peripheral lymphocytes. Drug. Chem. Toxicol. 2019, 42, 386–393. [Google Scholar] [CrossRef]
- Amin, M.M.; Tabatabaeian, M.; Chavoshani, A.; Amjadi, E.; Hashemi, M.; Ebrahimpour, K.; Klishadi, R.; Khazaei, S.; Mansourian, M. Paraben Content in Adjacent Normal-malignant Breast Tissues from Women with Breast Cancer. Biomed. Environ. Sci. 2019, 32, 893–904. [Google Scholar] [CrossRef]
- Fransway, A.F.; Fransway, P.J.; Belsito, D.V.; Yiannias, J.A. Paraben Toxicology. Dermatitis 2019, 30, 32–45. [Google Scholar] [CrossRef]
- Gardner, S. EU Tightens Restrictions on Three Substances Used in Cosmetics. Infant Care Products. Int. Environ. Rep. 2014, 37, 1362. [Google Scholar]
- Kaur, R.; Kaur, R.; Grover, A.; Rani, S.; Malik, A.K.; Kabir, A.; Furton, K.G. Fabric phase sorptive extraction/GC-MS method for rapid determination of broad polarity spectrum multi-class emerging pollutants in various aqueous samples. J. Sep. Sci. 2019, 42, 2407–2417. [Google Scholar] [CrossRef]
- Ozcan, S.; Levent, S.; Can, N.O.; Kozanli, M. A Novel HPLC Method for Simultaneous Determination of Methyl, Ethyl, n-propyl, Isopropyl, n-butyl, Isobutyl and Benzyl Paraben in Pharmaceuticals and Cosmetics. Comb. Chem. High Throughput Screen. 2021, 24, 352–365. [Google Scholar] [CrossRef]
- Ma, T.; Li, Z.; Jia, Q.; Zhou, W.H. Ultrasound-assisted temperature-controlled ionic liquid emulsification microextraction coupled with capillary electrophoresis for the determination of parabens in personal care products. Electrophoresis 2016, 37, 1624–1631. [Google Scholar] [CrossRef]
- Sanchis, Y.; Coscolla, C.; Yusa, V. Analysis of four parabens and bisphenols A, F, S in urine, using dilute and shoot and liquid chromatography coupled to mass spectrometry. Talanta 2019, 202, 42–50. [Google Scholar] [CrossRef]
- Alampanos, V.; Kabir, A.; Furton, K.G.; Roje, Z.; Vrcek, I.V.; Samanidou, V. Fabric phase sorptive extraction combined with high-performance-liquid chromatography-photodiode array analysis for the determination of seven parabens in human breast tissues: Application to cancerous and non-cancerous samples. J. Chromatogr. A 2020, 1630, 461530. [Google Scholar] [CrossRef] [PubMed]
- Rigkos, G.; Alampanos, V.; Kabir, A.; Furton, K.G.; Roje, Z.; Vrcek, I.V.; Panderi, I.; Samanidou, V. An improved fabric-phase sorptive extraction protocol for the determination of seven parabens in human urine by HPLC-DAD. Biomed. Chromatogr. 2021, 35, e4974. [Google Scholar] [CrossRef]
- Chen, L.X.; Wang, X.Y.; Lu, W.H.; Wu, X.Q.; Li, J.H. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef] [PubMed]
- Alexander, C.; Andersson, H.S.; Andersson, L.I.; Ansell, R.J.; Kirsch, N.; Nicholls, I.A.; O’Mahony, J.; Whitcombe, M.J. Molecular imprinting science and technology: A survey of the literature for the years up to and including 2003. J. Mol. Recognit. 2006, 19, 106–180. [Google Scholar] [CrossRef]
- Ashley, J.; Shahbazi, M.A.; Kant, K.; Chidambara, V.A.; Wolff, A.; Bang, D.D.; Sun, Y. Molecularly imprinted polymers for sample preparation and biosensing in food analysis: Progress and perspectives. Biosens. Bioelectron. 2017, 91, 606–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speltini, A.; Scalabrini, A.; Maraschi, F.; Sturini, M.; Profumo, A. Newest applications of molecularly imprinted polymers for exraction of contaminants from environmental and food matrices: A review. Anal. Chim. Acta 2017, 974, 1–26. [Google Scholar] [CrossRef]
- Lowdon, J.W.; Eersels, K.; Rogosic, R.; Heidt, B.; Dilien, H.; Redeker, E.S.; Peeters, M.; van Grinsven, B.; Cleij, T.J. Substrate displacement colorimetry for the detection of diarylethylamines. Sens. Actuator B Chem. 2019, 282, 137–144. [Google Scholar] [CrossRef]
- Feng, J.W.; Tao, Y.; Shen, X.L.; Jin, H.; Zhou, T.T.; Zhou, Y.S.; Hu, L.Q.; Luo, D.; Mei, S.R.; Lee, Y.I. Highly sensitive and selective fluorescent sensor for tetrabromobisphenol-A in electronic waste samples using molecularly imprinted polymer coated quantum dots. Microchem. J. 2019, 144, 93–101. [Google Scholar] [CrossRef]
- Wang, J.X.; Dai, J.D.; Xu, Y.Q.; Dai, X.H.; Zhang, Y.L.; Shi, W.D.; Sellergren, B.; Pan, G.Q. Molecularly Imprinted Fluorescent Test Strip for Direct, Rapid, and Visual Dopamine Detection in Tiny Amount of Biofluid. Small 2019, 15, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silverio, O.V.; So, R.C.; Elnar, K.J.S.; Malapit, C.A.; Nepomuceno, M.C.M. Development of dieldrin, endosulfan, and hexachlorobenzene-imprinted polymers for dye-displacement array sensing. J. Appl. Polym. Sci. 2017, 134, 11. [Google Scholar] [CrossRef]
- Chen, K.; Fu, Q.Q.; Ye, S.Y.; Ge, J.P. Multicolor Printing Using Electric-Field-Responsive and Photocurable Photonic Crystals. Adv. Funct. Mater. 2017, 27, 1702825. [Google Scholar] [CrossRef]
- Ge, J.P.; Yin, Y.D. Responsive Photonic Crystals. Angew. Chem. Int. Edit. 2017, 50, 1492–1522. [Google Scholar] [CrossRef]
- Hou, J.; Li, M.Z.; Song, Y.L. Recent advances in colloidal photonic crystal sensors: Materials, structures and analysis methods. Nano Today 2018, 22, 132–144. [Google Scholar] [CrossRef]
- Wang, H.F.; Gupta, S.K.; Xie, B.Y.; Lu, M.H. Topological photonic crystals: A review. Front Opto-Electron. 2020, 13, 50–72. [Google Scholar] [CrossRef]
- Joannopoulos, J.D.; Villeneuve, P.R.; Fan, S. Photonic crystals: Putting a new twist on light. Nature 1997, 386, 143–149. [Google Scholar] [CrossRef]
- Fenzl, C.; Hirsch, T.; Wolfbeis, O.S. Photonic Crystals for Chemical Sensing and Biosensing. Angew. Chem. Int. Edit. 2014, 53, 3318–3335. [Google Scholar] [CrossRef]
- Xu, J.S.; Shang, M.; Liu, J.; Chen, X.; Cao, Y.H. Simultaneous self-assembly of molecularly imprinted magnetic nanoparticles to construct a magnetically responsive photonic crystals sensor for bisphenol A. Sens. Actuator B Chem. 2021, 338, 129858. [Google Scholar] [CrossRef]
- Chiappini, A.; Pasquardini, L.; Bossi, A.M. Molecular Imprinted Polymers Coupled to Photonic Structures in Biosensors: The State of Art. Sensors 2020, 20, 5069. [Google Scholar] [CrossRef]
- Wang, X.H.; Chen, G.; Dong, Z.Q.; Zhu, Z.G.; Chen, C. Progress in molecular imprinted photonic crystals. Cailiao Gongcheng 2020, 48, 60–72. [Google Scholar] [CrossRef]
- Lin, Z.Z.; Li, L.; Fu, G.Y.; Lai, Z.Z.; Peng, A.H.; Huang, Z.Y. Molecularly imprinted polymer-based photonic crystal sensor array for the discrimination of sulfonamides. Anal. Chim. Acta 2020, 1101, 32–40. [Google Scholar] [CrossRef]
- Rizvi, A.S.; Murtaza, G.; Yan, D.; Irfan, M.; Xue, M.; Meng, Z.H.; Qu, F. Development of Molecularly Imprinted 2D Photonic Crystal Hydrogel Sensor for Detection of L-Kynurenine in Human Serum. Talanta 2020, 208, 120403. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.Z.; Chen, W.M.; Luo, Y.L.; Wang, Y.L.; Wang, Y.L.; Guo, H.S. Highly sensitive alpha-amanitin sensor based on molecularly imprinted photonic crystals. Anal. Chim. Acta 2020, 1093, 142–149. [Google Scholar] [CrossRef]
- Sai, N.; Wu, Y.T.; Sun, Z.; Yu, G.G.; Huang, G.W. A novel photonic sensor for the detection of chloramphenicol. Arab. J. Chem. 2019, 12, 4398–4406. [Google Scholar] [CrossRef] [Green Version]
- Hammoud, A.; Chhin, D.; Nguyen, D.K.; Sawan, M. A new molecular imprinted PEDOT glassy carbon electrode for carbamazepine detection. Biosens. Bioelectron. 2021, 180, 113089. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, Z.K.; Zeng, Q.S.; Huang, Y.M.; Gu, H.; He, J.H.; Liu, Y.Y.; Chen, S.L.; Sun, H.; Lai, J.P. Visual test for the presence of the illegal additive ethyl anthranilate by using a photonic crystal test strip. Microchim. Acta 2019, 186, 685. [Google Scholar] [CrossRef]
- Chen, S.L.; Sun, H.; Huang, Z.J.; Jin, Z.K.; Fang, S.Y.; He, J.H.; Liu, Y.Y.; Zhang, Y.; Lai, J.P. The visual detection of anesthetics in fish based on an inverse opal photonic crystal sensor Electronic supplementary information (ESI) available. RSC Adv. 2019, 9, 16831–16838. [Google Scholar] [CrossRef] [Green Version]
- Turner, N.W.; Piletska, E.V.; Karim, K.; Whitcombe, M.; Malecha, M.; Magan, N.; Baggiani, C.; Piletsky, S.A. Effect of the solvent on recognition properties of molecularly imprinted polymer specific for ochratoxin A. Biosens. Bioelectron. 2004, 20, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Haupt, K.; Dzgoev, A.; Mosbach, K. Assay system for the herbicide 2,4-dichlorophenoxyacetic acid using a molecularly imprinted polymer as an artificial recognition element. Anal. Chem. 1998, 70, 628–631. [Google Scholar] [CrossRef]
- Fan, J.; Qiu, L.L.; Zheng, W.X.; Meng, Z.H.; Xue, M.; Qiao, Y. Rapid self-assembly preparation of p-nitrophenol-molecular imprinted photonic crystal sensors. Microchem. J. 2021, 164, 105950. [Google Scholar] [CrossRef]
- Dehghani, Z.; Akhond, M.; Absalan, G. Carbon quantum dots embedded silica molecular imprinted polymer as a novel and sensitive fluorescent nanoprobe for reproducible enantioselective quantification of naproxen enantiomers. Microchem. J. 2021, 160, 105723. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Gu, H.; He, J.; Cui, A.; Wu, X.; Lai, J.; Sun, H. Rapid Screening of Butyl Paraben Additive in Toner Sample by Molecularly Imprinted Photonic Crystal. Chemosensors 2021, 9, 314. https://doi.org/10.3390/chemosensors9110314
Liu Y, Gu H, He J, Cui A, Wu X, Lai J, Sun H. Rapid Screening of Butyl Paraben Additive in Toner Sample by Molecularly Imprinted Photonic Crystal. Chemosensors. 2021; 9(11):314. https://doi.org/10.3390/chemosensors9110314
Chicago/Turabian StyleLiu, Yangyang, Hang Gu, Jiahua He, Anqi Cui, Xiaoyi Wu, Jiaping Lai, and Hui Sun. 2021. "Rapid Screening of Butyl Paraben Additive in Toner Sample by Molecularly Imprinted Photonic Crystal" Chemosensors 9, no. 11: 314. https://doi.org/10.3390/chemosensors9110314
APA StyleLiu, Y., Gu, H., He, J., Cui, A., Wu, X., Lai, J., & Sun, H. (2021). Rapid Screening of Butyl Paraben Additive in Toner Sample by Molecularly Imprinted Photonic Crystal. Chemosensors, 9(11), 314. https://doi.org/10.3390/chemosensors9110314




